Abstract

CO! You had me at hello The use of chiral biphenyl-based phosphoramidite ligands on rhodium provides an efficient [2+2+2] cycloaddition between terminal alkyl alkynes and alkenyl isocyanates. The cycloaddition proceeds through a CO migration pathway, and facilitates a rapid four-step asymmetric synthesis of indolizidine (−)-209D.
Keywords: CO migration, cycloaddition, indolizidine alkaloids, isocyanates, phosphoramidites
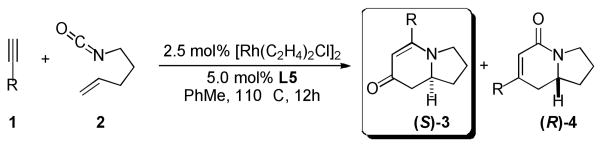
Indolizidine frameworks possessing an alkyl group substituted at the 5-position (indolizidine numbering)[i] represent a large class of naturally occurring compounds.[ii] Alkaloids ranging from structurally simple indolizidine 167B and 209D to more complex marine alkaloids such as cylindricines[iii] (Scheme 1) and the immunosuppressant FR901483[iv] all contain such ring systems. Most recently, Weinreb and coworkers described the first total synthesis of secu'amamine A, a novel tetracyclic alkaloid, via a 5-alkyl indolizinone as a late-stage intermediate.[v] Herein, we detail the development of an enantioselective rhodium-catalyzed [2+2+2] cycloaddition of terminal alkyl alkynes and alkenyl isocyanates to generate various 5-alkyl indolizinones (3). As part of a program directed toward developing a universal strategy to indolizidine alkaloids, the synthetic utility here is demonstrated by an expedient synthesis of (−)-209D.
Scheme 1.
[2+2+2] Cycloaddition strategies to the framework of various indolizidine alkaloids.
We have been exploring the use of neutral rhodium(I)/TADDOL-derived phosphoramidite complexes as enantioselective catalysts for various [2+2+2] cycloadditions, including reactions of terminal alkynes with isocyanates[vi] or carbodiimides.[vii] In previous studies, the use of terminal alkyl alkynes with these catalysts provide efficient cycloadditions to afford various bicyclic lactams 4 (Scheme 1) in good yields and enantioselectivities, while the 5-alkyl indolizinone cycloadducts 3, resulting from a CO migration process, can only be observed as minor components. In this work, we report a new Rh·L system to achieve a catalyst-controlled cycloaddition en route to 5-alkyl indolizinones 3.

To tune product selectivity through ligand design, we began our study by examining the cycloaddition of 1-octyne 1a and alkenyl isocyanate 2 with various phosphoramidite ligands[viii] (Table 1). Switching from TADDOL-derived ligands such as L1 to BINOL-derived L2 led to a complete inversion of product selectivity (entry 1 vs. 2) favoring the indolizinone 3a. Formation of 3 is thought to proceed through the initial metalacycle I followed by a CO migration process via II to arrive at III. Migratory insertion of the pendant alkene into Rh-N bond followed by reductive elimination gives rise to cycloadducts 3. Selectivity in the formation of two initial metalacycles (I vs. IV) is reflected in the product selectivity between 3 and 4. Despite the low yield and poor ee, the fundamental difference in product selectivity prompted further investigation into BINOL-derived phosphoramidites. Ligands possessing substitution at the 3,3′-positions of the BINOL backbone positively impact reaction efficiency toward the desired indolizinone 3a. Further exploration led to the discovery of GUIPHOS (L3). This TMS-substituted phosphoramidite L3 provides a much improved reaction with product selectivity ∼4:1, good chemical yield, and most importantly an excellent 96% ee for 3a (entry 3). Although the TMS-substituted biphenol-derived phosphoramidite L4 behaves no differently than GUIPHOS (entry 4), the corresponding ligand possessing tert-butyl groups at the 3,3′-positions (L5)[ix],[x] proved superior. Precatalyst [Rh(C2H4)2Cl]2 modified with L5 provides a clean reaction to furnish the desired indolizinone 3a with a good product ratio (6.2:1) in excellent yield and enantioselectivity (entry 5).
Table 1.
Ligand effect on product selectivity and enantioselectivity.[a]
 | ||||
|---|---|---|---|---|
| entry | L | 3 : 4[b] | yield (%) of 3[c] | ee (%) of 3[d] |
| 1 | L1 | 1 : 3.2 | 20 | 73[e] |
| 2 | L2 | 2.2 : 1 | 22 | 72 |
| 3 | L3 | 3.8 : 1 | 60 | 96 |
| 4 | L4 | 3.5 : 1 | 50 | 94 |
| 5 | L5 | 6.2 : 1 | 75 | 91 |
 | ||||
Conditions: 1 (2 equiv), 2 (0.27 mmol), Rh/L in PhMe (0.07 M) at 110 °C.
