Abstract
In the present investigation, we determined the chemotherapeutic efficacy of 9-bromonoscapine (Br-Nos), a more potent noscapine analog, on MCF10A, spontaneously immortalized human normal breast epithelial cells and MCF10A-CSC3, cigarette smoke condensate (CSC)-transformed cells. The results from cytogenetic analysis showed that Br-Nos induced polyploidy and telomeric association in MCF10A-CSC3 cells, while MCF10A cells remained unaffected. Our immunofluorescence data further demonstrated that MCF10A-CSC3 cells were susceptible to mitotic catastrophe on exposure to Br-Nos and failed to recover after drug withdrawal. MCF10A-CSC3 cells exhibited Br-Nos-induced aberrant multipolar spindle formation, which irreversibly impaired the alignment of replicated chromosome to the equatorial plane and finally culminated in cell death. Although MCF10A cells upon Br-Nos treatment showed bipolar spindles with some uncongressed chromosomes, these cells recovered fairly well after drug withdrawal. Our flow-cytometry analysis data reconfirmed that MCF10A-CSC3 cells were more susceptible to cell death compared to MCF10A cells. Furthermore, our results suggest that decreased levels of cdc2/cyclin B1 and cdc2 kinase activity are responsible for Br-Nos-induced mitotic cell arrest leading to cell death in MCF10A-CSC3 cells. This study thus explores the underlying mechanism of Br-Nos-induced mitotic catastrophe in CSC-transformed MCF10A-CSC3 cells and its potential usefulness as a chemotherapeutic agent for prevention of cigarette smoke-induced breast cancer growth.
Keywords: Cigarette smoke condensate, Breast cancer, 9-bromonoscapine, cell cycle, mitotic arrest, apoptosis
Introduction
Breast cancer is the most common cause of cancer-related deaths in women. Main-stream and side-stream cigarette smoke is a major threat to the health of both smokers and non-smokers. The carcinogens present in the cigarette smoke cause variety of cancers including larynx, oral cavity, pharynx, esophagus, pancreas, kidney, breast, bladder and colon cancer. Although lung cancer has been directly linked to cigarette smoking related deaths, its relationship to breast cancer has been an area of intense debate. Epidemiological studies reflect conflicting association between cigarette smoking and breast cancer risk [Lash and Aschengrau, 2002; Reynolds et al., 2004]. However, biological studies support the theory that cigarette smoke can play a role in breast carcinogenesis [Band et al., 2002; Narayan et al., 2004]. Chemical analysis of cigarette smoke indicates the presence of varying amounts of mammary carcinogens which could influence breast carcinogenesis [Hecht, 2002].
New strategies in combating cancer have been continuously developed which utilize combinations of conventional chemotherapeutic agents with radiation therapy [Lawrence et al., 2003]. Most of the chemotherapeutic agents act by creating DNA-strand breaks which, in turn, arrest or initiate apoptotic pathways in cancer cells. The majority of chemotherapeutic drugs can be divided into alkylating agents, antimetabolites, anthracyclines, plant alkaloids, topoisomerase inhibitors and monoclonal antibodies. Recent studies suggest that anti-microtubule agents can also induce apoptosis [Miller et al., 1999; Ye et al., 2001] and have potential for therapeutic applications for the treatment of cancer. These agents alter microtubule dynamics and interfere with cell cycle surveillance mechanisms to arrest the cells in mitosis. Microtubules also play a central role in biological processes such as intracellular transport, motility, morphogenesis and mitotic spindle formation during cell division [McIntosh et al., 2002]. Defects in the process of cell division have severe consequences leading to aneuploidy, genetic instability and eventually metastatic tumorigenesis [Jallepalli and Langauer, 2001]. Several new microtubule targeting agents have shown potent activity against proliferation of various cancer cells [Jordan et al., 1998]. One group of such agents (such as colchicines, nocadazole and vinca alkaloids) inhibits microtubule polymerization, while the other group of agents (taxoids and epothilones) promote microtubule polymerization. Nonetheless, they disrupt microtubule dynamics, block cell cycle progression at mitosis, and then cause cell death. Noscapine and its derivatives are another group of microtubule-modulating agents that possess anti-tumor activity [Ye et al., 1998; Zhou et al., 2003]. They subtly modulate microtubule dynamics, which is sufficient to activate mitotic checkpoints to arrest mitosis, but they do not overpolymerize or depolymerize microtubules. [Aneja et al., 2006a; Zhou et al., 2002]. Because they do not alter the total polymer mass of tubulin and are thus non-toxic, noscapinoids represent a novel class of microtubule-modulating agents and are suitable candidates for development as chemotherapeutic agents against rapidly dividing cancer cells.
We have previously shown that the spontaneously immortalized normal breast epithelial cell line, MCF10A, can be transformed in culture by treatment with cigarette smoke condensate (CSC) [(Narayan et al., 2004]. This study provided a link between cigarette smoking and the development of breast carcinogenesis. Since a successful chemotherapeutic application of a drug depends upon its efficacy of interfering with cell cycle and triggering the apoptotic pathway, we examined how a microrubule-modulating agent, 9-bromonoscapine (Br-Nos), can selectively initiate mitotic catastrophe and promote cell death in CSC-transformed MCF10A-CSC3 cells while sparing the normal MCF10A cells.
Materials and Methods
Maintenance of cells and treatment
The spontaneously immortalized human normal breast epithelial cell line, MCF10A, and CSC-transformed cell line, MCF10A-CSC3, were grown in DMEM/F-12 (50:50, v/v) (Mediatech, Cellgro, VA) medium supplemented with 5% horse serum (Sigma Chemical Co., St. Louis, MO) and growth factors as described earlier [Narayan et al., 2004].
Clonogenic assay
For clonogenic assay cells were trypsinized and a single cell suspension was prepared. Cells were plated at density of 3−5 × 102 cells per well (60 mm tissue culture plate). After the attachment cells were treated with different concentrations of Br-Nos for 48 h. Then the media containing Br-Nos was removed and supplemented with fresh medium. Cells were allowed to grow into colonies for additional 10 days. Colonies were fixed, stained with 0.025% (w/v) crystal violet and counted. Colonies containing less than twenty cells were not included in counting.
