Abstract
The authors present a case of subdural empyema in a macrocephalic patient. A 23-year-old male was admitted due to headache and fever. One month ago, he had mild head injury by his coworkers. Physical examination showed a macrocephaly and laboratory findings suggested purulent meningitis. Neuroimaging studies revealed a huge size of epidural space-occupying lesion. Under the impression of epidural abscess, operation was performed. Eventually, the lesion was located at subdural space and was proven to be subdural empyema. Later, histological examination of the specimen obtained by surgery demonstrated finings consistent with the capsule of the chronic subdural hematoma. Two weeks after operation, Propionibacterium acnes was isolated. The intravenous antibiotics were used for total of eight weeks under monitoring of the serum level of the C-reactive protein. Follow-up brain computed tomography (CT) scan showed the presence of significant amount of remaining subdural lesion. However, he has complained of minimal discomfort. It is suggested that the subdural empyema occurred with preexisting chronic subdural hematoma after head injury about one month prior to admission and it took a long time to treat Propionibacterium acnes subdural empyema with systemic antibiotics, at least over eight weeks.
Keywords: Subdural empyema, Chronic subdural hematoma, Propionibacterium acnes, C-reactive protein
INTRODUCTION
Subdural empyema (SE) has been the most imperative of all neurosurgical emergencies associated with high morbidity and mortality if not treated promptly and effectively. The common pathogens of SE are streptococci, staphylococci, and anaerobie bacteria6). Anaerobic streptococci have been shown to be the most common microorganisms isolated in SE11). Propionibacterium acnes (P. acnes) is a gram-positive, non-spore forming, anaerobic rod that is a normal inhabitant of the skin, anterior nose, conjunctiva, mouth, and upper respiratory tract. It is considered to manifest weak pathogenicity and is often contaminants of blood and tissue cultures. However, in recent years, infections of the central nervous system by P. acnes have been found increasingly more often in immunocompromised individuals, or following neurosurgical procedures and head trauma1,2,3,7). This report describes our experience with P. acnes subdural empyema in patient with macrocephaly caused by preexisting chronic subdural hematoma since childhood.
CASE REPORT
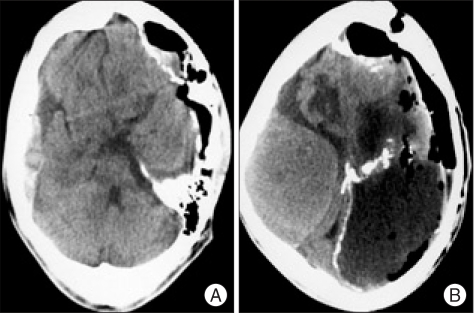
The patient was a 23-year-old male who presented with headache for a month. His family members stated that he had a long history of epileptic seizures and mental retardation since age four. And, they described that he had been physically assaulted to the head by his coworkers one month before admission. There had been no apparent history of infection and neurological surgery prior to admission. On physical examination, macrocephaly was noted and there was fever at 38.5℃ Neurological examination showed alert mentality, stiffness of neck and both lower extremities weakness of grade IV/V. After obtaining samples for routine blood tests including blood cultures, a lumbar puncture was performed that showed white blood cell count of 356/mm3 with 80% polymorphonuclear leukocytes. The cerebrospinal fluid (CSF) was slight turbid and the opening pressure was not measured. CSF and serum glucose concentrations were 10 mg/dL and 130 mg/dL, respectively; the CSF protein concentrations was over the 300 mg/dL. The CSF Gram's stain was negative, and culture remained sterile. A complete blood count revealed white blood cell count of 19,510 cells/mm with neutrophils 89%, a C-reactive protein (CRP) concentration of 205 mg/dL. Simple X-ray of