Abstract
Actions of lubiprostone, a selective type-2 chloride channel activator, on mucosal secretion were investigated in guinea pig small intestine and colon. Flat-sheet preparations were mounted in Ussing flux chambers for recording short-circuit current (Isc) as a marker for electrogenic chloride secretion. Lubiprostone, applied to the small intestinal mucosa in eight concentrations ranging from 1–3000 nM, evoked increases in Isc in a concentration-dependent manner with an EC50 of 42.5 nM. Lubiprostone applied to the mucosa of the colon in eight concentrations ranging from 1–3000 nM evoked increases in Isc in a concentration-dependent manner with an EC50 of 31.7 nM. Blockade of enteric nerves by tetrodotoxin did not influence stimulation of Isc by lubiprostone. Antagonists acting at prostaglandin (PG)E2, EP1–3, or EP4 receptors did not suppress stimulation of Isc by lubiprostone but suppressed or abolished PGE2-evoked responses. Substitution of gluconate for chloride abolished all responses to lubiprostone. The selective CFTR channel blocker, CFTR(inh)-172, did not suppress lubiprostone-evoked Isc. The broadly acting blocker, glibenclamide, suppressed (P < 0.001) lubiprostone-evoked Isc. Lubiprostone, in the presence of tetrodotoxin, enhanced carbachol-evoked Isc. The cholinergic component, but not the putative vasoactive intestinal peptide component, of neural responses to electrical field stimulation was enhanced by lubiprostone. Application of any of the prostaglandins, E2, F2, or I2, evoked depolarization of the resting membrane potential in enteric neurons. Unlike the prostaglandins, lubiprostone did not alter the electrical behavior of enteric neurons. Exposure to the histamine H2 receptor agonists increased basal Isc followed by persistent cyclical increases in Isc. Lubiprostone increased the peak amplitude of the dimaprit-evoked cycles.
Keywords: gastrointestinal tract, mucosal chloride secretion, enteric nervous system, prostaglandins, irritable bowel syndrome, cystic fibrosis transmembrane conductance regulator, ClC-2 channels
lubiprostone is classified as a prostone. Prostones are compounds derived from naturally occurring fatty acids associated with cell membranes. Lubiprostone is a bicyclic fatty acid, which is derived from a metabolite of prostaglandin E1 (PGE1) (36). The available evidence suggests that it acts directly to open and increase Cl− conductance in ClC-2 channels, which are expressed in the apical membranes of mucosal epithelial cells in the intestinal tract and epithelia elsewhere in the body.
Application of lubiprostone to confluent T84 cell monolayers stimulates, in a concentration-dependent manner, electrogenic Cl− secretion, which can be measured as increased short-circuit current (Isc) across the monolayer (16). Lubiprostone also activates ClC-2 channel currents in whole cell patch-clamp studies in human embryonic kidney (HEK)-293 cells that are stably transfected with recombinant human ClC-2 channels (16). Stimulation of Isc across confluent T84 cell monolayers occurs with an EC50 of 18 nM, and activation of the ClC-2 channels in transfected HEK-293 cells occurs also with a low EC50 of 17 nM. Low concentrations of lubiprostone <100 nM activate the ClC-2 channel in cultured cellular monolayers from Xenopus kidney (A6 cells), whereas concentrations greater than 100 nM activate the CFTR Cl− channel in the same monolayers (2).
Human recombinant ClC-2 channel currents recorded in HEK-293 expression systems are stimulated by agents, such as forskolin, that elevate intracellular cAMP and stimulate protein kinase A (PKA). Pretreatment with PKA inhibitors suppresses the stimulatory action of forskolin on the channels but does not suppress stimulation by lubiprostone (47). This can be interpreted as evidence for direct stimulation of the channel by lubiprostone in the HEK-293 cells rather than indirect stimulation by a second messenger.
The cellular physiology of ClC-2 channels in intestinal epithelial cells suggests that opening of these channels will elevate the intraluminal concentration of Cl− followed by movement of Na+ down its electrochemical gradient. As NaCl accumulates in the lumen, water moves across the mucosa down the osmotic gradient, and this increases the liquidity of the intestinal contents.
Lubiprostone-evoked opening of ClC-2 channels at the enterocyte level most likely stimulates intestinal glandular secretion at the organ system level of organization. As might be expected, oral administration of lubiprostone in conscious rats elevates NaCl and the volume of H2O in the lumen without changes in serum electrolytes (48). Moreover, we report here and elsewhere that lubiprostone stimulates electrogenic Cl− transport in preparations of the guinea pig small and large intestine and human small intestine in vitro in Ussing flux chambers (24, 46). The action of lubiprostone at ClC-2 channels to stimulate intestinal secretion of NaCl and H2O is likely to underlie its efficacy in treatment of chronic constipation and constipation-predominate irritable bowel syndrome in recent clinical trials (31–33).
The aim of the present work was to test a hypothesis that lubiprostone stimulates electrogenic Cl− transport (i.e., Isc) in mucosal preparations obtained from the guinea pig small and large intestine and enhances the paracrine action of the neuroimmune mediator histamine to activate a central pattern generator (CPG) in the enteric nervous system (ENS), which drives the timing of secretion. Some of the results have been published previously in an abstract from (24).
MATERIAL AND METHODS
Tissue preparation.
Adult male Hartley-strain guinea pigs (300–350 g) were stunned by a sharp blow to the head and exsanguinated from the cervical vessels according to a protocol approved by The Ohio State University Laboratory Animal Care and Use Committee and United States Department of Agriculture Veterinary Inspectors. Segments of ileum between 10 and 20 cm proximal to the ileocecal junction and segments of distal colon 5–10 cm orad to the anus were flushed with ice-cold Krebs solution and opened along the mesenteric border. The ileal preparations were “muscle-stripped” preparations from which the longitudinal and circular muscle layers together with the myenteric plexus were removed by microdissection. The submucosal plexus remained intact with the mucosa. The colon preparations were “whole-thickness” preparations with the intestinal wall intact. Four to six of the flat-sheet preparations were obtained from the ileum or colon of each animal for mounting in Ussing flux chambers. Whole thickness preparations of the colon (i.e., with muscularis externa intact) were used to explore the interactions of lubiprostone and histamine with the myenteric plexus present. Composition of the Krebs solution was (in mM): 120 NaCl, 6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.35 NaH2PO4, 14.4 NaHCO3, and 11.5 glucose. The Krebs solution in the Ussing chambers was bubbled with 95% O2-5% CO2 and buffered at pH 7.4.
