Abstract
Mammalian polyamine oxidases (PAO) catalyze the oxidation of N1-acetylspermine and N1-acetylspermidine to produce N-acetyl-3-aminopropanaldehyde and spermidine or putrescine. Structurally, PAO is a member of the monoamine oxidase family of flavoproteins. The effects of pH on kinetic parameters of mouse PAO have been determined to provide insight into the protonation state of the polyamine required for catalysis and the roles of ionizable residues in the active site in amine oxidation. For N1-acetylspermine, N1-acetylspermidine, and spermine, the kcat/Kamine-pH profiles are bell-shaped. In each case the profile agrees with that expected if the productive form of the substrate has a single positively charged nitrogen. The pKi-pH profiles for a series of polyamine analogs are most consistent with the nitrogen at the site of oxidation being neutral and one other nitrogen being positively charged in the reactive form of the substrate. With N1-acetylspermine as substrate, the value of kred, the limiting rate constant for flavin reduction, is pH dependent, decreasing below a pKa value of 7.3, again consistent with the requirement for an uncharged nitrogen for substrate oxidation. Lys315 in PAO corresponds to a conserved active site residue found throughout the monoamine oxidase family. Mutation of Lys315 to methionine has no effect on the kcat/Kamine profile for spermine, the kred value with N1-acetylspermine is only 1.8-fold lower in the mutant protein, and the pKa in the kred-pH profile with N1-acetylspermine shifts to 7.8. These results rule out Lys315 as a source of a pKa in the kcat/Kamine or kcat/kred profiles. They also establish that this residue does not play a critical role in amine oxidation by PAO.
The polyamines spermine and spermidine are essential for cell proliferation, with higher levels being found in rapidly growing cells (1, 2). This observation suggests that compounds which decrease the levels of polyamines in cells have potential as antineoplastic agents. Indeed, the polyamine biosynthetic pathway has been heavily studied with the goal of developing enzyme inhibitors (2–4). The pathway begins with the formation of putrescine from ornithine catalyzed by ornithine decarboxylase. Putrescine is then converted to spermine by two sequential reactions catalyzed by spermidine synthase, forming first spermidine and then spermine, using decarboxylated S-adenosylmethione as the propylamine donor in both steps. In the opposite direction, catabolism of spermine requires the sequential action of two enzymes (1). First, acetylation of spermine by spermidine/spermine N1-acetylspermine acetyltransferase forms N1-acetylspermine. This is then converted to spermidine by the flavoenzyme polyamine oxidase (PAO).1 The same two enzymes also catalyze the acetylation of spermidine to N1-acetylspermidine and the subsequent oxidation to putrescine. Very recently, several mammalian tissues have been found to contain a flavoenzyme capable of oxidizing spermine directly to spermidine (5–7); while referred to occasionally as PAO, it is more accurately a spermine oxidase.
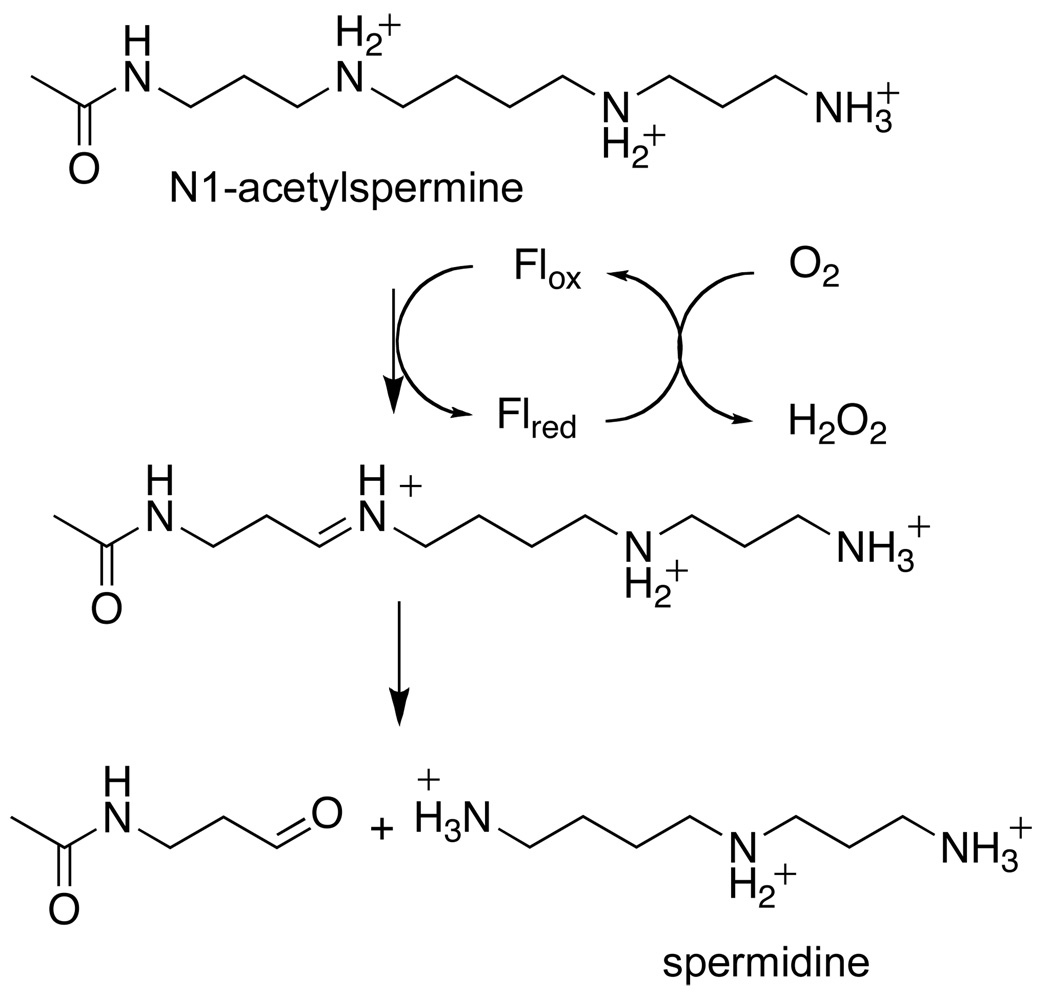
The reaction of mammalian PAO is shown in Scheme 1. The enzyme cleaves the exo carbon-hydrogen bond of its substrate, forming spermidine and N-acetyl-3-aminopropanaldehyde from N1-acetylspermine or putrescine and N-acetyl-3-aminopropanaldehyde from N1-acetylspermidine. There are also plant PAOs, of which the maize enzyme is the best-characterized (8–11). While the mammalian enzymes oxidize spermine to 3-aminopropanaldehyde and spermidine, the plant enzymes oxidize the endo bond of spermine to form propane-1,3-diamine and N-(3-aminopropyl)-4-aminobutyraldehyde (12). The structural bases for the difference in substrate specificity between polyamine and spermine oxidases and in the site of substrate oxidation between the plant and animal enzymes are not known.
Scheme 1.
The general reaction of flavin amine oxidases such as PAO can be divided into two half reactions. In the reductive half reaction a hydride equivalent is transferred from the substrate to the flavin, while the oxidative half reaction involves the oxidation of the reduced flavin by molecular oxygen, producing H2O2. The steady-state kinetic mechanism has previously been determined for mouse PAO (13). Consistent with the results for most flavoprotein oxidases (14), the kinetic pattern is ping-pong due to the reductive half reaction being effectively irreversible. Consequently, the kcat/Km value for the amine substrate includes the steps in the reductive half-reaction from amine binding through flavin reduction, while the kcat/Km value for oxygen is the second order rate constant for reoxidation of the reduced flavin. This simplifies analysis of the individual kinetic parameters, since the kcat/Km value for the amine substrate is independent of the oxygen concentration, while the rate constant for flavin reduction can readily be determined using rapid-reaction methods in the absence of oxygen.
The chemical mechanism of the reductive half-reaction of flavoprotein amine oxidases has been quite controversial (15). Oxidation of an amine substrate by an amine oxidase necessarily involves the removal of two protons and two electrons as the carbon-nitrogen single bond is converted to a double bond. The various mechanistic proposals for the flavin amine oxidases have included most of the possible combinations by which this can occur (15, 16). Cleavage of the carbon-hydrogen bond could occur by removal of the hydrogen as a proton, a hydrogen atom, or a hydride (17–19), with some mechanisms involving formation of an amineflavin adduct as an intermediate (19). In contrast, the hydrogen is generally proposed to be removed from the nitrogen as a proton, with the disagreement over when this occurs in the reaction. Thus, the proton could be lost to solvent before the amine binds to the protein (20) or to an active site base either before (21–23) or concurrent with cleavage of the carbon-hydrogen bond (21). Thus, establishing the catalytic mechanism of an amine oxidase necessarily requires knowledge of the timing of removal of hydrogens from both the carbon and the nitrogen. In addition, in the case of the proton on the nitrogen, loss of the proton from the bound substrate would require an active site base.
