Abstract
Stem cells hold great promise for regenerative medicine, but have remained elusive in many tissues because of a lack of adequate definitive markers. Progress in mouse genetics has provided the tools for characterization and validation of stem cell markers by functional and/or lineage tracing assays. The Wnt target gene Lgr5 has been recently identified as a novel stem cell marker of the intestinal epithelium and the hair follicle. In the intestine, Lgr5 is exclusively expressed in cycling crypt base columnar cells. Genetic lineage-tracing experiments revealed that crypt base columnar cells are capable of self-renewal and multipotency, thus representing genuine intestinal stem cells. In the stem cell niche of the murine hair follicle, Lgr5 is expressed in actively cycling cells. Transplantation and lineage tracing experiments have demonstrated that these Lgr5+ve cells maintain all cell lineages of the hair follicle throughout long periods of time and can build entire new hair follicles. Expression of Lgr5 in multiple other organs indicates that it may represent a global marker of adult stem cells. This review attempts to provide a comprehensive overview of the stem cell compartments in the intestine and skin with a focus on the cycling, yet long-lived and multipotent, Lgr5+ve stem cell populations.
Stem cells (SCs) are fundamental cornerstones in tissue biology, ensuring homeostatic self-renewal of tissues such as the skin, the blood, or the intestinal epithelium throughout life, but also presenting a reserve pool of cells that can be activated after tissue injury. A SC pool can be defined by a minimal set of two properties: the ability to maintain itself throughout long periods of time (self-renewal) and the potential to generate all differentiated cell types of the pertinent tissue (multipotency). When SCs divide, they are believed to undergo an asymmetric cell division into a new SC plus a committed daughter cell. The rapidly cycling daughter cells, also called transit-amplifying (TA) cells, then undergo a limited number of cell divisions before they terminally differentiate building tissue mass.
The gold standard for accurate identification of SCs is to assess their minimal properties, i.e., self-renewal and multipotency, in vitro and in vivo. Two strategies have been widely used in the identification of candidate SC populations. In the first, the transplantation strategy, SCs are isolated based on the expression of molecular markers followed by in vitro culture and/or transplantation into recipient animals. In the second strategy, SCs are genetically marked in situ, after which the introduced marker allows the visualization of the modified SC and its clonal offspring throughout time.
The identification of specific cell-surface markers has allowed the detection of pluripotential SCs in a number of tissues, including the bone marrow1 and the mammary gland,2,3 as well as the isolation and transplantation of these SCs. Hematopoietic (bone marrow) SCs (CD150+CD48−Sca-1+Lineage−c-kit+ in mice and CD34+CD38− in humans),1 which give rise to all types of mature blood cells, reside within specialized niches in the bone marrow but can also undergo hematopoiesis in the spleen and the liver during periods of hematopoietic stress. Mammary SCs and their TA progeny reside in the basement membrane of the mammary gland duct and have the capacity to form epithelial precursors that are committed to either a ductal or alveolar fate. Two groups recently identified and purified a population of mammary cells (Lin−, α6 or β1High, and CD24+), which are enriched for SCs.2,3 Transplantation of these cells tagged with a lacZ transgene into cleared fat pads of recipient animals unequivocally demonstrated that a single isolated mammary gland SC can regrow an entire mammary gland.
The lack of appropriate SC markers for many mammalian tissues, however, presents a formidable obstacle in the identification of individual SC types. Gene markers used for identification and discrimination of SCs should only be applied once they have been validated by lineage tracing and/or transplantation experiments. In the absence of definitive SC markers, surrogate markers of stemness such as preferential DNA label retention have been used. They are based on the assumption that SCs are quiescent, i.e., dividing much more slowly than their progeny. Alternatively, the immortal strand hypothesis proposed by Cairns4 35 years ago states that SCs selectively retain their original DNA strands, while donating the newly synthesized DNA strand to their TA daughters, thus retaining DNA labels. Little solid evidence exists to support the notion that quiescence and/or asymmetric cell division constitute central properties of mammalian SCs. Actually, surrogate markers such as label retention have recently been proven to be unreliable when compared with definitive independent markers.5,6
In this review, we summarize the progress made in the identification of adult SCs based on expression of the gene marker Lgr5. We will focus on the SCs of the intestine and the hair follicle (HF), and discuss the role of Wnt signaling in the control of SC maintenance in these tissues.