Product selectivity determined by 1H NMR of the unpurified mixture.
Isolated yield.
Determined by HPLC using a chiral stationary phase.
Other enantiomer.
Indolizidine 209D belongs to a family of 22 natural products, commonly referred to as gephyrotoxins, isolated from the skin secretions of neotropical frogs.[xi] Along with indolizidine 167B (Scheme 1), these two structurally simpler alkaloids have only been isolated in minute quantities from unidentified dendrobatid frogs found in a single population. Over the years, they have attracted much interest from the synthetic community, both to prepare them in greater quantities as well as a tool to validate new methodologies. [xii] In our own effort, the key intermediate 5-hexyl indolizinone 3a can be prepared conveniently by the cycloaddition protocol in one step and is suitable for scale-up (Scheme 2). The resulting vinylogous amide functionality readily undergoes a diastereoselective hydrogenation to afford enantioenriched amino alcohol 5 as a single diastereomer. Barton-McCombie deoxygenation via 6 completes the four-step enantioselective synthesis of (−)-209D, which also confirms the absolute configuration of 3a: [α]22D = -66.5° (c 1.0, CH2Cl2); lit.[10a] [α]26D = -80.4° (c 1.0, CH2Cl2). Considering that alkenyl isocyanate 2 can be prepared in one step from commercially available 5-hexenoic acid, this constitutes the shortest synthesis of 209D reported to date.[10]
Scheme 2.
Synthesis of indolizidine (−)-209D.
aSee entry 1, Table 2.
The newly developed Rh/phosphoramidite L5 catalyst promotes the enantioselective synthesis of 5-alkyl indolizinones very efficiently (Table 2). Alkyl alkynes bearing an array of functional groups including ester, chloride, silyl ether, Weinreb amide, unprotected terminal alkyne, and phenyl ring all react smoothly to afford cycloadducts in good product ratios and excellent enantioselectivities (entries 2 – 7). The cycloaddition is highly sensitive to both electronic and steric effects on the alkyne partner. The product selectivity shifts more toward formation of the bicyclic lactams 4 with electron-withdrawing substituents closer to the alkynyl center. For example, cycloaddition of 3-phenyl-1-propyne 1h gave a product ratio of 3:1 favoring the benzyl-substituted indolizinone 3h, instead of the ratio of 5:1 obtained with 1g (entry 8 vs. entry 7). In a more extreme case, cycloaddition of TIPS-protected propargyl alcohol 1i furnishes a 1.6:1 product mixture slightly favoring the indolizinone 3i (entry 9). On the other hand, reaction with the more sterically hindered alkyne 1j improves the product selectivity to provide the desired cycloadduct 3j in a high yield and excellent enantioselectivity (entry 10). In fact, bulky alkynes such as cyclohexyl and cyclopentyl acetylenes are among the best cycloaddition partners. The corresponding indolizinone products 3k and 3l can be obtained in high yields and enantioselectivities with excellent product ratios of 14:1 (entries 11, 12). Even more impressively, the rhodium catalyst modified by ligand L5 promotes the cycloaddition of tertiary alkyl-substituted alkynes to gain access to highly congested 5-alkyl indolizinones (entries 13, 14). For example, the MOM-protected cyclopentanol-substituted cycloadduct 3m can be produced in 60% yield with a slightly diminished 81% enantioselectivity as the only product. In general, cycloaddition with the tert-butyl substituted phosphoramidite L5 produces the best product selectivity and high overall reactivity, while the use of GUIPHOS (L3) usually gives the best level of enantiocontrol. Although GUIPHOS (L3) displays low reactivity toward most sterically hindered alkynes (3k: 44% yield, 95% ee; 3m: 23% yield, 80% ee), it does provide an efficient cycloaddition for the formation of tert-butyl substituted indolizinone 3n in a good chemical yield and enantioselectivity (entry 15). This protocol can also be applied to the synthesis of 5,9-dialkyl indolizinones (eq. 1). 1,1-Disubstituted alkenyl isocyanate 7 participates in the cycloadditions with 1-octyne 1a quite efficiently to provide the corresponding cycloadduct 8 in good product ratio and isolated yield. Interestingly, while the product selectivity stays relatively unchanged as those obtained with the unsubstituted alkenyl isocyanate 2, a profound effect on the enantioselectivity is observed. The use of GUIPHOS (L3) here provides a partial solution, improving the enantioselectivity significantly.
