SUMMARY
Heparan sulfates (HS) are extraordinarily complex extracellular sugar molecules that are critical components of multiple signaling systems controlling neuronal development [1-3]. The molecular complexity of HSs arises through a series of specific modifications, including sulfations of sugar residues and epimerizations of their glucuronic acid moieties. The modifications are introduced non-uniformly along protein-attached HS polysaccharide chains by specific enzymes (Fig.1A)[4]. Genetic analysis has demonstrated the importance of specific HS modification patterns for correct neuronal development [2, 3]. However, it remains unclear whether HS modifications provide a merely permissive substrate or whether they provide instructive patterning information during development. We show here with single cell resolution that highly stereotyped motor axon projections in C. elegans depend on specific modification patterns of HS present in the extracellular environment. We show that by manipulating extracellular HS modification patterns we can cell-specifically re-route axons, indicating that HS modifications are instructive. This axonal re-routing is dependent on the HS core protein lon-2/glypican and the axon guidance cue slt-1/Slit as well as its receptor eva-1. These observations suggest that a changed sugar environment instructs slt-1/Slit-dependent signaling via eva-1 to redirect axons. Our experiments provide genetic in vivo evidence for the “HS code” hypothesis which posits that specific combinations of HS modifications provide specific and instructive information to mediate the specificity of ligand/receptor interactions [3, 5, 6].
RESULTS
Distinct HS modification patterns are required by individual ventral cord motor neurons for correct axon pathfinding
The DA and DB classes of motor neurons are embryonically generated motor neurons (eMNs) in the ventral nerve cord (VNC) of C. elegans. Individual DA and DB motor neurons make differential, completely invariant choices as to whether to extend circumferentially along the right or left side of the animal (Fig.1B-D). This sidedness does not correlate with the membership of a motor neuron to a specific class (e.g. 2 DA neurons extend along the right side, and 7 DA neurons extend to the left side; Suppl. Table 1), nor position along the a/p axis (Fig.1B; Suppl. Table 1), lineage history (Suppl. Table 1) or any other unique cellular landmark in their environment (Suppl. Table 1). Intriguingly, there are no molecular markers that distinguish individual DA neurons or individual DB neurons from one another (i.e. no genes are known that are expressed in DB2 but not DB3) and no genes have been reported which control the cell-type specificity of the projection patterns. To test whether individual DA/DB motor neurons require distinct HS modifications for correct development, we genetically removed three major HS modifying enzymes, the C5-epimerase encoded by hse-5, the 6O-sulfotransferase, encoded by hst-6 and the 2O-sulfotransferase, encoded by hst-2 (Fig.1A, E). Since none of these enzymes have paralogs in C. elegans, we can assume that animals with null mutations in these genes completely lack C5-epimerization, 6O-sulfation or 2O-sulfation [7]. Removal of C5-epimerization or 2O-sulfation each result in mild defects in the sidedness of motor axon projections [7]. These mild defects show cell-type specificity with DA2, DB3, DB6 and DB7 being most affected by loss of 2O-sulfation (Fig.1E). Generation of double mutants as well as a triple mutant resulted in significantly enhanced mutant phenotypes, revealing cell-type specific redundancies in the requirement of HS modifications for individual motor axons (Fig.1F). For example, the DA2, DB3, and DB6 motor neurons display significantly enhanced defects in the hst-2 hst-6 and hse-5; hst-6 double mutant combinations, indicating that hst-6 function is redundant with both hse-5 and hst-2 in these motor neurons. However, in DA6 motor neurons hst-6 function is redundant with hse-5 but not with hst-2 while conversely, in DB5 motor neurons hst-6 is redundant with hst-2 but not with hse-5 (Fig.1F). The HS modification enzymes hst-6 and hst-2/hse-5 appear to act in different tissues during neuronal development with hst-6 acting in neuronal tissues and, hse-5/hst-2 in hypodermal tissues [7]. Thus, the observation that hst-6 and hse-5/hst-2 serve redundant functions in some neurons may seem non-intuitive given their disparate sites of action. However, HS has been demonstrated to act cell nonautonomously in for example ectodermal/mesodermal interactions [8]. It is therefore conceivable that differentially modified HS on neuronal and hypodermal tissues cooperate in a redundant manner to control axon routing.