The flavin amine oxidases can be divided into two structural classes, the MAO/PAO family (9, 24) and the D-amino acid oxidase (DAAO)/sarcosine oxidase family (25). No structure of a mammalian PAO has been described to date. However, structures are available for maize PAO and for S. cerevisiae spermine oxidase (Fms1) (26, 27). These enzymes both belong to the monoamine oxidase (MAO) family of flavoprotein amine oxidases (26). The sequences of mammalian PAOs align well with those of these and other members of this family (13, 26, 28). The available structures of members of the MAO family show that all contain a conserved lysyl residue in the active site (24, 26–30). This residue is part of a “Lys-H2O-N5” motif in which the lysyl side chain forms hydrogen bonds to the N5 of the isoalloxazine ring via an intervening water molecule. This lysyl residue has been proposed to be an active site base which accepts a proton from either the protonated amine of the substrate prior to its oxidation or from a water molecule to form hydroxide for hydrolysis of an imine intermediate (8, 24).
This paper describes the use of the effects of pH on the steady-state and reductive half-reaction kinetics of mouse PAO to probe substrate specificity and establish the protonation states of polyamines required for catalysis. In addition, the role the conserved lysyl residue plays in amine oxidation by this enzyme has been analyzed.
EXPERIMENTAL PROCEDURES
Materials
Spermine was purchased from Acros Organics (Geel, Belgium), and 1,8-diaminooctane and 1,12-diaminododecane were purchased from Aldrich (Milwaukee, WI). N1-Acetylspermine and N1-acetylspermidine were synthesized as previously described (31); N1-acetylspermidine was also purchased from Fluka (Switzerland).
Syntheses
N1-Acetyl-1,8-diaminododecane hydrochloride
1,8-Diaminooctane (3.5 mmol, 0.5 g) was added to 45 mL of ice-cold glacial acetic acid with magnetic stirring. The mixture was then heated to 55–60°C. A solution of 0.33 mL of acetic anhydride (1.0 equivalent) in 10 mL of glacial acetic acid was added dropwise with stirring over 1 h. The mixture was cooled to 25 °C and left overnight at that temperature. The residue after evaporation of the solvent under reduced pressure was dissolved in hot water, cooled and adjusted to acidic pH with 6 N HCl. The solvent was removed and the remaining solid was extracted twice with 50 mL 2-propanol; any insoluble material was discarded. The combined filtrates were concentrated and cooled to −10 °C. The crystals that formed were collected by filtration and recrystallized from 75 mL of hot 2-propanol. The yield was 0.21 g (32%). 1H NMR (CDCl3): δ 6.41 (1H, s), 2.79 (2H, t), 2.57 (2H, t), 1.98 (3H, s), 1.58 (2H, m), 1.37 (8H, t), 1.46 (2H, m), 1.09 (2H, s); HRMS (m + H) theor.: 187.19, found: 187.22.
N1-Acetyl-1,12-diaminododecane hydrochloride
N1-Acetyl-1,12-diaminododecane hydrochloride was synthesized by a similar procedure from 1,12-diaminododecane. The yield was 0.18 g (~ 31%). 1H NMR (CDCl3): δ 6.38 (1H, s), 2.74 (2H, t), 2.65 (2H, t), 1.95 (3H, s), 1.52 (2H, m), 1.38 (18H, m), 1.11 (1H, s); HRMS (m + H) theor.: 243.211, found: 243.209.
N1-Acetyl-N3-pentyl-1,3-diaminopropane
To 3-aminopropanol (1.0 g, 13 mmol) in 5 mL of dry acetonitrile was added dropwise one equivalent (2.9 g, 13 mmol) of (Boc)2O in 1.0 equivalent (1.85 ml, 13 mmol) of triethylamine. After 6 h at 25 °C an additional 0.5 equivalent (1.4 g) of (Boc)2O was added. The reaction was continued overnight until silica gel thin-layer chromatography in hexane/ethyl acetate (8:2) showed one major spot. The solvent was evaporated in vacuo. The brown residue was treated with 1 M KHSO4 (60 mL) and ether (100 mL). The solution was extracted with ether (4 × 25 mL), and the combined organic layers were washed with 50 mL each of 1 M KHSO4 and 1 M NaHCO3 and twice with a saturated solution of NaCl. The yellowish extract was dried (MgSO4) and the solvent removed under vacuum. The brown residue was chromatographed on silica using hexane/ethyl acetate (8:2) to give 1.25 g (54 %) N-Boc-3-aminopropanol. 1H NMR (CDCl3): δ 7.38 (1H, s), 3.72 (2H, m), 1.71 (2H, m), 2.55 (2H, t), 1.32 (9H, s).
The Boc-protected aminopropanol (1.0 g, 5.7 mmol) was added to 1.0 equivalent (0.65 g, 5.7 mmol) of methane sulfonyl chloride in 2 mL anhydrous pyridine. After 2 h at 25 °C, the reaction mixture was treated with 1 M NaHCO3 (30 mL) and ether (70 mL). The solution was extracted with ether (3 × 25 mL), and the combined organic layers were washed with a saturated NaCl solution (2 × 50 mL). A brownish crude product was obtained after evaporation of solvent. Silica gel column chromatography using hexane/ethyl acetate (8:2) as solvent yielded 0.75 g (52 %) N-Boc-3-amino-1-methanesulfonoxy-propane. 1H NMR (CDCl3): δ (ppm) 2.35 (3H, s), 4.21 (2H, t), 1.72 (2H, m), 2.81 (2H, t), 1.35 (9H, s).
N-Boc-3-Amino-1-methanesulfonoxy-propane (0.5 g, 2.0 mmol) was added to 1.0 equivalent (0.17 g, 2.0 mmol) of 1-aminopentane in 5 mL dry dichloromethane. After 5 h at 50 °C, the reaction mixture was cooled, washed with saturated Na2CO3 and extracted with ether. Silica gel chromatography using hexane/ethyl acetate (9:1) as solvent gave 0.32 g (67 %) N-(N-Boc-3’-aminopropyl)-1-aminopentane. 1H NMR (CDCl3): δ (ppm) 7.43 (1H, s), 2.71 (4H, m), 2.51 (2H, m), 1.73 (4H, m), 1.40 (4H, m), 1.33 (9H, s), 1.21 (3H, m), 1.12 (1H, s).
N-(N-Boc-3’-Aminopropyl)-1-aminopentane (0.325 g, 1.33 mmol) in dioxane (5 mL) was treated with 10 mL of 4 M HCl with stirring at 25 °C for 5 hr. After evaporation of the solvent under reduced pressure, the sticky residue was dissolved in distilled water (50 mL) and extracted with ether (3 × 20 mL). The aqueous layer was then passed through a C18 reverse phase column. The compound was eluted with 20% ethanol/water. Fractions were collected, flushed with N2 and lyophilized to afford 115 mg (60%) of N-(3’-aminopropyl)-1-aminopentane. 1H NMR (D2O): δ (ppm) 2.65 (6H, t), 1.67 (4H, m), 1.55 (4H, m), 1.28 (3H, s).
N-(3’-Aminopropyl)-1-aminopentane (0.115 g, 0.8 mmol) was added to 15 mL of cooled glacial acetic acid with stirring. The mixture was then heated to 55–60 °C. A mixture of 0.1 mL of acetic anhydride (1.05 equivalent) in 2 mL of glacial acetic acid was added with stirring over a 1 h period. The solution was stored at 25 °C overnight and then evaporated to dryness. The residue was dissolved in hot water, cooled and adjusted to acidic pH with 6 N HCl. After evaporation to dryness in vacuo, the resulting solid was extracted twice with 20 mL 2-propanol, and the insoluble residue was discarded. The combined filtrates were concentrated and cooled to −10 °C. The crystals that formed were collected by filtration and recrystallized from 30 mL of hot 2-propanol. The yield of N1-acetyl-N3-pentyl-1,3-diaminopropane was 50 mg (34 %). 1H NMR (CDCl3): δ (ppm) 6.38 (1H, s), 2.68 (4H, m), 2.49 (2H, m), 1.97 (3H, s), 1.75 (2H, m), 1.72 (2H, m), 1.44 (4H, m), 1.23 (3H, m), 1.15 (1H, s); HRMS (m + H) theor.: 187.121, found: 187.102.