Cytogenetic analysis
Conventional cytogenetic analysis was performed using the standard laboratory procedure [Multani et al., 2000]. In brief, Metaphase spreads from MCF10A and MCF10A-CSC3 cells were prepared after treatment with Br-Nos for 24 h followed by the addition of colcemid 2 h prior to trypsinization. Cells were trypsinized and centrifuged at 1,000 rpm, and incubated in 60 mM KCl for 15 min, centrifuged again, and finally fixed in methanol:glacial acetic acid (3:1, v/v). Giemsa staining was performed according to standard protocols. At least, 35 metaphases from each experiment were analyzed and the chromosomal ploidies as well as structural anomalies including telomeric association were recorded.
Immunofluorescence microscopy
Cells were grown on poly(L-lysine)-coated glass cover slips for immunofluorescence microscopy as described previously [Aneja et al., 2006a]. After treatment, cells were fixed with cold (−20°C) methanol for 5 min and then washed with PBS for 5 min. Nonspecific sites were blocked by incubating with 2% bovine serum albumin in PBS at 37°C for 15 min and incubated with a mouse monoclonal antibody against α-tubulin (Sigma-Aldrich Chem Co., St Louis, MO) for 2 h at 37°C. The microtubules were stained using the α-tubulin antibody (Sigma, St Louis, MO) that was labeled with a FITC-conjugated secondary antibody and the nucleus was stained using the DNA intercalating dye, propidium iodide. Cells were then washed and incubated with fluorescence isothiocyanate (FITC)-labeled goat anti-mouse IgG antibody (Jackson Immuno Research, Inc., West Grove, PA) for 1 h at 37°C. Cover slips were washed and then incubated with propidium iodide (PI) (0.5 µg/mL) for 15 min at room temperature before they were mounted with Aquamount (Lerner Laboratories, Pittsburgh, PA) containing 0.01 % 1,4-diazobicyclo(2,2,2)octane (Sigma). Cells were then examined using confocal microscopy for microtubule morphology and chromosomal organization (at least 100 cells were examined per condition). Propidium iodide staining of the nuclei was used to visualize fragmented pieces of DNA that are indicative of apoptotic bodies.
Fluorescence activated cell sorting (FACS)
For determining the cell cycle profile, cells were plated in 60 mm tissue culture dishes and grown until 60% confluence. Cells were treated with 25 µM Br-Nos for 24 h. After the treatment, cells were harvested at different time intervals, washed with ice-cold PBS and processed for FACS analysis as described previously [Jaiswal et al., 2002a]. The ranges for G0/G1, S, and G2/M phase arrested cells were established on the basis of the corresponding DNA content of the histograms.
Western blot analysis
The protein levels of Bax, Bcl2, Caspase-3, Cleaved product of Caspase-3, poly(ADP)ribose polymerase 1 (PARP-1), phospho-cdc2, cdc2, cyclin B1, and α-tubulin were determined by Western blot analysis with our previously described procedure [Narayan et al, 2004]. The anti-Bax, anti-Bcl2, anti-cdc2, anti-cyclin B1 antibodies were procured from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-procaspase-3 and active caspase-3, anti-PARP1, anti-phospho-cdc2, antibodies from Cell Signaling (Danvers, MA) and anti-α-tubulin antibody from Sigma Chem. Co., (St. Louis, MO), were used in these studies.
Results
MCF10A-CSC3 cells are more sensitive to the antiproliferative effects of Br-Nos than MCF10A cells
To determine the sensitivity of MCF10A-CSC3 and MCF10A cells to Br-Nos, we determined the IC50 of Br-Nos in these cell lines using a clonogenic assay [Balusu et al., 2007]. Cells were plated in 6-well plates and treated with varying concentrations of Br-Nos for 24 h. Then the media containing Br-Nos was removed and supplemented with fresh medium. After 10 days, cell colonies were counted in both control and treated groups. The data was plotted for the determination of IC50 of Br-Nos in these cell lines. The IC50 of Br-Nos in MCF10A-CSC3 cells (4.9 µM) was 2.0-fold lower than MCF10A cells (9.8 µM) (Fig. 1). These results indicated that MCF10A-CSC3 cells were more sensitive to Br-Nos treatment than MCF10A cells. In general, the anchorage-dependent colony forming efficiency of MCF10A-CSC3 cells was higher than MCF10A cells, which is consistent with our previous observations [Narayan et al., 2004].
Figure 1.
Clonogenic assay for the determination of IC50 of Br-Nos in MCF10A and MCF10A-CSC3 cells. MCF10A and MCF10A-CSC 3 cells were plated in 6-well plates and treated with Br-Nos for 48 h. After treatment, the drug was removed, and cells were washed and incubated with a fresh medium for 10 days. The visible colonies were stained and counted. The results were plotted on a common-log scale to determine the IC50. Data are mean ± SE of three different experiments.
Br-Nos treatment increases the diploid population (4N) of MCF10A-CSC3 cells
In previous studies, we have shown CSC-induced transformation of MCF10A cells and established a transformed cell line, MCF10A-CSC3, in culture [Narayan et al., 2004]. Since a novel noscapinoid, 9-bromonoscapine has been shown to selectively induce apoptosis in cancer cells and spares normal cells, we were inquisitive to know its effects on these paired cell lines: MCF10A and MCF10A-CSC3 and characterized them for any differential physiological characteristics and their responses to Br-Nos treatment. Moreover, IC50 data indicated that MCF10A-CSC3 cells are more sensitive to Br-Nos treatment as compared to MCF10A cells. MCF10A and MCF10A-CSC3 cells were treated with 20 µM of Br-Nos for 24 h, fixed, and processed for chromosome analysis (Table-1). Our results showed 68.5% diploid population in MCF10A cells with a lesser frequency of polyploidy (31.5%). However, after Br-Nos treatment for 24 h, diploid population of MCF10A cells increased to 84.4% with a further reduction of polyploidy (15.6%). On the other hand, MCF10A-CSC3 cells showed 44.1% diploid population and also exhibited 55.9% polyploidy, which did not change significantly after treatment with Br-Nos (Table 1). In addition, we observed a few MCF10A-CSC3 cells with fragmented DNA pieces and telomeric associations in treated group, which are indicative of apoptosis [Pathak et al., 1994], while MCF10A cells did not show any telomeric association (Table 1). This strongly suggested that MCF10A-CSC3 cells treated with Br-Nos were susceptible to mitotic catastrophe.