Intestinal SCs
The intestinal tract is covered by a simple epithelium whose primary function is digestion and absorption of nutrients. The intestinal epithelium is regenerated throughout adult life, with vigorous proliferation occurring in crypt compartments. In the mouse, the small intestinal epithelium is renewed every 5 days. This process is fueled by a SC compartment that has long been known to reside near the crypt bottom. The definitive identification of intestinal SCs, however, has been hampered by a lack of unique molecular markers.
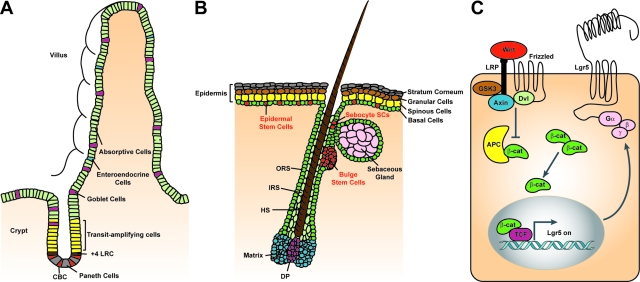
Two models regarding the exact identity of the intestinal SCs were formulated at approximately the same time, some 30 years ago: the +4 position model and the SC zone model. Both concepts are based on the assumption that every crypt contains approximately six independent SCs. The +4 position hypothesis has been based on the view that the crypt constitutes a tube of proliferating cells bounded from below by noncycling Paneth cells. The intestinal SCs would reside directly above these Paneth cells, at the so-called +4 position relative to the crypt bottom (Figure 1A). Potten and colleagues7,8 reported that these +4 cells retain DNA labels throughout long periods of time. In addition, the +4 cells are extremely radiation-sensitive, a property that would functionally protect the SC compartment from genetic damage.
Figure 1.
Epithelial SC compartments in the mammalian intestine and the skin and Wnt signaling in SCs. A: The intestinal crypt-villus unit. Intestinal epithelial SCs are thought to reside at the base of the crypt, either in the +4 position counting from the bottom of the crypt (brown) directly above the Paneth cells (gray), or as CBC cells (red) located between the Paneth cells. Recent evidence demonstrates that Lgr5 marks these rapidly cycling CBC cells, which can both self-renew and give rise to TA progenitor cells (yellow). The intestinal TA cells differentiate into four lineages—long-lived Paneth cells (gray) that occupy the base of the crypt and the enteroendocrine cells (blue), goblet cells (purple). and enterocytes (green) that move upwards and cover the villus. B: The skin. Three distinct niches for multipotent epidermal SCs exist—the bulge region of the HF, the base of the SG, and the basal layer of the IFE. SCs of these niches (red) are essential for the self-renewal of their pertinent tissue during normal homeostasis. The IFE SCs give rise to rapidly cycling keratinocytes that differentiate while moving upward to form the spinous layer, granular layer, and stratum corneum. Sebocyte SCs reside near the base of the SG. They give rise to proliferative progeny that differentiates into lipid-filled sebocytes. SCs of the HF are located within the bulge area. After the first and all subsequent hair cycles, bulge cells generate cells of the outer root sheath (ORS), which is thought to contain SCs that continue to migrate down to the follicle base and fuel the proliferative compartment of TA cells located at the base of the hair (matrix cells, blue) and adjacent to the dermal papilla (DP, purple). Matrix cells differentiate to form the various layers of the inner root sheath (IRS) and the hair shaft (HS). Lgr5+ve rapidly cycling cells reside in the bulge and the ORS of the growing HF and give rise to all of the newly formed cells of HF. C: Canonical Wnt signaling in SCs. SCs in the intestine and the skin are regulated by Wnt signaling. Wnt ligands bind to the Frizzled/LRP co-receptor complex and activate the canonical signaling pathway. Axin is recruited to the plasma membrane resulting in the inactivation of the APC destruction complex and subsequent stabilization of β-catenin. β-catenin translocates to the nucleus, where it binds Tcf transcription factors, thus activating the Wnt target gene Lgr5. Lgr5 encodes a seven-transmembrane protein with a large extracellular domain for ligand binding and a short cytoplasmic tail for coupling to G proteins.