Expression and purification of recombinant proteins
Mouse PAO was purified as previously described (32) with a few minor changes. The pellet resulting from the final 65% ammonium sulfate precipitation was resuspended in 50 mM potassium phosphate and 10% glycerol (pH 7.5) and dialyzed overnight with two buffer changes. The resulting protein sample was then centrifuged at 22,400xg for 30 min at 4 °C to remove precipitated protein. The purified protein was stored at −80 °C. The concentration of active enzyme was determined from the flavin visible absorbance spectrum, using an ɛ458 value of 10,400 M−1 cm−1.
The K315M mutation was introduced using the Stratagene QuikChange site-directed mutagenesis method and the mutagenic primer 5’-GGCTTCGGTACCAACAACATGATCTTCCTCGAGTTC −3’, which contains the K315M mutation (shown in bold) and a silent mutation at Leu318 that results in the addition of an AvaI site (underlined) used in screening colonies. The DNA sequence of the entire gene was carried out to ensure that no unwanted mutations occurred. Purification of the mutant enzyme was done using the same procedure as for wild type PAO.
Assays
Steady state kinetic assays were performed in air-saturated buffers on a computer-interfaced Hansatech (Hansatech Instruments) or YSI oxygen (Yellow Springs Instrument, Inc.) electrode. Assays were initiated by the addition of enzyme. All buffers contained 10% glycerol; 50 mM Tris-HCl, 50 mM CHES, and 50 mM CAPS were used for the pH ranges 7–8.5, 9.0–9.5, or 10–11 respectively.
Rapid-reaction kinetic experiments were conducted at 20 °C on an Applied Photophysics SX-18MV stopped-flow spectrophotometer. The night before the experiment, the instrument was flushed with anaerobic buffer followed by a solution of 36 nM glucose oxidase in 5 mM glucose, 50 mM Tris-HCl, pH 7.5. For enzyme solutions, anaerobic conditions were established by applying cycles of vacuum and argon, while substrate solutions were bubbled with argon. All buffers contained 10% glycerol and 5 mM glucose; 200 mM PIPES, 200 mM Tris-HCl, and 200 mM CHES were used for the pH ranges 6.5–6.9, 7–8.9, or 9.0–9.5, respectively. Glucose oxidase was added to all anaerobic solutions at a final concentration of 36 nM before loading them onto the stopped-flow spectrophotometer.
Data Analysis
Kinetic data were analyzed using the programs KaleidaGraph (Adelbeck Software, Reading, PA) and Igor (WaveMetrics, Lake Oswego, OR). Initial rate data obtained by varying the concentration of a single substrate were fit to the Michaelis-Menten equation. The effects of pH on kinetic parameters were fit to equations 1–3. Equation 1 applies for a kinetic parameter which decreases below pK1 due to the protonation of a single moiety. Equation 2 applies for a kinetic parameter which decreases above pK2 due to the protonation of a single moiety. Equation 3 applies for a kinetic parameter which decreases below pK1 due to protonation of a singe moiety and decreases above pK2 due to deprotonation of a single moiety. In all three equations, c is the pH-independent value. In each, y is the kinetic parameter being measured, c is the pH-independent value, K1 is the ionization constant for a residue which must be unprotonated, and K2 is the ionization constant for a residue which must be protonated.
| (1) |
| (2) |
| (3) |
Analysis of stopped-flow data was done using both KaleidaGraph and SPECFIT (Spectrum Software Associates, Marlborogh, MA). To determine the kinetic parameters for the reduction of wild type PAO by N1-acetylspermine, stopped-flow traces were fit to equation 4, which describes a triphasic exponential decay, where k1, k2, and k3 are first order rate constants, A1, A2, A3 correspond to the absorbance changes in each phase, and A∞ is the final absorbance. Equation 5 was used to fit the biphasic traces obtained for K315M PAO.
| (4) |
| (5) |
Calculation of polyamine protonation states
The microscopic pKa values for each of the nitrogens in N1-acetylspermine, N1-acetylspermidine, spermidine, and spermine were calculated using the method of Aikens et al. (33) and the values of Clark and Perrin (34). These values were then used to calculate the mole fractions with zero, one, two, three, or four charged nitrogens as a function of pH. A value of 0.15 was subtracted from each pKa value to correct for the difference between 25 and 30 °C.
RESULTS
kcat/Kamine-pH Profile
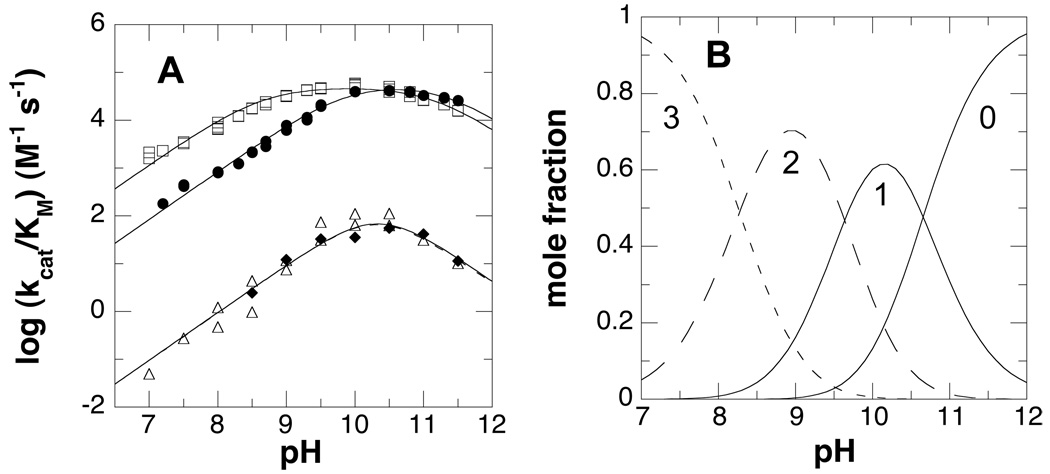
Previous steady-state kinetic studies at pH 7.6 indicated that the substrate preference for mouse PAO is N1-acetylspermine, N1-acetylspermidine, and then spermine, with spermine being a significantly slower substrate than the acetylated compounds (13). The effect of pH on the kinetic parameter kcat/Kamine was determined for each of these three substrates. The results are shown in Figure 1A, and the resulting pKa values are summarized in Table 1. The pH profiles for all three substrates exhibit decreases in activity at both low and high pH, consistent with a requirement for one moiety in the enzyme or substrate that must be protonated for substrate recognition and/or oxidation and one which must be unprotonated2. Both acetylated substrates have bell-shaped curves with two distinguishable pKa values. In contrast, with spermine the pH profile exhibits a sharp optimum so that the two pKa values are too close together to resolve; consequently, only the average of the two pKa values could be determined with this substrate.
Figure 1.
A, kcat/Kamine-pH profile of wild type PAO with N1-acetylspermine (□), N1-acetylspermidine (●), and spermine (△), and K315M PAO (◆) with spermine. The lines are from fits of the data to eq 3. B, Theoretical protonation states of N1-acetylspermine with no proton, 1 proton, 2 protons, or 3 protons.
Table 1.
pKa values for PAO substrates and inhibitors
| Kinetic parameter | pKa1 | pKa2 |
|---|---|---|
| Wild-type PAO | ||
| kcat/Km for N1-acetylspermine | 8.5 ± 0.1 | 11.2 ± 0.1 |
| kcat/Km for N1-acetylspermidine | 9.8 ± 0.1 | 11.3 ± 0.3 |
| kcat/Km for spermine | 10.3 ± 0.1 | 10.3 ± 0.1 |
| Ki for N1-acetyl-1,8-diaminooctane | - | 11.6 ± 0.1 |
| Ki for 1,8-diaminooctane | 9.3 ± 0.1 | 10.8 ± 0.1 |
| Ki for N1-acetyl-N3-pentyl-1,3-diaminopropane | 8.9 ± 0.1 | - |
| Ki for 1,12-diaminododecane | 10.0 ± 0.1 | 10.0 ± 0.1 |
| Ki for N1-acetyl-1,12-diaminododecane | - | 11.6 ± 0.1 |
| K315M PAO | ||
| kcat/Km for spermine | 10.3 ± 0.1 | 10.3 ± 0.1 |
Protonation states of polyamines as a function of pH
A likely basis for one or both of the pKa values in the kcat/Kamine-pH profiles is the protonation state of the substrates. A number of authors have determined the macroscopic pKa values for spermine (35–39), but we have been unable to find experimental values for N1-acetylspermine and N1-acetylspermidine. However, even the pKa values for spermine reflect protonations at multiple sites, in that the intrinsic pKa values of the individual nitrogens are not very different. Binding in the active site of PAO is likely instead to require that each nitrogen be either fully protonated or fully unprotonated. Consequently, the microscopic pKa values for each nitrogen were calculated for spermine, spermidine, N1-acetylspermine, and N1-acetylspermidine (Table S1). To determine the reliability of these pKa values, they were used to calculate the macroscopic pKa values for spermine and spermidine; in both cases, the values for all the nitrogens were consistent with published values (Table S2). The calculated microscopic pKa values were then used to determine the effect of pH on the mole fraction of each polyamine with an integral number of protons. Figure 1B shows the effect of pH on the mole fractions of N1-acetylspermine with zero, one, two, or three protons. The monoprotonated form is maximal at pH 10.1, in good agreement with the pH optimum in the kcat/Kamine-pH profile of 9.9, while the diprotonated form is maximal at pH 9.0. For spermine the pH maximum for the monoprotonated form is 10.4–10.5 (Table S2, Figure S1, and references (35, 38), similarly closer to the pH optima of 10.3 than is the maximum for the diprotonated form of 9.4–9.5. The agreement with N1-acetylspermidine is not as good, in that the optimum in the kcat/Kamine-pH profile is 10.5 while the calculated maximum for the monoprotonated form is 9.9 (Figure S1). However, a requirement for the diprotonated form of N1-acetylspermidine would contribute a single pKa of 9.1 for a group which must be protonated to the kcat/Kamine-pH profile, while a requirement for the uncharged form would contribute a single pKa value of 10.6 (Table S2 and Figure S1), in contrast to the observed bell-shaped profile. Thus, the kcat/Kamine-pH profiles are most consistent with the active form of the substrate having a single charge.
