Introduction
Manual medicine treatments (MMTs) are therapeutic applications of manually guided forces to improve not only structural imbalances but also physiologic function and to support homeostasis that has been altered by somatic dysfunction, physical trauma, inflammation and infection (Andersson et al 1999, Sucher et al 2005, Sucher 1993, Jones 2004, Meltzer et al 2007). Many MMTs used clinically by osteopaths, chiropractors, physical therapists, massage therapists and Rolfers share similar --and at times identical-- biophysical maneuvers despite dissimilar nomenclature.
The most commonly utilized MMT across these disciplines is myofascial release (MFR) in which manually guided forces are applied to superficial and deep fascia to restore normal function. (Jones 2004). Reports extol favorable clinical outcome post-MFR, yet cellular and molecular mechanisms responsible for its efficacy are lacking.
Fibroblasts, the principle cell type within the fascia, are uniquely poised to serve simultaneously as the mechanosensor (possibly via activation of stretch activated calcium channels, SACC) as well as the mechanotransmitter (via secretion of pro- and anti-inflammatory cytokines) of MFR. Clinician-directed strain enhancement of ATP-dependent cytokine secretion may underlie the clinical anti-inflammatory benefits seen post-MFR such as improved range of motion (ROM), decreased edema, and reduced analgesic requirements.
Additionally, strain-mediated increases in fibroblast proliferation and subsequent chronic increased anti-inflammatory cytokine production may account clinically for long-term benefits despite short-term treatment.
Fibroblast strain direction (uni- vs. multiaxial) specifically appears key in differentiating cellular cytokine secretion and proliferative responses to MMTs, suggesting the existence of optimal strain patterns to treat a myriad of somatic dysfunctions.
Our goal is to develop, refine and improve an in vitro fibroblast culture model, useful for unraveling the cellular and molecular mechanisms responsible for manual medicine-induced improvement in cell function.
Study 1: Modeling Repetitive Motion Strain and Counterstrain (Meltzer et al 2007)
Objective
To investigate human fibroblast proliferation and interleukin secretory profiles in response to modeled repetitive motion strain (RMS) and modeled counterstrain (CS). We hypothesized that the RMS model would increase fibroblast proliferation and proinflammatory interleukin secretion, while the CS model would reverse these effects.
Methods
Cultured human fibroblasts were exposed in vitro to one of three conditions: an 8-hour RMS
a 60-second CS, or
an 8-hour RMS followed by a 60-second CS.
Data on fibroblast proliferation and interleukins present in conditioned media were obtained immediately after RMS, at 24 hours after RMS (24RMS), at 24 hours after CS (24CS), and at 24 hours after RMS and CS (24RMS+CS).
Cytokine protein array and enzyme-linked immunosorbent assay were used in data analysis. Fibroblast proliferation was measured colorimetrically with a cell proliferation assay as well as by cell counting.
Results
Fibroblasts that underwent RMS secreted several proinflammatory interleukins 24 hours after strain cessation, with substantially increased secretion of IL-1α, IL-1β, IL-2, IL-3, IL-6, and IL-16.
At 24 hours after strain cessation, fibroblasts subjected to RMS also secreted increased amounts of the anti-inflammatory IL-1ra, and they displayed 15% less proliferation, compared with baseline cells (P<.05).
Fibroblasts that underwent CS, when analyzed at 24 hours after CS, did not display increased interleukin secretion or proliferation. However, they did display a 44% reduction in proinflammatory IL-3 secretion when compared with baseline cells (P<.05). The use of 24RMS+CS did not induce interleukin secretion in fibroblasts that were analyzed 24 hours after the combined exposure. However, cells in the 24RMS+CS group did display a 46% reduction in proinflammatory IL-6 secretion compared with RMS alone (24RMS; P<.05), as well as a 51% increase in proliferation compared with the 24RMS group (P<.05) (Meltzer et al 2007).
Study #2: Is Strain Direction Important in Determining Cellular Function Outcome? (Eagan et al 2007)
Objective
Manual medicine treatments (MMTs) rely on biophysical techniques that use manually guided forces in numerous strain directions to treat injuries and somatic dysfunctions. Although clinical outcomes post-MMT are positive, the underlying cellular mechanisms responsible remain elusive. We previously described an in vitro model of strain-induced tissue injury and MMTs. Using this model, the current study sought to determine if strain direction (equibiaxial [EQUI] vs heterobiaxial [HETERO]) differentially regulates human fibroblast function.