The second (SC zone) hypothesis was put forward after the identification of a unique cell type populating the crypt bottom. These so-called crypt base columnar (CBC) cells are small cycling cells interwedged between the Paneth cells (Figure 1A). Originally based on morphological considerations9 but recently also on clonal marking techniques,10 Bjerknes and Cheng11 proposed that these CBC cells represent the true intestinal SCs. Neither of these two hypotheses on intestinal SC identity, however, was supported by direct evidence for stemness such as lineage tracing and/or transplantation.
Skin SCs
The skin epidermis and its appendages—the HFs and associated sebaceous glands (SGs)—serve as a protective barrier against external environmental insults and prevent dehydration. These tissues undergo continuous turnover during adult life and engage in wound repair in response to injury. Homeostasis of the skin is ensured by epidermal SCs. Three distinct niches for multipotent epidermal SCs exist—the bulge region of the HF, the base of the SG, and the basal layer of the interfollicular epidermis (IFE) (Figure 1B).12,13,14 SCs in these niches have the ability to self-renew and to generate daughter cells that differentiate along the lineages of the HF, the SG, and the IFE, respectively. Under conditions in which tissue homeostasis is disrupted (e.g., during wound healing or after engraftment), epidermal SCs are capable of generating daughter cells that differentiate along any of the epidermal lineages.15,16,17,18
The HF undergoes a tightly regulated pattern of cyclical regeneration with phases of active growth (anagen), regression (catagen), and rest (telogen).19 Long-lived, infrequently dividing HF SCs reside in the bulge region of the permanent part of the HF.13,14,17,18 When activated to form a new hair during anagen, a cluster of SCs at the base of the bulge exits the niche, becomes proliferative, and generates all of the epithelial cells of the newly formed HF.17,18 Mouse HF SCs residing in the bulge are characterized by expression of the CD34 cell surface marker and expression of cytokeratin 15 (K15)16,17,20 and can retain DNA labels throughout long periods of time.18,21
The IFE is composed of a single layer of proliferative cells that gives rise to stratified layers of terminally differentiated keratinocytes, which are sloughed off from the skin surface and continually replaced by younger cells moving outwards. Genetic lineage tracing analysis has revealed that the IFE contains a population of SCs that is distinct from those of the HF and responsible for maintaining normal homeostasis.15 After wounding, however, bulge SCs are activated and contribute to the formation of new IFE.
Even though the existence of SCs in the epidermis has been known for years, their exact identity has remained elusive. Classically, it was proposed that a small number of slow-cycling epidermal SCs resided in the basal layer of the epidermis and were organized in epidermal proliferating units.12 These SCs were thought to undergo infrequent symmetrical divisions that gave rise to a large number of transiently amplifying (TA) cells, which differentiated after a limited number of divisions. However, recent studies have reported the occurrence of asymmetrical division in basal layer cells22,23 and suggested that, contrary to the stem/TA-cell model, all cells within the basal layer have SC abilities. These cells comprise a single population of progenitor cells, the so-called committed progenitor cells, which maintain the epidermis by undergoing an unlimited number of symmetrical as well as asymmetrical divisions.
