Abstract
Plasmodium falciparum NDH2 (pfNDH2) is a non-proton pumping, rotenone-insensitive alternative enzyme to the multi-subunit NADH:ubiquinone oxidoreductases (Complex I) of many other eukaryotes. Recombinantly expressed pfNDH2 prefers coenzyme CoQ0 as an acceptor substrate, and can also use the artificial electron acceptors, menadione and dichlorophenol–indophenol (DCIP). Previously characterized NDH2 inhibitors, dibenziodolium chloride (DPI), diphenyliodonium chloride (IDP), and 1-hydroxy-2-dodecyl-4(1H)quinolone (HDQ) do not inhibit pfNDH2 activity. Here, we provide evidence that HDQ likely targets another P. falciparum mitochondrial enzyme, dihydroorotate dehydrogenase (pfDHOD), which is essential for de novo pyrimidine biosynthesis.
Keywords: Type II NADH dehydrogenase, NDH2, Dibenziodolium chloride, DPI, Diphenyliodonium chloride, IDP, 1-Hydroxy-2-dodecyl-4(1H)quinolone, HDQ, Dihydroorotate dehydrogenase, DHOD, Ubiquinone, CoQ, CoQn
Malaria is a vector-born disease caused by a protozoan parasite of the genus Plasmodium. It accounts for 300–500 million cases each year and over a million deaths, mostly of children under the age of five in sub-Saharan Africa.1 Given the emergence and spread of resistance to current antimalarials such as chloroquine, pyrimethamine, sulfadoxine, and atovaquone, the identification of novel drugs and drug targets in the Plasmodium parasite is critical for effective disease control.2–12 The electron transport chain (ETC), a component of which is targeted by the commonly used drug, atovoquone, was examined to expand the repertoire of exploitable chemotherapeutic targets.13 Although the ETC is generally well conserved across species, the first component (Complex I) of the Plasmodium falciparum ETC is DNA sequence divergent and relatively uncharacterized. The Complex I of eukaryotes is typically composed of a multi-subunit NADH:Ubiquinone oxidoreductase that oxidizes NADH in a rotenone-sensitive manner. In contrast, P. falciparum encodes an alternative single polypeptide non-proton pumping enzyme that is rotenone-insensitive. Alternative NADH dehydrogenase (NDH2) enzymes are flavoproteins that catalyze the transfer of electrons from NADH to ubiquinone (CoQn), using a ping-pong mechanism, in order to maintain a pool of oxidized NADH for reductive metabolic pathways, such as glycolysis or the TCA cycle.14 NDH2 enzymes are found throughout the plant kingdom and in some bacteria, yeast, and protists.15–23 These enzymes are characterized by their lack of a transmembrane domain, the presence of a conserved GxGxxG motif, and their insensitivity to Complex I inhibitors such as rotenone and piericidin A.24 Like other NDH2 proteins, the respiratory chain NDH2 of P. falciparum (pfNDH2) is a single polypeptide, approximately 52 kDa in size.14
In an effort to explore targets along the ETC in P. falciparum, specific inhibitors for each component of the chain must be identified and characterized. While there are specific inhibitors to Complex III (cytochrome bc1), such as antimycin A and atovaquone, the specificity of inhibitors to alternative Complex I, pfNDH2, has been debated.25, 26 Recombinant pfNDH2 has been difficult to express and purify, and previous biochemical studies have been confounded by the use of crude or partially purified lysate in enzymatic assays.14, 26, 27 In this study, biochemically active pfNDH2 was recombinantly expressed in Escherichia coli in order to thoroughly evaluate substrate and inhibitor specificity. Full-length protein was expressed in-frame with a C-terminal 6xHis tag. The presence of detergent (0.5% Triton X-100) was critical for purification of active enzyme. The enzymatic activity of pfNDH2 was measured by chemical quantification of NAD+ using an assay adapted from Putt et al. in which addition of acetophenone base, followed by incubation at 100 °C with formic acid, yields a product with strong fluorescence emission at 444 nm when excited at 372 nm (Supplemental Scheme 1).28 The pH dependence of the pfNDH2 reaction was assessed using this fluorescence-based assay at fixed concentrations of NADH and CoQ0 (0.1 mM for both substrates). pfNDH2 was maximally active at a pH range between 7.0 and 9.0 (Supplemental Fig. 1). Given these data, a HEPES buffer equilibrated to pH 8.0 was used in subsequent kinetics assays.
