Abstract
A Cu-catalyzed method for efficient boron-copper addition processes involving acyclic and cyclic disubstituted aryl olefins are reported. Reactions are promoted with 0.5–5 mol % of a readily available N-heterocyclic carbene (NHC) complex; the presence of MeOH promotes in situ protonation of the C–Cu bond and leads to efficient catalyst turnover, constituting a net Cu-catalyzed hydroboration process. Reactions proceed in >98:<2 site-selectivity, and furnish secondary organoborane isomers that complement those obtained through reactions of boron-hydride reagents or by Rh- or Ir-catalyzed hydroborations (benzylic secondary C–B bonds). Initial observations regarding processes catalyzed by chiral NHC complexes, delivering products in up to 99:1 enantiomeric ratio, are disclosed.
Development of efficient and stereoselective catalytic hydroboration reactions is a compelling objective in chemical synthesis;1 a number of challenges, however, are yet to be addressed. For example, reactions of disubstituted olefins, furnishing regioisomers not accessible through the existing methods, have not been introduced. As part of studies regarding the development of N-heterocyclic carbenes (NHCs)2 for facile site- and enantioselective synthesis,3 we have begun to probe the ability of the derived metal complexes as catalysts for C–B bond formation. Herein, we disclose a protocol for catalytic boron-copper addition to acyclic and cyclic aryl olefins. Reactions are promoted by 0.5–5 mol % of a readily available NHC–Cu complex, proceed with >98:<2 site-selectivity, and afford boronate isomers that complement those obtained through transformations with borohydride reagents or catalyzed by Rh-and Ir-based catalysts.1,4 With chiral NHC complexes, Cu-catalyzed hydroborations proceed with high enantioselectivity [enantiomeric ratio (er) values up to 99:1].
Our studies were guided by previous disclosures on boron-copper addition to styrenes with stoichiometric amounts of an NHC–Cu complex and bis(pinacolato)diboron (1), affording secondary C–Cu and primary C–B bonds. Development of a catalytic version, where protonation of the C–Cu bond constitutes net hydroboration,5 required us to identify an efficient procedure with the substantially less reactive disubstituted alkenes to obtain chiral alkylboranes.
We first established that treatment of (E)-β-methylstyrene with Cu complex 2 (1 equiv), diborane 1 and NaOt-Bu delivers a complex mixture, only 20% of which is boronate 5 (after quench; entry 1, Table 1). We then noted a report on reactions of α,β-unsaturated esters with 1, promoted by phosphine–Cu complexes.6 An alcohol additive was used to protonate the resulting C–Cu bond to generate a catalytically active Cu–alkoxide, enhancing reaction efficiency. Such considerations led us to determine that subjection of (E)-β-methylstyrene to 0.5 mol % 2 and NaOt-Bu, 1.1 equivalents of 1 in the presence of 2.0 equivalents of MeOH results in >98% conversion to 5 at 22 °C within ten minutes (entry 2, Table 1); 5 is isolated in >98% yield and >98% site-selectivity. It should be noted that reaction of (E)-β-methylstyrene with BH3•THF or 9-BBN delivers the alternative boronate regioisomer preferably (benzylic C–B; 88:12 and 95:5, respectively, at 22 °C).
Table 1.
Cu-Catalyzed Hydroboration of (E)-β-Methylstyrene with Various Lewis Basesa
 | |||||
|---|---|---|---|---|---|
| entry | NHC or phoshine; mol % |
additive; equiv |
mol % CuCl |
time | conv (%) b |
| 1 | 2; 100 | none | – | 10 min | 20 |
| 2 | 2; 0.5 | MeOH; 2.0 | – | 10 min | >98 |
| 3 | 3; 1.0 | MeOH; 2.0 | – | 10 min | 65 |
| 4 | 3; 1.0 | MeOH; 2.0 | – | 24 h | 77 |
| 5 | 4; 1.0 | MeOH; 2.0 | – | 10 min | 13 |
| 6 | 4; 1.0 | MeOH; 2.0 | – | 24 h | 20 |
| 7 | PPh3; 1.0 | MeOH; 2.0 | 1.0 | 24 h | <10 |
| 8 | PCy3; 1.0 | MeOH; 2.0 | 1.0 | 24 h | 50 |
| 9 | PPh2(CH2)2PPh2; 1.0 | MeOH; 2.0 | 1.0 | 24 h | 75 |
 | |||||
Reactions under N2 atm; >98:<2 site-selectivity in all cases.