Table-1.
Metaphase analysis of MCF10A and MCF10A-CSC3 cell lines after treatment with Br-Noscapine.
| Treatment | No. of cells analyzed |
% Normal diploid cells |
% Polyploidy | % Cells with telomeric association |
|---|---|---|---|---|
| MCF10A | ||||
| Control | 54 | 68.5 | 31.5 | 0 |
| Br-Noscapine | 32 | 84.4 | 15.6 | 0 |
| MCF10A-CSC3 | ||||
| Control | 34 | 44.1 | 55.9 | 0 |
| Br-Noscapine | 36 | 41.7 | 58.3 | 12.1 |
The MCF10A and MCF10A-CSC3 cell lines were treated with 20 µM Br-Noscapine for 24 h and then processed for metaphase analysis. Data are the mean of 32–54 samples in each group.
Br-Nos disrupts spindle architecture
We found that the MCF10A-CSC3 cells are more susceptible to aneuploidy as compared to MCF10A cells after treatment with Br-Nos. To test further whether Br-Nos can induce spindle abnormality and mitotic catastrophe in MCF10A-CSC3 cells, we employed immunofluorescence microscopy to assess its effect on microtubule morphology, nuclear morphology and chromosomal organization. MCF10A and MCF10A-CSC3 cells were treated with 25 µM of Br-Nos for 24 h and processed for immunofluorescence analysis. We observed that both untreated MCF10A and MCF10A-CSC3 cells exhibited normal radial microtubule arrays in interphase cells (Fig. 2A). However, Br-Nos treatment showed a remarkable difference in the spindle organization. Both MCF10A and MCF10A-CSC3 cells arrested transiently in mitosis with aberrant spindle morphology. The normal breast epithelial cells, MCF10A, showed a bipolar spindle apparatus with a few congressed chromosomes at one or both poles, whereas, MCF10A-CSC3 cells had multipolar spindles (number of poles greater than 3). The quantitation of these poles in MCF10A and MCF10A-CSC3 cells after treatment with Br-Nos showed 90% and 7.5% of bipolar spindle apparatus in MCF10A and MCF10A-CSC3 cells, respectively. A significant increase of 77% in multi-polar spindle apparatus consisting of more than 4 poles in MCF10A-CSC3 cells was observed as compared to MCF10A (Fig. 2B). This perhaps, is partly explainable in MCF10A cells where improper congression of chromosomes at the metaphase plate might be due to attenuation of microtubule dynamics. But the precise mechanism for the emergence of multipolar phenotype in malignant transformed cells is not yet clear.
Figure 2.
Panel A, Br-Nos disrupts spindle architecture in MCF10A and MCF10A-CSC3 cells. Confocal micrographs of MCF10A and CSC transformed MCF10A-CSC3 cells treated with 25 µM of Br-Nos for 24 h. Intact normal radial arrays of microtubules and typical characteristic of cellular morphologies of MCF10A cells are visible at beginning of treatment. After 24 h of Br-Nos treatment, mitotic figures were evident in both MCF10A and MCF10A-CSC3 cells. Bipolar spindle formation with uncongressed chromosomes was also abundant in MCF10A cells while multipolar spindle were predominantly present in MCF10A-CSC3 cells. Panel B, quantitation of multipolar spindle in MCF10A and MCF10A-CSC3 cells in Br-Nos treated group. Multipolarity in MCF10A cells (almost none) and MCF10A-CSC3 cells were assessed by counting the number of poles (bipolar, 3 poles, 4 poles and > 4 poles). Data are mean ± SE of three different experiments.
Br-Nos causes irreversible mitotic arrest in MCF10A-CSC3 cells
To determine whether Br-Nos-induced mitotic arrest in MCF10A-CSC3 cells was a reversible phenomenon, we treated MCF10A and MCF10A-CSC3 cells with 25 µM of Br-Nos for 24 h. After treatment the drug was removed and fresh medium was supplemented as outlined in Figure 3A. Cells were then allowed to grow in fresh medium for different intervals. One set of cells was collected immediately after the treatment, which served as a zero time withdrawal control (Fig. 3B, upper and lower left panels). Cells were stained and processed for immunofluorescence microscopy as described in materials and methods. MCF10A cells (Fig. 3B, upper panel left) showed bipolar spindles with uncongressed chromosomes, whereas, MCF10A-CSC3 cells (Fig. 3B, lower panel left) exhibited pronounced multipolar spindles. After 12 h of drug withdrawal, MCF10A and MCF10A-CSC3 cells did not show a significant change in the microtubular and mitotic architecture. However, after 24 h of Br-Nos withdrawal, MCF10A cells showed recovery from mitotic arrest and normal cytokinesis was observable. Normal radial microtubule arrays were evident (Fig 3B, upper panel right). On the other hand, MCF10A-CSC3 cells still showed aberrant multipolar spindles (Fig. 3B, lower panel right) which perhaps have committed to cell death. DNA fragmentation was observable indicating cell death. This indicated that even after drug removal MCF10A-CSC3 cells exhibited irreversible mitotic arrest followed by mitotic castarophe or apoptotic cell death. The quantitative analysis of the poles after 24 h of drug withdrawal further showed a complete absence of multipolar spindle apparatus in MCF10A cells, while MCF10A-CSC3 cells still showed a predominance of multipolar spindle apparatus (Fig. 3C).
Figure 3. Br-Nos irreversibly induces spindle abnormalities in MCF10A-CSC3 cells.
Panel A shows the protocol of the experiment. Panel B, Immunoflorescence micrographs of MCF10A and CSC-transformed MCF10A-CSC3 cells treated with 25 µM of Br-Nos for 24 h. After treatment, the drug was removed; cells were washed and supplemented with fresh medium. Cells were allowed to grow for additional 12h and 24 h. Intact normal radial arrays of microtubules, typical characteristic of cellular morphologies of MCF10A cells were visible at time 0. At 24 h post drug treatment, mitotic figures with bipolar spindles and a few uncongressed chromosomes were abundant in MCF10A cells while multipolar spindle were predominantly present in MCF10A-CSC3 cells. Panel C, quantitation of multipolar spindle formation in MCF10A and MCF10A-CSC3 cells after the 24 h of withdrawal of Br-Nos. Multipolarity in MCF10A cells (almost none) and MCF10A-CSC3 cells were assessed by counting the number of poles (bipolar, 3 poles, 4 poles and > 4 poles). Data is representative of three different experiments.