Ussing flux chambers.
Ussing flux chambers were equipped with pairs of Ag/AgCl electrodes via Krebs-agar bridges connected to calomel half cells for measurement of transmural potential difference (PD). A second pair of electrodes was connected to an automated voltage-clamp apparatus, which compensated for the solution resistance between the PD-sensing bridges. The flat-sheet preparations were mounted between halves of Ussing chambers, which had a total cross-sectional area of 0.64 cm2. The tissues were bathed on both sides with 10 ml of Krebs solution and maintained at 37°C by circulation from a temperature-controlled water bath. The current necessary to change the transepithelial PD by 2.5 mV (small intestine) or 8 mV (colon) was passed periodically to monitor tissue conductance, calculated according to Ohm's law, and used together with responses to carbachol at the end of the experiment for evaluation of tissue viability. Isc was monitored by electronic voltage-clamp amplifiers (DVC-1000, World Precision Instruments, Sarasota, FL). Concentration-response relations were obtained by adding lubiprostone (1–3000 nM) to the serosal or mucosal compartment of the chamber. Lubiprostone-evoked changes in Isc were calculated as ΔIsc. Data were normalized to the cross-sectional area of the preparations.
Transmural electrical field stimulation (EFS) of intramural neurons in the Ussing chamber preparations was accomplished, according to the method of Cooke et al. (10, 11), by passing electrical current between a pair of aluminum foil electrodes placed on the submucosal surface at the intersection between the two halves of the Ussing chamber. The foil electrodes were connected to Grass SD 88 stimulators (Grass Instruments, Quincy, MA).
Electrophysiological recording.
Our methods for intracellular recording from neurons in the myenteric and submucosal plexuses are described in detail elsewhere (53). Transmembrane electrical potentials were recorded with conventional “sharp” microelectrodes filled with 2% biocytin in 2 M KCl buffered with 0.05 M Tris at pH 7.4. Resistances of the electrodes ranged between 80 and 120 MΩ. The preamplifiers (M-767, World Precision Instruments) were equipped with a bridge circuit for intraneuronal injection of electrical current. Constant current rectangular pulses were driven by Grass SD9 stimulators (Grass Instrument Division; Astro-Med, Warwick, RI). Chart records were made on Astro-Med thermal recorders. All data were recorded initially in digital format on magnetic tape for later analysis.
Statistics.
Student's t-test was used to calculate the statistical significance of differences between paired or unpaired data. When more than two means were compared, one-way analysis of variance was used to determine significance of differences among means. When significant differences were indicated by analysis of variance, multiple-range testing with the Newman-Keuls test was performed. Values of P < 0.05 were considered statistically significant. Continuous curves for concentration-response relationships were constructed with the following least-squares fitting routine using SigmaPlot software (SPSS, Chicago, IL): V = Vmax/[1 + (EC50/C)nH], where V is the observed increased Isc, Vmax is the maximal response, C is the corresponding drug concentration, EC50 is the concentration that induces the half-maximal response, and nH is the apparent Hill coefficient.
Chemicals.
SPI-0211 was obtained from Sucampo Pharmaceuticals (Bethesda, MD) as frozen aliquots of 2 mM solutions in 100% DMSO. DMSO, obtained as frozen aliquots from Sucampo Pharmaceuticals, was used to dilute lubiprostone and to test vehicle effects. Tetrodotoxin, bumetanide, DIDS, carbachol, prostaglandin E2, vasoactive intestinal peptide (VIP), CFTR(inh)-172, and glibenclamide were purchased from Sigma (St. Louis, MO). Dimaprit and 5-nitro-2-2(-henylpropylamino) benzoic acid (NPPB) were purchased from Tocris, Ellisville, MO. The prostaglandin receptor antagonists GW627368X, AH6809, and SC-19220 were purchased from Cayman Chemical, Ann Arbor, MI. Stock solutions were prepared in Krebs solution, deionized H2O or DMSO. Volumes added to 10-ml bath solutions did not exceed 10 μl.
RESULTS
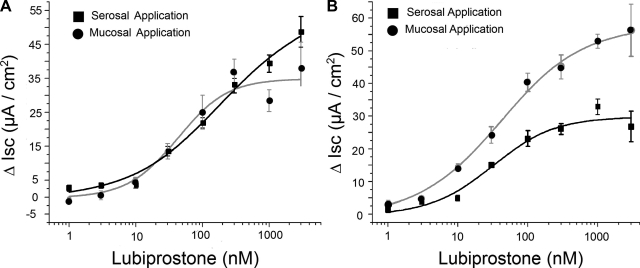
Changes in peak Isc were recorded as markers for electrogenic Cl− secretion in Ussing chambers to test the hypothesis that lubiprostone stimulates Cl− secretion in the whole bowel. Lubiprostone applied to the serosal side of six muscle-stripped ileal preparations in eight concentrations ranging from 1–3000 nM evoked increases in Isc from 2.6 ± 0.9 to 50.26 ± 4.5 μA/cm2 in a concentration-dependent manner, with an EC50 of 227.2 nM for serosal side application, and an increase in Isc from 0.78 ± 0.7 to 37.8 ± 7.9 μA/cm2, with an EC50 of 42.5 nM for mucosal side application (Fig. 1A). Lubiprostone applied to the serosal side of six muscle-stripped colonic preparations in eight concentrations ranging from 1 to 3000 nM evoked increases in Isc from 1.3 ± 0.7 to 26.8 ± 4.7 μA/cm2 in a concentration-dependent manner, with an EC50 of 31.7 nM for serosal side application, and an increase in Isc from 3.4 ± 0.9 μA/cm2 to 58.9 ± 12.3 μA/cm2, with an EC50 of 48.9 nM for mucosal side application (Fig. 1B). There was no significant time lag for the lubiprostone-evoked responses to reach their peak irrespective of mucosal or serosal side application.
Fig. 1.
Cumulative concentration-response curves for lubiprostone-evoked increases in short-circuit current (Isc) in guinea pig ileum (A) and colon (B). Lubiprostone applied to the serosal side compartment of the Ussing chambers evoked increases in Isc from 2.6 ± 0.9 to 50.26 ± 4.5 μA/cm2 in a concentration-dependent manner, with an EC50 of 227.2 nM, and an increase in Isc from 0.78 ± 0.7 to 37.8 ± 7.9 μA/cm2, with an EC50 of 42.5 nM for mucosal side application. Lubiprostone applied to the serosal side of colonic preparations evoked increases in Isc from 1.3 ± 0.7 to 26.8 ± 4.7 μA/cm2 in a concentration-dependent manner, with an EC50 of 31.7 nM for serosal side application, and an increase in Isc from 3.4 ± 0.9 to 58.9 ± 12.3 μA/cm2, with an EC50 of 48.9 nM for mucosal side application. Values are expressed as means ± SE, n = 6 animals in each series.