Similar to other CoQn-utilizing enzymes, NDH2 homologs show differential substrate specificities for various CoQn. To gauge the substrate specificity of recombinant pfNDH2, the concentration of NADH was held constant, and in excess at 200 µM, while the substrates, CoQ0, CoQ1, CoQ4, CoQD, menadione, and DCIP, were varied from 0.5 µM to 2 mM. The Km,app and kcat for the various substrates were calculated based on best fit Michaelis–Menten kinetic curves, and are reported in Table 1 (Supplemental Fig. 2).
Table 1.
Kinetic properties of purified pfNDH2 in the presence of electron-accepting substrates
| Electron acceptor | kcat (1/min)a | Kma,b |
|---|---|---|
| CoQ0 | 20.0 ± 0.3 | 104.3 ± 6.6 |
| CoQ1 | 0.9 ± 0.1 | 2.2 ± 1.2 |
| CoQ4 | n.a. | n.a. |
| CoQD | n.a. | n.a. |
| Menadione | 4.6 ± 0.2 | 239.3 ± 22.4 |
| DCIP | 1.9 ± 0.0 | 4.6 ± 0.4 |
Km and kcat values and standard deviations were calculated from three independent experiments. n.a., enzyme activity not observed.
For each Km determination, the concentration of either the electron acceptor or NADH was varied while the other substrate was fixed at 500 µM. Km and kcat values were determined using best-fit Michaelis–Menton curves.
Among the CoQn substrates, only CoQ0 and CoQ1 showed detectable catalysis with pfNDH2. CoQ0 afforded the maximal catalytic rate; however, the Km,app was significantly higher for CoQ0 than CoQ1, reducing the catalytic efficiency (kcat/Km,app) of the substrate. Electron acceptors with longer carbon chains such as CoQ4, and CoQD were not efficiently utilized by pfNDH2. It should be noted that in vivo, pfNDH2 is predicted to be membrane-associated, however, the in vitro enzyme assay is carried out in the absence of membrane components. CoQ0 does not have the carbon tail of the endogenous substrate, but the catalytic rings of the molecule are intact.29 The observed differences in kcat may be due to differential binding affinities of CoQn in the absence of mitochondrial membrane association. The inorganic electron acceptor, DCIP, functioned with similar efficiency to CoQ0, albeit with increased substrate specificity and lower catalytic rate. In comparison, the efficiency of menadione was significantly reduced due to an increase in the Km,app.
In the absence of a crystal structure for NDH2 enzymes, predictions for the flavin-binding region and binding sites for NADH and CoQn have been based on sequence and structural similarities to other redox enzymes.30 The biochemical relevance of these predictions, however, has not yet been demonstrated. An N-terminal truncation product of pfNDH2 (ND214) was expressed and purified in order to determine if the conserved GxGxxG domains are necessary for catalytic activity. Truncated pfNDH2 showed significantly less activity compared to full-length product arguing that the N-terminal region of the enzyme is critical for full catalytic activity (data not shown).
NDH2 has been implicated as an activator of the plant-derived antimalarial, artemisinin.31 A knockout screen using Saccharamyces cerevisiae homozygous deletion strains showed that deletion of two S. cerevisiae NDH2 genes resulted in artemisinin resistance. Although expression of pfNDH2 in S. cerevisiae NDH2 knockout strains partially restored artemisinin sensitivity, and over-expression of the S. cerevisiae NDH2 genes was shown to increase artemisinin sensitivity, no biochemical link between pfNDH2 and artemisinin has yet been established.31 Therefore artemisinin was tested for either activation or inhibition of pfNDH2 activity. We found that arteminsin, even at high concentrations, did not perturb catalysis by pfNDH2 (Table 2). These results suggest that artemisinin may act via an indirect mechanism with NDH2 rather than by direct enzyme binding.
Table 2.