Br-Nos induces apoptosis in MCF10A-CSC3 cells
Tumor cells respond to chemotherapeutic agents by initiating apoptosis [Wang et al., 1999]. To determine the quantitative effect of Br-Nos treatment on cell cycle and apoptosis, we treated MCF10A and MCF10A-CSC3 cells with varying concentrations of Br-Nos for 24 h and then processed them for FACS analysis. The untreated MCF10A and MCF10A-CSC3 cells showed a typical distribution of cell population in G0/G1, S, and G2/M phases of cell cycle (Fig 4A and B, respectively). There was an increased G0/G1 phase arrest in both MCF10A and MCF10A-CSC3 cell lines treated with 10 µM of Br-Nos. However, the G0/G1 phase arrest declined and S phase arrest was increased in MCF10A cells treated with 25 µM of Br-Nos. On the other hand, after 25 µM Br-Nos treatment, MCF10A-CSC3 cells were arrested in S and G2/M phases, with the G2/M phase arrest being more prominent (Fig. 4B). The sub-G1 cell population, which is an indicator of apoptosis in FACS analysis, was 7–10% in MCF10A cells (Fig. 4A). However, the sub-G1 population in MCF10A-CSC3 cells increased in a dose-dependent manner, and was 44% at 25 µM of Br-Nos treatment (Fig. 4B). These results indicate that Br-Nos treatment induces pronounced apoptosis in MCF10A-CSC3 cells compared to MCF10A cells.
Figure 4. Br-Nos induces differently the cell cycle perturbation and apoptosis in MCF10A and MCF10A-CSC3 cell lines.
Br-Nos inhibits cell cycle progression at mitosis in MCF10A cells followed by the appearance of sub-G1 population, indicative of apoptosis. Panel A depicts analysis of cell cycle distribution in MCF10A cells treated with varying concentrations (0–25 µM) of Br-Nos for 24 h as determined by FACS analysis. Panel B represents analysis of cell cycle distribution in MCF10A-CSC3 cells treated with varying concentrations (0–25 µM) of Br-Nos for 24 h as determined by FACS analysis. The number of sub-G1 phase cells is calculated from the 100% of total number of cells in each well. Data are mean ± SE of three different estimations.
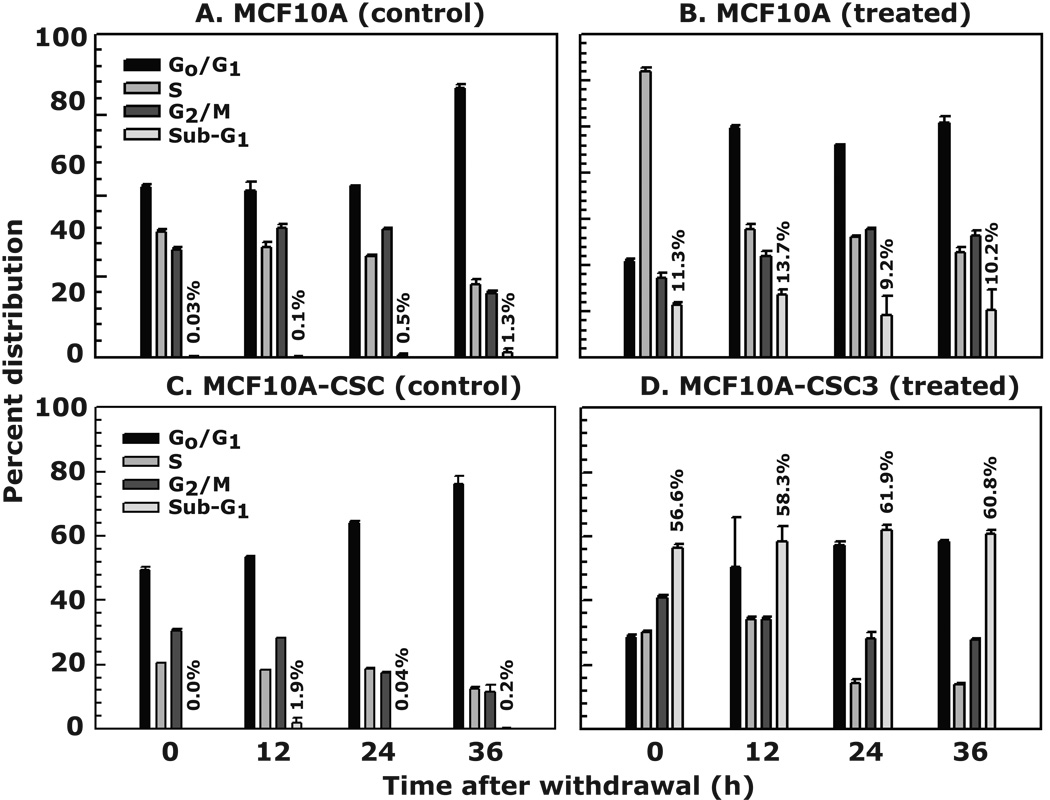
To test whether increased mitotic arrest in MCF10A-CSC3, which presumably leads to apoptosis, is irreversible, we treated both cell lines with 25 µM of Br-Nos for 24 h. After treatment, the drug containing medium was replaced with fresh medium. Cells were harvested at different intervals and processed for FACS analysis. Our results showed a normal distribution of cells in various phases of cell cycle progression in control untreated MCF10A cells (Fig. 5A), while in the Br-Nos treated group, cells significantly arrested in S phase before drug withdrawal. After drug withdrawal, the MCF10A cells recovered from the arrest and resumed normal cell cycle, exhibiting up to 10% sub-G1 population at 36 h post-withdrawal (Fig. 5B). However, the MCF10A-CSC3 cells after drug withdrawal continued to arrest in the G2/M phase and showed 60% sub-G1 population compared to untreated controls (Fig. 5, compare C with D, respectively). The extent of apoptosis in MCF10A-CSC3 cells remained in the same range at all time points after the withdrawal of the drug. This suggests that after a 24 h Br-Nos treatment, MCF10A-CSC3 cells committed to apoptosis, and thus could not recover after the drug was withdrawn.