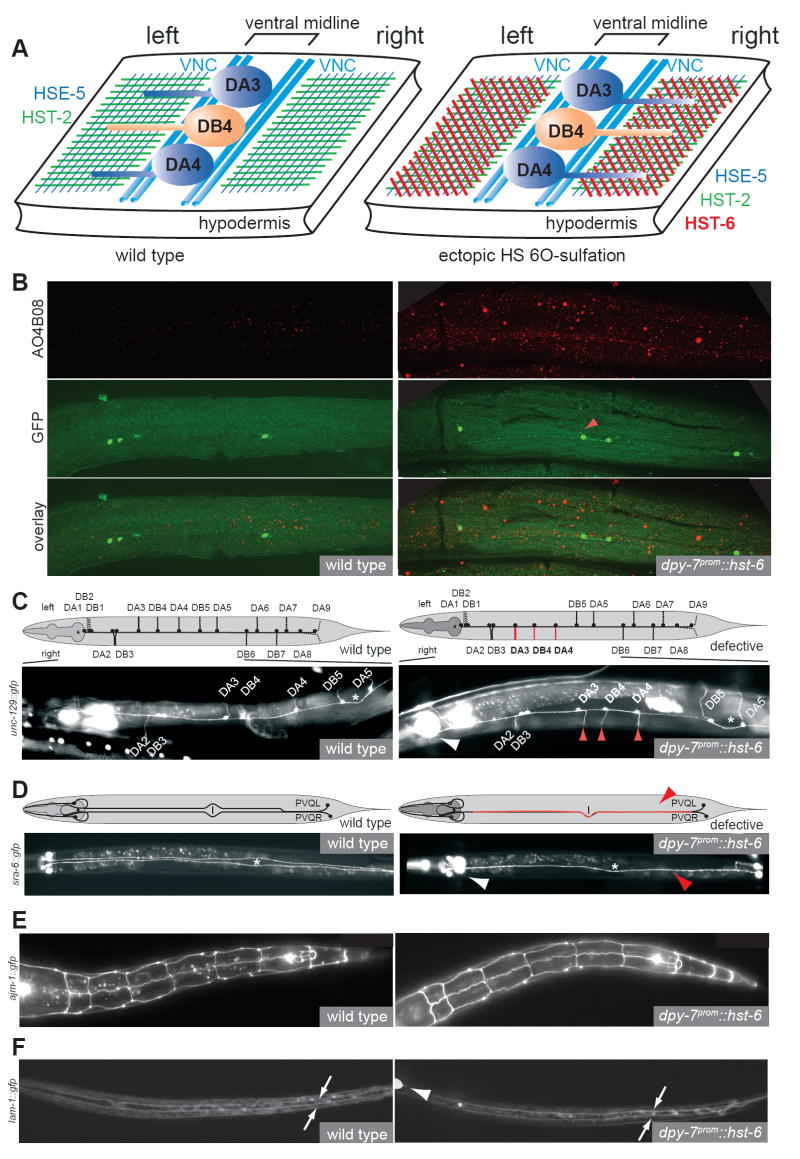
Fig.1. Individual DA/DB motor neurons require distinct combinations of HS modifications.
A: Heparan sulfate is a polymer composed of highly modified disaccharide repeats that is attached to a core protein. Shown is a characteristic HS disaccacharide repeat unit consisting of a hexuronic acid (circle) and a glucosamine (hexagon) residue and enzymes that modify this structure. Large blue vs. black circles indicate iduronic acid vs. glucuronic acid, respectively. Small circles in black, green and, red indicate modifications as color coded in the disaccharide repeat. HSE-5: HS C5 epimerase; HST-X: HS XO-sulfotransferase (X=2, 6).
B: The upper panel shows the circumferential axonal trajectory of exemplary DA, DB and DD-type eMNs. eMNs are distinguished by their axonal projection patterns and by the neurotransmitter, which they express. The lower panel shows a schematic “open book” representation of the 2- to 3-fold embryonic stage when eMN axon outgrowth occurs, illustrating that individual DA, DB eMNs show a distinct sidedness of circumferential axonal growth. The eMN cell bodies shown here lie on top of the junctions between bilaterally symmetry P1/2, P3/4, P5/6, P7/8, P9/10 and P11/12 hypodermal cells. More anteriorly and posteriorly positioned eMNs are not shown (see Experimental Procedures). The only other axon that populates the VNC at that stage is the axon of the AVG pioneer neuron. See Supplementary Table 1 for a systematic comparison of individual motor neuron class members.
C: Visualizing the DA and DB motor axons with an unc-129∷gfp expressing transgene.
D: The left/right asymmetric, circumferential DA, DB axon projection patterns are highly stereotyped. We focus our scoring on the DA2 to DA6 and DB3 to DB7 motor neurons since those express the gfp reporter transgene most reliably. “% defect.” indicates percentage of animals with a defective sidedness of the respective DA or DB axon as indicated.
E: Loss of HS modifications affect DA and DB axon choices in a cell-type specific manner. All mutants used are null mutants [7, 30]. Single mutants were statistically compared to wild type (see D.); ns = not significantly different; * P<0.05, ** P<0.005.
F: Comparison of effects of single, double and, triple HS mutants on specific motor axons. Double mutants were statistically compared to the more severe of two respective single mutants; ns = not significantly different; * P<0.05, ** P<0.005, *** P<0.0005. The defects in both DA2 and DB3 are weakly statistically significant when comparing the triple hst-2 hst-6 hse-5 mutant and the syndecan null mutant (p=0.04 and 0.03, respectively). However, these differences are not significant between the hst-2 hst-6 double mutant and either the sdn-1 mutant or the hst-2 hst-6 hse-5 triple mutant suggesting that the phenotype of the sdn-1 and the hst-2 hst-6 hse-5 triple mutant is very similar. See methods for statistics.
In contrast, the defects in hse-5; hst-2 double mutants are no more severe than the defects in the most severe (hst-2) of the single mutants (Fig.1F). This last finding is consistent with analyses in other cellular contexts that demonstrate that hse-5 and hst-2 can genetically act in the same pathway [7]. Finally, no further enhancement is observed in the triple mutant combination compared to the hst-2 hst-6 double null mutant (Fig.1F). We conclude that individual members of the same class of motor neurons (DA or DB) that make apparently identical guidance choices at the ventral midline of C. elegans are dependent on different combinations of HS modifications.
Removal of the HS core protein syndecan (sdn-1) recapitulates the defects observed in hse-5; hst-2 hst-6 triple mutants, both in terms of penetrance and cell-type specificity (Fig.1E, F). In contrast, neither null mutations in the two C. elegans glypican HS core proteins (gpn-1 and lon-2; [9, 10]) nor mutations in the secreted HS core proteins unc-52 (perlecan; [11]) or cle-1 (collagen XVIII; [12]) display significant defects in eMN left/right projection patterns (Suppl. Fig.1A). These results suggest that the majority of HS that controls DA/DB motor axon choices may be attached to the syndecan core protein.
