Abstract
Although various non-viral transfection methods are available, cell-toxicity, low transfection efficiency and high-cost remain hurdles for in vitro gene delivery in cultured primary endothelial cells. Recently, unprecedented transfection efficiency for primary endothelial cells has been achieved due to the newly developed nucleofection technology that utilizes a combination of novel electroporation conditions and specific buffer components that stabilize the cells in the electrical field. Despite its superior transfection efficiency and cell viability, high cost of the technology has discouraged the cardiovascular researchers to liberally adopt this new technology. Here, we report that a phosphate-buffered saline (PBS)-based nucleofection method can be used for efficient gene delivery into primary endothelial cells and other types of cells. Comparative analyses of transfection efficiency and cell viability for primary arterial, venous, microvascular and lymphatic endothelial cells were performed by using PBS. Compared to the commercial buffers, PBS can support equally remarkable nucleofection efficiency to both primary and non-primary cells. Moreover, PBS-mediated nucleofection of siRNA showed more than 90% knockdown of the expression of target genes in primary endothelial cells. Together, we demonstrate that PBS can be an unprecedented economical alternative for the high-cost buffers for nucleofection of various primary and non-primary cells.
Keywords: electroporation, nucleofection, primary endothelial cells, phosphate-buffered saline
Introduction
Although various gene delivery methods for primary endothelial cells have been developed based on calcium phosphate, cationic liposome, synthetic amphiphiles, DEAE-dextran or electroporation [1–7], cell toxicity, poor transfection efficiency and cost have limited them to be widely utilized in the cardiovascular community. However, recently developed nucleofection technology has been proven to be a new electroporation method with superior transfection efficiency and cell viability for various primary cultures, including primary endothelial cells [8–12]. The nucleofection technology utilizes a combination of optimized electroporation condition and special proprietary buffers that stabilize the cells in the electrical field, which reportedly allows a direct electro-transfer of nucleotides into the nuclei of the cells [8–12]. Typically, nucleofection of primary endothelial cells can support > 70% transfection efficiency and > 80% cell viability when commercial kits are used. Despite the outstanding transfection efficiency and cell viability, the accessibility to the new technology has been limited due to high cost for the nucleofection kits that supplies the proprietary special buffers. Here, we report that phosphate-buffered saline (PBS) solution can be used to yield comparable transfection efficiency with a tolerable decrease in cell viability.
Material and Methods
Cell isolation and culture
Primary human umbilical arterial and venous endothelial cells (HUAECs and HUVECs, respectively) were purchased from Lonza (Basel, Switzerland). HUAECs were cultured in the EGM-MV medium (CC-3125, Lonza) and HUVECs in the EGM-2 medium (CC-3162, Lonza). Primary human dermal blood vascular and lymphatic endothelial cells (BECs and LECs, respectively) were isolated from neonatal foreskins and cultured in endothelial basal medium (EBM, from Lonza) with 20% FBS and other supplements as previously described [13]. All primary cells were cultured on fibronectin (10 μg/ml, Sigma)-coated culture plates. L6 rat skeletal muscle cells (ATCC) and thyroid cancer cells (TPC1) (a gift from Dr. James Fagin, Memorial Sloan Kettering Cancer Center, NY) were cultured in DMEM with 10% FCS and antibiotics. Phosphate-buffered saline (PBS) was purchased from Mediatech, Inc. (Manassas, VA).
Transfection using Lipofectamine 2000 and Polyethylenimine
Polyethylenimine (PEI, m.w. ~25K, Sigma) was dissolved in sterile water to make a stock solution (100 mg/ml), which was further diluted at 1:100 to make a working solution (1 mg/ml). A round glass cover-slip was placed in each well of a 12-well plate and then briefly coated with fibronectin (10 μg/ml). Primary LECs (passage 6) were then seeded at 30~40% cell-confluency on the cover-slips in 1 ml of culture media. After 24-hours, 0.5 μg of a GFP-expressing vector (pmaxGFP, Lonza) was mixed with 0.5 μg (1:1) or 1.5 μg (1:3) of Lipofectamine 2000 (Invitrogen Corp. Carlsbad, CA), or with 1.5 μg (1:3) of PEI at room temperature for 20 minutes before addition onto LECs in serum-containing culture media. Transfection efficiency and cell viability were measured after 48-hours.
