Abstract
In brain regions that have been implicated in the reinstatement of drug-seeking, the prelimbic cortex has emerged as a critical regulator of relapse behaviours. Here, the effects of prelimbic cortex dopamine (DA) D1 receptor antagonism on drug-seeking produced by heroin-paired cues, or by a single priming dose of heroin are examined. Rats lever-pressed daily for i.v. heroin discretely paired with a conditioned stimulus during 3-h sessions for a period of 2 wk, followed by extinction and reinstatement of drug-seeking by previously heroin-paired cues (tone+light) or heroin-priming injections (0.25 mg/kg) in the absence of heroin reinforcement. Intracranial infusion of the DA D1 receptor antagonist, SCH 23390 (0.02−2.0 μg/side), into the prelimbic cortex potently and dose dependently attenuated heroin-seeking in response to either cue presentations or a priming dose of heroin. These results suggest that DA D1 receptors regulate prefrontal cortex pathways necessary for the reinstatement of heroin-seeking.
Keywords: Dopamine, heroin, prelimbic, reinstatement, relapse
Introduction
Relapse to drug-taking following periods of abstinence constitutes the major impediment in the treatment of addiction. Relapse can be triggered by stimuli previously associated with the drug (Childress et al. 1999), exposure to the drug itself (Jaffe et al. 1989), or stress (Sinha et al. 2000). Using the reinstatement model of relapse in animals, exposure to conditioned cues, stress, or a drug-priming injection will reinstate extinguished drug-seeking as defined by responding on a previously drug-paired operandum in the absence of drug reinforcement (Shaham et al. 2003). A number of studies have explored the neural circuitry for relapse to drug-seeking, primarily for psychostimulants and opiates (Feltenstein & See, 2008; Shalev et al. 2002). While several key brain regions have been implicated in the reinstatement of drug-seeking, the prelimbic region of the medial prefrontal cortex has emerged as a critical component of the relapse circuitry across different modalities of reinstatement and different classes of abused drugs (Kalivas & Volkow, 2005). The results from animal models of relapse coincide well with in-vivo brain-imaging studies, in which the prefrontal cortex shows activation in human drug addicts during presentation of drug-associated cues and other trigger factors (Volkow et al. 2004).
While it has been established that the prelimbic cortex is important for drug-seeking, the specific cortical neurotransmitter regulation during reinstatement has not been well explored. Prefrontal dopamine (DA) innervation is a critical regulator of cognitive and motivational domains (Seamans & Yang, 2004), and prefrontal cortex DA dysfunction has been suggested to play a major role in psychopathologies, including addiction (Volkow et al. 2002). Some evidence has implicated medial prefrontal DA activity during reinstatement of cocaine-seeking in that DA receptor blockade reduced cocaine-primed (Sun & Rebec, 2005) or stress-induced (Capriles et al. 2003) reinstatement, while DA infusion alone into the prelimbic cortex produced reinstatement of cocaine-seeking (McFarland & Kalivas, 2001). However, while the prelimbic cortex has been implicated in cue-induced reinstatement (McLaughlin & See, 2003), the role of prefrontal DA receptor modulation of cue-induced relapse remains unknown.
It has recently been reported that the neurocircuitry underlying reinstatement of heroin-seeking showed overlapping, yet distinct differences with the neurocircuitry for reinstatement of cocaine-seeking (Rogers et al. 2008). The prelimbic cortex was one of the key brain regions necessary to maintain reinstatement produced by either heroin-associated cues or heroin priming. While opiates, including heroin, act primarily via stimulation of the μ-opioid receptor, heroin activation of μ-opioid receptors indirectly stimulates DA release. Dopaminergic mechanisms presumably play a key role in relapse in opiate addiction, probably through DA D1 receptor activation, as D1 receptors mediate prefrontal cortex neuronal excitability important for cognitive and motivational functions (Vijayraghavan et al. 2007). Thus, the present study examined the impact of acute DA D1 receptor blockade in the prelimbic cortex on heroin-seeking triggered by heroin-paired cues or heroin-priming injections.
Methods
Subjects
Male Sprague–Dawley rats (n = 31, initial weight 250−275 g; Charles River, USA) were individually housed on a 12-h reversed light/dark cycle (lights on 18:00 hours). Animals were given water ad libitum and maintained on 25 g standard rat chow per day during chronic heroin self-administration and then placed on ad-libitum feeding for the remainder of the experiment. Rats were habituated to handling and allowed to adapt for a minimum of 4 d prior to the start of the experiment. Housing and care of the rats were carried out in accordance with the ‘Guide for the Care and Use of Laboratory Rats’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina.