Manipulating HS modification patterns redirect motor axon projections
Our present work with DA/DB motor neurons together with previous work shows that the function of HS requires their extensively modified polysaccharide chains (Fig.1A)(reviewed in [3, 13]). However, it is unclear whether HS modifications constitute a merely permissive environment or whether they are sufficient to guide growing axons. The question of permissiveness versus instructiveness represents one of the key problems in the HS field [3, 13].
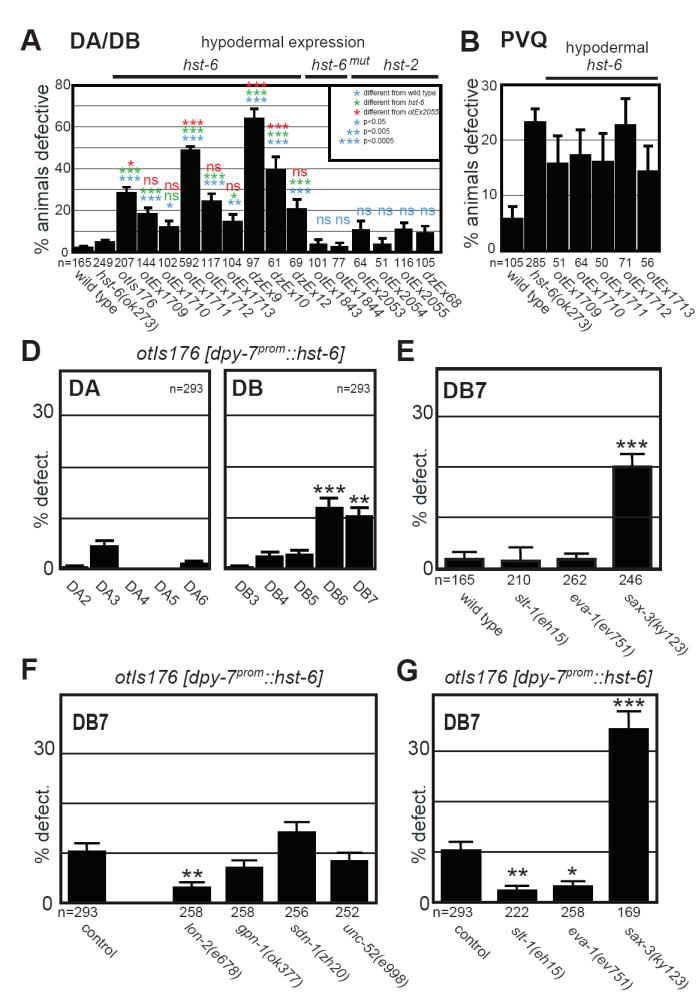
To address whether HS modifications are sufficient to guide axons, i.e. serve instructive functions, we manipulated HS modification patterns by inducing ectopic 6O-sulfation in the hypodermal cells that underlie the DA/DB motor neurons (Fig.2A). The 6O-sulfotransferase hst-6 does not appear to be expressed in hypodermal cells [7](Fig.2A) and as shown above, DA/DB axon choices are unaffected by removal of hst-6 alone (Fig.1E). To alter HS modification patterns presented by hypodermal cells, we generated multiple transgenic C. elegans lines that aberrantly express hst-6 in an otherwise wild-type background under control of the hypodermis-specific dpy-7 promoter [14]. Worms expressing this construct show an altered status of HS modification patterns. First, we detect novel staining patterns with an antibody that recognizes HS epitopes comprising specific oligosaccharides rather than individual modifications (Fig.2B) [15]. This antibody labels the anterior intestine and, possibly some neurons and basement membranes in wild-type animals (not shown). However, increased staining is observed consistently in the hypodermis upon misexpression of hst-6 in this tissue. This increased staining pattern is not observed in non-transgenic but otherwise isogenic controls (Fig.2B). And second, biochemical analyses of HS from transgenic worms expressing hst-6 in the hypodermis display an increase of 6O-sulfated relative to 2O-sulfated HS disaccharides (Suppl. Fig.2). Each transgenic line shows a significant number of animals in which the projections of individual DA and DB motor neurons are re-routed (Fig.2C, 3A; Suppl. Fig.3). These defects are not the result of apparent changes in cell fate or structure of hypodermal cells misexpressing hst-6 (Fig.2E) nor are they due to changes in eMN positioning in the VNC (data not shown). Moreover, we cannot detect obvious disruptions of the basement membranes surrounding eMNs and therefore consider it unlikely that ectopic hst-6 expression grossly disrupts midline structures (Fig.2F).
Fig.2. Manipulating HS modification patterns redirects motor axons.
A: Schematic view of the ventral cord with HS modifications in the hypodermis indicated by colored hatchings. Note that unlike in larvae, the embryonic midline, defined as a physical barrier between the left and right ventral fascicles, is not constituted by a hypodermal evagination but by motor neuron cell bodies [31].
B: Immunostaining with the AO4B08 HS specific antibody [15] of transgenic animals, which ectopically express the HS modification enzyme hst-6 in the hypodermis (right panels; otIs176). Increased hypodermal staining observed in transgenic animals. This increased staining is not seen in isogenic controls, which do not ectopically express hst-6 (left panels). Intestinal staining (not shown) serves as an internal staining control as does an unrelated synthetic antibody (MPB49), which shows no staining at all. Note that the antibody AO4B08 does not recognize individual HS modifications but rather an oligosaccharide, which requires the activities of both, hst-2 and hst-6 [15]. Since only hst-2 but not hst-6 appears to be expressed in the hypodermis [7], the observed staining of the hypodermis following misexpression of hst-6 in this tissue are consistent with the known specificities of AO4B08. Note the aberrant projection of DB6 in the hst-6 misexpressing line (red arrowhead).