Figure 5. Br-Nos induces irreversible apoptosis in MCF10A-CSC3 cells.
The protocol for experimental procedure is given in Figure 2, Panel A. The MCF10A and MCF10A-CSC3 cells were treated with 25 µM of Br-Nos for 24 h. After 24 h of treatment Br-Nos was removed and supplemented with fresh medium. Cells were allowed to grow for additional 36 h. Cell cycle analysis was determined by FACS at various time intervals after withdrawal of Br-Nos. The percent distribution of G0/G1, S and G2/M phase cells does not include the sub-G1 cells. The number of sub-G1 phase cells is calculated from the 100% of total number of cells in each well. Data are mean ± SE of three different estimations.
Br-Nos-induced irreversible mitotic arrest in MCF10A-CSC3 cells is due to inactivation of cdc2/cyclin B1
The molecular mechanisms of cell cycle arrest and apoptosis in MCF10A and MCF10A-CSC3 cells upon treatment with Br-Nos are not known. It is well-appreciated that increased levels of cdc2 and cyclin B1 serve as a marker for G2/M phase arrest and the sequential entry into mitosis requires activation of cdc2 kinase, while the exit from mitosis needs inactivation of cdc2 kinase activity [Murray, 1989]. The cdc2/cyclin B1 kinase is inactivated in G2/M phase-arrested cells after phosphorylation at serine-14 and tyrosine-15 residues of the cdc2 protein [O'Connor, 2002]. To test the hypothesis that sustained activation of cdc2 should be maintained in Br-Nos-induced mitotic arrest, we treated MCF10A and MCF10A-CSC3 cells with 25 µM of Br-Nos for 24 h, removed the drug and followed the time course as described in Figure 6A. We examined Br-Nos-induced irreversible mitotic arrest leading to cell death in MCF10A-CSC3 cells by determining cdc2/cyclin B1 levels. The phospho-cdc2 levels were dramatically reduced after 24 h of treatment (indicated as 0 h time point, see protocol in Fig. 6A) with Br-Nos in both MCF10A and MCF10A-CSC3 cell lines. The effect was more pronounced in MCF10A-CSC3 cells than in MCF10A cells (Fig. 6B, compare lane 1 with 5 and 9 with 13, respectively). The total cdc2 level was reduced only in MCF10A cells, not as much in MCF10A-CSC3 cells (Fig.6B, compare lane 1 with 5 and 9 with 13, respectively). A similar effect was observed on the level of cyclin B1. Once Br-Nos was withdrawn from the cells and they were incubated further for recovery, the total phospho-cdc2 and cyclin B1 levels were decreased in untreated MCF10A and MCF10A-CSC3 cells in a time-dependent manner (Fig. 6B, compare lane 1–4 and 9–12, respectively). In Br-Nos treated MCF10A cells, total cdc2, phospho-cdc2 and cyclin B1 levels were recovered and maintained up to 36 h (Fig. 6B, lanes 5–8), while in MCF10A-CSC3 cells, these protein levels did not recover at all (Fig. 6B, lanes 13–16). In fact, the level of phospho-cdc2 and cyclin B1 were drastically reduced after only 12 h of drug withdrawal (Fig. 6B, compare lane 13 with 14–16). These results suggest correlation between decreased cyclin B1 levels and the irreversible effect of Br-Nos on the apoptosis of MCF10A-CSC3 cells.
Figure 6. Effect of Br-Nos on cell cycle-related proteins.
Panel A describes the protocol for experimental procedure. The MCF10A and MCF10A-CSC3 cells were treated with 25 µM of Br-Nos for 24 h, after the treatment Br-Nos was removed and supplemented with fresh medium. Cells were allowed to grow for additional 36 h. The cells were harvested at various time intervals after withdrawal of Br-Nos and lysates were prepared for western blot analysis. Panel B shows the protein levels of cdc2, cyclin B1, phospho-cdc2 and α-tubulin in MCF10A and MCF10A-CSC3 cells. The quantification of the protein bands is shown on the top of each autoradiogram as a fold-change of control. Results are representative of three different experiments.
Br-Nos-induced apoptosis in MCF10A-CSC3 cells is due to activation of apoptosisrelated gene products
To further investigate the underlying mechanism of Br-Nos-induced apoptosis in MCF10A-CSC3 cells, we examined the effect of drug on some apoptosis-regulatory molecules (Fig. 7A). Our results showed that Bax and Bcl2 protein levels remain unaltered in both MCF10A and MCF10A-CSC3 cells after the withdrawal of Br-Nos for up to 36 h, except untreated MCF10A-CSC3 cells showed some increase in Bax levels (Fig. 7B). Next, we examined the protein levels of caspase-3 and activated caspase-3 in both MCF10A and MCF10A-CSC3 cells after withdrawal of Br-Nos treatment. Our results showed an active caspase 3 (cleaved caspase-3) product after 24 h of treatment in both MCF10A and MCF10A-CSC3 cells (Fig. 7B, compare lane 1 with 5 and 9 with 13, respectively), which is shown as 0 h withdrawal (Fig. 7A). However, as the cells were allowed to recover after withdrawal of Br-Nos, there was an absence of cleaved caspase-3 in MCF10A cells, which, however, persisted in MCF10A-CSC3 cells for an additional 24 h (Fig. 7B, compare lane 5 with 6–8 and 13 with 14– 16, respectively). The activation of caspase-3 generates a product of 17 and 19 kDa protein fragments. A similar pattern of cleavage was observed with PARP-1. The cleaved PARP-1 recovered in both MCF10A and MCF10A-CSC3 cells after 24 h and 36 h respectively, after drug withdrawal. These result indicated that MCF10A-CSC3 cells showed a sustained activation of caspase-3 and cleavage of PARP-1 for longer periods of time which perhaps might be associated with Br-Nos-induced apoptosis in MCF10A-CSC3 cells compared to MCF10A cells.