Figure 2 (see also Fig. 7A) shows that blockade of enteric neuronal conduction by 1 μM TTX, applied to the serosal side, had no significant effect on stimulation of Isc by serosally applied 300 μM lubiprostone (with TTX Isc = 28.5 ± 3.5 μA/cm2, n = 12 preparations; without TTX Isc = 32.8 ± 2.1 μA/cm2, n = 6 ileal preparations, P = 0.30). Substitution of gluconate for Cl− in the bathing solution abolished all responses to 300 nM lubiprostone when it was applied to the mucosal or serosal side of six ileal preparations (Fig. 2). Bumetanide (100 μM), an inhibitor of the basolateral Na/K/2Cl cotransporter, partially suppressed the increases in Isc evoked by 300 nM lubiprostone (Fig. 2). NPPB (200 μM), a nonspecific Cl− channel blocker, suppressed basal Isc from 244.8 ± 9.7 to 231.0 ± 24.3 μA/cm2 when added to the serosal compartment and from 214.6 ± 7.2 to 196.6 ± 14.5 μA/cm2 when added to the mucosal compartment. NPPB (200 μM) partially suppressed the increases in Isc evoked by 300 nM lubiprostone from 231.3 ± 9.1 μA/cm2 to 208 ± 9 μA/cm2 (ΔIsc = 22.4 ± 1.6 μA/cm2, Fig. 3) for trials in the serosal compartment and from 172 ± 8.08 μA/cm2 to 145.8 ± 7.6 (ΔIsc = −26.8 ± 5.6 μA/cm2, Fig. 3) in the mucosal compartment each for six muscle-stripped preparations from six animals.
Fig. 2.
Effects of tetrodotoxin (TTX), Cl-free media or bumetanide on lubiprostone-evoked Isc responses in guinea pig ileum. Placement of 1 μM TTX in the serosal compartment evoked a small negative change in Isc. Pretreatment with TTX (1 μM), applied to the serosal side, did not significantly alter the responses evoked by 300 nM lubiprostone (Lubi.) in the serosal compartment of the Ussing chamber (P = 0.30). Substitution of gluconate for Cl− in the serosal or mucosal compartment evoked small increases in Isc. Substitution of gluconate for Cl− in both compartments abolished all responses to 300 nM lubiprostone when applied in the serosal or mucosal compartment. Bumetanide (100 μM) in the mucosal compartment, an inhibitor of the basolateral Na/K/2Cl cotransporter, suppressed increases in Isc evoked by 300 nM lubiprostone when it was applied on either the mucosal (P < 0.01) or serosal side (P < 0.01). The responses to serosal and mucosal applications of lubiprostone were not different (P > 0.05) when bumetanide was present in the mucosal compartment. Bumetanide or TTX were applied 5 min before application of lubiprostone. Values are expressed as means ± SE, each mean is data for preparations from 6–12 animals. **P < 0.01; ***P < 0.001.
Fig. 3.
Effects of 5-nitro-2-2(-henylpropylamino) benzoic acid (NPPB) and DIDS on lubiprostone-evoked Isc in guinea pig ileum. NPPB (200 μM), a nonspecific chloride channel blocker, or DIDS (250 μM), a specific inhibitor of cellular anion permeability, was applied into the serosal or mucosal compartment of the Ussing chamber 5 min before adding 300 nM lubiprostone. NPPB abolished lubiprostone-evoked ΔIsc. DIDS did not alter lubiprostone-evoked increases in Isc. DIDS or NPPB was applied 5 min before application of lubiprostone. Values are expressed as means ± SE, n = 6 animals. ***P < 0.001.
DIDS, which acts specifically to suppress cellular anion permeability, had no significant effect on lubiprostone-evoked increases in Isc when applied to the serosal or mucosal compartment of the Ussing chambers (Fig. 3). CTFR(inh)-172 (10 μM), which is a selective and high-affinity inhibitor of the CFTR (9), did not suppress stimulation of Isc by 300 nM lubiprostone when applied to the mucosal or serosal compartment (Fig. 4). Application of the sulfonylurea glibenclamide (100 μM), which is a widely used and nonselective Cl− channel blocker that is also an open-channel blocker of the CFTR chloride channel (41), suppressed lubiprostone-evoked Isc by 59% and 47% when applied to the serosal or mucosal side, respectively (Fig. 4).
Fig. 4.
Effects of CTFR(inh)-172 and glibenclamide on lubiprostone-evoked Isc in guinea pig ileum. CTFR(inh)-172 (10 μM), is an inhibitor of CFTR with a Ki = 300 nM, or glibenclamide (100 μM), a nonspecific blocker of CFTR and ClC-2 channels, was applied into the serosal or mucosal compartment of the Ussing chamber 5 min before adding 300 nM lubiprostone. CTFR(inh)-172 did not significantly reduce the amplitude of lubiprostone-evoked Isc. Glibenclamide suppressed lubiprostone-evoked Isc by 59% and 47% when applied to the serosal or mucosal side, respectively. Values are expressed as means ± SE, n = number of animals. *P < 0.001.
Application of 10 nM VIP, which is a neurotransmitter released from secretomotor neurons at neuroepithelial junctions in the small and large intestine (44), evoked increases in Isc in a manner typical of earlier reports (Fig. 5A 1). Stimulation of Isc was unaffected by the presence of 1 μM TTX in the chamber, indicating a direct action of VIP at its receptors on the enterocytes, rather than stimulation of secretomotor neurons (Fig. 5, A1 and B). When 1 μM TTX and 300 nM lubiprostone were placed into the chamber before application of 10 nM VIP, the VIP-evoked increases, which were starting from a lubiprostone-evoked increase in baseline Isc, were reduced significantly (Fig. 5, A2 and B).
Fig. 5.