C: Axonal misrouting of DA/DB motor neurons in animals with ectopic hst-6 expression in the hypodermis (right panel, otEx1711). Dashed lines indicate neurons that were not scored and, affected neurons are in red. See Fig.3 and Suppl. Fig.3 for quantification of defects.
D: Midline crossover defects of PVQ interneurons in animals with ectopic hst-6 expression in the hypodermis (right panel, otEx1711).
E: Hypodermal cell fate as well as cell morphology, as visualized by normal AJM-1 expression and localization patterns, is not visibly affected by ectopic hst-6 expression (right panel, otIs176).
F: Basement membrane topology, as visualized by a lam-1∷gfp reporter (kind gift of David Sherwood)[32], is not visibly affected by ectopic hst-6 expression (right panel, otIs176). Arrows point to the basement membrane that ensheathes the ventral midline, and which visually appears most concentrated on each side of the midline as a result of optical sectioning. Left panels represent wild type controls in all cases. White arrowheads point to pharyngeal expression of gfp denoting the presence of the hypodermal hst-6 misexpression array and red arrowheads indicate axonal routing errors. All views are ventral aspects with anterior to the left.
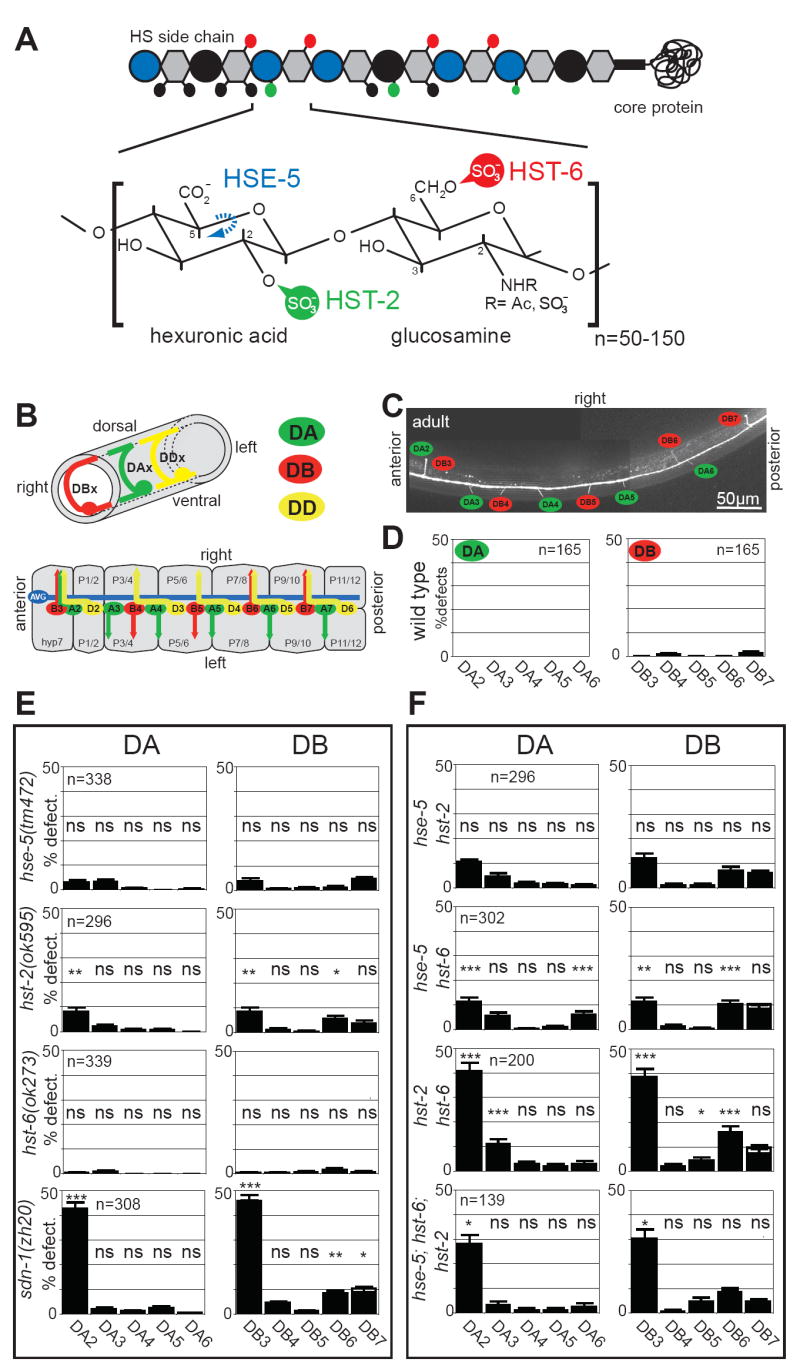
Fig.3. Genetic analyses of hst-6-dependent axonal misrouting phenotypes.
A: Quantification of DA/DB motor neuron defects in different transgenic strains that express the indicated constructs from the hypodermal specific dpy-7 promoter. Shown is the percentage of animals with any number (≥1 per animal) of DA or DB axon sidedness defects.
B: Quantification of midline cross over defects in PVQ interneurons in different transgenic strains that express hst-6 from the hypodermal specific dpy-7 promoter.
C: Effect of ectopic hst-6 expression on individual DA and DB neurons. One representative line is shown here, 5 more extrachromosomal lines are shown in Suppl. Fig.2B. Shown is the percentage of animals with defects in individual DA/DB motor axons projections as indicated.
D: Defects in DB7 motor axon projection patterns in different mutants of the Slit/Robo signaling pathway as indicated. Shown is the percentage of animals with defects in DB7 motor axon projections.