Figure 7. Effect of Br-Nos on cell cycle- and apoptosis-related proteins.
Panel A describes the protocol for experimental procedure. The MCF10A and MCF10A-CSC3 cells were treated with 25 µM of Br-Nos for 24 h, after the treatment Br-Nos was removed and supplemented with fresh medium. Cells were allowed to grow for additional 36 h. The cells were harvested at various time intervals after withdrawal of Br-Nos and lysates were prepared for western blot analysis. Panel B depicts the protein levels of Bax, Bcl2, casapse 3, activated caspase-3, cleaved product of PARP1 and α-tubulin in MCF10A and MCF10A-CSC3 cells. The quantification of the protein bands is shown on the top of each autoradiogram as a fold-change of control. Results are representative of three different experiments.
Discussion
In the present study, we have examined the molecular mechanisms by which the non-toxic microtubule-modulating noscapinoid, Br-Nos, might induce apoptosis in CSC-transformed breast epithelial cells. Microtubule disrupting agents have been targeted for therapeutic applications in the treatment of cancer. These compounds exert their action by primarily blocking mitosis leading to cell death. Noscapine and its derivatives have emerged as potent anti-tumor agents [Ye et al., 1998; Zhou et al., 2003; Aneja et al., 2006a; 2006b]. However, one major issue that remains is whether these microtubule disrupting agents can exert their toxic effects selectively on cancer cells. The safety of a drug is assessed based on minimal toxicity to the normal cells versus cancer cells. To test this hypothesis we examined the effect of Br-Nos on paired breast cancer cells, MCF10A (near-normal) and MCF10A-CSC3 (malignant tranformant). Spontaneously immortalized human normal breast epithelial cell line, MCF10A, upon exposure to CSC, acquires a transformed phenotype and exhibits an anchorage independent colony forming efficiency, which is a characteristic of cell transformation [Narayan et al., 2004]. This change in phenotypic characteristic establishes a link between cigarette smoking and the development of breast cancer. The malignant transformation of MCF10A cells on exposure to CSC led to acquisition of resistance towards cell death, partly due the increased Bcl2/Bax ratio [Narayan et al., 2004; Cheng et al., 2001; Zhang et al., 2000] and impaired DNA repair capacity of cells [Narayan et al., 2004; Kundu et al., 2007]. One of the transformation characteristics of CSC-transformed MCF10A-CSC3 cells was its increased polyploidy upon Br-Nos treatment. We found an enhanced polyploidy and telomeric association in MCF10A-CSC3 cells, while the MCF10A cells remained normal. Telomeres play a functional role in cell survival and are implicated in the control of mitosis [Greider and Blackburn, 1996; Kishi et al, 2001]. During the cell cycle progression from G0/G1→S→G2/M phase the chromosomes under go large scale movement and rearrangement. If the chromosomes are not properly aligned then there can be catastrophic consequence leading to mitotic arrest, aneuploidy and cell death. The abnormal chromosomal segregation can lead to mitotic catastrophe due to defects in cell cycle and spindle check points. When cells fail to arrest before or at mitosis they segregate with aberrant chromosomes, leading to activation of apoptotic pathway. Mitotic catastrophe is considered as one of the mechanisms for preventing aneuploidy, which is the hallmark for carcinogenesis. Mitotic catastrophe is regulated by the cell cycle specific kinases, cell cycle checkpoint proteins, survivin, p53, caspases and Bcl2-family members. The mitotic catastrophe is characterized by the presence of multiple micronuclei and decondensed chromosomes [Swanson et al., 1995]. It is accompanied by mitochondrial release of proapoptotic proteins, caspase activation and DNA degradation; however, it is fundamentally different from apoptosis where chromatin condensation is a hallmark. Mitotic catastrophe can be induced by microtubule-depolymerzing as well as DNA damaging agents [Roninson et al., 2001]. The increased polyploidy in MCF10A-CSC3 cells could be a result of telomeric association causing increased genomic instability [Gilley et al., 2005], which is also supported by studies in yeast [Galitski et al., 1999]. The MCF10A and MCF10A-CSC3 cells were further analyzed by immunofluorescence studies and our results indicated that Br-Nos treatment induced bipolar spindle formation with a few uncongressed chromosomes in MCF10A cells, while it exhibited significantly aberrant multipolar spindle morphologies in MCF10A-CSC3 cells. The MCF10A arrested resumed normal cell cycle upon drug removal, while the MCF10A-CSC3 cells with aberrant multipolar spindle formation did not recover upon drug withdrawal. The clonogenic survival assay further supported that the reduced number of colonies of MCF10A-CSC3 cells after Br-Nos treatment are due to a sustained mitotic arrest that culminates in cell death. The irreversible mitotic catastrophe in MCF10A-CSC3 leads them to cell death.
The withdrawal effect of the Br-Nos treatment on MCF10A-CSC3 cells resulted in an elevated level of Bax expression, indicating that cells are more susceptible for apoptotic stimuli. On the other hand, MCF10A cells showed a reduced level of Bax as compared to MCF10A-CSC3 cells, indicating that these cells are more resistant to apoptotic stimuli. In previous studies, it has been found that p53 level also increases when cells were exposed to noscapine for longer period. In these studies, the p53 level was associated with the increased Bax/Bcl2 ratio [Aneja et al., 2007], suggesting that the noscapine induced apoptosis might be dependent on p53. Several p53-inducible genes, which participate in apoptosis, have been identified. These genes are mdm-2 [Oliner et al., 1992], GADD45 [Kastan et al., 1992], Bax [Miyashita and Reed, 1995], and p21 [Xiong et al., 1993]. It has been demonstrated that p53 can up-regulate the proapoptotic gene Bax [Miyashita and Reed, 1995], and possibly, transcriptionally repress the antiapoptotic gene Bcl-2 [Haldar et al., 1994]. Therefore, p53-mediated up-regulation of Bax and concomitant down-regulation of Bcl-2 are important for deciding the cellular fate between apoptosis and cell survival.