PAO pH Dependence of Inhibition
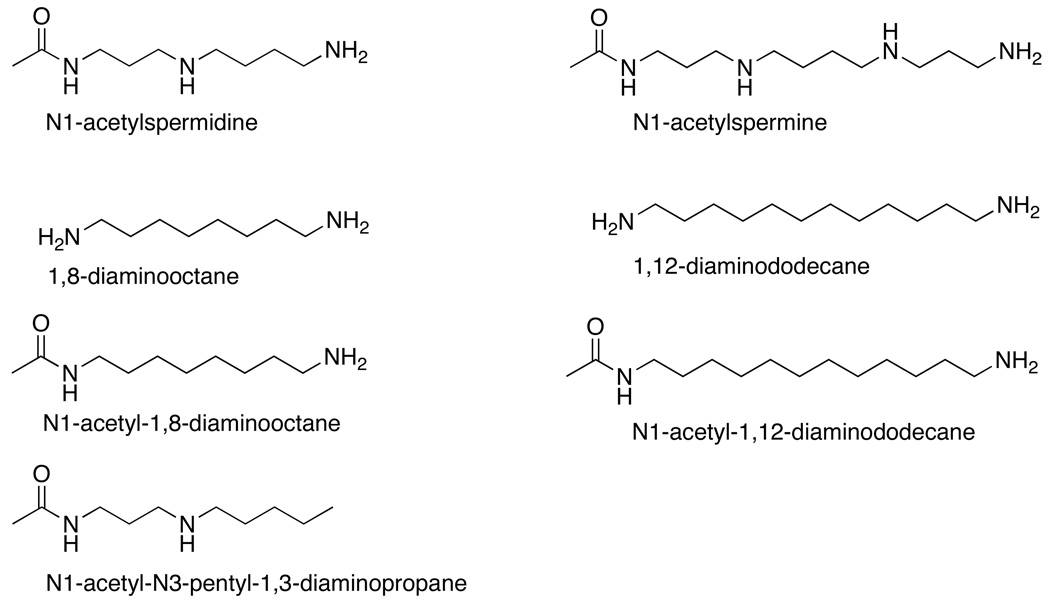
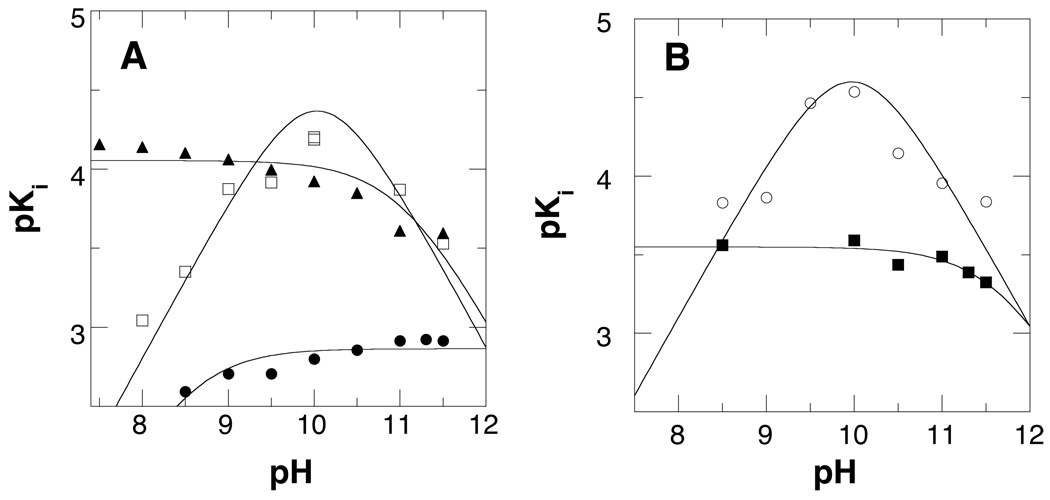
While the kcat/Kamine-pH profiles are consistent with the monoprotonated forms of the substrate being preferred, they do not establish which nitrogen in each substrate must be charged. In addition, the kcat/Kamine-pH profiles reflect both binding and catalysis. To determine the preferred protonation states of individual nitrogens in substrates for productive binding, analogues lacking one or more nitrogens (Scheme 2) were characterized as inhibitors. 1,8-Diaminooctane, N1-acetyl-1,8-diaminooctane, and N1-acetyl-N3-pentyl-1,3-diaminopropane all mimic the substrate N1-acetylspermidine. Each is a competitive inhibitor versus the amine (data not shown). The pKi-pH profile for 1,8-diaminooctane is bell-shaped with the tightest binding at pH 10 (Figure 2A, Table 1), consistent with binding requiring that one nitrogen on the inhibitor be protonated and one be unprotonated. With N1-acetyl-1,8-diaminooctane the only protonatable group is the amino group on carbon 8; in this case the pKi-pH profile shows a decrease at high pH with a pKa value of 11.6 ± 0.1, but no decrease at low pH (Figure 2A). This is most consistent with the acidic pKa in the pH profile for N1-acetylspermine being due to a need for N9 to be charged. N1-Acetyl-N3-pentyl-1,3-diaminopropane also has only one protonatable nitrogen group, located at the 4 position, the site of oxidation in N1-acetylspermidine. In this case, the pKi-pH profile shows a decrease at low pH with a pKa value of 8.9 ± 0.1, but no decrease at high pH, indicating that the N4 nitrogen must be unprotonated. These results are consistent with the active form of N1-acetylspermidine being the monoprotonated species in which the N9 nitrogen is protonated and the N4 nitrogen is unprotonated.
Scheme 2.
Figure 2.
pKi-pH profile of wild type PAO with (A) N1-acetyl-N3-pentyl-1,3-diaminopropane (●), 1,8-diaminooctane (□), and N1-acetyl-1,8-diaminooctane (▲) and (B) N1-acetyl-1,12-diaminododecane (■) and 1,12-diaminododecane (○). The lines for N1-acetyl-1,8-diaminooctane and N1-acetyl-1,12-diaminododecane are from fits to eq 1, for N1-acetyl-N3-pentyl-1,3-diaminopropane to eq 2, and for 1,8-diaminooctane and 1,12-diaminododecane to eq 3.