Nucleofection of plasmids and siRNA duplexes
Lung Microvascular Endothelial Cell kit (VPB-1003, Lonza) was used for nucleofection of all primary endothelial cells. One million primary endothelial cells (passages 5~7) grown to 80~90% confluency were trypsinized and harvested by centrifugation at 1000 rpm at 4°C for 5 minutes. Supernatant was thoroughly removed and cell pellets were resuspended in 100 μl of the nucleofection reagent, PBS or plain EBM solution without any serum or additives. The pmaxGFP vector (1 μg) or siRNA duplexes (20 pmol) for Prox1 and/or COUP-TFII were mixed with cells and subsequently transferred into either nucleofection cuvettes (Lonza) or generic electroporation cuvettes (Bio-Rad, 2-mm gap). We found that generic electroporation cuvettes with a 2-mm gap were as good as nucleofection cuvettes supplied in the nucleofection kits. All primary endothelial cells were nucleofected by using the S-05 program in the nucleofection device (Nucleofector I) and pre-warmed 500 μl culture media was immediately added into the cells. Transfected cells were seeded onto 10 cm culture plates, in which 2~3 glass cover-slips were pre-placed and coated with fibronectin. After nucleofection, no media change was performed for 48-hours to retain all dead and live cells for assessment of cell viability. Transfection efficiency and total RNA/protein yields were subsequently determined as below.
Measurement of transfection efficiency and cell viability
Two days after transfection of pmaxGFP, the cover slips were collected and stained/mounted with DAPI-containing mounting solution (Vector Shield). At least five optical fields of GFP-positive cells were captured and percentages of the GFP-positive cells were calculated by counting at least 400 nuclei. Separately, both floating (dead) and adherent (live) cells were collected and centrifuged, and cell pellets were resuspended in 1 ml of PBS, of which 10 μl was stained with trypan blue (Sigma) to count live versus dead cells. Cell viability was calculated by percentage of trypan-blue negative (live) cells over total number of cells counted (> 500). The remaining cells ( > 100,000) were used for flow cytometry analysis for GFP-signals.
Results and Discussion
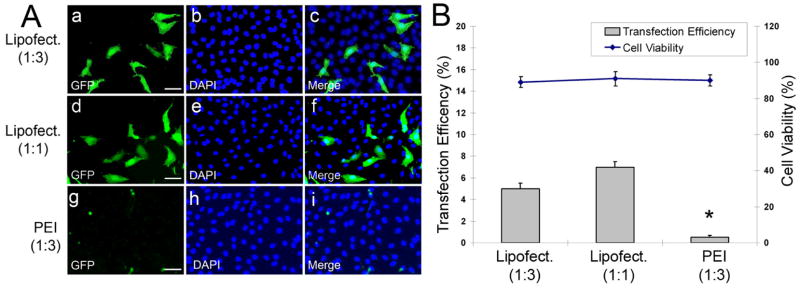
We first evaluated the transfection efficiency of two widely-used transfection reagents, Lipofectamine 2000 [14] and PEI [15], for primary human dermal lymphatic endothelial cells (LECs, passage 6). 0.5 μg of pmaxGFP was mixed with either 1.5 μg (1:3) or 0.5 μg (1:1) of Lipofectamine 2000, or with 1.5 μg (1:3) of PEI and subsequently added onto LECs grown in 1 ml of serum-containing culture media in a well of a 12-well plate. Two days later, we determined transfection efficiency and cell viability (Figure 1A,B). While most (> 90%) of the transfected cells were viable by both methods, transfection efficiency was less than 10% for all cases.
Figure 1.
Transfection of primary human dermal lymphatic endothelial cells (LECs) using Lipofectamine and PEI. (A) Primary LECs were transfected with 0.5 μg of pmaxGFP that was mixed with 1.5 μg (a–c) or 0.5 μg (d–f) of Lipofectamine 2000 or with 1.5 μg (g–i) of PEI. After 48-hours, transfected cells were stained with DAPI. GFP-positive cells (a,d,g), DAPI-stained nuclei (b,e,h) and merged images (c,f,i) were shown. Bar: 100 μm. (B) Transfection efficiency of Lipofectamine or PEI was expressed by a percentage of GFP positive cells over all DAPI-positive nuclei ( > 400). Cell viability was determined by counting trypan blue-negative live cells over all cells counted ( > 500). Asterisk indicates p < 0.05 in reference to the first two conditions.