skull showed macrocephaly and multiple calcifications at frontal area (Fig. 1). A precontrast computerized tomography (CT) scan demonstrated a lentiform-shaped high density lesion confined over left frontal convexity bordered with underlying cortex by a thick hyperdense calcified rim and another separated large left hemispheric isodense space-occupying lesion surrounded by hyperdense border with multiple calcifications (Fig. 2A, B). Under the impression of epidural abscess associated with pyogenic meningitis, he was started on intravenous antibiotics such as cefotaxime and gentamycin empirically and surgical decompression was planned. While waiting for operation, the patient's level of consciousness deteriorated (Glasgow Coma Scale score of 6) and his left pupil was dilated and not reactive to light. The patient underwent urgent surgery via a left craniotomy. There were no abnormal findings in epidural space and the dura was very tight. A thick, hard, and yellowish capsule was identified during dural opening and this was firmly adhered to overlying dura. As soon as the capsule was incised and dissected, huge amount of purulent materials was gushed out. Also, inner capsule of the lesion was firmly adhered to underlying cortex and couldn't be removed completely. A drain catheter was placed in the subdural space. Histological examination of the removed outer capsule revealed inflammatory changes with prominent neutrophil infiltration (Fig. 3A, B). Immediate postoperative CT scan showed preoperative midline shifting was improved but sizable amount of empyema still remained and occurrence of new contralateral large amount of EDH (Fig. 4A, B). Postoperative neurological examination revealed decrease in left pupil diameter and sluggish response to light but he was stuporous. Postoperative antibiotic therapy consisted with cefotaxime, vancomycin, amikacin, and clindamycin with injection of vancomycin into the subdural space. There were no bacteria found in the culture of the blood, urine, and sputum. There was no evidence of any other source of infection, including otorhinologic or gastrointestinal disease. Two weeks after operation, P. acnes was isolated from the purulent materials and the CRP concentration was 154 mg/dL. The antibiotic regimen was changed to vancomycin and imipenem for next six weeks. Thereafter, follow-up results of CRP were 98 mg/dL, 64 mg/dL, 27 mg/dL, 16 mg/dL, and <10 mg/dL, respectively third, fourth, fifth, sixth, and since seventh week. The patient was discharged four months after operation with both lower extremity weaknesses. One year after operation, follow-up brain CT scan showed large amount of subdural empyema still remained (Fig. 5), but he complained of minimal discomfort.
Fig. 1.

The lateral view of the skull showing macrocephaly and multiple calcifications.
Fig. 2.
Preoperative precontrast computed tomography scans revealing lentiform-shaped high density lesion at the left frontal area (A) and a large space-occupying lesion with isodensity over the left cerebral hemisphere (B).
Fig. 3.
Immediate postoperative computerized tomography scans showing improvement of preoperative midline shifting (A) and new occurrence of a large amount of epidural hematoma at right cerebral convexity (B).
Fig. 4.
Photomicrograph of the resected outer capsule of subdural empyema showing thick, fibrous membrane suggesting the membrane of the chronic subdural hematoma (A) (H & E, ×40) and revealing inflammatory changes with prominent neutrophil infiltration (B) (H & E, ×200).
Fig. 5.
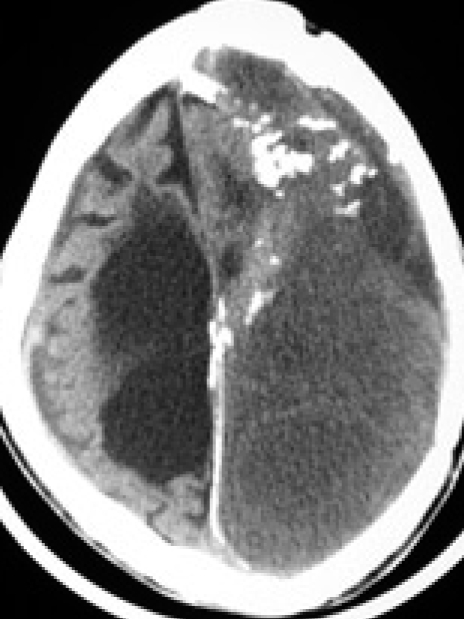
Computed tomography scan at postoperative one year demonstrating still remaining significant amount of subdural fluid collection at left cerebral convexity.