Effects of lubiprostone on carbachol or vasoactive intestinal peptide (VIP) stimulation of Isc in guinea pig ileum. In the presence of TTX, application of the muscarinic receptor agonist carbachol or VIP evoked increases in Isc, which reflected the direct actions at receptors for acetylcholine or VIP on the intestinal epithelium. Lubiprostone (300 nM) or vehicle was applied in the serosal compartment of the Ussing chamber 5 min after 1 μM TTX. Carbachol (1 μM) or VIP (10 nM) was applied in the serosal side of the chamber 6 min after application of lubiprostone. A: sample traces illustrate Isc responses to carbachol (A3, A4) or VIP (A1, A2) in the presence of TTX with or without lubiprostone pretreatment. B: lubiprostone augmented the stimulatory action of carbachol. Lubiprostone increased baseline Isc but suppressed the stimulatory action of VIP at its receptors on the epithelium. Values are expressed as means ± SE, n = 6 animals. **P < 0.01; ***P < 0.001.
Application of 1 μM carbachol, which is a muscarinic cholinergic receptor agonist in the small and large intestine (29, 30), evoked increases in Isc in a manner typical of earlier reports (Fig. 5A3). Stimulation of Isc was unaffected by the presence of 1 μM TTX in the chamber, indicating a direct action of carbachol on the enterocytes, rather than stimulation of secretomotor neurons (Fig. 5, A1 and B). When 1 μM TTX and 300 nM lubiprostone were placed into the chamber before application of 1 μM carbachol, carbachol-evoked increases in Isc were enhanced significantly (Fig. 5, A4 and B).
Transmural electrical stimulation.
Transmural EFS was used to activate the secretomotor innervation of the mucosal epithelium with the aim of exploring how lubiprostone might influence neurogenic electrolyte secretion. Transmural EFS evoked characteristic biphasic increases in Isc as reported by Cooke, Shonnard, and Wood (12) and Fang et al. (23) (Fig. 6). The first phase (i.e., phase 1) mainly reflects stimulation of cholinergic submucosal secretomotor neurons and action of acetylcholine (ACh) at muscarinic receptors expressed by the enterocytes (10). The second phase (i.e., phase 2) reflects Cl− secretion evoked by release of peptidergic/cholinergic neurotransmitters (15). Our protocol was to evoke the biphasic increases in Isc with EFS, add 300 nM lubiprostone in the bathing solution in the mucosal or serosal compartment of the chamber, and apply EFS a second time 20 min later. The presence of lubiprostone in either the mucosal or serosal compartment significantly increased the magnitude of the first phase of the biphasic response (Fig. 6, A and B). The magnitude of the second phase of the response was slightly but not significantly suppressed (P > 0.05) by lubiprostone (Fig. 6, A and B).
Fig. 6.
Action of lubiprostone on Isc evoked by transmural electrical field stimulation (EFS) in guinea pig ileum. Transmural EFS in evoked biphasic increases in Isc. The first phase 1 and the second phase 2 represent cholinergically evoked and putative peptidergic/cholinergic-evoked chloride secretion, respectively. Lubiprostone (300 nM) was applied into either the serosal or mucosal compartment of the Ussing chambers 20 min after EFS. When baseline Isc returned to a stable level, EFS was repeated. A: sample traces illustrating the Isc response to EFS before and after application of lubiprostone. B: lubiprostone significantly increased the magnitude of phase 1 of the response to EFS but had no significant effect on phase 2. Values are expressed as means ± SE, n = 6 animals. **P < 0.01; ***P < 0.001 (compared with response to EFS before lubiprostone).
Histamine.
Exposure of full-thickness preparations of guinea pig colon to histamine in Ussing chambers evokes recurrent cycles of elevated Isc that are driven by a CPG in the ENS and known to be initiated by excitatory action of histamine at the histamine H2 receptor subtype expressed by enteric neurons (13, 14, 49). Exposure to leukotrienes or to prostaglandins evokes similar recurrent Isc cycles (25, 26). The aim of this phase of the study was to investigate the action of lubiprostone on the histamine-evoked cyclic Isc behavior.
Exposure of full-thickness preparations of guinea pig colon to the histamine H2 receptor agonist, dimaprit (50 μM), in the serosal compartment evoked an increase in baseline Isc followed by rhythmic, cyclically occurring elevations of Isc (Fig. 7A). The action of dimaprit to increase baseline Isc was reversed to Isc values below baseline by addition of TTX (Fig. 7, A and B). Addition of lubiprostone, with dimaprit and TTX present, restored baseline Isc (Fig. 7, A and B). Application of carbachol at this point in the protocol stimulated Isc and served as a control for tissue viability.
Fig. 7.
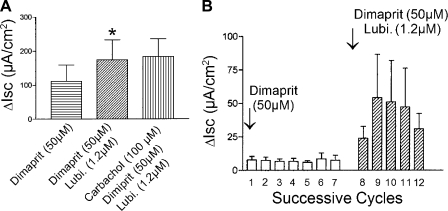
Lubiprostone action on cyclical changes in Isc evoked by the histamine H2 receptor agonist, dimaprit, in full-thickness preparations of guinea pig colon. A: exposure to dimaprit, in the serosal compartment of the Ussing chamber, evoked an initial increase in baseline Isc followed by cyclically occurring elevations of Isc superimposed on the elevated baseline. Blockade of intramural neuronal excitability by TTX in the presence of dimaprit reduced Isc to a subbaseline level and eliminated the cyclic changes in Isc. Lubiprostone (Lubi.) applied in the presence of dimaprit and TTX reversed the effects of neural blockade and elevated Isc beyond its previous baseline value. Carbachol, which was applied as a control for viability of the preparation, evoked maximal elevation of Isc when applied in the presence of dimaprit, TTX, and lubiprostone. B: quantitative data for the action of dimaprit alone, TTX action on the dimaprit response, action of lubiprostone in the presence of dimaprit and TTX, and action of carbachol. Values are expressed as means ± SE, n = 7 animals. ***P < 0.001 (compared with response to dimaprit alone).
The rhythmic Isc cycles evoked by dimaprit ranged from 91 ± 27 to 143 ± 24 μA/cm2 at a frequency of 0.20 ± 0.04 cycles/min for eight preparations (Fig. 7, A and B). The cyclic activity was abolished by 0.2 μM TTX, indicating that a functional enteric nervous system was necessary for the activity (Fig. 7, A and B). The mean amplitude of the dimaprit-evoked cycles was 109 ± 7 μA/cm2, and it increased to 135 ± 5 μA/cm2 (P < 0.009) after application of lubiprostone (Fig. 8, A and B). The cycle frequency was 0.20 ± 0.04 cycles/min and doubled in the presence of 1.2 μM lubiprostone, to 0.44 ± 0.5 cycles/min (P < 0.0001) (Fig. 8B).
Fig. 8.