The SG develops late in mouse embryogenesis in the upper portion of the HF. Its major role is the generation of terminally differentiated sebocytes, which degenerate to release lipids and sebum to lubricate the skin surface. Mature sebocytes are constantly regenerated during adult life, requiring a continuous source of cells. Several studies support the view that slow-cycling progenitors/SCs exist within the SG, maintaining this structure independently of the HF bulge.16,17,21 Expression of the transcriptional repressor Blimp-1 specifically marks progenitor cells at the base of the SG, which in genetic lineage tracing experiments give rise to all cells of the SG.24
Wnt Signaling in Adult SCs of the Intestine and HF
SCs are believed to reside within restricted tissue microenvironments known as niches. This belief is based on the observation that transplanted SCs survive and grow only in particular tissue locations. SC niches generate signals that regulate the maintenance of SCs and regulate their function, controlling the crucial choice between self-renewal and the initiation of differentiation. The SC niche plays an important role in regulating the number of daughter cells that retain SC identity and in blocking expansion of the SC pool, thus preventing both exhaustion of the SC pool and tumor formation. Interestingly, the ability to culture isolated SCs has shown that the niche is not necessary for SC survival in vitro.2,3
The Wnt signaling pathway plays an important and perhaps universal role in the maintenance and the activation of proliferation of SC reservoirs.25 There is strong genetic evidence that Wnt signaling plays critical roles in the regulation of epithelial SCs in the intestinal tract2,26 and the HF.14 Progenitors at the bottom of the intestinal crypt accumulate nuclear β-catenin, a hallmark of active Wnt signaling.27 Abrogation of Wnt signaling by removal of Tcf4 or β-catenin or by overexpression of the Wnt inhibitor Dickkopf 1 (Dkk-1) results in a complete loss of proliferation, supporting the notion that Wnt signaling is the dominant force behind proliferative activity in intestinal crypts.26,28,29 Mutational activation of the Wnt pathway by loss of the tumor suppressor gene APC results in adenomatous transformation of the epithelium,30 and is the principal cause of colon cancer in humans.31,32
Canonical Wnt signaling has also been implicated in the regulation of HF bulge SCs.33 Inhibition of the Wnt pathway by ectopic expression of Dkk-1 or conditional ablation of β-catenin results in loss of HFs in adult skin.34,35 In the converse experiment, constitutive activation of β-catenin in transgenic mice results in de novo hair morphogenesis.36 Transient, low-level expression of activated β-catenin induces quiescent SCs to re-enter the cell cycle.37 Overexpression of Tcf3 on the other hand suppresses all three epithelial lineages in the skin,38 underscoring that SC fate in skin is intricately linked to changes in Wnt signaling. A current model for Wnt regulation of HF SCs states that when levels of nuclear β-catenin are low, Tcf3 can act to maintain bulge SCs in their undifferentiated state, but as the levels rise, SCs exit the niche, activate Wnt target genes, proliferate, and differentiate along the HF lineage. Wnt signaling in SCs of the skin appears to be critical not only for regulating tissue homeostasis but also for wound repair.39,40 Elevated levels of Wnt signaling have been reported in wounded skin, leading to the induction of de novo HF formation from epidermal SCs.39
Lgr5 Marks Adult Intestinal SCs
Insights into the molecular machinery controlling self-renewal and cancer allow a rational approach to the identification of SCs and their molecular markers. The unique and instrumental role of the Wnt signaling pathway in the physiology of the intestine suggested that one or more Wnt target genes could present SC markers. The genetic program inappropriately activated in human colon cancer cells on APC loss is equivalent to that physiologically expressed in the proliferative cells of healthy crypts.27 This intestinal Wnt target gene program consists of a core of ∼80 Wnt target genes.41 Even though the great majority of these genes are expressed throughout the proliferative crypt compartment, a subset of these genes shows a much more restricted expression. One of these latter genes is Lgr5/Gpr49 (Figure 1C).