Table 2.
Enantioselective synthesis of 5-alkyl indolizinones.[a]
 | |||
|---|---|---|---|
| entry | Major Product | 3:4 ratio[b], yield (%)[c] and ee (%)[d] of 3 | |
| 1[e] |  |
6 : 1 66, 91 |
|
| 2 |  |
5 : 1 66, 90 |
|
| 3 |  |
5 : 1 57, 94 |
|
| 4 |  |
5 : 1 62, 90 |
|
| 5 | 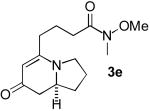 |
5 : 1 54, 90 |
|
| 6 |  |
5 : 1 55, 91 |
|
| 7 |  |
5 : 1 56, 91 |
|
| 8 |  |
3 : 1 52, 90 |
|
| 9 |  |
1.6 : 1 44, 87 |
|
| 10 |  |
8 : 1 72, 91 |
|
| 11 |  |
14 : 1 86, 91 |
|
| 12 |  |
14 : 1 87, 89 |
|
| 13 |  |
>20 : 1 60, 81 |
|
| 14 |  |
10 : 1 67, 79 |
|
| 15[f] |  |
6 : 1 66, 88 |
|
Conditions: 1 (2 equiv), 2 (0.27 mmol), Rh/L in PhMe (0.07 M) at 110 °C.
Product selectivity determined by 1H NMR of the unpurified mixture.
Isolated yield.
Determined by HPLC using a chiral stationary phase.
1.4 mmol scale (2) at 100 °C.
GUIPHOS (L3) used as the ligand.
 |
(1) |
In conclusion, we have developed an efficient catalyst system that promotes a cycloaddition between terminal alkyl alkynes and alkenyl isocyanates involving a CO migration process. This previously unattainable process allows access to various 5-alkyl indolizinones including an enantioselective synthesis of indolizidine (−)-209D. Further studies on the reaction scope as well as applications to the synthesis of alkaloids are ongoing.
Experimental Section
General procedure: In an inert atmosphere (N2) glove box, a flame-dried round bottom flask was charged with [Rh(C2H4)2Cl]2 (2.6 mg, 0.0068 mmol) and the phosphoramidite ligand L5 (5.8 mg, 0.0136 mmol), and was fitted with a flame-dried reflux condenser. The system was sealed with a standard septum. Upon removal from the glove box, 1.0 ml toluene was added via syringe and the resulting yellow solution was stirred at ambient temperature under argon flow for 5 minutes. To this solution was added a solution of alkyne 1 (0.54 mmol) and isocyanate 2 (30 mg, 0.27 mmol) in 2 ml of toluene via syringe. After an additional 1 ml of toluene was added to wash down the remaining residue, the resulting solution was heated to 110 °C in an oil bath, and maintained at reflux for 12 h. The reaction mixture was cooled to ambient temperature, concentrated in vacuo, and purified by column chromatography.
Supplementary Material
Footnotes
We thank NIGMS (GM080442), Eli Lilly, Boehringer Ingelheim and Johnson & Johnson for support. TR is a fellow of the Alfred P. Sloan Foundation and thanks the Monfort Family Foundation for a Monfort Professorship.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- i.Crabb TA, Newton RF, Jackson D. Chem Rev. 1971;71:109–126. [Google Scholar]
- ii.a) Michael JP. Nat Prod Rep. 2005;22:603–626. doi: 10.1039/b413748p. [DOI] [PubMed] [Google Scholar]; b) Michael JP. Nat Prod Rep. 2007;24:191–222. doi: 10.1039/b509525p. [DOI] [PubMed] [Google Scholar]; c) Michael JP. Nat Prod Rep. 2008;25:139–165. doi: 10.1039/b612166g. [DOI] [PubMed] [Google Scholar]
- iii.For a recent review on cylindricines, see: Weinreb SM. Chem Rev. 2006;106:2531–2549. doi: 10.1021/cr050069v.
- iv.For total syntheses, see: Snider BB, Lin H. J Am Chem Soc. 1999;121:7778–7786.Scheffler G, Seike H, Sorensen EJ. Angew Chem Int Ed. 2000;39:4593–4596. doi: 10.1002/1521-3773(20001215)39:24<4593::aid-anie4593>3.0.co;2-x.Ousmer M, Braun NA, Bavoux C, Perrin M, Ciufolini MA. J Am Chem Soc. 2001;123:7534–7538. doi: 10.1021/ja016030z.Maeng J, Funk RL. Org Lett. 2001;3:1125–1128. doi: 10.1021/ol015506g.Kan T, Fujimoto T, Ieda S, Asoh Y, Kitaoka H, Fukuyama T. Org Lett. 2004;6:2729–2731. doi: 10.1021/ol049074w.