Action of lubiprostone to enhance Isc responses evoked by the histamine H2 receptor agonist, dimaprit, in whole-thickness preparations from guinea pig colon. A: exposure to dimaprit, in the mucosal-side compartment of the Ussing chamber, stimulated basal Isc. Application of lubiprostone significantly enhanced the dimaprit-evoked stimulation of Isc (*P < 0.05; data for preparations from 7 animals). Carbachol, which was applied as a control for viability of the preparation, evoked maximal elevation of Isc when applied in the presence of dimaprit and lubiprostone. B: quantitative data for the amplitude of 7 successive “Isc spikes” recorded after application of dimaprit in the mucosal-side compartment for preparations from 7 animals. Application of lubiprostone, in the presence of dimaprit, resulted in significant enhancement of the cyclically occurring “Isc spikes”.
Neuronal action.
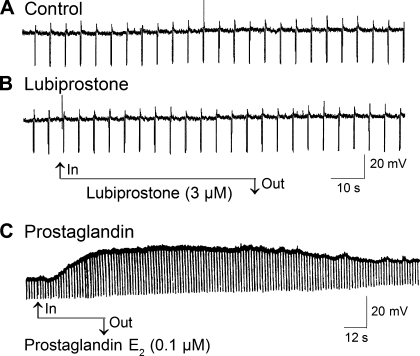
We impaled ganglion cells in the submucosal plexus of guinea pig small intestine with “sharp” glass microelectrodes and applied lubiprostone with the aim of investigating any possible actions on the excitability of enteric neurons. Application of lubiprostone in concentrations in a range of 0.3–30 μM did not evoke any change in the resting membrane potential nor the input resistance of nine neurons with S-type electrophysiological behavior and uniaxonal morphology (Fig. 9, A and B). Membrane potential before lubiprostone was −57.5 ± 7.0 mV and −57.5 ± 6.1 mV after lubiprostone (P > 0.05).
Fig. 9.
Neither resting membrane potential nor input resistance of submucosal secretomotor neurons was altered by lubiprostone as determined by microelectrode recording. A: control. B: absence of any action of lubiprostone on resting membrane potential or neuronal input resistance. C: application of prostaglandin E2 resulted in depolarization of the membrane potential and increased input resistance in an AH-type neuron. Downward deflections are electrotonic potentials evoked by repetitive injection of hyperpolarizing current pulses. An increase in the amplitude of the electrotonic potentials reflects increased input resistance, which is a marker for closing of membrane channels and increased ionic conductance. Lack of effect of lubiprostone on the amplitude of the electrotonic potentials indicates that it does not alter the neuronal input resistance. Prostaglandin E2 decreased membrane conductance in the neuron.
Prostaglandins.
In preparations from rats and humans, lubiprostone was reported to contract the stomach longitudinal muscle coat and inhibit neuronally mediated contractions of the colonic circular muscle coat. These actions were reported to be suppressed by an EP1 receptor antagonist and an EP4 receptor antagonist, respectively, which suggest that lubiprostone might act like a prostaglandin to stimulate EP receptors (3, 4). We tested this suggestion by comparing the electrophysiological responses of submucosal ganglion cells evoked by prostaglandins with responses evoked by lubiprostone. In contrast to lubiprostone, which did not alter neuronal excitability as described above, application of prostaglandins E2, F2, or I2 evoked depolarization of the resting membrane potential in association with increased input resistance in neurons with AH-type electrophysiological behavior and multipolar Dogiel II morphology and decreased input resistance in neurons with S-type electrophysiological behavior and uniaxonal morphology (Fig. 9C). Details of properties of enteric neurons classified as AH- and S-types are reviewed elsewhere (49–51). The PGE2-evoked membrane depolarization was concentration-dependent with an EC50 of 5.3 ± 0.8 nM, and the EC50 for PGI2 was 374.1 ± 39.3 nM and was 941.0 ± 240.9 nM for PGF2 (Fig. 10).
Fig. 10.
Concentration-response relationship for membrane depolarization evoked by prostaglandin E2 and its analogs in submucous plexus neurons. A and B: concentration-response relations for PGE2, PGF2, and PGI2. The curves were plotted with the least-squares fitting routine: V = Vmax/[1 + (EC50/C)nH], where V is the observed membrane depolarization, EC50 is the concentration which induces the half-maximal response, and nH is the apparent Hill coefficient. Each point represents 5–12 neurons.
We compared the actions of lubiprostone and prostaglandin E2 to stimulate Isc and found that 1.2 μM lubiprostone evoked changes in Isc that were about four times greater that the changes evoked by 2 μM PGE2 (Fig. 11, A and B). Pretreatment with a PGE2 receptor antagonist (SC-19220) suppressed the stimulation of Isc by PGE2. SC-19220 did not reduce significantly the stimulation of Isc by lubiprostone (Fig. 11B). Pretreatment with an EP1–3 antagonist (AH6890) or an EP4 antagonist (GW627368X) abolished stimulation of Isc by PGE2 (Fig. 11B). Neither AH6890 nor GW627368X reduced significantly the stimulation of Isc by lubiprostone (Fig. 11A).
Fig. 11.
Actions of mucosal application of prostaglandin receptor antagonists on stimulation of Isc by lubiprostone and prostaglandin E2 in full-thickness preparations of distal colon. A: neither the PGE2 receptor antagonist SC-19220, the EP1–3 receptor antagonist AH6809 nor the EP4 receptor antagonist GW627368X suppressed stimulation of Isc by lubiprostone when applied to the mucosa in preparations from 7 animals (P < 0.05). B: PGE2 receptor antagonist SC-19220, the EP1–3 receptor antagonist AH6890 and the EP4 receptor antagonist GW627368X each significantly suppressed (P > 0.05) or abolished stimulation of Isc by prostaglandin PGE2 in preparations from 3 animals.
DISCUSSION
The concentration dependence and EC50 values in the low nanomolar range for stimulation of Isc by lubiprostone in our study are reminiscent of the results of Bao et al. and Cuppoletti et al. (2, 16), who reported that application of low nanomolar concentrations of lubiprostone to confluent epithelial cell monolayers stimulated electrogenic Cl− secretion and Isc across the monolayers. Our finding that application of lubiprostone to either the mucosal or submucosal side of the preparations stimulated Isc was unexpected because the ClC-2 channels, on which lubiprostone is believed to act, are reported to be expressed in the apical membrane and should be opened by mucosal but not serosal application. Nevertheless, this was not the case for the guinea pig intestine or for T84 cell monolayers (16). The paths over which serosally applied lubiprostone reached the apical membranes on the mucosal side in our study are unclear. A leak pathway through “edge damage,” which has often been a concern for tissues set up in Ussing flux chambers, might be an explanation. On the other hand, DMSO, which was the vehicle, readily penetrates epithelia (e.g., skin) and might have transported lubiprostone from serosal to mucosal sides of the tissue.