E: Effect of genetic removal of HS core proteins on projection errors in DB7 motor axons as a result of ectopic hst-6 expression. Shown is the percentage of animals with defects in DB7 motor neuron projections. Statistic comparisons are made to the transgenic control. The projection pattern of DB7 in animals that misexpress hst-6 in the hypodermis and are also mutant for lon-2, is statistically indistinguishable from non-transgenic, wild-type animals.
F: Effect of removal of genes in the Slit/Robo signaling pathway on projection errors in DB7 motor axons as a result of ectopic hst-6 expression. Shown is the percentage of animals with defects in DB7 motor axon projections. Statistic comparisons are made to the transgenic control. The projection pattern of DB7 in animals that misexpress hst-6 in the hypodermis and are also mutant for slt-1 or eva-1, is statistically indistinguishable from non-transgenic, wild-type animals. Statistical significance is indicated by: ns, not significantly different, * P<0.05, ** P<0.005, *** P<0.0005 in all panels.
The axonal misrouting defects we observe are the result of hst-6 activity as the introduction of a premature stop codon into the hst-6 expression construct abolishes its ability to induce axon misrouting (Fig.3A). Furthermore, no comparable misrouting defects can be observed upon dpy-7prom-driven overexpression of the HS 2O-sulfotransferase hst-2 (Fig.3A), which is normally expressed in both neuronal and hypodermal tissues [7]. These hst-2 expressing transgenes are functional: first, the transgenes are able to effectively rescue neuronal hst-2 loss of function phenotypes (Suppl. Fig.4A); second, using an antibody recognizing specific HS epitopes we detect increased staining in transgenic, hst-2-overexpressing strains (Suppl. Fig.4B); third, biochemical analyses of hst-2 overexpressing strains display increased 2O-sulfation relative to 6O-sulfation compared to wild type (Suppl. Fig.2). Together, our results argue that the effects of ectopic hst-6 expression are due to the regiospecific addition of a sulfate moiety to the 6O-hydroxyl group of the glucosamine residue, rather than the unspecific addition of a negative charge.
Examining the axon choices of individual DA and DB motor axons, we find that the defects induced by ectopic 6O-sulfation are cell-type specific with all different transgenic lines displaying qualitatively similar misrouting patterns. Interestingly, there seems to be a graded response of DB neurons to misexpressed hst-6 with the more posterior neurons being more strongly affected (Fig.3C; Suppl. Fig.3). In contrast, within the DA class of eMNs only DA3 exhibits significant defects. Ectopic hypodermal 6O-sulfation also causes axon guidance defects in a selected number of other neurons in the nervous system. For example, axon midline guidance of the PVQ interneurons is affected (Fig.2D, 3B), while many neurons, such as touch neurons, amphid, phasmid and, RMEV neurons display no apparent defects (Suppl. Table 2). Notably, PVQ interneurons are also affected by loss of endogenous, neuronally acting hst-6 [7]. The addition of 6O-sulfation in the wrong location (hypodermis) can therefore have the same detrimental effect for an axon as not having 6O-sulfation in the correct place (neurons). Taken together, manipulating HS modifications in the hypodermis through addition of novel HS modification patterns can redirect axonal projections of individual neurons.
Ectopic 6O-sulfation-induced motor axon misrouting requires Slit/slt-1 and its co-receptor eva-1
Most DA/DB motor neurons that respond to ectopic HS 6O-sulfation require hst-6 for normal development in the absence of hst-2 function (compare Fig.1E, F and 3C). The exception is DB7 suggesting that DB7 axonal projections are normally independent of 6O-sulfated HS. However, upon hst-6 expression in the hypodermis, DB7 is misrouted suggesting that DB7 can be positively instructed by ectopic 6O-sulfated HS. We thus focus our genetic analyses here on the DB7 motor neuron.
We first asked which HS core protein would bear the ectopic 6O-sulfated HS presented by the hypodermis that misdirects the DB7 motor neuron. To this end, we systematically removed the genes coding for four canonical HS core proteins in the C. elegans genome, lon-2/glypican, gpn-1/glypican, sdn-1/syndecan, or unc-52/perlecan, respectively, and tested the effect on DB7 motor neuron projection patterns in animals expressing hst-6 in the hypodermis. We find that removal of lon-2/glypican but neither of the other HS core proteins suppresses the axonal misrouting of DB7 to a level that is statistically indistinguishable from wild type (Fig. 3E). As lon-2/glypican normally acts in the hypodermis [10], our data suggests that the ectopic HS structures in the hypodermis that redirect DB7 may be attached to the lon-2/glypican HS core protein.
We next sought to address how alterations in HS modification patterns exert an impact on the DB7 motor axon guidance decision. As HS may play a role in determining ligand/receptor binding specificities in vitro (reviewed in [3]), we reasoned that changing HS modification patterns may reprogram the responsiveness of motor axons to defined cues. To address this possibility, we asked whether a specific axon guidance cue is required for the misrouting of individual motor axons induced by ectopic 6O-sulfation. The guidance cue slt-1/Slit is known to require different HS modifications in distinct cellular contexts in C. elegans [7]. Slit-mediated axon growth at the fly midline and, in vertebrate tissue explants are also known to require the presence of HS, albeit of unknown modification status [16-18]. The ligand slt-1/Slit signals through the sax-3/Robo of the Robo family of guidance receptors [19]. More recently, EVA-1, a conserved transmembrane protein with extracellular domains that display similarity to sugar binding domains has been identified as an additional Slit/SLT-1 receptor that is required for Slit/SLT-1 signaling and physically interacts with SLT-1 [20].