Heroin self-administration
Procedures for heroin self-administration have been previously described in detail (Rogers et al. 2008). Self-administration occurred in standard operant chambers linked to a data collection programme (Med Associates Inc., USA). Rats underwent initial lever training on a fixed ratio 1 (FR1) schedule of food pellet reinforcement with an active or inactive lever available during a 15-h overnight training session. Two days after lever training, rats were implanted with jugular catheters and intracranial guide cannulae. In brief, animals were anesthetized (ketamine and xylazine, 66 and 1.33 mg/kg; equithesin, 0.5 ml/kg i.p.) and catheters were implanted into the right jugular vein and secured with sutures. Immediately following catheter surgery, animals were placed into a stereotaxic frame and bilateral stainless-steel guide cannulae (26-gauge) were inserted into the medial prefrontal cortex at the following coordinates: +3.0 A/P, ±0.7 M/L, −1.0 D/V. Catheters were flushed once daily for 4 d after surgery with an antibiotic solution of cefazolin (100 mg/ml; Schein Pharmaceuticals, USA) and heparinized saline (70 U/ml; Elkins-Sinn, USA). For the duration of the experiment, each subject received 0.1 ml heparinized saline (10 U/ml) prior to self-administration and cefazolin +70 U/ml heparinized saline following each session. To verify catheter patency, rats occasionally received a 0.12 ml infusion of methohexital sodium (10.0 mg/ml i.v.; Eli Lilly and Co., USA), a short-acting barbiturate that produces a rapid loss of muscle tone.
Five to seven days after surgery, rats began self-administration of heroin (diacetylmorphine HCl, National Institute on Drug Abuse, USA) along a FR1 schedule in daily 3-h sessions at an initial dose of 50 μg/50 μl per infusion for 2 d, followed by 10−12 d of self-administration at a dose of 25 μg/50 μl per infusion. The houselight signalled the initiation of the session and remained illuminated throughout the entire session for all experimental phases. Active lever responses resulted in a 2-s activation of the infusion pump and a 5-s presentation of a conditioned stimulus (CS) complex, which consisted of a white cue-light and a tone (78 dB, 4.5 kHz). Following each infusion, responding on the active lever had no consequences during a 20-s time-out period. Inactive lever presses had no consequences, but were recorded. After the last day of self-administration, rats experienced daily 3-h extinction sessions, in which responses on either the active or inactive lever were recorded, but resulted in no programmed consequences (i.e. no infusion and no CS presentation). Animals continued under extinction conditions until they reached a criterion of a minimum of 10 d and ≤25 lever presses per session for two consecutive days.
Reinstatement testing
Following extinction, rats underwent six reinstatement tests, using a counterbalanced, within-subjects design, with a minimum of 2 d of extinction between each test. Subjects were tested in two cohorts that allowed for testing of vehicle and two doses of SCH 23390 (in μg/0.5 μl) for each set of reinstatement tests [0, 0.02, 0.1 (n = 16) and 0, 0.2, 2.0 (n = 15)]. Three cue reinstatement tests were conducted first, followed by three heroin-primed reinstatement tests. Work by my group has previously used similar designs in order to avoid the effects of non-contingent drug injections on subsequent cue reinstatement (Kippin et al. 2006; Rogers et al. 2008). Immediately prior to each reinstatement test, the rat received either intracranial vehicle or SCH 23390. Injection cannulae (33-gauge) were inserted to a depth of 2 mm below the tip of the guide cannulae (prelimbic cortex) just prior to placement into the chamber. Injection cannulae were connected to 10 μl syringes (Hamilton Co., USA) mounted on an infusion pump (Harvard Apparatus, USA). SCH 23390 hydrochloride (Sigma-Aldrich, USA) or PBS vehicle (pH 7.0 for both) were infused at a volume of 0.5 μl/side over a 2-min time-period. This volume has been previously used in many studies of intracranial drug effects in the prefrontal cortex, including SCH 23390 (Capriles et al. 2003; Duvauchelle et al. 1998). Injection cannulae were left in place for 1 min prior to and after the infusion.