Inhibition of in vitro enzyme activity and in vivo parasite proliferation
| Compound | IC50 (µM) of enzyme activitya |
IC50 (µM) of P. falciparum proliferationa,b |
|||
|---|---|---|---|---|---|
| pfNDH2 | pfDHOD | scDHOD | DD2 | DD2-scDHOD | |
| Atovaquone | >10 | >10 | >10 | 0.002 ± 0.0002 | >10 |
| Antimycin A | >10 | >10 | >10 | 3.4 ± 1.8 mg/mLc | >10 |
| HDQ | >10 | 4.0 ± 0.1 | >10 | 0.24 ± 0.09 | >10 |
| DPI | >10 | >10 | >10 | 2.7 ± 0.5 | 3.0 ± 0.6 |
| IDP | >10 | >10 | >10 | 12.4 ± 2.0 | 10.6 ± 3.8 |
| Rotenone | >10 | >10 | >10 | >10 | >10 |
| Flavone | >10 | >10 | >10 | >10 | >10 |
| Artemisinin | >10 | >10 | >10 | 0.01 ± 0.0006 | 0.006 ± 0.0001 |
IC50 values and standard deviations were calculated from three independent experiments.
IC50 values for DD2 and DD2-scDHOD parasite strains are based upon [3H]-hypoxanthine dose–effect curves.
IC50 values are given in mg/mL as antimycin A was a mixture of the components A1, A2, A3, and A4.
Previously described inhibitors of Complex I and NDH2 were evaluated for inhibition of recombinant pfNDH2 activity. Inhibition of enzymatic activity was assessed in the presence of excess NADH (200 µM) and CoQ0 approximately equivalent to the Km,app (100 µM). As expected, Complex I inhibitors had no effect on pfNDH2 enzyme activity (Table 2). Eschemann et al. identified 1-hydroxy-2-dodecyl-4(1H)quinolone (HDQ) as an inhibitor of yeast (Yarrowia lipolytica) NDH2 by isolating mitochondrial membrane fractions in lieu of purified protein.14 In addition, HDQ was shown to be a potent inhibitor of P. falciparum parasite proliferation.14, 32 Dibenziodolium chloride (DPI) and diphenyliodonium chloride (IDP) have been reported to inhibit pfNDH2 activity in crude lysate fractions (IC50 = 0.24 ± 0.03 and 5.99 ± 0.36, respectively), and both show efficacy against whole parasite proliferation.26 It has been suggested that the antimalarial mechanisms of DPI, IDP, and HDQ may be attributed to the inhibition of pfNDH2 activity, however, dose–effect profiles using purified recombinant pfNDH2 did not corroborate these findings. In fact, these compounds did not inhibit pfNDH2 activity at concentrations of up to 10 µM (Table 2). Both DPI and IDP are well-known flavoprotein oxidoreductase inhibitors, suggesting that previous observations of reduction in NADH consumption using crude parasite lysate may have been due to inhibition of a different flavoenzyme-dependent reaction.33–38
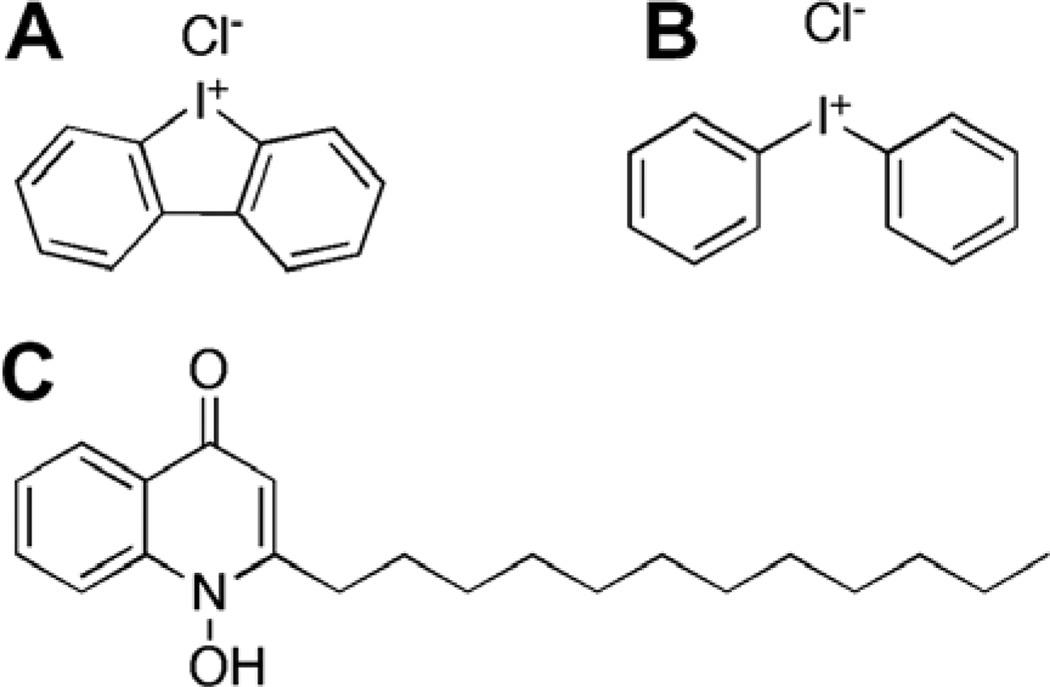