DA/DB motor neurons and in particular DB7 do not require slt-1 or eva-1 to make their correct guidance decision at the midline while its canonical receptor sax-3/Robo is involved in routing all DA/DB motor neurons (Fig.3D, Suppl. Fig.1B). These findings are consistent with previous findings demonstrating that sax-3/Robo has functions that are independent of its canonical ligand slt-1/Slit [7, 19] and imply that this slt-1-independent function of sax-3/Robo is likewise independent of eva-1. Intriguingly, loss of slt-1 significantly suppresses the axonal projection defects in DB7 as a result of ectopically expressing hst-6 in the hypodermis (Fig.3F). This suppression does not result from changes in expression levels of either slt-1 or its canonical receptor sax-3 (Suppl. Fig.5). Thus a possible interpretation of our data is that ectopic 6O-sulfated HS attached to lon-2/glypican instructs slt-1 to misroute the DB7 motor axon by possibly promoting the interaction of SLT-1 with a cognate receptor on motor neurons, thereby misrouting an extending axon.
We next asked whether the slt-1/Slit receptor eva-1 could serve as this cognate receptor in the context of DB7 misrouting. Indeed, genetic removal of eva-1 significantly suppresses the hst-6-dependent axonal misrouting of DB7 (Fig.3F). With eva-1 expressed in eMNs [20], this genetic data is in accord with a scenario where HS of a specific modification status instructs slt-1 to interact with eva-1 leading to an axonal misrouting response of DB7. The misrouting of DB7 must be largely sax-3-independent because the penetrance of defects in the hst-6 misexpression strain (10.2%) and sax-3 loss of function animals (19.8%) are additive in the strain carrying the hst-6-misexpressing transgene in conjunction with the sax-3 null mutation (34.3%)(compare Fig.3D and F). This last finding implies potential sax-3-independent functions for eva-1.
DISCUSSION
Our analysis of the projection patterns of embryonic motor axons in C. elegans reveals that superficially similar neuron types are programmed to extend their axons in distinct directions through diverse mechanisms. In addition to a large number of guidance cues and intracellular signaling molecules, which are involved in patterning motor axon projections at the worm midline (data not shown), HS modifications play a crucial role in determining the projection patterns of individual motor axons. Our findings indicate that HS modifications have specific and instructive functions, likely through mediating specific ligand/receptor interactions (Fig.4).
Fig.4. A model for the role of HS in redirecting axonal projections.
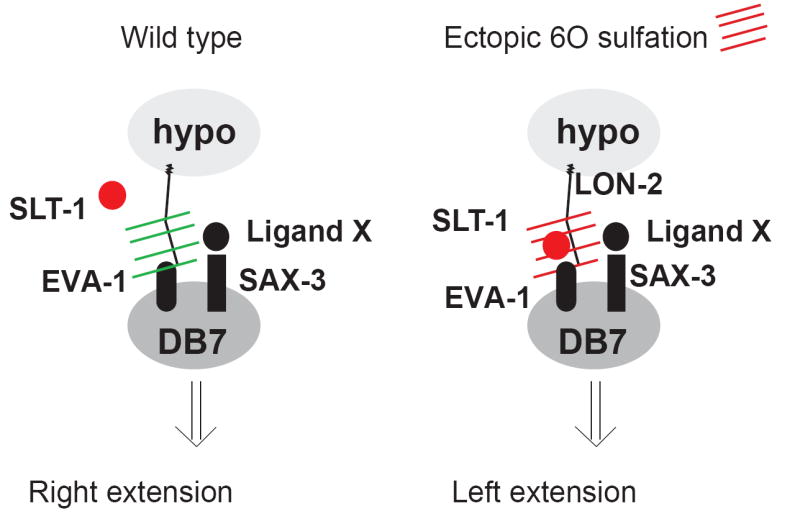
A possible interpretation of the genetic data presented in Fig.3. In wild-type animals, slt-1 or eva-1 is not required for DB7 neuron projections (as slt-1 and eva-1 mutants show no axonal defects; Fig.3D), while sax-3 does normally have a role in DB7 axon extension (Fig.3D). In wild-type animals, SAX-3 may therefore be interacting with an as yet unknown ligand X. Upon introducing ectopic 6O-sulfation patterns (red hatches), an interaction of the SLT-1 protein with EVA-1 may be promoted, which supersedes the effect normally induced by SAX-3/Ligand X. We emphasize that these conclusions are based on purely genetic arguments and that more indirect mechanistic models can be envisioned which are not shown here.
This interpretation takes the broadly accepted concept into account that extracellular HS molecules not only promote, but are often essential for the activity of specific ligand/receptor systems [3]. In support of this notion, in vitro experiments suggest that differentially modified HS can promote signaling through distinct signaling systems [21, 22]. Together with our finding that both the removal of enzymes that modify HS molecules as well as the ectopic addition of HS modifications through misexpression of a HS modifying enzyme causes misrouting defects, we propose that the local modification status of HS in the vicinity of extending motor axons impinges on which ligand/receptor system is engaged (Fig.4). This model is consistent not only with the effects observed upon removal of HS modifying enzymes, but also with the genetic analyses of animals with ectopic expression of hst-6 in the hypodermis. In this scenario, the introduction of a novel HS modification pattern which is attached to a specific core protein in the hypodermis (LON-2) aberrantly promotes the interaction of the repulsive SLT-1 cue with a receptor molecule, possibly EVA-1, thereby causing a mis-orientation of motor axon pathfinding (Fig.4). This is supported by our genetic data demonstrating that genetic removal of lon-2, slt-1 or eva-1 suppresses the misrouting defect in DB7 to levels that are statistically indistinguishable from wild type (Fig.3D-F). However, we note that this model is not the only way to interpret the genetic interaction of ectopic hst-6 with lon-2, slt-1, or eva-1. Indirect effects are conceivable and can not be ruled out by genetic analysis alone.