During the 3-h cue reinstatement tests, each active lever press resulted in the CS presentation in the absence of heroin reinforcement. For heroin-primed reinstatement, a single, non-contingent dose of heroin (0.25 mg/kg s.c.) was administered immediately prior to the 3-h session, during which lever responses had no programmed consequences. After completion of all testing, the rats were anaesthetized with equithesin and transcardially perfused with PBS and 10 % formaldehyde solution. The brains were dissected and stored in 10 % formaldehyde solution prior to sectioning in order to verify cannulae placements.
Locomotor activity
In order to assess the specificity of SCH 23390 effects, locomotor responses were measured in a novel environment after SCH 23390 infusion in a subset of animals from the low-dose cohort after all reinstatement testing was completed. Animals (n = 5 per group) were infused with vehicle or SCH 23390 at the highest dose of 2.0 μg/side immediately prior to being placed in a Plexiglas open-field apparatus. Each chamber was equipped with a Digiscan monitor (Omnitech Electronics, USA) containing 16 photobeams (eight on each horizontal axis) that tabulated total distance (cm) travelled. Beam breaks were detected by a Digiscan analyser and recorded by DigiPro software (version 1.4).
Data analysis
Reinstatement of responding from extinction levels and the effects of SCH 23390 on cue-induced and heroin-primed reinstatement were analysed using one-way analysis of variance (ANOVA), followed by pairwise comparisons with the Student–Newman–Keuls test. Locomotor activity was analysed with a two-way ANOVA (group × time). Analyses were considered statistically significant at p < 0.05. All data are presented as mean ± s.e.m.
Results
No differences were found in responding between the two experimental cohorts for heroin self-administration, extinction responding, or response to intracranial vehicle infusions. Responding during the last 2 d of heroin self-administration on the active lever was 69.32 ± 12.77, while responding on the inactive (non drug-paired) lever was 3.55 ± 0.79. The number of heroin infusions for the last 2 d of self-administration was 22.74 ± 1.82, which resulted in an average of 1.64 ± 0.13 mg/kg per session when adjusted for body weight.
Figure 1 shows lever responding for extinction and during cue-induced and heroin-primed reinstatement of drug-seeking after intracranial infusions of vehicle or SCH 23390. Significant group differences were found for cue-induced reinstatement for both the low-dose (F3,60 = 4.77, p < 0.01) and high-dose (F3,56 = 6.59, p < 0.001) cohorts (Fig. 1a, b). Compared to extinction, responding on the previously heroin-paired lever in the presence of cues significantly reinstated drug-seeking following vehicle infusion at a magnitude similar to that previously found for animals with a history of heroin self-administration (Rogers et al. 2008). SCH 23390 at doses ranging from 0.1 to 2.0 blocked the increase in responding over baseline extinction. When compared to responding after vehicle infusion, post-hoc analyses for vehicle vs. SCH 23390 showed significant differences at both the 0.2 and 2.0 doses (p < 0.05). For heroin-primed reinstatement, significant group differences were found for both the low-dose (F3,60 = 5.65, p < 0.005) and high-dose (F3,56 = 8.02, p < 0.001) cohorts (Fig. 1c, d). Responding on the previously heroin-paired lever after a priming injection of heroin produced robust drug-seeking as previously reported (Rogers et al. 2008). While the lower doses of SCH 23390 had no significant effects, the two higher doses (0.2 and 2.0) blocked heroin-primed reinstatement (p < 0.05). Comparison of vehicle vs. SCH 23390 infusions showed significant differences from vehicle at both the 0.2 and 2.0 doses (p < 0.05). In contrast to responding on the previously heroin-paired (active) lever, inactive lever responding showed no significant differences between groups for either cohort, except for a slight, but significant, reduction during cue-induced reinstatement following the 2.0 μg dose of SCH 23390 (p < 0.05).
Fig. 1.
Responses (mean ± s.e.m.) on the previously heroin-associated active (■) and non-heroin-associated inactive (□) levers at the end of extinction (Ext) trials (mean of the last 2 d) and following vehicle or SCH 23390 (0.02−2.0 μg/side) infusions into the prelimbic cortex immediately prior to testing. Lever responses are indicated for conditioned-cued reinstatement after (a) low and (b) high doses and for heroin-primed reinstatement after (c) low and (d) high doses. Significant differences are noted for reinstatement over extinction levels (Student–Newman–Keuls test, * p < 0.05), and between vehicle and SCH 23390 (Student–Newman–Keuls test, † p < 0.05).