The chemical structure of HDQ is similar to that of the CoQn substrate (Fig. 1) and thus, we speculated that its mode of action might be related to another CoQn-dependent enzyme, P. falciparum Type II dihydroorotate dehydrogenase (pfDHOD). pfDHOD is a mitochondrial flavoenzyme that catalyzes the oxidation of dihydroorotate (l-DHO) using a FMN cofactor that is re-oxidized by CoQn.39 The malaria parasite relies upon pfDHOD as it catalyzes the rate-limiting step for de novo pyrimidine biosynthesis.40 Inhibition of pfDHOD activity by HDQ was assessed in the presence of excess l-DHO (500 µM) and CoQ0 approximately equivalent to the Km,app (115 µM), by coupling the assay to the chromogen, DCIP (Supplemental Scheme 2). HDQ inhibited pfDHOD activity with an IC50 of 4.0 ± 0.1 µM (Table 2).
Figure 1.
Chemical structures of (A) DPI, (B) IDP, and (C) HDQ.
A DD2 transgenic P. falciparum strain expressing a Type I cytoplasmic DHOD from S. cerevisiae (scDHOD) was used to pinpoint the antimalarial mechanism of HDQ (Supplemental methods). Painter et al. had previously shown that the role of the mitochondrial electron potential in the asexual stage of P. falciparum growth was to maintain a pool of CoQn in order to sustain pfDHOD activity and subsequent de novo pyrimidine biosynthesis.40 It was demonstrated that addition of exogenous scDHOD results in a bypass of the endogenous electron transport chain through Complex III.40 ScDHOD utilizes fumarate or NAD+ rather than CoQn to reoxidize the flavin (FMN) prosthetic group in the second half-reaction of the redox process.41–45 Therefore, inhibition of scDHOD activity was assessed in the presence of excess l-DHO (500 µM) and fumarate approximately equivalent to the Km,app (115 µM), by coupling the assay to the chromogen, DCIP (Supplemental Scheme 2). None of the compounds tested inhibited scDHOD enzyme activity. Proliferation of the scDHOD-expressing transgenic strain was unaffected by HDQ at a drug concentration of up to 10 µM, while the parental strain showed half-maximal growth inhibition at 0.24 ± 0.09 µM(Table 2) (Supplemental Fig. 3). This rescued growth phenotype associated with the addition of exogenous yeast DHOD argues that pfDHOD is the likely in vivo target of HDQ rather than pfNDH2 as formerly described.
The results of this study have shown that previously characterized NDH2 inhibitors are not effective inhibitors of pfNDH2. Such an observation is not unexpected since inhibitors for alternative rotenone-insensitive NADH dehydrogenases are rare and non-specific.46 The essentiality of pfNDH2 remains undetermined, therefore additional work is required to identify specific inhibitors of pfNDH2 for further biochemical characterization.47
Supplementary Material
Acknowledgments
The authors thank Ralph Mazitschek for suggestions and advice. This work was supported by NICHD K12-HD000850 (J.D.D.), and by the NSF Graduate Research Fellowship Program (V.P.), and Harvard Malaria Initiative (D.F.W.).
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2008.11.071.