Similar studies were conducted with analogues of N1-acetylspermine and spermine. 1,12-Diaminododecane shows a bell-shaped pKi-pH profile with an average pKa value of 10.0 ± 0.1 (Figure 2B). This matches well the kcat/Kamine-pH profile for spermine (Table 1), consistent with productive binding requiring a substrate with a single positive charge. The pKi-pH profile for the N1-acetylspermine analogue N1-acetyl-1,12-diaminododecane shows a decrease at high pH with a single pKa value of 11.6 ± 0.1 (Figure 2B), confirming that a nitrogen not located next to the site of CH bond cleavage must be protonated for catalysis.
pH Dependence of Flavin Reduction
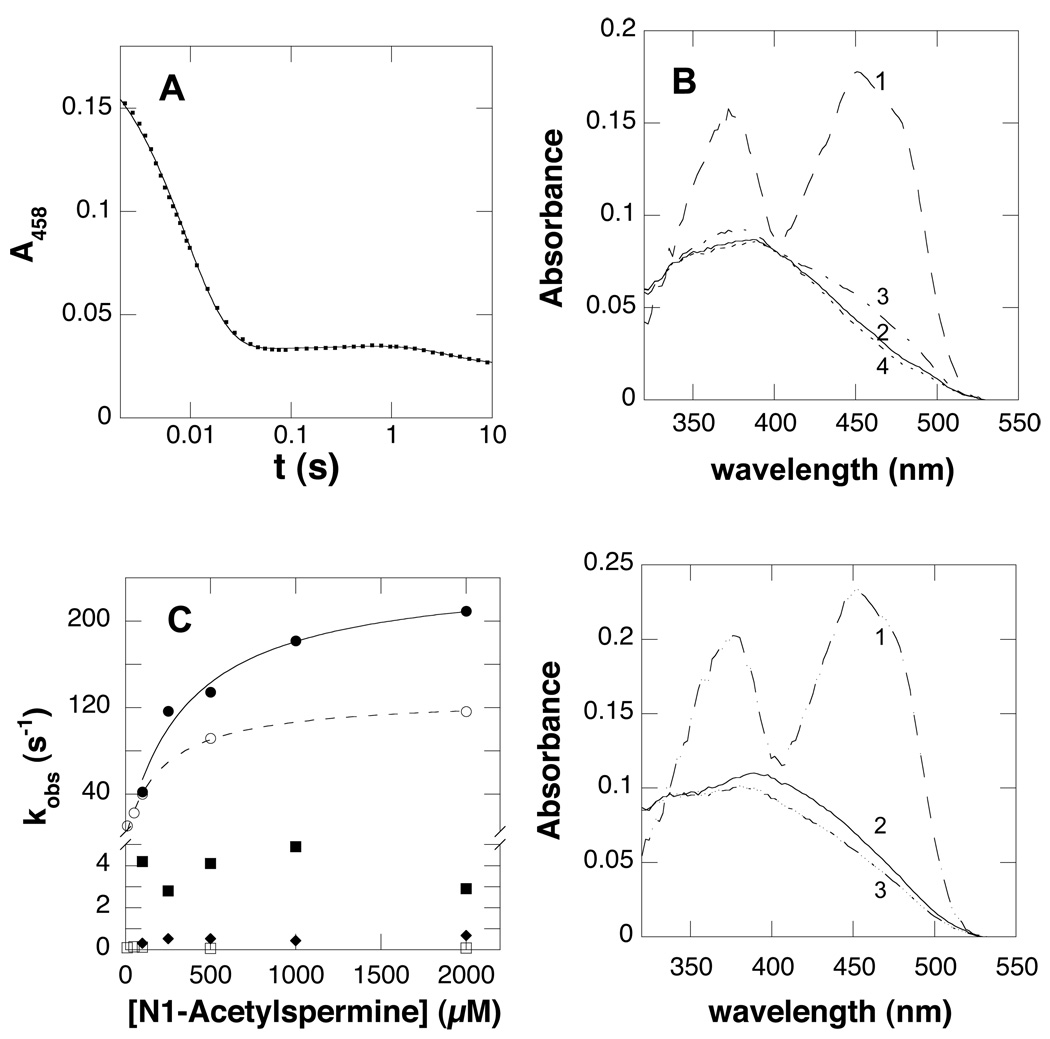
To address the effect of pH on catalysis rather than binding, stopped-flow spectroscopy was used to determine the rate constant for flavin reduction by N1-acetylspermine as a function pH. Reactions were carried out at 20 °C instead of 30 °C because much of the reaction occurred in the dead time of the instrument at the higher temperature. Over the pH range 6.5–9.5, the flavin absorbance at 458 nm showed the same behavior: an initial decrease in absorbance, a slower, slight increase in absorbance, and finally a slow decrease in absorbance (Figure 3A). Data could not be obtained at pH 10 and above due to enzyme instability. The same kinetic behavior was seen when the reaction was monitored from 320–600 nm by photodiode array spectroscopy; this approach also allowed the spectra of the intermediates to be obtained (Figure 3B). The initial fast phase of the reaction accounts for the majority of the change in amplitude and has a rate constant that is dependent on substrate concentration (Figure 3C). This phase can be attributed to the rapid and reversible binding of N1-acetylspermine with no detectable change in the flavin spectrum, followed by flavin reduction. The slowest two rate constants are independent of substrate concentration and slower than kcat (Figure 3C); therefore, they are not relevant to catalysis.
Figure 3.
The reduction of PAO by 1 mM N1-acetylspermine at pH 7.5, 20 °C. (A) Absorbance changes at 458 nm upon reduction of 20 µM wild-type PAO by 1 mM N1-acetylspermine. The line is from a fit to eq 4. (B) Absorbance spectra of flavin intermediates observed in the reductive half reaction of wild-type PAO. (C) The dependence of the individual rate constants on the N1-acetylspermine concentration for wild-type (first phase(●), second phase (■) and third phase (◆)) and K315M (first phase (○) and second phase (□)) PAO. The lines are from fits of the concentration dependence of the rate constant for the first phase to the Michaelis-Menten equation. (D) Absorbance spectra of the flavin intermediates observed in the reductive half reaction of K315M PAO.
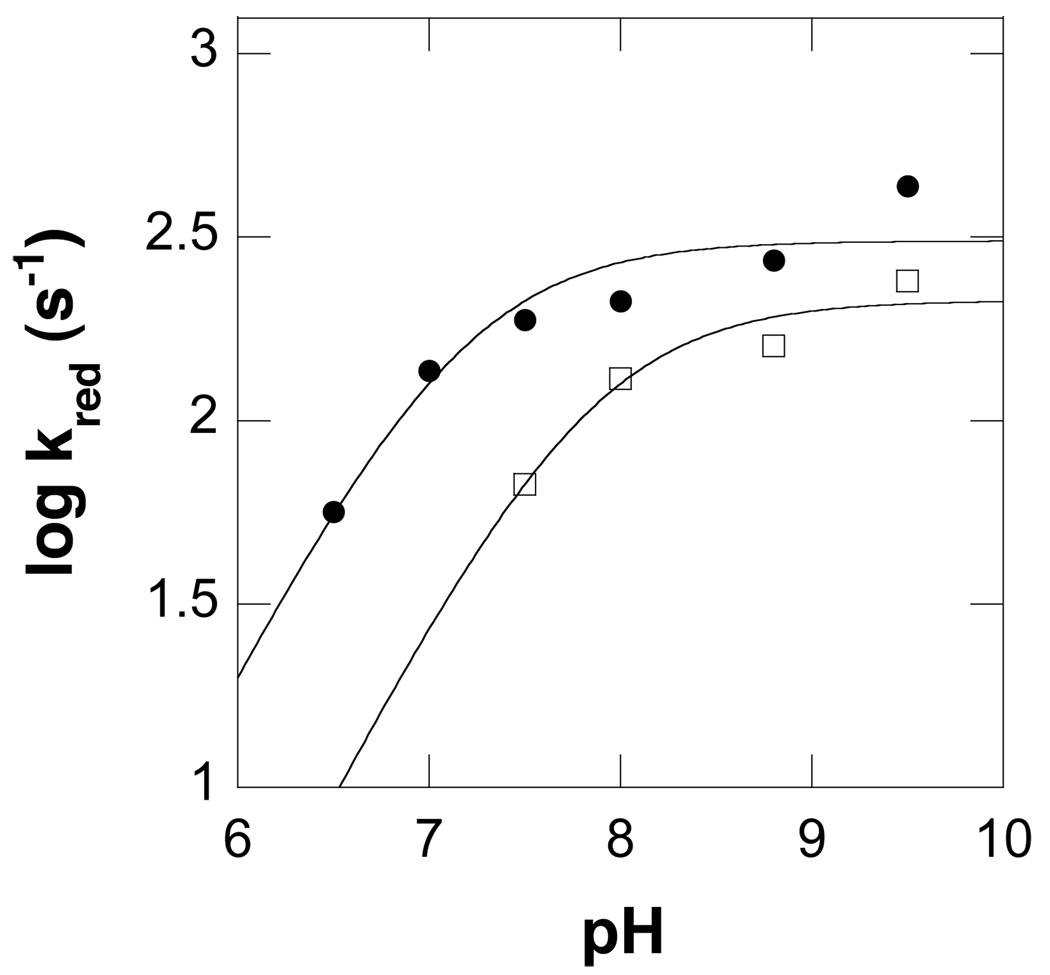
The effect of pH on the rate constant for reduction of PAO at saturating concentrations of N1-acetylspermine (kred) is shown in Figure 4. The value of this kinetic parameter shows a decrease at acidic pH with a pKa of 7.3 ± 0.1, indicating that a group in the ES complex must be unprotonated for reduction. Flavin reduction is significantly faster than kcat over the pH range investigated, so that the oxidative half reaction is rate-limiting for turnover with this substrate.