With this caveat in mind, the finding that ectopic expression of an HS modifying enzyme and the dependence of the resulting phenotype on a specific axon guidance molecule (slt-1) and one of its receptors (eva-1) provides additional support for a concept in which the enormous molecular complexity embedded with HS molecules may carry not merely permissive but also instructive information content. It has been speculated before that the molecular diversity of HS molecules could provide a code that regulates the regiospecific activity of individual axon patterning systems [3, 5-7]. Specifically, in the HS code hypothesis regiospecific HS modification patterns determine the responsiveness of neurons to individual ligand/receptor patterning cues. In accordance with this hypothesis, the removal of individual modifications cause highly specific nervous system patterning defects [7, 23-25](this study). Furthermore, we show that the removal of combinations of HS modifications uncovers cell-specific redundancies in the requirements for HS modifications (Fig.2), indicating that the presumptive HS code may be degenerate. And lastly, as pointed out previously [13], an important additional criterion for the existence of such a code is that HS modifications act instructively, and not merely as permissive co-factors. A genetic test to determine the difference between instructiveness and permissiveness is to ask whether a given gene not only displays a specific loss of function phenotype, but whether a gain-of-function phenotype of the gene upon misexpression can also instruct a novel phenotype [26]. Keeping in mind that these experiments provide genetic rather than biochemical evidence for the action of HS, our results clearly show that we can redirect the DB7 neuron upon misexpression of the HS 6O-sulfotransferase hst-6. We can detect the molecular correlate of this hst-6 misexpression in the hypodermis by using both an HS specific antibody and biochemical analyses of HS disaccharides (Fig.2B, Suppl. Fig.2). Moreover, we find that this novel phenotype is dependent on a specific HS core protein (lon-2/glypican) and that aberrant 6O-sulfation appears to activate slt-1 signaling through the slt-1 receptor eva-1 (Fig.3E, F). Further biochemical studies are warranted to corroborate these genetic results and to gain mechanistic insight into how HS can misdirect axons.
EXPERIMENTAL PROCEDURES
Neuroanatomical scoring
All animals were scored at 20°C in a mixed population of mid-larval to adult stages. DA/DB were scored with unc-129∷gfp (evIs82b) and PVQ with sra-6∷gfp (oyIs14). GFP expression in the DA/DB neurons of evIs82b transgenic animals is variable in DA7, DA8 and DA9; moreover, projection patterns of DA1, DB1 and DB2 are often not easy to follow on the single cell level and we therefore excluded these neuron types from our analysis. Individual motor neurons were identified based on their cell body position and the dorsal projection patterns. In each figure, the error bars denote the standard error of proportion and asterisks indicate statistical significance: * P<0.05, ** P<0.005, *** P<0.0005; n.s. not significant. Statistical significance was calculated using the z-test. If multiple comparisons were made, p-values were subjected to the Bonferroni correction.
Transgenes
For ectopic hypodermal expression of HS modifying enzymes, the complete hst-6 and hst-2 loci were fused to the dpy-7 promoter [14] by PCR fusion [27]. For the dpy-7prom∷hst-6mut construct, the template for PCR fusion was genomic DNA from OH281 [mgIs18; lon-2(e678)hst-6(ot17)], which harbors a premature stop codon in hst-6 [28]. All constructs were injected at 2.5 ng/μl into evIs82b (Is[unc-129∷gfp]) using pceh-22∷gfp at 50 ng/μl as injection marker and pBS at 50 ng/μl as carrier.
Immunohistochemistry
Worms were prepared for immunohistochemistry using a freeze crack protocol [29] followed by 20min fixations in ice cold acetone and methanol. Worms were then incubated for 30min in PBS containing 10% donkey serum and in the same buffer at 4C over night with antibody AO4B08 or HS4C3 (1:5)[15,34]. The immunohistochemical stain was developed using the monoclonal anti-VSV antibody P5D4 (dilution 1:250) and Alexa labeled (555) donkey anti mouse antibodies (dilution 1:250).