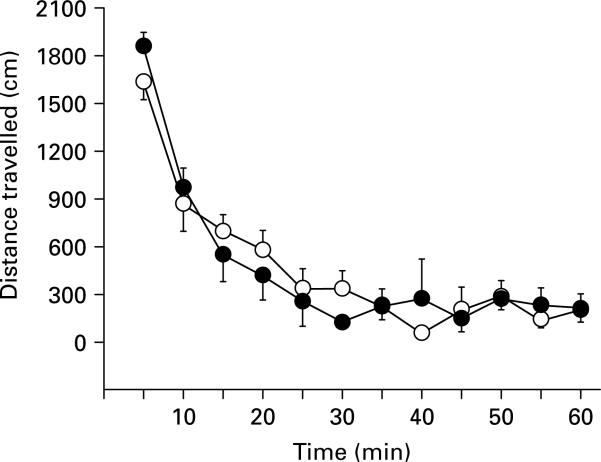
In order to further assess possible non-specific effects of DA D1 receptor blockade, the impact of SCH 23390 on general locomotor activity in response to a novel environment was examined. Upon exposure to the novel environment, animals with either vehicle or SCH 23390 (2.0 μg/side) infusions in the prelimbic cortex exhibited robust locomotor activity that decreased over the 1-h test session (Fig. 2). A significant main effect for time was found (F11,88 = 36.37, p < 0.001), but no significant effect of group (F1,8 = 0.01, p = 0.98), or a group × time interaction (F11,88 = 0.83, p = 0.61), thus indicating no effect of the highest dose of SCH 23390 on general locomotor activity.
Fig. 2.
Locomotor activity in a novel environment after bilateral vehicle ( ) or 2.0 μg SCH 23390 (
) or 2.0 μg SCH 23390 ( ) infusions into the prelimbic cortex. Locomotor activity (mean ± s.e.m.) is shown in distance (cm) as measured by photobeam breaks across 5-min sample bins following vehicle or SCH 23390 infusions. No significant differences were found between groups.
) infusions into the prelimbic cortex. Locomotor activity (mean ± s.e.m.) is shown in distance (cm) as measured by photobeam breaks across 5-min sample bins following vehicle or SCH 23390 infusions. No significant differences were found between groups.
Discussion
The present results demonstrate that both heroin-paired cue and heroin-primed reinstatement of heroin-seeking depend upon intact prelimbic cortex DA D1 receptor function. This DA D1 receptor dependency is particularly noteworthy, in that doses of SCH 23390 that produced significant attenuation of reinstatement were relatively low, with profound reduction of both forms of reinstatement at doses ≥0.2 μg/side, and significant attenuation of cue-induced reinstatement, even at 0.1 μg/side. Prior studies with intracranial cortical infusions of SCH 23390 have not gone below 0.25 μg/side, including studies with cocaine-induced reinstatement (Capriles et al. 2003; Sun & Rebec, 2005). It is unlikely that the attenuation of lever responding was due to non-specific impairment of function, as there were no notable effects on inactive lever responding or general locomotor activity. Inactivation of the prelimbic cortex via intracranial infusions of GABA agonists has also been shown to have no effect on locomotor activity or self-administration of food (McFarland & Kalivas, 2001). Furthermore, SCH 23390 at doses of 0.3 and 0.6 μg/side attenuated cue-induced reinstatement of heroin-seeking, but failed to affect non-drug reinforced behaviours when infused into the nucleus accumbens, a region more clearly linked to locomotor activity than the prelimbic cortex (Bossert et al. 2007).