Our results with lubiprostone at the whole organ level are consistent with the interpretation of results acquired by others at the cellular level with ClC-2-transfected HEK-293 cells and confluent T84 and A6 cell monolayers (2, 16). Lubiprostone opens epithelial Cl− channels, and this was reflected as stimulation of Isc in our Ussing chamber studies with both small and large intestinal preparations. Reduction of Cl− in the bathing medium, suppression of the basolateral Na/K/2Cl cotransporter, and application of the potent nonspecific Cl− channel blockers, NPPB and glibenclamide, each suppressed lubiprostone-evoked increases in Isc and are consistent collectively with opening of Cl− channels by lubiprostone. Stimulation of Isc by lubiprostone appeared not to involve opening of CFTR Cl− channels on the basis of failure of CFTR(inh)-172, which is thought to be a selective inhibitor of CFTR (9), to suppress stimulation of Isc by lubiprostone.
We did not specifically identify the involved Cl− channel. Nevertheless, discoveries (16) that intestine-derived T84 cells express the ClC-2 channel in their apical membranes and findings that lubiprostone in low nanomolar concentrations selectively increases conductance in the channels without any action on the CFTR Cl− channel, when expressed in HEK-293 and A6 cells, interpreted together with lack of suppression of lubiprostone action by CFTR(inh)-172, suggest that the action of lubiprostone on Isc in our study was most likely at the ClC-2 channel. Moreover, glibenclamide is reported to be nearly as effective in blocking ClC-2 as CFTR (2), which suggests that suppression of lubiprostone-evoked Isc by glibenclamide might have reflected blocking action at ClC-2 and/or CFTR.
We interpret the stimulatory action of lubiprostone on Isc to be a reflection of a direct action on the mucosal epithelium because it was unaffected by the nerve-blocking agent, TTX. Had its action been an indirect effect attributable to stimulation of submucosal secretomotor neurons and release of excitatory neurotransmitters at the neuroepithelial junctions, then TTX, which blocks conduction of impulses in axons of enteric neurons, would have suppressed the response. Moreover, absence of evidence for any excitatory action of lubiprostone on submucosal ganglion cells is consistent with a nonneuronal mechanism of action. It is unlikely also that the action reflected stimulation of paracrine release of mediators from nonneural sources, such as enteric mast cells, enteroendocrine cells, and/or enterochromaffin cells. Microelectrode studies in enteric neurons document that release of these kinds of paracrine mediators (e.g., histamine, serotonin, and eicosanoids) in food allergies and inflammatory states is reflected by excitation of the neurons (51, 52). Stimulation of Isc by lubiprostone was not accompanied by excitation of the neurons in our microelectrode studies, which suggests that its action was not mediated by release and action of paracrine mediators at the epithelial level.
Transmural EFS.
Transmural electrical stimulation of guinea pig flat-sheet intestinal preparations, in the present study, evoked increases in Isc in a similar manner to observations in a variety of other species including mice (8), rabbit (27), and human (28). The biphasic responses to EFS in the guinea pig reflect the release of multiple neurotransmitters from secretomotor neurons at neuroepithelial junctions and release of transmitters from neurons that provide excitatory synaptic input to secretomotor neurons. ACh and VIP are main neurotransmitters released by the secretomotor neurons. The action of ACh is at muscarinic M3 receptors, and signal transduction involves elevation of cytosolic Ca2+ in the enterocytes and activation of PKC (10, 17, 19, 40). Signal transduction for VIP differs from that for ACh and involves stimulation of adenylate cyclase, elevation of cAMP, and stimulation of PKA in the enterocytes (22, 45). The first phase of the biphasic response to EFS in the guinea pig is mediated mainly, if not entirely, by the release of ACh and is mimicked by application of the muscarinic agonist carbachol. The second phase has a peptidergic component that, most likely, includes VIP as a major contributor (15).
Lubiprostone, in the present study, enhanced the first phase of the EFS-evoked response and marginally suppressed the second phase. Its actions on responses to carbachol, which represents the first phase, and VIP, which represents the second phase, were discordant in that responses to carbachol were enhanced and responses to VIP were suppressed. These discordant effects likely reflect a direct action of lubiprostone on the epithelium because lubiprostone had no direct action on the secretomotor neurons.
Phase 1 of EFS-evoked Isc responses in the guinea pig ileum is mediated by the release of ACh and its action at muscarinic M3 receptors expressed by the enterocytes (6, 34). Signal transduction for M3-mediated activation of Cl− secretion involves elevation of Ca2+ in the enterocytes and stimulation of PKC (19). Absence of evidence for opening of ClC-2 channels by elevation of intracellular Ca2+ makes ClC-2 an unlikely candidate for explanation of the enhancing effects of lubiprostone on the muscarinic-evoked first phase. In salivary glands, ClC-2 channels are inwardly rectifying channels that are opened by membrane hyperpolarization and not opened by elevation of intracellular Ca2+ (1). An explanation for the action of lubiprostone on phase 1 of the EFS-evoked Isc responses might be that direct opening of ClC-2 channels by lubiprostone adds a Cl− secretory component, which becomes synergistic with a separate Ca2+-activated Cl− component, both of which enhance cholinergically evoked Cl− secretion recorded as elevated Isc.
Suppression by lubiprostone of VIP stimulation of Isc might be explained by opening of the same set of channels by both substances. Both lubiprostone and VIP open ClC-2 channels in the apical membranes. Lubiprostone does this by directly opening the channels. On the other hand, channel opening by VIP is by a different metabotropic mechanism operating through stimulation of PKA and channel phosphorylation (17, 40). Activation of transfected ClC-2 channels by lubiprostone is reported to be independent of PKA in HEK-293 cells (16). Lubiprostone opening of the same ClC-2 channels that are required for stimulation of Isc by VIP can be expected to blunt the response to VIP whether it is released as a neurotransmitter or exogenously applied, as was found in our study.
Histamine.