References and notes
- 1.World malaria situation in 1994. Part I. Population at risk. Wkly Epidemiol. Rec. 1997;72(36):269–274. [PubMed] [Google Scholar]
- 2.Cadigan FC, Jr, Sadudee N, Bourke AT, Gould DJ, Winter PE. Trans. Roy. Soc. Trop. Med. Hyg. 1968;62:562. doi: 10.1016/0035-9203(68)90145-4. [DOI] [PubMed] [Google Scholar]
- 3.Mahoney LE. Lancet. 1968;2:1139. doi: 10.1016/s0140-6736(68)91606-1. [DOI] [PubMed] [Google Scholar]
- 4.Peters W. Lancet. 1969;2:54. doi: 10.1016/s0140-6736(69)92618-x. [DOI] [PubMed] [Google Scholar]
- 5.Young MD, Moore DV. Am. J. Trop. Med. Hyg. 1961;10:317. doi: 10.4269/ajtmh.1961.10.317. [DOI] [PubMed] [Google Scholar]
- 6.Charles LJ, Van Der Kaay HJ, Vincke IH, Brady J. Bull. World Health Organ. 1962;26:103. [PMC free article] [PubMed] [Google Scholar]
- 7.Clyde DF, Shute GT. Trans. Roy. Soc. Trop. Med. Hyg. 1954;48:495. doi: 10.1016/0035-9203(54)90085-1. [DOI] [PubMed] [Google Scholar]
- 8.Young MD, Contacos PG, Stitcher JE, Millar JW. Am. J. Trop. Med. Hyg. 1963;12:305. doi: 10.4269/ajtmh.1963.12.305. [DOI] [PubMed] [Google Scholar]
- 9.Hess U, Timmermans PM, Jones M. Am. J. Trop. Med. Hyg. 1983;32:217. doi: 10.4269/ajtmh.1983.32.217. [DOI] [PubMed] [Google Scholar]
- 10.Rumans LW, Dennis DT, Atmosoedjono S. Lancet. 1979;2:580. doi: 10.1016/s0140-6736(79)91633-7. [DOI] [PubMed] [Google Scholar]
- 11.Vleugels MP, Wetsteyn JC, Meuwissen JH. Trop. Geogr. Med. 1982;34:263. [PubMed] [Google Scholar]
- 12.Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Mol. Microbiol. 1999;33:704. doi: 10.1046/j.1365-2958.1999.01515.x. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya AB. Mitochondrial Physiology as a Target for Atovaquone and Other Antimalarials. In: Sherman IW, editor. Malaria: Parasite Biology, Pathogenesis, and Protection. Washington, DC: American Society for Microbiology; 1998. pp. 355–368. [Google Scholar]
- 14.Eschemann A, Galkin A, Oettmeier W, Brandt U, Kerscher S. HDQ (1-hydroxy-2-dodecyl-4(1H)quinolone), a high affinity inhibitor for mitochondrial alternative NADH dehydrogenase: evidence for a ping-pong mechanism. J. Biol. Chem. 2005;280:3138. doi: 10.1074/jbc.M411217200. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson AG, Svensson AS, Knoop V, Grohmann L, Brennicke A. Plant J. 1999;20(1):79. doi: 10.1046/j.1365-313x.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 16.de Vries S, Grivell LA. Eur. J. Biochem. 1988;176:377. doi: 10.1111/j.1432-1033.1988.tb14292.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerscher SJ, Okun JG, Brandt U. J. Cell Sci. 1999;112:2347. doi: 10.1242/jcs.112.14.2347. [DOI] [PubMed] [Google Scholar]
- 18.Melo AM, Duarte M, Moller IM, Prokisch H, Dolan PL, Pinto L, Nelson MA, Videira A. J. Biol. Chem. 2001;276:3947. doi: 10.1074/jbc.M008199200. [DOI] [PubMed] [Google Scholar]
- 19.Bjorklof K, Zickermann V, Finel M. FEBS Lett. 2000;467:105. doi: 10.1016/s0014-5793(00)01130-3. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita K, Otofuji A, Iwahashi M, Toyama H, Adachi O. FEMS Microbiol. Lett. 2001;204:271. doi: 10.1111/j.1574-6968.2001.tb10896.x. [DOI] [PubMed] [Google Scholar]
- 21.Gomes CM, Bandeiras TM, Teixeira M. J. Bioenerg. Biomembr. 2001;33:1. doi: 10.1023/a:1005630221892. [DOI] [PubMed] [Google Scholar]
- 22.Bandeiras TM, Salgueiro C, Kletzin A, Gomes CM, Teixeira M. FEBS Lett. 2002;531:273. doi: 10.1016/s0014-5793(02)03514-7. [DOI] [PubMed] [Google Scholar]