Supplementary Material
Acknowledgments
We thank Q. Chen and M. Attreed for expert technical assistance, the CGC, J. Culotti, Y. Jin, C. Bargmann, and C. Li for providing strains, D. Hall and R. Durbin for providing embryonic electron micrographs, Rachel Freemont for initial antibody stains, A. Boyanov for EM image analysis and several colleagues, J. Culotti and members of the Hobert and Bülow labs for comments on the manuscript. This work was funded in part by the Muscle Dystrophy Association, the McKnight Foundation and by NIH grants 2R01NS039996-05 and 5R01NS050266-03 (to O.H.), 5R01HD055380 to H.E.B. and 5T32NS07098 to R.A.T.. H.E.B is an Alfred P. Sloan Fellow. O.H. is an Investigator of the HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004;5:923–935. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- 2.Van Vactor D, Wall DP, Johnson KG. Heparan sulfate proteoglycans and the emergence of neuronal connectivity. Curr Opin Neurobiol. 2006;16:40–51. doi: 10.1016/j.conb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Bülow HE, Hobert O. The Molecular Diversity of Glycosaminoglycans Shapes Animal Development. Annu Rev Cell Dev Biol. 2006 doi: 10.1146/annurev.cellbio.22.010605.093433. [DOI] [PubMed] [Google Scholar]
- 4.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 5.Turnbull J, Powell A, Guimond S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 2001;11:75–82. doi: 10.1016/s0962-8924(00)01897-3. [DOI] [PubMed] [Google Scholar]
- 6.Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem. 2000;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 7.Bülow HE, Hobert O. Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron. 2004;41:723–736. doi: 10.1016/s0896-6273(04)00084-4. [DOI] [PubMed] [Google Scholar]
- 8.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–124. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 9.Hudson ML, Kinnunen T, Cinar HN, Chisholm AD. C. elegans Kallmann syndrome protein KAL-1 interacts with syndecan and glypican to regulate neuronal cell migrations. Dev Biol. 2006;294:352–365. doi: 10.1016/j.ydbio.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Gumienny TL, MacNeil LT, Wang H, de Bono M, Wrana JL, Padgett RW. Glypican LON-2 is a conserved negative regulator of BMP-like signaling in Caenorhabditis elegans. Curr Biol. 2007;17:159–164. doi: 10.1016/j.cub.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 11.Rogalski TM, Williams BD, Mullen GP, Moerman DG. Products of the unc-52 gene in Caenorhabditis elegans are homologous to the core protein of the mammalian basement membrane heparan sulfate proteoglycan. Genes Dev. 1993;7:1471–1484. doi: 10.1101/gad.7.8.1471. [DOI] [PubMed] [Google Scholar]
- 12.Ackley BD, Kang SH, Crew JR, Suh C, Jin Y, Kramer JM. The basement membrane components nidogen and type XVIII collagen regulate organization of neuromuscular junctions in Caenorhabditis elegans. J Neurosci. 2003;23:3577–3587. doi: 10.1523/JNEUROSCI.23-09-03577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt CE, Dickson BJ. Sugar codes for axons? Neuron. 2005;46:169–172. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilleard JS, Barry JD, Johnstone IL. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol. 1997;17:2301–2311. doi: 10.1128/mcb.17.4.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurup S, Wijnhoven TJ, Jenniskens GJ, Kimata K, Habuchi H, Li JP, Lindahl U, van Kuppevelt TH, Spillmann D. Characterization of anti-heparan sulfate phage display antibodies AO4B08 and HS4E4. J Biol Chem. 2007;282:21032–21042. doi: 10.1074/jbc.M702073200. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Steigemann P, Molitor A, Fellert S, Jackle H, Vorbruggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Hu H. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci. 2001;4:695–701. doi: 10.1038/89482. [DOI] [PubMed] [Google Scholar]
- 19.Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans Slit Acts in Midline, Dorsal-Ventral, and Anterior-Posterior Guidance via the SAX-3/Robo Receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa K, Wrana JL, Culotti JG. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science. 2007;317:1934–1938. doi: 10.1126/science.1144874. [DOI] [PubMed] [Google Scholar]
- 21.Guimond SE, Turnbull JE. Fibroblast growth factor receptor signalling is dictated by specific heparan sulphate saccharides. Curr Biol. 1999;9:1343–1346. doi: 10.1016/s0960-9822(00)80060-3. [DOI] [PubMed] [Google Scholar]
- 22.Irie A, Yates EA, Turnbull JE, Holt CE. Specific heparan sulfate structures involved in retinal axon targeting. Development. 2002;129:61–70. doi: 10.1242/dev.129.1.61. [DOI] [PubMed] [Google Scholar]
- 23.Kinnunen T, Huang Z, Townsend J, Gatdula MM, Brown JR, Esko JD, Turnbull JE. Heparan 2-O-sulfotransferase, hst-2, is essential for normal cell migration in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:1507–1512. doi: 10.1073/pnas.0401591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratt T, Conway CD, Tian NM, Price DJ, Mason JO. Heparan sulphation patterns generated by specific heparan sulfotransferase enzymes direct distinct aspects of retinal axon guidance at the optic chiasm. J Neurosci. 2006;26:6911–6923. doi: 10.1523/JNEUROSCI.0505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin D, Karlsson F, Tian N, Pratt T, Bullock SL, Wilson VA, Price DJ, Mason JO. Specific modification of heparan sulphate is required for normal cerebral cortical development. Mech Dev. 2003;120:1481–1488. doi: 10.1016/j.mod.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Held LI. Imaginal Discs: The Genetic and Cellular Logic of Pattern Formation. Cambridge University Press; 2002. [Google Scholar]
- 27.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- 28.Bülow HE, Berry KL, Topper LH, Peles E, Hobert O. Heparan sulfate proteoglycan-dependent induction of axon branching and axon misrouting by the Kallmann syndrome gene kal-1. Proc Natl Acad Sci U S A. 2002;99:6346–6351. doi: 10.1073/pnas.092128099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duerr JS. T.C.e.R. Community. WormBook. WormBook; Immunohistochemistry. [Google Scholar]
- 30.Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A. Syndecan regulates cell migration and axon guidance in C. elegans. Development. 2005;132:4621–4633. doi: 10.1242/dev.02042. [DOI] [PubMed] [Google Scholar]
- 31.Durbin RM. Studies on the development and organisation of the nervous system of Caenorhabditis elegans. Cambridge, UK: University of Cambridge; 1987. p. 148. [Google Scholar]
- 32.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Toyoda H, Kinoshita-Toyoda A, Fox B, Selleck SB. Structural analysis of glycosaminoglycans in animals bearing mutations in sugarless, sulfateless, and tout-velu. Drosophila homologues of vertebrate genes encoding glycosaminoglycan biosynthetic enzymes. J Biol Chem. 2000;275:21856–21861. doi: 10.1074/jbc.M003540200. [DOI] [PubMed] [Google Scholar]
- 34.Ten Dam GB, Kurup S, van de Westerlo EM, Versteeg EM, Lindahl U, Spillmann D, van Kuppevelt TH. 3-O-sulfated oligosaccharide structures are recognized by anti-heparan sulfate antibody HS4C3. J Biol Chem. 2006;281:4654–4662. doi: 10.1074/jbc.M506357200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.