Figure 4.
pH dependence of kred for wild type (●) and K315M (□) PAO with N1-acetylspermine at 20 °C. The lines are from fits of the data to eq 2.
K315M PAO
Figure 5 shows the relative positions of the FAD and the conserved active site lysine in the structures of several members of the MAO structural family. Based on sequence alignment, PAO Lys315 corresponds to this conserved residue. The location of this lysine with respect to the flavin makes it a potential source of a pKa in the kcat/Kamine- and kred-pH profiles. Consequently, the mutation K315M was introduced into PAO. The circular dichroism spectrum of the mutant protein did not reveal any significant changes compared to wild-type PAO, suggesting the K315M mutation does not affect the overall folding of the protein or the flavin environment (data not shown). The KM value for N1-acetylspermine is less than 10 µM for the mutant protein (data not shown), so the kcat/Kamine-pH profile for this mutant was only determined using the substrate spermine. The kcat/Kspermine value for K315M PAO is identical to that for wild-type PAO over the entire pH range (Figure 1A, Table I), resulting in an identical pKa of 10.3. Thus, Lys315 is not critical for polyamine oxidation, and ionization of Lys315 does not contribute to the kcat/Kamine-pH profile.
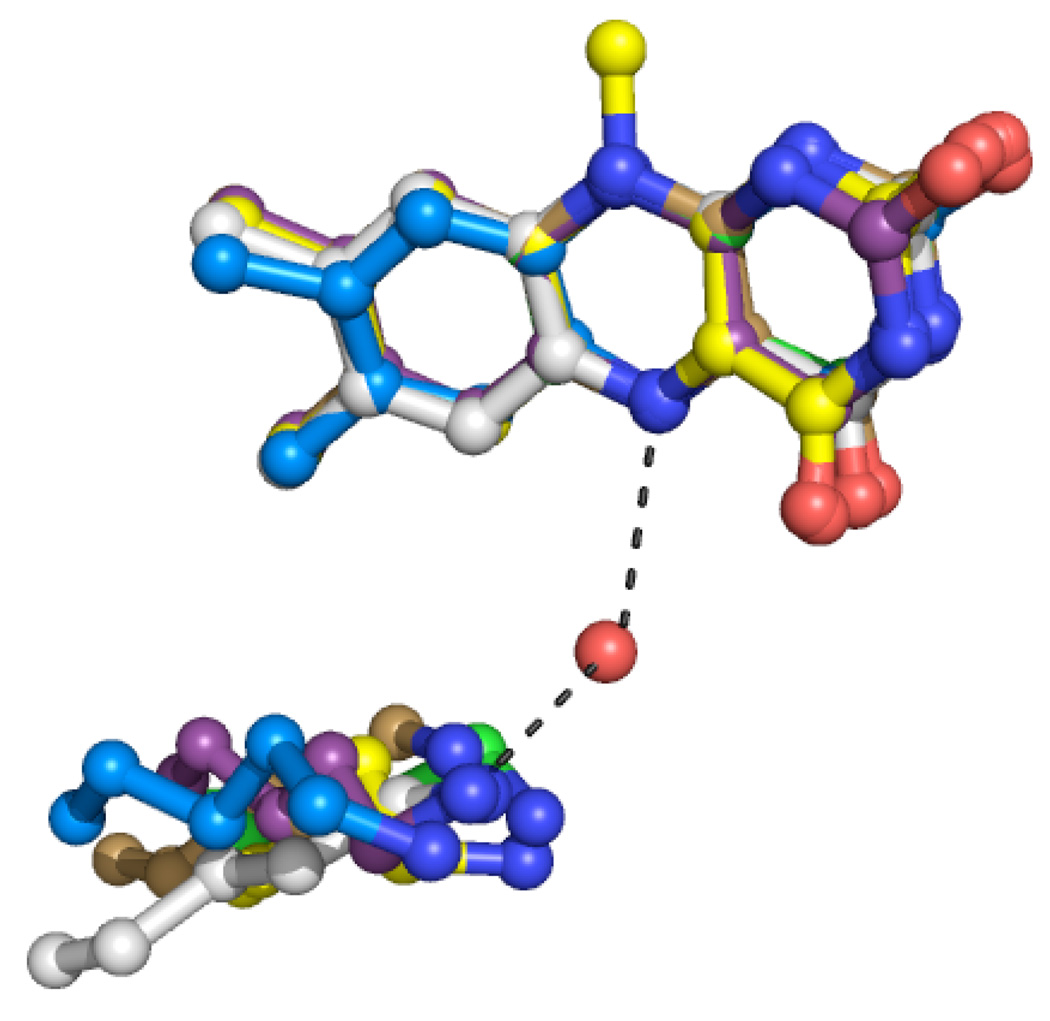
Figure 5.
Relative positions of the conserved active site lysine and the FAD in human MAO A (blue carbons), human MAO B (purple carbons), maize PAO (yellow carbons), S. cerevisiae spermine oxidase Fms1 (green carbons), Calloselasma rhodostoma L-amino acid oxidase (gray carbons), and human LSD1 (light brown carbons). This figure was composed from PDB files 2BXS (MAO A), 1OJ9 (MAO B), 1H83 (maize PAO), 1RSG (Fms1), 1F8S (L-amino acid oxidase), and 2HKO (LSD1). To generate the figure, the atoms of the central pyrazine rings of the FAD cofactors were overlaid. The water molecule shown is from maize PAO.
To further investigate the role of Lys315 in catalysis, the effect of pH on the value of kred with N1-acetylspermine was determined for the mutant protein. The changes in the flavin spectrum upon reduction of K315M PAO are biphasic, with a fast phase exhibiting a large change in absorbance and a slower phase exhibiting a smaller amplitude (Figure 3C). As with the wild-type enzyme, the rate constant for the fast phase shows a dependence on substrate concentration, while the rate constant for the slow phase is independent of substrate concentration and is slower than the kcat value for the mutant protein (Figure 3C). At pH 9.5, the value of kred is not decreased substantially from the value for the wild-type enzyme (240 s−1 versus 440 s−1), indicating that this mutation does not have a significant effect on the reductive half reaction. The kred-pH profile for K315M PAO is similar to that observed for the wild-type enzyme, with a basic shift in the pKa to 7.8 ± 0.1 (Figure 4).
DISCUSSION
The protonation state of the amine substrate required for productive binding has been a subject of controversy among those studying flavin amine oxidases. For example, Harris et al. (21) proposed for DAAO that a coupled deprotonation/dehydrogenation of the protonated substrate occurs in which a proton is transferred to the solvent. However, Denu and Fitzpatrick (40) reported that DAAO does not show a solvent isotope effect, leading to the conclusion that the amino group of the substrate must be uncharged. Further evidence for the amine being in the neutral form was provided by measurement of 15N isotope effects for DAAO, which indicated that the amino group must be unprotonated for catalysis (20). Zhao and Jorns (41) subsequently concluded from studies of monomeric sarcosine oxidase that the amine substrate within the enzyme-substrate complex must be unprotonated for flavin reduction. The situation with the MAO/PAO family has been less clear. Jones et al. (23) concluded that uncharged inhibitors bind MAO A better, but the predominant species of the amine in the pH range of 7–9 is protonated and therefore must be the substrate. In contrast, Dunn et al. (42) concluded from kinetic isotope and pH studies with MAO A that deprotonation of the amine is required for catalysis. The studies reported here clearly show that mammalian PAO requires that the N4 nitrogen next to the site of CH bond cleavage be unprotonated for CH bond cleavage to occur. This is consistent with the results of Dunn et al. (42) with MAO, establishing that the enzymes of the MAO/PAO family all require an unprotonated nitrogen for amine oxidation, as do the members of the DAAO/sarcosine oxidase family. This requirement for a substrate with a neutral nitrogen extends the mechanistic similarities of these two structural classes of flavin amine oxidases, a clear example of convergent evolution of enzyme mechanisms.
Comparison of the effect of pH on the protonation state of each substrate with its kcat/Kamine-pH profiles establishes that the monoprotonated forms are required for catalysis. More specifically, the pKi-pH profiles for the inhibitors establish that these pKas can be attributed to specific nitrogens in the substrates. In N1-acetylspermidine, the nitrogen next to the carbon being oxidized must be unprotonated and the N10-nitrogen must be protonated. N1-Acetylspermine and spermine are more complicated due to the increased number of nitrogens. For N1-acetylspermine the N4-nitrogen must be unprotonated, but the data for the inhibitors do not establish whether it is the N10- or N14-nitrogen that must be protonated. However, the kcat/Kamine values for N-acetylspermine and N1-acetylspermidine are identical at the pH optimum, suggesting that it is the N10-nitrogen that is protonated, as is the case for N1-acetylspermidine. A similar case can be made for spermine. These results suggest that both protonated and unprotonated forms of the substrate can bind, but only the protonated form can react. Thus, the pKas in the kcat/Kamine-pH profiles are due to the substrate and not an ionizable residue within the active site of the enzyme.