DISCUSSION
Our macrocephalic patient had a huge amount of subdural empyema surrounded by thick capsule. Although P. acnes was isolated from culture of the purulent materials and it is reported that the clinical course of this infection was indolent, we thought that the amount of the subdural empyema was too much to be tolerable for a long time, and probably there had been another pathology within intracranial cavity7,9). The chronic subdural hematoma must be differentiated from the causes of childhood macrocephaly10). If undiagnosed and untreated, as a result of a gradual growth of the hematomas, it is possible to occur with atrophy in underlying cerebral hemisphere and to be accompanied by macrocephaly such as our patient10). Also, it has been reported that calcification and ossification occur in 0.8-10% of chronic subdural hematoma patients5,6). Postoperative histological examination of resected capsule revealed thick, fibrous, connective tissue membrane with chronic type inflammatory infiltrations and numerous necrotic amorphous fragments and degradation products of red blood cells within lumen under the membrane. It was suggested that these findings are indication of chronic nature of the disease. Therefore, it is presumed that the patient have had chronic subdural hematoma since childhood based on physical and radiological examination and histological findings despite lack of apparent clinical information. There is a possibility that head injury one month prior to admission may have been the provoking factor for occurrence of his subdural empyema on circumstance having had chronic subdural hematoma. However, portals of entry for microbes couldn't be identified correctly despite thorough investigation. Postoperatively, he had been treated with empirical intravenous antibiotics such as cefotaxime, vancomycin, amikacin, and clindamycin until isolation of P. acnes at two weeks after operation. When P. acnes was isolated in our patient, at first we couldn't believe that result because it had been known to be a benign commensal and being two-week delay in the culture study. However, given his huge amount of subdrual empyema and relatively long history of present symptom opposing that most subdural empyemas are acute in onset, rapid in clinical course, it might be possible to consider P. acnes as the causative organism. Thereafter, antibiotics were changed to vancomycin plus imipenem for a next six weeks. At present, there is no guideline for optimal duration of systemic antibiotics management for P. acnes. However, most reports recommend the usage of systemic antibiotics over six weeks1,2,3). However, the most important factor determining duration of antibiotics management must be the patient's clinical condition. CRP is an acute phase protein synthesized by hepatocytes and levels rarely rise above 10 mg/dL in healthy subjects4). We decided the value of CRP as the important parameter of clinical condition and followed every one week. And, scheduled brain CT scans were followed every two weeks. Six weeks after operation, the CRP value was declined to nearly normal level and neurological status was improved gradually despite the brain CT scan results that showed still considerable amount of subdural fluid with mixed density. Thus, there was a discrepancy between the patient's clinical conditions and neurorimaging findigns. One year after operation, follow-up brain CT scan revealed that there was still a significant amount of subdural fluid remained but the patient complained of minimal symptoms.
CONCLUSION
As in our case, it is possible that a huge amount of subdural empyema can be accumulated in subdural space with preexisting chronic subdural hematoma that may be a suitable media for P. acnes, especially after head trauma. Also, it takes a long time to treat P. acnes subdural empyema with systemic antibiotics, at least over six weeks, and because there is an apparent discrepancy between patient's clinical course and neuroimaging findings, the most important parameters determining management duration are the patient's neurological status and the laboratory value of the CRP rather than neuroimaging results.
References
- 1.Barkhoudarian G, Hoff JT, Thompson BG. Propionibacterium infection associated with bovine pericardium dural allograft. J Neurosurg. 2005;103:182–185. doi: 10.3171/jns.2005.103.1.0182. [DOI] [PubMed] [Google Scholar]
- 2.Chu RM, Tummala RP, Hall WA. Focal intracranial infections due to Propionibacterium acnes: report of three cases. Neurosurgery. 2001;49:717–720. doi: 10.1097/00006123-200109000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Ghalayini SR, Likhith AM, Golash A. Propionibacterium acnes causing delayed subdural empyema-a case report and review of literature. J Clin Neurosci. 2004;11:677–679. doi: 10.1016/j.jocn.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Huntley JS, Kelly MB. C-reactive protein: a valuable acute investigation. A case of pneumococcal meningitis presenting as ankle pain. Emerg Med J. 2005;22:602–603. doi: 10.1136/emj.2003.012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ide M, Jimbo M, Yamamoto M, Umebra Y, Hagiwara S. Asymptomatic calcified chrnonic subdural hematoma. Report of three cases. Neurol Med Chir (Tokyo) 1993;33:559–563. doi: 10.2176/nmc.33.559. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Son EI, Kim DW, Kim SP. Calcified chronic subdural hematoma associated with intracerebral hematoma. J Korean Neurosurg Soc. 2003;34:177–178. [Google Scholar]
- 7.Ramos JM, Esteban J, Soriano F. Isolation of Propionibacterium acnes from central nervous system infections. Anaerobe. 1995;1:17–20. doi: 10.1016/s1075-9964(95)80366-1. [DOI] [PubMed] [Google Scholar]
- 8.Ryu S, Lim M, Harsh IV GR. Management of epidural abscess and subdural empyema. Oper Tech Neurosurg. 2005;7:182–187. [Google Scholar]
- 9.Sawauchi S, Saguchi T, Miyazaki Y, Ikeuchi S, Ogawa T, Yuhki K, et al. Infected subdural hematoma. J Clin Neurosci. 1998;5:233–237. doi: 10.1016/s0967-5868(98)90048-0. [DOI] [PubMed] [Google Scholar]
- 10.Vertinsky AT, Barnes PD. Macrocephaly, increased intracranial pressure, and hydrocephalus in the infant and young child. Top Magn Reson Imaging. 2007;18:31–51. doi: 10.1097/RMR.0b013e3180d0a753. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz N, Kiymaz N, Ylimaz C, Bay A, Yuca SA, Mumcu C, et al. Surgical treatment outcome of subdural empyema: A clinical study. Pediatr Neurosurg. 2006;42:293–298. doi: 10.1159/000094065. [DOI] [PubMed] [Google Scholar]