Methods
Cultured human fibroblasts were strained EQUI at 10% beyond their resting length for 48 hours followed by assessment of cell morphology, proliferation, and cytokine secretion via protein cytokine array and enzyme-linked immunosorbent assay (ELISA). These observations were then compared with those obtained previously for HETERO fibroblasts.
Results
No alterations in cell morphology were seen in EQUI fibroblasts despite our report of such changes in HETERO cells.
Fibroblasts secretion profiles for 60 cytokines (via cytokine protein array) showed that in EQUI strained cells, fractalkine significantly increased (121%), whereas macrophage-derived chemoattractant/chemokine and pulmonary and activation-regulated chemokine significantly decreased (32% and 10%, respectively) compared with nonstrained cells (p<.05).
The EQUI fibroblasts, when compared with HETERO fibroblasts, exhibited a significant decrease in proliferation (22%), inflammatory interleukin 6 secretion (75%, measured by ELISA), and macrophage-derived chemoattractant/chemokine secretion (177%, measured by ELISA, p<.05) (Eagen et al 2007)
Conclusions
These divergent observations in HETERO vs EQUI strained fibroblasts may underlie the relative efficacies of MMTs carried out in different tissue strain directions. We are currently modeling MMTs such as myofascial release to further investigate this.
From these two studies, it is clear that strain direction, frequency and duration, impact important fibroblast physiological functions known to mediate pain, inflammation and ROM. Clinical translation of these studies is important in order to definitively identify cause and effect of manual medicine treatments.
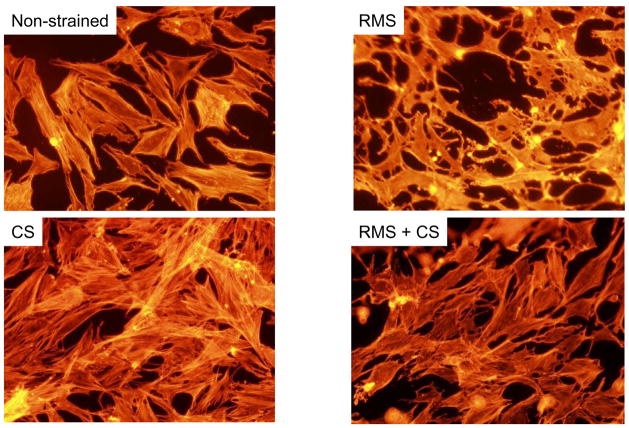
FIGURE 1.
Effects of RMS and CS on fibroblast morphology and actin stress fiber architecture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson GBJ, Lucente T, Davis AM, et al. Comparison of osteopathic spinal manipulation with standard care for patients with low back pain. N Engl J Med. 1999;341:1426–31. doi: 10.1056/NEJM199911043411903. [DOI] [PubMed] [Google Scholar]
- Eagan T, Meltzer K, Standley PR. Biophysical strain regulation of inflammatory interleukins in human fibroblasts. J Manip Physiol Therap. 2007;30(8):584–592. doi: 10.1016/j.jmpt.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Jones TA. Rolfing. Phys Med Rehab Clin N Am. 2004;15:799–809. doi: 10.1016/j.pmr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Meltzer K, Schad J, King H, Stoll S, Standley PR. In vitro Human Fibroblast Model of Repetitive Motion Strain (RMS) and Direct OMT (DOMT): Roles for Proliferation and Apoptosis. J Am Osteopath Assoc. 2007;107:527–537. [PubMed] [Google Scholar]
- Sucher BM, Hinriches RN, Welcher RL, et al. Manipulative treatment of carpal tunnel syndrome: biomechanical and osteopathic intervention to increase the length of the transverse carpal ligament: part 2. Effect of sex differences and manipulative “priming”. J Am Osteopath Assoc. 2005;105(3):135–43. [PubMed] [Google Scholar]
- Sucher BM. Myofascial release of carpal tunnel syndrome. J Am Osteopath Assoc. 1993;93(1):92–4. 100–1. [PubMed] [Google Scholar]