Conversion to the desired product by analysis of 400 MHz 1H NMR spectra of unpurified reaction mixtures. Mes = (2,4,6)-trimethylphenyl; B(pin) = pinacolatoboron.
In the presence of MeOD, 5-d1 is isolated in >98% yield and as a single diastereomer (eq 1, >98:<2 dr, >98% D incorporation). The efficient and stereoselective C–D bond formation underlines an important advantage of the present approach (vs processes involving borohydrides)7: diastereoselective capture of the intermediate C–Cu bond with other classes of (e.g., C-based) electrophiles might constitute the development of additional catalytic and versatile protocols.
 |
(1) |
When unsaturated 3, a complex related to the one employed in previously reported studies,5 serves as the catalyst (entries 3–4, Table 1), additions are less efficient. Processes promoted by alkyl-substituted complex 4 (entries 5–6, Table 1) proceed even less readily (20% conv in 24 h). The above observations point to the structural requirements for an effective Cu-based chiral catalyst for enantioselective hydroboration (see below for details). It is further noteworthy that phosphine-based catalysts are substantially less effective than NHC complexes (see entries 7–9 of Table 1). The superior activity of the more strongly σ-donating2 NHC-Cu systems (vs P-based variants) is consistent with theoretical studies indicating that the olefin substrate serves predominantly as a π-Lewis acid in this class of transformations.8
A range of aryl alkenes can be used (Table 2). Reaction with (Z)-β-methylstyrene is less efficient (entry 1; vs the E isomer); further conversion is not observed after 24 hours. The observed low activity is presumably due to effective Cu–olefin coordination requires aryl-alkene conjugation (lowering of alkene π* and more effective back-bonding),8 a conformation that suffers from allylic strain. When cyclic Z-alkenes indene (entry 2, Table 2) and dihydronaphthalene (entry 3) are used, however, complete conversion is achieved. The lower activity of p-methoxy-(E)-β-methylstyrene (entry 4) versus p-trifluoromethyl-(E)-β-methylstyrene and (E)-β-methylstyrene is consistent with the aforementioned electronic requirements.8 Sterically hindered olefins (entries 6–7) undergo hydroboration with high efficiency and without detectable loss of site-selectivity.
Table 2.
Cu-Catalyzed Hydroboration of β-Substituted Aryl Olefinsa
 | |||||
|---|---|---|---|---|---|
| entry | substrate | mol % 2 |
time | conv (%)b | yield (%)c |
| 1 | 5.0 | 10 min | 49 | 41 | |
| 2 | 1.0 | 24 h | >98 | 92 | |
| 3 | 0.5 | 10 min | >98 | 97 | |
| 4 |  |
3.0 | 10 min | >98d | 95 |
| 5 |  |
1.0 | 5 h | >98 | 91 |
| 6 |  |
1.0 | 10 min | 70e | 66 |
| 7 |  |
5.0 | 3 h | 93 | 88 |
Reactions under N2 atm; >98:<2 site-selectivity in all cases.
Determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures.
Yield of pure products.
1.5 equiv 1 used.
30% of Z-alkene recovered.