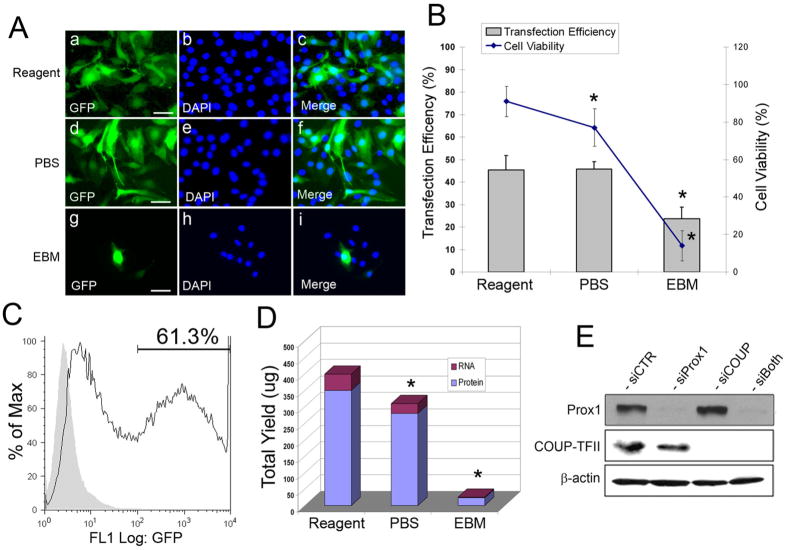
We then investigated transfection efficiency and cell viability of primary endothelial cells by using nucleofection. We harvested primary LECs (passage 6) from 80~90% confluent culture plates (10-cm) and resuspended in either the nucleofection reagent from the HMVEC-L kit (Lonza), PBS or endothelial basal medium (EBM). Cells were nucleofected with 1 μg of pmaxGFP by using the S-05 program of the Amaxa nucleofection device (Lonza). After 48-hours, we determined transfection efficiency and cell viability (Figure 2A,B). Surprisingly, the nucleofection and PBS yielded largely comparable transfection efficiency (40~50%) based on the number of GFP-positive cells. In comparison, Nucleofection using plain EBM solution supported only about 10~20% transfection efficiency. Moreover, while the nucleofection reagents showed the highest cell viability ( > 90%), PBS supported a reduced cell viability (70~ 80%) and most of cells died with EBM-mediated nucleofection (Figure 2A,B). In agreement with visual counting of GFP-positive cells, we also performed flow cytometry analyses to count GFP-positive cells and found that PBS-nucleofected LECs showed 61.3% GFP-positive cells counting from ~ 70,000 cells (Figure 2C). We then determined the yields of total RNA and whole cell lysates from reagents versus PBS-nucleofected cells. While reagent-mediated nucleofection yielded ~50 μg of total RNA and ~350 μg of whole cell lysate, PBS-mediated nucleofection provided ~30 μg of total RNA and ~280 μg of whole cell lysate (Figure 2D). These data suggest that although the nucleofection reagent better protects the cells in the electric field and thus provides higher cell viability, PBS-mediated nucleofection proves to be good enough for routine transfection experiments. Furthermore, we also performed knockdown of genes by using PBS-mediated transfection of siRNA duplexes into primary LECs. We nucleofected one million cells with siRNA duplexes against Prox1 and/or COUP-TFII, two important transcriptional regulators expressed in LECs. Cell-viability and RNA/protein yields were comparable to those of PBS-mediated nucleofection of a plasmid vector (data not shown). Moreover, western blotting analyses revealed that PBS-mediated nucleofection of siRNA efficiently knockdown ~ 90% of Prox1 and/or COUP-TFII proteins in primary LECs (Figure 2E).
Figure 2.
Nucleofection of primary human dermal lymphatic endothelial cells. (A) Primary LECs were nucleofected by using the Amaxa Lung Microvascular Endothelial Cell kit reagent (a–c), PBS (d–f) or endothelial basal media (EBM) (g–i). After 48-hours, nucleofected cells were stained with DAPI. GFP-positive cells (a,d,g), DAPI-stained nuclei (b,e,h) and merged images (c,f,i) were shown. Bar: 100 μm. (B) Transfection efficiency and cell viability for the kit reagent, PBS and EBM were expressed by a percentage of GFP positive cells and trypan blue-negative live cells, respectively. Asterisks indicate p < 0.05 in reference to the reagent condition. (C) PBS-mediated nucleofected LECs were further analyzed by flow cytometry. pmaxGFP-transfected LECs were compared against LECs transfected with a non-GFP vector (pcDNA3). About 61.3% cells showed GFP-signal above the background control level. (D) Yields of total RNA and whole cell lysate were compared for the reagent, PBS and EBM-nucleofected LECs. Asterisks indicate p < 0.05 in reference to the reagent condition. (E) Primary LECs were transfected with siRNA against Prox1 and/or COUP-TFII by using PBS-mediated nucleofection. After 48-hours, the steady-state level of Prox1 and COUP-TFII proteins were determined by western blotting analyses. siCTR, a negative control siRNA targeting the firefly luciferase; siProx1, siRNA for Prox1; siCOU, siRNA for COUP-TFII; siBoth, mixture of siRNAs for Prox1 and COUP-TFII.