The Lgr5 gene encodes an orphan G protein-coupled receptor characterized by a large leucine-rich extracellular domain and seven transmembrane domain. The protein is closely related to TSH, FSH, and LH receptors,42 which have glycoprotein ligands. Lgr5 was initially identified as a Wnt target gene expressed in colon cancer,27,41 but has also been recently shown to be overexpressed in cancers of the ovary and liver.43,44 Lgr5 displays a complex expression pattern during embryogenesis, yet expression in most tissues subsides around birth (N. Barker et al, unpublished data). In adult mice, Lgr5 expression is restricted to rare, scattered cells in the intestine, HF, eye, brain, mammary gland, reproductive organs, and stomach.2 Mice deficient for Lgr5 exhibit a malformation of the tongue and the lower jaw, causing newborns to swallow air, leading to early neonatal death.45
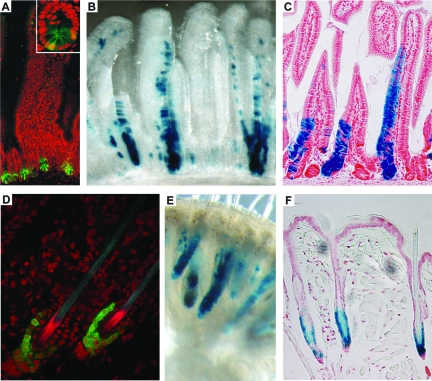
In situ hybridization on small intestinal tissues revealed that Lgr5 expression is restricted to the crypt bottom, a pattern reminiscent of the CBC cells mentioned above (Figure 2A). A knock-in allele, in which LacZ was intergrated just N-terminal to the first transmembrane domain of Lgr5, confirmed the Lgr5 expression pattern in CBC cells of the small intestine and at the base of the colonic crypt.2 Of note, the Lgr5-positive CBC cells are actively dividing with a cell cycle time in the order of 1 day.
Figure 2.
Lgr5 expression in the small intestine and the HF. A: Lgr5 expression is restricted to CBC cells interspersed between the Paneth cells at the base of the crypt (inset) of the small intestine of Lgr5-GFP knockin mice. Magnification = ×20 (inset ×60). B and C: Lineage tracing in Lgr5-GFP-Ires-CreERT2/Rosa26-LacZ mice induced for 5 days with low-dose tamoxifen. Whole-mount photograph (B) and section (C) showing a ribbon of blue cells extending from a single CBC cell at the base of the crypt to the tip of the villus. This demonstrates that Lgr5+ve CBC cells are capable of generating the entire villus epithelium. All epithelial cell types are present within the blue ribbons, proving that the Lgr5+ve CBC cells are multipotent. Similar observations at much later time points (6 to 14 months after induction) prove that Lgr5+ve CBC cells are long-lived, allowing the conclusion that they are the true intestinal SC. Magnification = ×16 (B); ×10 (C). D: The Lgr5 gene is exclusively expressed in the lower bulge and the secondary germ of telogen HFs of Lgr5-GFP knockin mice, whereas in anagen and catagen HF Lgr5+ve cells occur in the lower ORS (not shown). Magnification = ×60. E and F: Whole-mount photograph (E) and section (F) from Lgr5-GFP-Ires-CreERT2/Rosa26-LacZ mice 6 months after induction with low-dose tamoxifen at postnatal day 20 (when all HFs are in telogen). All blue cells necessarily originate from Lgr5+ve telogen HF cells. Blue cells occur in all parts of the newly formed HF below the SG, proving that Lgr5+ve HF cells are multipotent as well as long-lived. (Adapted from Barker et al.5) Magnification = ×16 (E); ×20 (F).
To visualize live CBC cells and to follow their potential progeny, a second knock-in allele with a cassette encoding green fluorescent protein (GFP) and a tamoxifen-inducible version of the Cre recombinase enzyme integrated into exon 1 of Lgr5 was generated.2 The GFP pattern observed in the Lgr5-GFP mice faithfully recapitulated the Lgr5-LacZ expression pattern (Figure 2A). Lgr5-GFP mice were crossed with the Cre-activatable R26R-LacZ reporter, allowing fate mapping of the Lgr5+ve cells. Injection of tamoxifen should activate the otherwise inactive CreERT2 recombinase enzyme expressed uniquely in CBC cells. This would allow irreversible marking of these cells by Cre-mediated excision of the roadblock sequence in the R26R-LacZ reporter. The activated LacZ reporter then acts as a genetic mark in the CBC cells and their potential progeny.