- 23.Bandeiras TM, Salgueiro CA, Huber H, Gomes CM, Teixeira M. Biochim. Biophys. Acta. 2003;1557:13. doi: 10.1016/s0005-2728(02)00374-2. [DOI] [PubMed] [Google Scholar]
- 24.Melo AM, Bandeiras TM, Teixeira M. Microbiol. Mol. Biol. Rev. 2004;68:603. doi: 10.1128/MMBR.68.4.603-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidya AB, Painter HJ, Morrisey JM, Mather M. W. Trends Parasitol. 2008;24:8. doi: 10.1016/j.pt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Biagini GA, Viriyavejakul P, O’Neill PM, Bray PG, Ward SA. Antimicrob. Agents Chemother. 2006;50:1841. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krungkrai J, Kanchanarithisak R, Krungkrai SR, Rochanakij S. Exp. Parasitol. 2002;100:54. doi: 10.1006/expr.2001.4674. [DOI] [PubMed] [Google Scholar]
- 28.Putt KS, Hergenrother PJ. Anal. Biochem. 2004;326:78. doi: 10.1016/j.ab.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 29.de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. FEMS Microbiol. Lett. 2002;207:13. doi: 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 30.Fisher N, Bray PG, Ward SA, Biagini GA. Trends Parasitol. 2007;23:305. doi: 10.1016/j.pt.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Mo W, Shen D, Sun L, Wang J, Lu S, Gitschier JM, Zhou B. PLoS Genet. 2005;1:e36. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleh A, Friesen J, Baumeister S, Gross U, Bohne W. Antimicrob. Agents Chemother. 2007;51:1217. doi: 10.1128/AAC.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross AR, Jones OT. Biochem. J. 1986;237:111. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, Nathan CF. FASEB J. 1991;5:98. doi: 10.1096/fasebj.5.1.1703974. [DOI] [PubMed] [Google Scholar]
- 35.Sanders SA, Eisenthal R, Harrison R. Eur. J. Biochem. 1997;245:541. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 36.Tew DG. Biochemistry. 1993;32:10209. doi: 10.1021/bi00089a042. [DOI] [PubMed] [Google Scholar]
- 37.Luetjens CM, Bui NT, Sengpiel B, Munstermann G, Poppe M, Krohn AJ, Bauerbach E, Krieglstein J, Prehn JH. J. Neurosci. 2000;20:5715. doi: 10.1523/JNEUROSCI.20-15-05715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majander A, Finel M, Wikstrom M. J. Biol. Chem. 1994;269:21037. [PubMed] [Google Scholar]
- 39.Malmquist NA, Gujjar R, Rathod PK, Phillips MA. Biochemistry. 2008;47:2466. doi: 10.1021/bi702218c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Nature. 2007;446:88. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 41.Taylor ML, Taylor WH, Eames DF, Taylor CD. J. Bacteriol. 1971;105:1015. doi: 10.1128/jb.105.3.1015-1027.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Plas J, Hellingwerf KJ, Seijen HG, Guest JR, Weiner JH, Konings WN. J. Bacteriol. 1983;153:1027. doi: 10.1128/jb.153.2.1027-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skophammer RG, Servin JA, Herbold CW, Lake JA. Mol. Biol. Evol. 2007;24:1761. doi: 10.1093/molbev/msm096. [DOI] [PubMed] [Google Scholar]
- 44.Denis-Duphil M. Biochem. Cell. Biol. 1989;67:612. doi: 10.1139/o89-094. [DOI] [PubMed] [Google Scholar]
- 45.Jordan DB, Bisaha JJ, Picollelli MA. Arch. Biochem. Biophys. 2000;378:84. doi: 10.1006/abbi.2000.1823. [DOI] [PubMed] [Google Scholar]
- 46.Fang J, Beattie DS. Biochemistry. 2002;41:3065. doi: 10.1021/bi015989w. [DOI] [PubMed] [Google Scholar]
- 47.Kawahara K, Mogi T, Tanaka TQ, Hata M, Miyoshi H, Kita K. J. Biochem. 2008 doi: 10.1093/jb/mvn161. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.