- v.Liu P, Hong S, Weinreb SM. J Am Chem Soc. 2008;130:7562–7563. doi: 10.1021/ja802700z. [DOI] [PubMed] [Google Scholar]
- vi.a) Yu RT, Rovis T. J Am Chem Soc. 2006;128:12370–12371. doi: 10.1021/ja064868m. [DOI] [PubMed] [Google Scholar]; b) Lee EE, Rovis T. Org Lett. 2008;10:1231–1234. doi: 10.1021/ol800086s. [DOI] [PMC free article] [PubMed] [Google Scholar]; For our first report in this area, see: Yu RT, Rovis T. J Am Chem Soc. 2006;128:2782–2783. doi: 10.1021/ja057803c.
- vii.Yu RT, Rovis T. J Am Chem Soc. 2008;130:3262–3263. doi: 10.1021/ja710065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- viii.a) de Vries AHM, Meetsma A, Feringa BL. Angew Chem Int Ed. 1996;35:2374–2376. [Google Scholar]; b) Feringa BL. Acc Chem Res. 2000;33:346–353. doi: 10.1021/ar990084k. [DOI] [PubMed] [Google Scholar]; c) Alexakis A, Burton J, Vastra J, Benhaim C, Fournioux X, van den Heuvel A, Leveque J, Maze F, Rosset S. Eur J Org Chem. 2000:4011–4028. [Google Scholar]; d) Panella L, Feringa BL, de Vries JG, Minnaard AJ. Org Lett. 2005;7:4177–4180. doi: 10.1021/ol051559c. [DOI] [PubMed] [Google Scholar]
- ix.Phosphoramidites based on Biphen (3,3′-di-tert-butyl-5,5′,6,6′-tetramethyl-1,1′-biphenyl-2,2′-diol) have been prepared and used successfully in asymmetric hydroformylation and hydrogenation: Hua Z, Vassar VC, Choi H, Ojima I. Proc Natl Acad Sci USA. 2004;101:5411. doi: 10.1073/pnas.0307101101.Giacomina F, Meetsma A, Panella L, Lefort L, de Vries AHM, de Vries JG. Angew Chem Int Ed. 2007;46:1497–1500. doi: 10.1002/anie.200603930.
- x.Both enantiomers of the bisphenol required for the synthesis of L5 are commercially available.
- xi.a) Daly JW. Fortschr Chem Org Naturst. 1982;41:205. doi: 10.1007/978-3-7091-8656-5_6. [DOI] [PubMed] [Google Scholar]; b) Aronstam RS, Daly JW, Spande TF, Narayanan TK, Albuquerque EX. Neurochem Res. 1986;11:1227. doi: 10.1007/BF00965950. [DOI] [PubMed] [Google Scholar]
- xii.For asymmetric syntheses of indolizidine 209D, see: Polniaszek RP, Belmont SE. J Org Chem. 1990;55:4688–4693.Jefford CW, Wang JB. Tetrahedron Lett. 1993;34:3119–3122.Åhman J, Somfai P. Tetrahedron Lett. 1995;36:303–306.Åhman J, Somfai P. Tetrahedron. 1995;51:9747–9756.Nukui S, Sodeoka M, Sasai H, Shibasaki M. J Org Chem. 1995;60:398–404.Jefford CW, Sienkiewicz K, Thornton SR. Helv Chim Acta. 1995;78:1511–1524.Takahata H, Kubota M, Ihara K, Okamoto N, Momose T, Azer N, Eldefrawi AT, Eldefrawi ME. Tetrahedron: Asymmetry. 1998;9:3289–3301.Chênevert R, Ziarani GM, Morin MP, Dasser M. Tetrahedron: Asymmetry. 1999;10:3117–3122.Yamazaki N, Ito T, Kibayashi C. Org Lett. 2000;2:465–467. doi: 10.1021/ol990389z.Back TG, Nakajima K. J Org Chem. 2000;65:4543–4552. doi: 10.1021/jo000080p.Kim G, Jung S, Kim W. Org Lett. 2001;3:2985–2987. doi: 10.1021/ol0163171.Reddy PG, Baskaran S. J Org Chem. 2004;69:3093–3101. doi: 10.1021/jo035258x.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