A low-dose tamoxifen pulse resulted in activation of the R26R-LacZ reporter stochastically and at low frequency in CBC cells. These occasional LacZ-positive CBC cells then gave rise to parallel ribbons of blue cells that emanated from the crypt bottom, reaching the tips of adjacent villi after 5 days (Figure 2, B and C). At time points up to 14 months after induction, the appearance and frequency of actively renewing blue ribbons remained unchanged. All four differentiated cell types of the intestinal epithelium (enterocytes, goblet cells, Paneth cells, and enteroendocrine cells) were present in the clonal blue ribbons. Bjerknes and Cheng10 previously reported the existence of different types of long-lived epithelial clones in the intestine: columnar (enterocyte) clones, mucous (goblet) clones, and mixed clones. The clones induced by Lgr5+ve CBC cells belonged exclusively to the mixed variety. The lineage tracing demonstrated that CBC cells self-renew and give rise to all intestinal epithelial lineages throughout very long periods of time, thus fulfilling the minimal definition of stemness, i.e., long-term self renewal and multipotency.
Additionally, Lgr5-GFP mice were intercrossed with Apcmin mice to examine the expression of Lgr5 in the premalignant adenomas, which spontaneously arise in the intestines as a result of chronic activation of the Wnt signaling pathway.31,32 In contrast to other Wnt target genes, which exhibit a uniform high-level expression throughout these tumors, Lgr5-GFP expression was restricted to a small number of cells within large adenomas. It is therefore tempting to speculate that Lgr5 not only marks the normal intestinal SCs, but also a limited population of tumor-initiating/propagating cells thought to exist within colon cancers—the cancer SCs.
Lgr5 Marks HF SCs
Microarray profiling of genes preferentially expressed in the bulge of telogen HFs showed that Lgr5 mRNA is also highly enriched in bulge SCs.17 Lgr5 expression is restricted to an actively cycling cell population of the bulge and the secondary germ of the telogen HF (Figure 2D).46 Interestingly, Lgr5 is also expressed in the lower outer root sheath of the anagen HF.46 This is consistent with previous reports suggesting that SCs from the bulge migrate along the outer root sheath to the base of the follicle, where they become activated to proliferate by signals originating from the dermal papilla.47 Indeed, Lgr5+ve cells localized in the secondary germ of late telogen/pre-anagen state HFs are positive for Ki-67 and enter S phase suggesting that Lgr5+ve cells are the first cells activated after anagen-initiating signals, reminiscent of a model for SC trafficking. Consistent with the properties of Lgr5+ve intestinal SCs, Lgr5+ve HF SCs do not retain label unlike previously described bulge SCs,16,17,20 thus representing a novel actively cycling SC population in the HF.
In telogen hair, Lgr5+ve cells express the known bulge SC markers CD34 and K15. In anagen HFs, however, Lgr5+ve cells are distinct from CD34/K15-positive bulge cells, which remain localized to the bulge. Importantly, Lgr5high CD34+ double-positive keratinocytes exhibited enhanced in vitro colony-forming efficiency when compared to Lgr5low CD34+ keratinocytes. Cultured Lgr5high cells constituted the most potent population in regenerating HF in vivo when grafted onto the back of nude mice.
Lineage tracing using Lgr5-GFP mice in combination with the R26R-LacZ reporter along the lines discussed above verified that Lgr5+ve HF cells constitute a novel, long-lived HF SC population. Lgr5+ve cells of the bulge and the secondary germ of telogen HF give rise to all cell lineages of the newly formed HF during the following hair cycle phases throughout long periods of time (up to 6 months) (Figure 2, E and F). Interestingly, the upper permanent part of the HF, the SG, and the IFE never contained any Lgr5 progeny suggesting that under normal homeostatic conditions, Lgr5+ve cells are true HF SCs. Similar to previous studies, when expanded in vitro followed by transplantation, Lgr5+ve cells can give rise to sebocytes.
In conclusion, Lgr5 marks an actively cycling SC population in the HF that under normal conditions maintains the cycling part of the HF and can contribute to all HF structures including the bulge and isthmus, previously known SC/progenitor compartments.46 Lgr5+ve HF cells are self-renewing and multipotent, thus exhibiting the minimal SC characteristics defined at the beginning of this review.