Cell cycle is a precisely regulated cellular event controlled primarily by cyclin dependent kinases (Cdks) and cyclins [Lew and Kornbluth, 1996]. Cdc2 has been known to play a central role in orchestrating this process. Cdc2 kinase activity is regulated at multiple levels by its binding with cyclin A or cyclin B, by phosphorylation/dephosphorylation, and by Cdk inhibitors. The increased level of these proteins has been associated with responses to several apoptotic stimuli such as DNA-damaging agents [Jaiswal and Narayan, 2002b; 2004], tumor necrosis factor-α [Meikanrantz et al., 1994], radiation [Porter et al., 2000], transforming growth factor-β [Choi et al., 1999], and microtubule-interfering agents [Ye et al., 1998; Aneja et al., 2006a, Zhou et al., 2002]. Activation of these proteins leads to entry into mitosis while inactivation of these proteins could trigger the release from mitosis. The aberrant expression/activation of cdc2 and certain types of cdk2, especially regulated by cyclin A or cyclin B, can result in activation of the apoptotic pathways. However, data from variety of systems have generated lot of controversy about the general applicability of this model. Other reports suggest that cdc2 activation is not required for the DNA-damage induced apoptosis [Norbury et al., 1994; Ongkeko et al., 1995]. The results described in the present study show that Br-Nos-induced mitotic arrest in MCF10A cells, was a reversible phenomenon, while CSC-transformed MCF10A-CSC3 cells showed an irreversible mitotic arrest that finally led to apoptosis. The most prevalent effect of Br-Nos is thus on mitotic events which ultimately decide cellular fate. The western blot analysis showed that cdc2, cyclin B1 and phospho-cdc2 levels decreased after Br-Nos treatment which started recovering after drug removal. The phospho-cdc2 levels are indirectly the measure of cdc2 kinase activity. The increased level of phospho-cdc2 reflects the inactivation of cdc2-kinase, which further suggests that cells are arrested in the G2/M phase. This is in contrast with the finding that sustained activation of cdc2 is required for noscapine-induced apoptosis [Ye et al., 2001]. However, it is quite possible that modification at 9th position in the parent compound to halogenate it could drastically alter the overall scenario of its mode of action. Thus, different microtubule disrupting agents can have different effects on cdc2 and cyclin B1 and apoptosis of different cancer cells. For example, the microtubule disrupting agent, paclitaxel, induces apoptosis in breast cancer cells and does not require cdc2 activity [Henley et al., 2007]. On the other hand, paclitaxel stimulates accumulation and activation of cdc2 during apoptosis of ovarian carcinoma cells [Chadebech et al., 2000]. In support of our studies, the reduced level of cdc2 and cyclin B1 is also implicated in baicalein (a bioactive flavonoid)- induced apoptosis of bladder cancer cell lines [Chao et al., 2007].
The Br-Nos withdrawal from treated MCF10A-CSC3 cells should reduce apoptosis, at least to those cells which have not committed to apoptosis. Interestingly, these cells showed reduced levels of phospho-cdc2 and cyclin B1 and proceeded in to cell cycle, but still maintained a high level apoptosis. This suggest that in MCF10A-CSC3 cells after Br-Nos treatment, progressing through G2/M with abnormal telomeric DNA, which instead of following the G0/G1→S phase progression, they are entering into apoptosis. Furthermore, it appears that the irreversible degradation of cyclin B1 is more important phenomenon in Br-Nos-induced apoptosis instead of supporting the mitotic arrest of MCF10A-CSC3 cells, which is indicated by recent findings [Gascoigne and Taylor, 2008]. In these studies, it has been suggested that the anti-mitotic drugs determine the fate of cells by two competing networks: (i) involving the degradation of cyclin B1, and (ii) activation of caspases. Both these pathways lead to cell death. Other studies also support our findings in which the gradual decrease in cyclin B1 level has been linked with mitotic arrest and eventually to cell death [Weaver and Cleveland, 2005; Brito and Rieder, 2006]. Thus, the irreversible decreased levels of cdc2 and cyclin B1 are perhaps responsible for Br-Nos-induced death of MCF10A-CSC3 cells. These results indicate that Br-Nos can be useful chemotherapeutic approach for the prevention of CSC-induced breast cancer growth with minimum or no effect on the normal breast epithelial cells.
Acknowledgements
The financial support for these studies was provided to Satya Narayan by the grants from NCI-NIH (CA-100247) and Flight Attendant Medical Research Institute.
References
- Aneja R, Lopus M, Zhou J, Vangapandu SN, Ghaleb A, Yao J, Nettles JH, Zhou B, Gupta M, Panda D, Chandra R, Joshi HC. Rational design of the microtubule-targeting anti-breast cancer drug EM015. Cancer Res. 2006a;66:3782–3791. doi: 10.1158/0008-5472.CAN-05-2962. [DOI] [PubMed] [Google Scholar]
- Aneja R, Vangapandu SN, Lopus M, Viswesarappa VG, Dhiman N, Verma A, Chandra R, Panda D, Joshi HC. Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem Pharmacol. 2006b;72:415–426. doi: 10.1016/j.bcp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balusu R, Jaiswal AS, Armas ML, Kundu CN, Bloom LB, Narayan S. Structure/function analysis of the interaction of adenomatous polyposis coli with DNA polymerase beta and its implications for base excision repair. Biochemistry. 2007;46:13961–13974. doi: 10.1021/bi701632e. [DOI] [PubMed] [Google Scholar]
- Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadebech P, Truchet I, Brichese L, Valette A. Up-regulation of cdc2 protein during paclitaxel-induced apoptosis. Int J Cancer. 2000;87:779–786. [PubMed] [Google Scholar]
- Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–3048. doi: 10.1158/1535-7163.MCT-07-0281. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2,BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Choi KS, Eom YW, Kang Y, Ha MJ, Rhee H, Yoon JW, Kim SJ. Cdc2 and Cdk2 kinase activated by transforming growth factor-beta1 trigger apoptosis through the phosphorylation of retinoblastoma protein in FaO hepatoma cells. J Biol Chem. 1999;274:31775–31783. doi: 10.1074/jbc.274.45.31775. [DOI] [PubMed] [Google Scholar]
- Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR. Ploidy regulation of gene expression. Science. 1999;285:251–254. doi: 10.1126/science.285.5425.251. [DOI] [PubMed] [Google Scholar]
- Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14:111–122. doi: 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Gilley D, Tanaka H, Herbert BS. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol. 2005;37:1000–1013. doi: 10.1016/j.biocel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996;274:92–97. doi: 10.1038/scientificamerican0296-92. [DOI] [PubMed] [Google Scholar]
- Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54:2095–2097. [PubMed] [Google Scholar]
- Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ Mol Mutagen. 2002;39:119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- Henley D, Isbill M, Fernando R, Foster JS, Wimalasena J. Paclitaxel induced apoptosis in breast cancer cells requires cell cycle transit but not Cdc2 activity. Cancer Chemother Pharmacol. 2007;59:235–249. doi: 10.1007/s00280-006-0262-1. [DOI] [PubMed] [Google Scholar]
- Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002a;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- Jaiswal AS, Narayan S. SN2 DNA-alkylating agent-induced phosphorylation of p53 and activation of p21 gene expression. Mutat Res. 2002b;500:17–30. doi: 10.1016/s0027-5107(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Jaiswal AS, Narayan S. Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J Cell Biochem. 2004;93:345–357. doi: 10.1002/jcb.20156. [DOI] [PubMed] [Google Scholar]
- Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- Jordan A, Hadfield JA, Lawrence NJ, McGown AT. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev. 1998;18:259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kishi S, Wulf G, Nakamura M, Lu KP. Telomeric protein Pin2/TRF1 induces mitotic entry and apoptosis in cells with short telomeres and is down-regulated in human breast tumors. Oncogene. 2001;20:1497–1508. doi: 10.1038/sj.onc.1204229. [DOI] [PubMed] [Google Scholar]
- Kundu CN, Balusu R, Jaiswal AS, Gairola CG, Narayan S. Cigarette smoke condensate-induced level of adenomatous polyposis coli blocks long-patch base excision repair in breast epithelial cells. Oncogene. 2007;26:1428–1438. doi: 10.1038/sj.onc.1209925. [DOI] [PubMed] [Google Scholar]
- Lash TL, Aschengrau A. A null association between active or passive cigarette smoking and breast cancer risk. Breast Cancer Res Treat. 2002;75:81–84. doi: 10.1023/a:1019625102365. [DOI] [PubMed] [Google Scholar]
- Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Grishchuk EL, West RR. Chromosome-microtubule interactions during mitosis. Annu Rev Cell Dev Biol. 2002;18:193–219. doi: 10.1146/annurev.cellbio.18.032002.132412. [DOI] [PubMed] [Google Scholar]
- Meikrantz W, Gisselbrecht S, Tam SW, Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci USA. 1994;91:3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, 3rd, Johnson KR, Willingham MC, Fan W. Apoptotic cell death induced by baccatin III, a precursor of paclitaxel, may occur without G(2)/M arrest. Cancer Chemother Pharmacol. 1999;44:444–452. doi: 10.1007/s002800051117. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Multani AS, Ozen M, Narayan S, Kumar V, Chandra J, McConkey DJ, Newman RA, Pathak S. Caspase-dependent apoptosis induced by telomere cleavage and TRF2 loss. Neoplasia. 2000;2:339–345. doi: 10.1038/sj.neo.7900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW. Cell biology: the cell cycle as a cdc2 cycle. Nature. 1989;342:14–15. doi: 10.1038/342014a0. [DOI] [PubMed] [Google Scholar]
- Narayan S, Jaiswal AS, Kang D, Srivastava P, Das GM, Gairola CG. Cigarette smoke condensate-induced transformation of normal human breast epithelial cells in vitro. Oncogene. 2004;23:5880–5889. doi: 10.1038/sj.onc.1207792. [DOI] [PubMed] [Google Scholar]
- Norbury C, MacFarlane M, Fearnhead H, Cohen GM. Cdc2 activation is not required for thymocyte apoptosis. Biochem Biophys Res Commun. 1994;202:1400–1406. doi: 10.1006/bbrc.1994.2086. [DOI] [PubMed] [Google Scholar]
- O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–44. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Ongkeko W, Ferguson DJ, Harris AL, Norbury C. Inactivation of Cdc2 increases the level of apoptosis induced by DNA damage. J Cell Sci. 1995;108:2897–2904. doi: 10.1242/jcs.108.8.2897. [DOI] [PubMed] [Google Scholar]
- Pathak S, Risin S, Brown NM, Berry K. Telomeric association of chromosomes is an early manifestation of programmed cell death. Int J Oncol. 1994;4:323–328. doi: 10.3892/ijo.4.2.323. [DOI] [PubMed] [Google Scholar]
- Porter LA, Singh G, Lee JM. Abundance of cyclin B1 regulates gamma-radiation-induced apoptosis. Blood. 2000;95:2645–2650. [PubMed] [Google Scholar]
- Reynolds P, Hurley S, Goldberg DE, Anton-Culver H, Bernstein L, Deapen D, Horn-Ross PL, Peel D, Pinder R, Ross RK, West D, Wright WE, Ziogas A. Regional variations in breast cancer among california teachers. Epidemiology. 2004;15:746–754. doi: 10.1097/01.ede.0000134863.45834.50. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–313. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Swanson PE, Carroll SB, Zhang XF, Mackey MA. Spontaneous premature chromosome condensation, micronucleus formation, and non-apoptotic cell death in heated HeLa S3 cells. Ultrastructural observations.Am J Pathol. 1995;146:963–971. [PMC free article] [PubMed] [Google Scholar]
- Wang LG, Liu XM, Kreis W, Budman DR. The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol. 1999;44:355–361. doi: 10.1007/s002800050989. [DOI] [PubMed] [Google Scholar]
- Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Zhou J, Landen JW, Bradbury EM, Joshi HC. Sustained activation of p34(cdc2) is required for noscapine-induced apoptosis. J Biol Chem. 2001;276:46697–46700. doi: 10.1074/jbc.C100550200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- Zhou J, Gupta K, Aggarwal S, Aneja R, Chandra R, Panda D, Joshi HC. Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol. 2003;63:799–807. doi: 10.1124/mol.63.4.799. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yao J, Joshi HC. Attachment and tension in the spindle assembly checkpoint. J Cell Sci. 2002;115:3547–3555. doi: 10.1242/jcs.00029. [DOI] [PubMed] [Google Scholar]