The requirement for the monoprotonated substrate provides a potential mechanism of discrimination against spermine by PAO, since an acetyl moiety would prevent the terminal nitrogen from ionizing and thereby result in a very large increase in the fraction of substrate in the correctly protonated form. Although the kcat/Kamine values are essentially identical at pH 10 for the two natural substrates N1-acetylspermine and N1-acetylspermidine, at the physiological pH of approximately 8.2 (43), N1-acetylspermine is the far better substrate at physiological pH. Compared with N1-acetylspermidine, N1-acetylspermine has a broader pH profile (Figure 1A). This is most readily explained by a difference in the forward commitments of the two substrates, with N1-acetylspermine being a more sticky substrate.
The kred-pH profile for wild type PAO shows a pKa of 7.3 with N1-acetylspermine as substrate. The pH profile for kred reports on the protonation states of ionizable groups in the enzyme-substrate complex required for reduction. The decrease in activity at acidic pH can be attributed to the substrate bound to the enzyme. The incorrectly protonated form of the substrate must be able to bind but not to react. If one assumes that substrate binding is at equilibrium, a likely oversimplification, the difference between the pKa of the reactive nitrogen when bound to the enzyme and free in solution of 2.1 establishes that the correctly protonated form binds about 100-fold more tightly than the form with N4 protonated. Monomeric sarcosine oxidase and MAO A show similar perturbations of the amine pKa upon binding (41, 42), suggesting that the active sites of these enzymes also preferentially bind the form of the substrate with the critical nitrogen in its neutral form.
Although numerous mechanisms have been put forth for flavin amine oxidases, most recent data support the mechanism as direct hydride transfer. Kinetic studies using 15N isotope effects have ruled out the possibility of a polar nucleophilic addition mechanism (44, 45). The 15N isotope effects are consistent with a single electron transfer mechanism, but the failure to detect any intermediate with a natural substrate for any flavin amine oxidase and the very unfavorable redox change for single electron transfer from an amine to an oxidized flavin provide arguments against such a mechanism. Reduction of wild-type PAO by N1-acetylspermine shows multiple phases, with the rate constant for the fastest phase reflecting amine oxidation, while the slower phases are likely due to product release from reduced enzyme, a step that is not significant during turnover in the presence of oxygen. More critically the flavin spectrum showed no intermediate between fully oxidized and fully reduced flavin during reduction of wild type PAO by N1-acetylspermine over the entire pH range studied, indicating that oxidation of the amine substrate to the imine occurs in a single step. This result is consistent with what has been observed with other flavin amine oxidases (21, 46–48).
The conserved active site lysyl residue in flavin amine oxidases provides a potential source of a pKa in the kcat/Kamine profile. The role of this residue has previously been examined in several members of this family. In maize PAO, when Lys300 is similarly mutated to a methionine, a 1400-fold decrease in kred is observed, suggesting an important but undefined role for this residue in substrate oxidation (8). The corresponding K661A mutation in human LSD1 completely abolished demethylase activity (49). In contrast, the substitution of methionine for this lysine in PAO results in no change in the kcat/Kspm value or the pH profile with spermine, and the rate constant for flavin reduction by N1-acetylspermine shows only a 1.8-fold decrease at pH 9.5. This rules out Lys315 acting as an active site base in mouse PAO or playing any other critical role in the reductive half-reaction. The kred-pH profile for K315M PAO shows a slight basic shift in the pKa as compared to that for wild type PAO; this can be attributed to a change in the active site environment due to the loss of the charged lysine. The reasons for the differences in the effects of mutating this residue among the different flavin amine oxidases is not apparent. It may be that this residue plays a critical role in positioning the flavin or otherwise stabilizing the active site structure, and that different flavin amine oxidases simply tolerate the loss of this interaction more than others.
In conclusion, the present study establishes the protonation state of the amine required for productive binding to PAO, and presumably for the other members of the MAO/PAO family. The results will be of use in further studies of the mechanism of amine oxidation, for interpretation of the effects of site-directed mutagenesis, for design of inhibitors, and for understanding the different substrate specificities and reactivities of polyamine and spermine oxidases. The results rule out a critical role for Lys315 in polyamine oxidation and further support hydride transfer from the neutral amine as the mechanism of flavin amine oxidases.
Supplementary Material
Footnotes
This work was supported in part by grants from the NIH (R01 GM58698 to P.F.F. and T32 GM065088 to M.H.P.) and The Welch Foundation (A-1245 to P.F.F.)
Abbreviations used: PAO, polyamine oxidase; MAO, monoamine oxidase; LSD1, lysine-specific histone demethylase 1; Fms1, S. cerevisiae spermine oxidase; DAAO, D-amino acid oxidase.
The presence of three or four nitrogen atoms in N1-acetylspermine or spermine which have pKa values in the accessible range should yield more complex pH profiles than simple bell shapes, with slopes of ± 2 at pH values where additional nitrogens are incorrectly protonated. However, no improvement in the data fitting was seen when an additional pKa was incorporated into the equation. Simulations of the effect of the additional nitrogen(s) showed that the effect was negligible over the pH range used here.
Supporting Information Available: Comparison of calculated and measured macroscopic pKa values for polyamines, calculated microscopic pKa values used to calculate the macroscopic pKa values and the mole fractions of the individual polyamines, and the calculated mole fractions of the different protonated forms of spermine and N1-acetylspermidine. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Tabor CW, Tabor H. Polyamines. Ann. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 2.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Ann. Rev. Pharmacol. Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 3.Lin PKT, Dance AM, Bestwick C, Milne L. The biological activities of new polyamine derivatives as potential therapeutic agents. Biochem. Soc. Trans. 2003;31:407–410. doi: 10.1042/bst0310407. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Casero RA., Jr Mammalian polyamine catabolism: a therapeutic target, a pathological problem, or both? J. Biochem. (Tokyo) 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 5.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 2002;367:665–675. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem. Biophys. Res. Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 7.Cervelli M, Polticelli F, Federico R, Mariottini P. Heterologous expression and characterization of mouse spermine oxidase. J. Biol. Chem. 2003;278:5271–5276. doi: 10.1074/jbc.M207888200. [DOI] [PubMed] [Google Scholar]
- 8.Polticelli F, Basran J, Faso C, Cona A, Minervini G, Angelini R, Federico R, Scrutton NS, Tavladoraki P. Lys300 plays a major role in the catalytic mechanism of maize polyamine oxidase. Biochemistry. 2005;44:16108–16120. doi: 10.1021/bi050983i. [DOI] [PubMed] [Google Scholar]
- 9.Binda C, Mattevi A, Edmondson DE. Structure-function relationships in flavoenzyme dependent amine oxidations. A comparison of polyamine oxidase and monoamine oxidase. J. Biol. Chem. 2002;277:23973–23976. doi: 10.1074/jbc.R200005200. [DOI] [PubMed] [Google Scholar]
- 10.Binda C, Angelini R, Federico R, Ascenzi P, Mattevi A. Structural bases for inhibitor binding and catalysis in polyamine oxidase. Biochemistry. 2001;40:2766–2776. doi: 10.1021/bi002751j. [DOI] [PubMed] [Google Scholar]
- 11.Bellelli A, Angelini R, Laurenzi M, Federico R. Transient kinetics of polyamine oxidase from Zea mays L. Arch. Biochem. Biophys. 1997;343:146–148. doi: 10.1006/abbi.1997.0129. [DOI] [PubMed] [Google Scholar]
- 12.Sebela M, Radová A, Angelini R, Tavladoraki P, Frébort I, Pec P. FAD-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci. 2001;160:197–207. doi: 10.1016/s0168-9452(00)00380-0. [DOI] [PubMed] [Google Scholar]