Conclusion and Perspectives
Throughout the past few years, there has been impressive progress in the identification of mammalian SCs and the elucidation of the mechanisms that regulate SC function. Lgr5 presents a novel marker that specifically identifies SC populations of the intestinal epithelium and the HF. Lgr5-positive SCs in the intestine and the HF are actively cycling, contradicting the common assumption that SCs are quiescent. Taken together, these observations call for a reinvestigation of the relationship between label retention and SCs.
As recently proposed for olfactory neural SCs,48 quiescent SCs may represent a reserve population activated upon tissue damage, living side-by-side with cycling SCs that under normal conditions maintain homeostasis. Such a situation may exist in the HF, where Lgr5+ve SCs appear to represent SC populations more ready to respond to stimulating signals than their quiescent bulge neighbors.
It is currently less clear if a similar situation may exist in the intestine. Recently, Bmi-1 was reported to specifically mark undifferentiated cells at the crypt bottom of the proximal small intestine, located predominantly at the +4 position but also occasionally intermingled with Paneth cells.49 Lineage tracing using Bmi-1-CreERT2 mice in combination with the R26R-LacZ/YFP reporter suggested that these Bmi-1-positive cells exhibit SC properties and may therefore be identical to Potten’s +4 cells.7,8 However, these Bmi-1-positive cells reportedly are actively in cycle. Moreover, the apparent lack of Bmi-1 expression and lineage tracing in the distal small intestine and colon suggests the existence of Bmi-1-negative intestinal SCs. It remains to be addressed if Bmi-1 expression overlaps with Lgr5 in CBC cells, thus marking a subpopulation of Lgr5+ve intestinal SCs.
The finding that SCs are rapidly cycling implies that these cells go through many hundreds of cell divisions during the lifetime of a mouse without apparent SC exhaustion, telomere collapse, loss of genetic information, or excessive malignant transformation. It will be interesting to identify and understand the underlying mechanisms that allow these novel SCs to prevent and/or repair acquired mutations. Expression of high levels of telomerase could be one such mechanism. Telomerase activity is essential to prevent telomere shortening and cellular senescence, thereby ensuring sustained division potential. Indeed, expression of telomerase in individual cells of the lower small intestinal crypt has been reported.50 Of note, telomerase-null mice do not exhibit any intestinal phenotype until the fifth to sixth mouse generation, indicating that telomere regeneration is not absolutely required until this point, presumably because of the unusual length of mouse chromosomes.
Lgr5 exhibits a highly restricted expression in a variety of other tissues, i.e., the eye, brain, mammary gland, reproductive organs, and stomach.2 These findings together with ongoing lineage tracing experiments support the notion, that Lgr5+ve cells may represent SCs in the mammary gland and the stomach epithelium (N. Barker and H. Clevers, unpublished data). Thus, Lgr5 may represent a general marker for adult SCs.
The molecular function of Lgr5 is currently unknown. It is predicted to be a receptor for a peptide ligand of unknown nature. Given the close association of Lgr5 expression with stemness, it is tempting to speculate that the ligand controls crucial aspects of SC behavior.
Footnotes
Address reprint requests to Hans Clevers or Andrea Haegebarth, Hubrecht Institute and University Medical Center Utrecht, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands. E-mail: clevers@niob.knaw.nl and a.haegebarth@niob.knaw.nl.
Supported by the Koningin Wilhelmina Fonds (grant to H.C.), the Louis Jeantet Foundation (grant to H.C.), and an EMBO Long-Term Fellowship (to A.H.).
H.C. is an inventor on several patents involving Wnt target genes.
References
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Cheng H, LeBlond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat. 1981;160:77–91. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H. The intestinal Wnt/TCF signature. Gastroenterology. 2007;132:628–632. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Liang SG, Hsueh AJ. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol. 1998;12:1830–1845. doi: 10.1210/mend.12.12.0211. [DOI] [PubMed] [Google Scholar]
- McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–426. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37:528–533. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]
- Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24:9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]