The recurrent cycles of elevated Isc evoked by histamine H2 receptor stimulation reflected cyclic increases in electrogenic Cl− secretion that were essentially the same as described in earlier reports (13, 14, 49). The timing, spontaneously recurrent nature, and persistence of the secretory pattern resembled the recurrent patterns of behavior of the intestinal musculature in stretched flat-sheet preparations, rhythmic segmentation-like muscular movements evoked by mucosal exposure to fatty acids, and the recurrent propulsive contractile waves that continue during sustained distension of intestinal segments (50). Outputs from neural networks with CPGs behave in like manner. CPGs in the ENS are postulated to underlie the sustained rhythmic behavior found in intestinal preparations in vitro (50). CPGs are well-known neural circuits, which generate organized and repetitive motor patterns independent of sensory input in many kinds of independent integrative nervous systems like the ENS (37–39).
Guinea pig intestinal preparations in Ussing chambers exhibit a persistent basal Isc, which reflects the tonic discharge of secretomotor neurons in the submucosal plexus (7). TTX blocks the secretomotor activity and accounts for the fall in baseline Isc after TTX (see Figs. 5A, 7A). Neural blockade by TTX in the networks, which are responsible for pattern generation, stops the recurrent secretory behavior evoked by histamine H2 receptor agonists (see Fig. 7A). Lubiprostone appeared not to influence the output of the CPG in the preparations of the present study. On the other hand, it enhanced the peak amplitude of each cycle by elevating the basal Isc on which each Isc cycle was superimposed.
Prostaglandins.
Our results do not support the suggestion in the reports of Bassil et al. (3, 4) that actions of lubiprostone on gastrointestinal motility and perhaps mucosal secretion reflect stimulation of prostaglandin EP receptors. We found that intraneuronal microelectrodes recorded excitatory responses (i.e., membrane depolarization and spike discharge) in submucosal ganglion cells in response to each of three different prostaglandins. The excitatory responses to each of these prostaglandins were similar to the responses to prostaglandin E2 in myenteric neurons reported by others for guinea pig ileum (18). Unlike the prostaglandins, lubiprostone had no effect on the electrophysiology of the enteric neurons in the present study. This and failure of TTX to suppress the secretory responses to lubiprostone are inconsistent with a prostaglandin-like action of lubiprostone on secretomotor neurons.
Intramural prostanoids are released by a variety of cell types in the intestine (e.g., mast cells, activated phagocytes, endothelial cells, and several others). Prostaglandins D2, E2, F2α, and I2 are known stimulators of intestinal Cl− and bicarbonate secretion (5, 20, 21, 34, 43). These kinds of prostaglandin-evoked secretory responses reflect interplay of actions on both epithelial cells and enteric neurons (18, 30). Failure of TTX to suppress responses to lubiprostone suggests that it did not release prostaglandins to act on secretomotor neurons, nor did it act directly to stimulate the neurons.
A suggestion that the action of lubiprostone to stimulate Isc reflects direct stimulation of prostaglandin receptors expressed by enterocytes is not supported by our results. Support would require that prostaglandin receptor antagonists suppress responses to both a prostaglandin and lubiprostone. We found this not to be the case. Responses to PGE2 were suppressed or abolished by three different selective prostaglandin receptor antagonists. The same receptor antagonists did not suppress significantly the stimulation of Isc by lubiprostone.
Conclusions.
Lubiprostone stimulates electrogenic Cl− secretion in the intestinal mucosa, which is expected to increase the liquidity (i.e., H2O content) of the luminal contents in the functional animal. Elevation of luminal liquidity in patients treated with lubiprostone is likely to be a contributing factor in its efficacy in treatment of chronic constipation and constipation-predominant irritable bowel syndrome (31–33). The Cl− channels involved in the action of lubiprostone are probably ClC-2 channels. The action is not mediated by stimulation of enteric neurons and differs from the actions of prostaglandins.
GRANTS
This work was supported by National Institutes of Health grants RO1 DK 37238 and RO1 DK 57075 to J. Wood, KO8 DK60468 to Y. Xia, a Pharmaceutical Manufacturers of America Foundation Postdoctoral Fellowship to S. Liu, and a grant-in-aid from Sucampo Pharmaceuticals.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arreola J, Park K, Melvin JE, Begenisich T. Three distinct chloride channels control anion movements in rat parotid acinar cells. J Physiol 490: 351–362, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G234–G251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassil AK, Thangiah R, Borman RA, Jarvie EM, Vivekanandan S, Lalude O, Sung EZ, Baragwanath P, Wong LS, Nwokolo CU, Lee K, Sanger GJ. Effect of lubiprostone on rat and human colon muscle: possible involvement of prostaglandin EP receptors (Abstract). Gastroenterology 132: A455, M2123, 2007. [Google Scholar]
- 5.Calderaro V, Giovane A, De Simone B, Camussi G, Rossiello R, Quagliuolo L, Servillo L, Taccone W, Giordano C, Balestrieri C. Arachidonic acid metabolites and chloride secretion in rabbit distal colonic mucosa. Am J Physiol Gastrointest Liver Physiol 261: G443–G450, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Carey HV, Tien XY, Wallace LJ, Cooke HJ. Muscarinic receptor subtypes mediating the mucosal response to neural stimulation of guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 253: G323–G329, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Carey HV, Cooke HJ. Tonic activity of submucosal neurons influences basal ion transport. Life Sci 44: 1083–1088, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Carey HV, Cooke HJ. Influence of enteric nerves on jejunal function of the piebald-lethal mouse (Abstract). Gastroenterology 86: A1040, 1984. [Google Scholar]
- 9.Caci E, Caputo A, Hinzpeter A, Arous N, Fanen P, Sonawane N, Verkman AS, Ravazzolo R, Zegarra-Moran O, Galietta LJ. Evidence for direct CFTR inhibition by CFTR(inh)-172 based on Arg347 mutagenesis. Biochem J 413: 135–142, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cooke HJ Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 246: G263–G267, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Cooke HJ, Shonnard K, Highison G, Wood JD. Effects of neurotransmitter release on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 245: G745–G750, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Cooke HJ, Shonnard K, Wood JD. Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 245: G290–G296, 1983. [DOI] [PubMed] [Google Scholar]
- 13.Cooke HJ, Wang YZ, Reddix R, Javed N. Cholinergic and VIP-ergic pathways mediate histamine H2 receptor-induced cyclical secretion in the guinea pig colon. Am J Physiol Gastrointest Liver Physiol 268: G465–G470, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Cooke HJ, Wang YZ, Rogers R. Coordination of Cl− secretion and contraction by a histamine H2-receptor agonist in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 265: G973–G978, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Cooke HJ Neural and humoral regulation of small intestinal electrolyte transport. In: Physiology of the Gastrointestinal Tract. New York: Raven Press, 1987, p. 1307–1350.