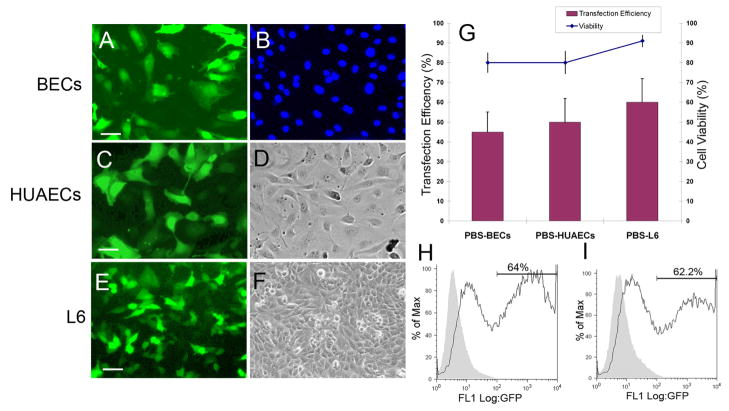
We further asked if PBS-mediated nucleofection can also be applied to other types of primary endothelial cells and tested human dermal blood vascular endothelial cells (BECs), human umbilical arterial endothelial cells (HUAECs) and human umbilical venous endothelial cells (HUVECs). Primary BECs and HUAECs from 80~90% confluent plates (10-cm) were nucleofected in 100 μl of PBS with 1 μg of pmaxGFP. We found that their transfection efficiency (40~50%) and cell viability (70~80%) were largely compatible to those of PBS-nucleofected LECs (Figure 3A–D, G). We also observed that compared PBS, the nucleofection reagent provided a 10~20% higher cell viability with largely similar transfection efficiency to primary BECs and HUAECs As in the case of LECs (data not shown). Moreover, we asked whether the more amount of DNA increases nucleofection efficiency and nucleofected HUAECs with 1 or 4 μg of pmaxGFP in PBS. Interestingly, flow cytometry analyses demonstrated that both cases yielded 62~64% transfection efficiency (Figure 3H,I), suggesting that 1 μg is a saturating amount of DNA per 1 million primary cells. We also found the similar nucleofection efficiency and cell viability with HUVECs (data not shown). Taken together, these data demonstrate that PBS may be adopted as an alternative nucleofection buffer for various primary human endothelial cells despite total 10~30% cell death and that PBS-mediated nucleofection can be an efficient and low-cost transfection method to deliver plasmid vectors and siRNA into various primary endothelial cells.
Figure 3.
PBS-based nucleofection of primary human dermal blood vascular endothelial cells (BECs), primary HUVECs and L6 rat myoblasts. Primary BECs (A,B), HUAECs (C,D) and L6 rat myoblasts were nucleofected with 1 μg of pmaxGFP and after 48-hours, images of GFP-transfected cells (A,C,E), DAPI-stained nuclei (B) and bright field (D,F) of the cells were captured. Bars: 100 μm. (G) Transfection efficiency and cell viability of PBS-based nucleofected cells were expressed by a percentage of GFP positive cells and trypan blue-negative live cells by counting > 400 cells. (H,I) Flow cytometry analyses of PBS-based nucleofected HUAECs with 1 μg (H) and 4 μg (I) of pmaxGFP.