The current results of prefrontal DA D1 receptor-dependent control of the reinstatement of heroin-seeking support earlier studies that have utilized non-selective pharmacological means of disrupting prelimbic cortex function prior to reinstatement. Reinstatement of drug-seeking produced by conditioned cues and drug priming has been shown to be attenuated by intra-prelimbic cortex infusion of GABA receptor agonists (McFarland & Kalivas, 2001; Rogers et al. 2008) or a sodium channel blocker (McLaughlin & See, 2003). Since D1 receptors are localized on both pyramidal and non-pyramidal neurons, the exact mechanism of D1 modulation remains unclear. Activation of D1 receptors in the prefrontal cortex increases neuronal excitability (Thurley et al. 2008), and D1 receptor function is critical for reward-motivated attentional processing (Granon et al. 2000). Thus, heightened D1 receptor tone is probably required to maintain sufficient activation of the cortical circuitry that drives reinstatement of heroin-seeking. During heroin self-administration, activation of μ-opioid receptors disinhibits GABA neurons in the ventral teg-mental area, consequently increasing DA release in the prefrontal cortex (Kelley et al. 1980; Noel & Gratton, 1995). These prolonged actions of heroin on prefrontal DA function not only alter D1 receptor activity, but probably play a critical role in the associative learning of cue–drug interactions that can trigger drug desire during abstinence. Although there are no published reports of increased cortical DA measured under relapse conditions, the current data suggest that such an increase occurs under conditions of cue- or drug-activated relapse. D1 receptor antagonism in the pre-frontal cortex during reinstatement probably works downstream of midbrain μ-receptor activity, via blockade on cortical glutamatergic neurons (Wirkner et al. 2004; Young & Yang, 2005). However, a recent report suggests that co-localized μ-opioid and DA D1 receptors in the cortex can form hetero-oligomers (Juhasz et al. 2008), which may provide an additional point of modulation by D1 receptor blockade in subjects with a history of chronic heroin intake.
The prelimbic cortex primarily projects to the nucleus accumbens core, as well as several limbic regions (Voorn et al. 2004). Cocaine-seeking during relapse has been shown to depend upon a prefrontal cortical-striatal glutamatergic pathway (Kalivas & Volkow, 2005), and this pathway has recently been demonstrated to be activated during reinstatement of heroin-seeking (LaLumiere & Kalivas, 2008). DA D1 receptors in the prelimbic cortex probably play a critical role in modulating these corticostriatal projections for multiple stimuli (cues, drugs, stress) across different classes of abused drugs. In addition, prefrontal DA D2 receptors may also play a role in drug-seeking, as reported for cocaine-primed reinstatement (Sun & Rebec, 2005). Thus, future exploration of prefrontal cortex DA receptor-mediated drug-seeking will provide new insights for developing anti-relapse pharmacotherapy for addiction.
Acknowledgements
The author thanks Jason Rogers and Anthony Carnell for technical assistance and data collection. This research was supported by National Institute on Drug Abuse grants DA10462 and DA15369, and NIH grant C06 RR015455.
Footnotes
Statement of Interest
None.
References
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. Journal of Neuroscience. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvauchelle CL, Fleming SM, Kornetsky C. Prefrontal cortex infusions of SCH 23390 cause immediate and delayed effects on ventral tegmental area stimulation reward. Brain Research. 1998;811:57–62. doi: 10.1016/s0006-8993(98)00952-4. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. British Journal of Pharmacology. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. Journal of Neuroscience. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Juhasz JR, Hasbi A, Rashid AJ, So CH, George SR, O'Dowd BF. Mu-opioid receptor heterooligomer formation with the dopamine D1 receptor as directly visualized in living cells. European Journal of Pharmacology. 2008;581:235–243. doi: 10.1016/j.ejphar.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L, Iversen SD. Interactions between D-ala-met-enkephalin, A10 dopaminergic neurones, and spontaneous behaviour in the rat. Behavioural Brain Research. 1980;1:3–24. doi: 10.1016/0166-4328(80)90043-1. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology. 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. Journal of Neuroscience. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Noel MB, Gratton A. Electrochemical evidence of increased dopamine transmission in prefrontal cortex and nucleus accumbens elicited by ventral tegmental mu-opioid receptor activation in freely behaving rats. Synapse. 1995;21:110–122. doi: 10.1002/syn.890210204. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacological Reviews. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Thurley K, Senn W, Luscher HR. Dopamine increases the gain of the input-output response of rat prefrontal pyramidal neurons. Journal of Neurophysiology. 2008;99:2985–2997. doi: 10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Krause T, Koles L, Thummler S, Al-Khrasani M, Illes P. D1 but not D2 dopamine receptors or adrenoceptors mediate dopamine-induced potentiation of N-methyl-d-aspartate currents in the rat prefrontal cortex. Neuroscience Letters. 2004;372:89–93. doi: 10.1016/j.neulet.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Young CE, Yang CR. Dopamine D1-like receptor modulates layer- and frequency-specific short-term synaptic plasticity in rat prefrontal cortical neurons. European Journal of Neuroscience. 2005;21:3310–3320. doi: 10.1111/j.1460-9568.2005.04161.x. [DOI] [PubMed] [Google Scholar]