- 16.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekkers JA, Akkermans LM, Kroese AB. Effects of the inflammatory mediator prostaglandin E2 on myenteric neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 272: G1451–G1456, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson KE, Frizzell RA, Sekar MC. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol 225: 291–298, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Diener M, Bridges RJ, Knobloch SF, Rummel W. Neuronally mediated and direct effects of prostaglandins on ion transport in rat colon descendents. Naunyn Schmiedebergs Arch Pharmacol 337: 74–78, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Diener M, Nobles M, Schmitt C, Rummel W. Characterization of the antisecretory action of prostaglandin D2 in the rat colon. Acta Physiol Scand 145: 19–24, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Eklund S, Brunsson I, Jodal M, Lundgren O. Changes in cyclic 3′5′-adenosine monophosphate tissue concentration and net fluid transport in the cat's small intestine elicited by cholera toxin, arachidonic acid, vasoactive intestinal polypeptide and 5-hydroxytryptamine. Acta Physiol Scand 129: 115–125, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Hu HZ, Gao N, Liu S, Wang GD, Wang XY, Xia Y, Wood JD. Neurogenic secretion mediated by the purinergic P2Y1 receptor in guinea-pig small intestine. Eur J Pharmacol 536: 113–122, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Fei G, Liu S, Wang GD, Qu MH, Wang XY, Zou F, Du Y, Xia Y, Bohn L, Wood JD. Stimulation of mucosal secretion by lubiprostone in guinea-pig small intestine and colon (Abstract). Gastroenterology 132: A683, W1206, 2007. [Google Scholar]
- 25.Frieling T, Becker K, Rupprecht C, Dobreva G, Haussinger D, Schemann M. Leukotriene-evoked cyclic chloride secretion is mediated by enteric neuronal modulation in guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol 355: 625–630, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Frieling T, Rupprecht C, Dobreva G, Schemann M. Differential effects of 5 inflammatory mediators on ion secretion in the guinea-pig colon. Comp Biochem Physiol A Physiol 118: 341–343, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Hubel KA The effects of electrical field stimulation and tetrodotoxin on ion transport by the isolated rabbit ileum. J Clin Invest 62: 1039–1047, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubel KA, Shirazi S. Human ileal ion transport in vitro: changes with electrical field stimulation and tetrodotoxin. Gastroenterology 83: 63–68, 1982. [PubMed] [Google Scholar]
- 29.Javed NH, Cooke HJ. Acetylcholine release from colonic submucous neurons associated with chloride secretion in the guinea pig. Am J Physiol Gastrointest Liver Physiol 262: G131–G136, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Javed NH, Wang YZ, Cooke HJ. Neuroimmune interactions: role for cholinergic neurons in intestinal anaphylaxis. Am J Physiol Gastrointest Liver Physiol 263: G847–G852, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Johanson JF, Drossman DA, Panas R, Wahle A, Ueno R. Clinical trial: phase 2 trial of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Ther 27: 685–696, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Johanson JF, Morton D, Geenen J, Ueno R. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of lubiprostone, a locally-acting type-2 chloride channel activator, in patients with chronic constipation. Am J Gastroenterol 103: 170–177, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Johanson JF, Ueno R. Lubiprostone, a locally acting chloride channel activator, in adult patients with chronic constipation: a double-blind, placebo-controlled, dose-ranging study to evaluate efficacy and safety. Aliment Pharmacol Ther 25: 1351–1361, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Kachur JF, Sturm BL, Gaginella TS, Noronha-Blob L. Regulation of guinea pig ileal electrolyte transport by M3-muscarinic acetylcholine receptors in vitro. Mol Pharmacol 38: 836–840, 1990. [PubMed] [Google Scholar]
- 35.Keenan CM, Rangachari PK. Contrasting effects of PGE2 and PGD2: ion transport in the canine proximal colon. Am J Physiol Gastrointest Liver Physiol 260: G481–G488, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol 41: 345–351, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol 15: R685–R699, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996. [DOI] [PubMed] [Google Scholar]
- 40.McCabe RD, Dharmsathaphorn K. Mechanism of VIP-stimulated chloride secretion by intestinal epithelial cells. Ann NY Acad Sci 527: 326–345, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Melin P, Hosy E, Vivaudou M, Becq F. CFTR inhibition by glibenclamide requires a positive charge in cytoplasmic loop three. Biochim Biophys Acta 1768: 2438–2446, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Park K, Begenisich T, Melvin JE. Protein kinase A activation phosphorylates the rat ClC-2 Cl− channel but does not change activity. J Membr Biol 182: 31–37, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Rask-Madsen J, Bukhave K, Beubler E. Influence on intestinal secretion of eicosanoids. J Intern Med Suppl 732: 137–144, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Reddix R, Kuhawara A, Wallace L, Cooke HJ. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. J Pharmacol Exp Ther 269: 1124–1129, 1994. [PubMed] [Google Scholar]
- 45.Schwartz CJ, Kimberg DV, Sheerin HE, Field M, Said SI. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest 54: 536–544, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun X, Xia Y, Liu S, Wang YZ, Qu MH, Wang XY, Wang GD, Needleman B, Mikami DJ, Melvin WS, Bohn LM, Ueno R, Wood JD. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in the human jejunum (Abstract). Gastroenterology 844: A122 2008. [Google Scholar]
- 47.Tewari KP, Malinowska DH, Sherry AM, Cuppoletti J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am J Physiol Cell Physiol 279: C40–C50, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Ueno R, Osama H, Habe T, Engelke K, Patchen M. Oral SPI-0211 increases intestinal fluid secretion and chloride concentration without altering serum electrolyte levels (Abstract). Gastroenterology 126: A298-AM1109, 2004. [Google Scholar]
- 49.Wang YZ, Cooke HJ. H2 receptors mediate cyclical chloride secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 258: G887–G893, 1990. [DOI] [PubMed] [Google Scholar]
- 50.Wood JD Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Wood JD Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 127: 635–657, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Wood JD Cellular neurophysiology of enteric neurons. In: Physiology of the Gastrointestinal Tract. San Diego: Elsevier Academic, 2006, p. 629–664.
- 53.Zafirov DH, Cooke HJ, Wood JD. Elevation of cAMP facilitates noradrenergic transmission in submucous neurons of guinea-pig ileum. Am J Physiol Gastrointest Liver Physiol 264: G442–G446, 1993. [DOI] [PubMed] [Google Scholar]