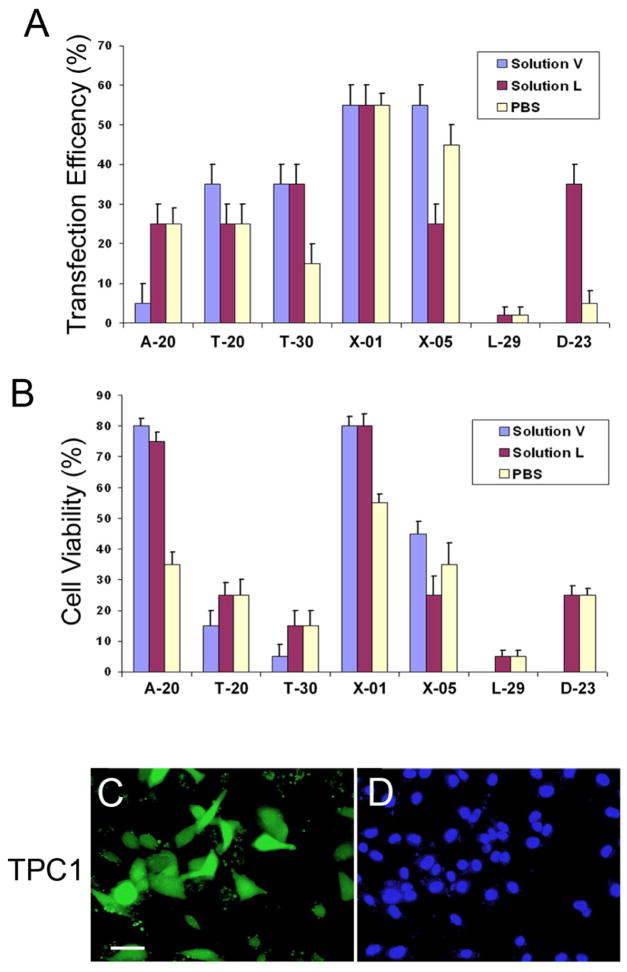
We next applied PBS-mediated nucleofection to non-primary cell types. L6 rat skeletal muscle cells have been shown to be nucleofected up to 60% by using Nucleofector Kit R (VCA-1001, Lonza). One million L6 cells from 80~90% confluent culture plates were resuspended in 100 μl PBS and subjected to nucleofection program X-05 in the presence of 1 μg of pmaxGFP. After 48-hours, transfection efficiency and cell viability were determined to be ~60% and ~90%, respectively (Figure 3E–G). In addition, we took human thyroid cancer cells TPC1, whose nucleofection condition has not been established, and employed the Amaxa Cell Line Optimization Nucleofector Kit (VCO-1001, Lonza) to determine the optimal nucleofection condition. Following the manufacturer protocols, one million TPC1 cells were nucleofected in Solution V, Solution L or PBS by using seven different nucleofection programs. We found that with a few exceptions, PBS could support transfection efficiency and viability largely compatible to those of Solutions V and L, and that X-01 was the best condition for all the three solutions (Figure 4). Together, we found that PBS-mediated nucleofection can also be applied to non-primary cell lines, proving an excellent transfection method for various cell types.
Figure 4.
Determination of the optimal conditions of PBS-based nucleofection for human thyroid cancer cells (TPC1). Human thyroid cancer cells TPC1 were nucleofected with 2 μg of pmaxGFP using Solution V, Solution L or PBS and different nucleofection programs (A-20, T-20, T-30, X-01, X-05, L-29 and D-23). After 48-hours of nucleofection, nucleofection efficiency (A) and cell viability (B) of each condition were measured. Images of GFP-transfected cells (C) and bright field (D) of TPC1 nucleofected using PBS and the X-01 program were shown. Bars: 100 μm.
In this report, we demonstrated that PBS can be successfully used as an alternative nucleofection solution that provides excellent transfection efficiency with a tolerable loss of cell viability for various primary endothelial cells and non-primary cells. Although PBS-nucleofection yielded excellent transfection efficiency, the commercially available nucleofection solutions indeed provided a better cell viability (10~20% higher) to the cells tested, especially primary endothelial cells, during electroporation. Moreover, we found that when primary endothelial cells are overly confluent or contaminated by mycoplasma, culture condition are not optimal, or the cells are under various stresses, PBS-mediated nucleofection results in much higher post-nucleofection cell death. Thus, it is important to maintain the optimal culture condition for primary endothelial cells and use cell cultures less than 95~100% confluency, which may correspond to 24~48 hours post expanding/splitting of endothelial cells. Moreover, we have not investigated the effect of PBS-mediated nucleofection on other cell biology and behaviors beyond gene expression studies. It could be possible that PBS-mediated nucleofection may cause undefined or unnoticeable damages to the cells, although we did not find distinct alterations in cell morphology and adhesion. Nonetheless, we found that PBS can be successfully utilized as a nucleofection reagent for most of routine in vitro studies with a substantial economical merit.