- 13.Wu T, Yankovskaya V, McIntire WS. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J. Biol. Chem. 2003;278:20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 14.Bright HJ, Porter DJT. Flavoprotein oxidases. In: Boyer P, editor. The Enzymes, 3rd Ed., Vol. XII. 3 ed. New York: Academic Press; 1975. pp. 421–505. [Google Scholar]
- 15.Fitzpatrick PF. Substrate dehydrogenation by flavoproteins. Acc. Chem. Res. 2001;34:299–307. doi: 10.1021/ar0000511. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick PF. Insights into the mechanisms of flavoprotein oxidases from kinetic isotope effects. J. Labelled Comp. & Radiopharm. 2007;50:1016–1025. doi: 10.1002/jlcr.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scrutton NS. Chemical aspects of amine oxidation by flavoprotein enzymes. Nat. Prod. Rep. 2004;21:722–730. doi: 10.1039/b306788m. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G, Jorns MS. Spectral and kinetic characterization of the Michaelis charge transfer complex in monomeric sarcosine oxidase. Biochemistry. 2006;45:5985–5992. doi: 10.1021/bi0600852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmondson DE, Binda C, Mattevi A. Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B. Arch. Biochem. Biophys. 2007;264:269–276. doi: 10.1016/j.abb.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz KA, Rishavy MA, Cleland WW, Fitzpatrick PF. Nitrogen isotope effects as probes of the mechanism of D-amino acid oxidase. J. Am. Chem. Soc. 2000;122:12896–12897. [Google Scholar]
- 21.Harris CM, Pollegioni L, Ghisla S. pH and kinetic isotope effects in D-amino acid oxidase catalysis: Evidence for a concerted mechanism in substrate dehydrogenation via hydride transfer. Eur. J. Biochem. 2001;268:5504–5520. doi: 10.1046/j.1432-1033.2001.02462.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, Song H, Chen Z, Mathews S, Jorns MS. Monomeric sarcosine oxidase: role of histidine 269 in catalysis. Biochemistry. 2002;41:9751–9764. doi: 10.1021/bi020286f. [DOI] [PubMed] [Google Scholar]
- 23.Jones TZ, Balsa D, Unzeta M, Ramsay RR. Variations in activity and inhibition with pH: the protonated amine is the substrate for monoamine oxidase, but uncharged inhibitors bind better. J. Neural Transm. 2007;114:707–712. doi: 10.1007/s00702-007-0675-y. [DOI] [PubMed] [Google Scholar]
- 24.Pawelek PD, Cheah J, Coulombe R, Macheroux P, Ghisla S, Vrielink A. The structure of L-amino acid oxidase reveals the substrate trajectory into an enantiomerically conserved active site. EMBO J. 2000;19:4204–4215. doi: 10.1093/emboj/19.16.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trickey P, Wagner MA, Jorns MS, Mathews FS. Monomeric sarcosine oxidase: structure of a covalently flavinylated amine oxidizing enzyme. Structure. 1999;7:331–345. doi: 10.1016/s0969-2126(99)80043-4. [DOI] [PubMed] [Google Scholar]
- 26.Binda C, Coda A, Angelini R, Federico R, Ascenzi P, Mattevi A. A 30 Å long U-shaped catalytic tunnel in the crystal structure of polyamine oxidase. Structure. 1999;7:265–276. doi: 10.1016/s0969-2126(99)80037-9. [DOI] [PubMed] [Google Scholar]
- 27.Huang Q, Liu Q, Hao Q. Crystal structures of Fms1 and its complex with spermine reveal substrate specificity. J. Mol. Biol. 2005;348:951–959. doi: 10.1016/j.jmb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Binda C, Newton-Vinson P, Hubalek F, Edmondson DE, Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002;9:22–26. doi: 10.1038/nsb732. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Yang Y, Wang F, Wan K, Yamane K, Zhang Y, Lei M. Crystal structure of human histone lysine-specific demethylase 1 (LSD1) Proc. Natl. Acad. Sci. USA. 2006;103:13956–13961. doi: 10.1073/pnas.0606381103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma J, Yoshimura M, Yamashita E, Nakagawa A, Ito A, Tsukihara T. Structure of rat monoamine oxidase A and its specific recognitions for substrates and inhibitors. J. Mol. Biol. 2004;338:103–114. doi: 10.1016/j.jmb.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 31.Gawandi V, Fitzpatrick PF. The synthesis of deuterium-labeled spermine, N1-acetylspermine and N1-acetylspermidine. J. Labelled Comp. & Radiopharm. 2007;50:666–670. doi: 10.1002/jlcr.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royo M, Fitzpatrick PF. Mechanistic studies of mouse polyamine oxidase with N1,N12-bisethylspermine as a substrate. Biochemistry. 2005;44:7079–7084. doi: 10.1021/bi050347k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aikens D, Bunce S, Onasch F, Parker R, 3rd, Hurwitz C, Clemans S. The interactions between nucleic acids and polyamines. II. Protonation constants and 13C-NMR chemical shift assignments of spermidine, spermine, and homologs. Biophys. Chem. 1983;17:67–74. doi: 10.1016/0301-4622(83)87015-x. [DOI] [PubMed] [Google Scholar]
- 34.Clark J, Perrin DD. Prediction of the strengths of organic bases. Q. Rev. Chem. Soc. 1964;18:295–320. [Google Scholar]
- 35.Frassineti C, Ghelli S, Gans P, Sabatini A, Moruzzi MS, Vacca A. Nuclear magnetic resonance as a tool for determining protonation constants of natural polyprotic bases in solution. Anal. Biochem. 1995;231:374–382. doi: 10.1006/abio.1995.9984. [DOI] [PubMed] [Google Scholar]
- 36.Kimberly M, Goldstein JH. Determination of pKa values and total proton distribution pattern of spermidine by carbon-13 nuclear magnetic resonance titrations. Anal. Chem. 1981;53:789–793. [Google Scholar]
- 37.Lomozik L, Gasowska A, Bolewski L. Copper (II) ions as a factor interfering in the interaction between bioligands in systems with adenosine and polyamines. J. Inorg. Biochem. 1996;63:191–206. [Google Scholar]
- 38.Bencini A, Bianchi A, Garcia-Espana E, Micheloni M, Remirez JA. Proton coordination by polyamine compounds in aqueous solution. Coord. Chem. Rev. 1999;188:97–156. [Google Scholar]
- 39.da Silva JA, Felcman J, Lopes CC, Lopes RSC, Villar JDF. Study of the protonation/deprotonation sequence of two polyamines: bis-[(2S)-2-pyrrolidinylmethyl] ethylenediamine and spermidine by 1H and 13C nuclear magnetic resonance. Spectrosc. Lett. 2002;35:643–661. [Google Scholar]
- 40.Denu JM, Fitzpatrick PF. Intrinsic primary, secondary, and solvent kinetic isotope effects on the reductive half-reaction of D-amino acid oxidase: Evidence against a concerted mechanism. Biochemistry. 1994;33:4001–4007. doi: 10.1021/bi00179a029. [DOI] [PubMed] [Google Scholar]
- 41.Zhao G, Jorns MS. Ionization of zwitterionic amine substrates bound to monomeric sarcosine oxidase. Biochemistry. 2005;44:16866–16874. doi: 10.1021/bi051898d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn RV, Marshall KR, Munro AW, Scrutton NS. The pH dependence of kinetic isotope effects in monoamine oxidase A indicates stabilization of the neutral amine in the enzyme-substrate complex. FEBS Journal. 2008;275:3850–3858. doi: 10.1111/j.1742-4658.2008.06532.x. [DOI] [PubMed] [Google Scholar]
- 43.Dansen TB, Wirtz KWA, Wanders RJA, Pap EHW. Peroxisomes in human fibroblasts have a basic pH. Nat. Cell Biol. 1999;2:51–53. doi: 10.1038/71375. [DOI] [PubMed] [Google Scholar]
- 44.Ralph EC, Anderson MA, Cleland WW, Fitzpatrick PF. Mechanistic studies of the flavoenzyme tryptophan 2-monooxygenase: Deuterium and 15N kinetic isotope effects on alanine oxidation by an L-amino acid oxidase. Biochemistry. 2006;45:15844–15852. doi: 10.1021/bi061894o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ralph EC, Hirschi JS, Anderson MA, Cleland WW, Singleton DA, Fitzpatrick PF. Insights into the mechanism of flavoprotein-catalyzed amine oxidation from nitrogen isotope effects on the reaction of N-methyltryptophan oxidase. Biochemistry. 2007;46:7655–7664. doi: 10.1021/bi700482h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JR, Edmondson DE. Structure-activity relationships in the oxidation of para-substituted benzylamine analogues by recombinant human liver monoamine oxidase A. Biochemistry. 1999;38:13670–13683. doi: 10.1021/bi990920y. [DOI] [PubMed] [Google Scholar]
- 47.Ghisla S, Massey V. L-Lactate oxidase. In: Muller F, editor. Chemistry and Biochemistry of Flavoenzymes, Vol. II. Boca Raton: CRC Press; 1991. pp. 243–289. [Google Scholar]
- 48.Emanuele JJ, Jr, Fitzpatrick PF. Mechanistic studies of the flavoprotein tryptophan 2-monooxygenase. 1. Kinetic mechanism. Biochemistry. 1995;34:3710–3715. doi: 10.1021/bi00011a028. [DOI] [PubMed] [Google Scholar]
- 49.Lee MB, Winder C, Cooch H, Shiekhattar R. An essential role for CoREST in nucleosomal histone3 lysine4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.