Acknowledgments
The authors thank Juneyong Lee for his technical assistance. This study is supported by the March of Dimes Foundation, American Heart Association, American Cancer Society and NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ear T, Giguere P, Fleury A, Stankova J, Payet MD, Dupuis G. High efficiency transient transfection of genes in human umbilical vein endothelial cells by electroporation. J Immunol Methods. 2001 Nov 1;257(1–2):41–9. doi: 10.1016/s0022-1759(01)00445-8. [DOI] [PubMed] [Google Scholar]
- 2.Kotnis RA, Thompson MM, Eady SL, Budd JS, Bell PR, James RF. Optimisation of gene transfer into vascular endothelial cells using electroporation. Eur J Vasc Endovasc Surg. 1995 Jan;9(1):71–9. doi: 10.1016/s1078-5884(05)80228-x. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani AC, Gale KM, Pemberton KD, Crossman DC, Tuddenham EG, McVey JH. Efficient gene transfer into human umbilical vein endothelial cells allows functional analysis of the human tissue factor gene promoter. Br J Haematol. 1994 Sep;88(1):122–8. doi: 10.1111/j.1365-2141.1994.tb04987.x. [DOI] [PubMed] [Google Scholar]
- 4.Sipehia R, Martucci G. High-efficiency transformation of human endothelial cells by Apo E-mediated transfection with plasmid DNA. Biochem Biophys Res Commun. 1995 Sep 5;214(1):206–11. doi: 10.1006/bbrc.1995.2276. [DOI] [PubMed] [Google Scholar]
- 5.Tanner FC, Carr DP, Nabel GJ, Nabel EG. Transfection of human endothelial cells. Cardiovasc Res. 1997 Sep;35(3):522–8. doi: 10.1016/s0008-6363(97)00151-x. [DOI] [PubMed] [Google Scholar]
- 6.Teifel M, Heine LT, Milbredt S, Friedl P. Optimization of transfection of human endothelial cells. Endothelium. 1997;5(1):21–35. doi: 10.3109/10623329709044156. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen EB, van der Veen AY, Hoekstra D, Engberts JB, Halie MR, van der Meer J, et al. Transfection of small numbers of human endothelial cells by electroporation and synthetic amphiphiles. Eur J Vasc Endovasc Surg. 1999 Jan;17(1):9–14. doi: 10.1053/ejvs.1998.0677. [DOI] [PubMed] [Google Scholar]
- 8.Thiel C, Nix M. Efficient transfection of primary cells relevant for cardiovascular research by nucleofection. Methods Mol Med. 2006;129:255–66. doi: 10.1385/1-59745-213-0:255. [DOI] [PubMed] [Google Scholar]
- 9.Schakowski F, Buttgereit P, Mazur M, Marten A, Schottker B, Gorschluter M, et al. Novel non-viral method for transfection of primary leukemia cells and cell lines. Genet Vaccines Ther. 2004 Jan 12;2(1):1. doi: 10.1186/1479-0556-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trompeter HI, Weinhold S, Thiel C, Wernet P, Uhrberg M. Rapid and highly efficient gene transfer into natural killer cells by nucleofection. J Immunol Methods. 2003 Mar 1;274(1–2):245–56. doi: 10.1016/s0022-1759(02)00431-3. [DOI] [PubMed] [Google Scholar]
- 11.Martinet W, Schrijvers DM, Kockx MM. Nucleofection as an efficient nonviral transfection method for human monocytic cells. Biotechnol Lett. 2003 Jul;25(13):1025–9. doi: 10.1023/a:1024157508492. [DOI] [PubMed] [Google Scholar]
- 12.Distler JH, Jungel A, Kurowska-Stolarska M, Michel BA, Gay RE, Gay S, et al. Nucleofection: a new, highly efficient transfection method for primary human keratinocytes*. Exp Dermatol. 2005 Apr;14(4):315–20. doi: 10.1111/j.0906-6705.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003 Feb;162(2):575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, et al. Advanced transfection with Lipofectamine 2000 reagent: primary neurons, siRNA, and high-throughput applications. Methods. 2004 Jun;33(2):95–103. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Kichler A, Leborgne C, Coeytaux E, Danos O. Polyethylenimine-mediated gene delivery: a mechanistic study. J Gene Med. 2001 Mar-Apr;3(2):135–44. doi: 10.1002/jgm.173. [DOI] [PubMed] [Google Scholar]