Cu-catalyzed hydroboration of allylic carbonate 6 delivers α-carbamylboronate 7 in 76% yield without complications arising from allylic substitution9 (<2%; 400 MHz 1H NMR analysis); oxidation with H2O2 affords the derived cyclic carbonate in 61% overall yield. Allylic acetate 8, methyl ether 9 and heterocyclic 10 are isolated in 82%, 96% and >98% yield, respectively. With allylic alcohol 11, use of MeOH is not needed: 12 is obtained in 80% yield (after acid workup and product purification).10
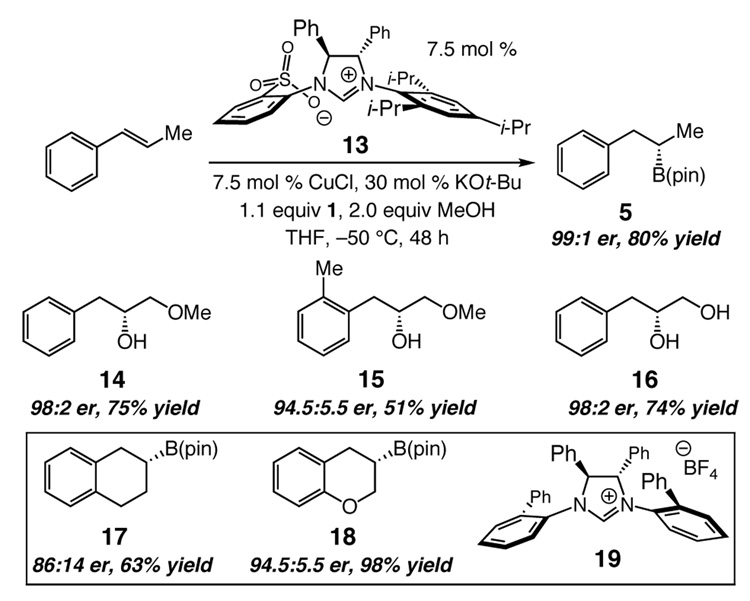
The efficiency of 2 in initiating additions to aryl-substituted alkenes bodes well for the development of Cu-catalyzed enantioselective hydroborations. We have thus established that reaction of (E)-β-methylstyrene with 7.5 mol % of bidentate imidazolinium salt 13 delivers boronate 5 in 99:1 er, >98:<2 site-selectivity and 80% yield. α-Hydroxymethyl ethers 14–15 and 16 are obtained in 98:2, 94.5:5.5, and 98:2 er, respectively, after oxidative workup. Thus far, reactions of cyclic olefins (17–18) proceed with slightly lower selectivity (86:14 and 94.5:5.5 er, respectively); optimal results are achieved through the use of monodentate NHC derived from 19.11 The enantiomerically enriched secondary alkyl-boronates obtained method can be used for stereoselective C–N12a and C–C12b bond formation, allowing access to an assortment of enantiomerically enriched compounds.
Development of copper-boron additions to other classes of olefins and related processes are in progress.
Supplementary Material
Experimental procedures and spectral, analytical data for all products (PDF). This material is available on the web: http://www.pubs.acs.org
Scheme 1.
Cu–Catalyzed Hydroboration of Allylic Esters, Ethers and Alcohols
Scheme 2.
Cu–Catalyzed Enantioselective Hydroboration Reactionsa
aSee SI for experimental details; oxidation conditions for 14–16: H2O2, 2.0 N aqueous NaOH; MeOH not used for 16. >98:<2 site-selectivity in all cases.
Acknowledgment
We thank the NSF (CHE-0715138) and the NIH (GM-47480) for support. Y. L. is grateful for an AstraZeneca Graduate Fellowship. Mass spectrometry facilities at Boston College are supported by the NSF (DBI-0619576).
References
- 1.For a review on catalytic enantioselective hydroboration, see: Carroll A-M, O’Sullivan TP, Guiry P. J. Adv. Synth. Catal. 2005;347:609–631.
- 2.Glorious F, editor. N-Heterocyclic Carbenes in Transition Metal Catalysis. Berlin, Heidelberg: Springer-Verlag; 2007. [Google Scholar]
- 3.(a) Van Veldhuizen JJ, Campbell JE, Giudici RE, Hoveyda AH. J. Am. Chem. Soc. 2005;127:6877–6882. doi: 10.1021/ja050179j. [DOI] [PubMed] [Google Scholar]; (b) Larsen AO, Leu W, Nieto-Oberhuber C, Campbell JE, Hoveyda AH. J. Am. Chem. Soc. 2004;126:11130–11131. doi: 10.1021/ja046245j. [DOI] [PubMed] [Google Scholar]; (c) Brown MK, May TL, Baxter CA, Hoveyda AH. Angew. Chem., Int. Ed. 2007;46:1097–1100. doi: 10.1002/anie.200604511. [DOI] [PubMed] [Google Scholar]
- 4.For Rh-catalyzed enantioselective hydroborations of disubstituted styrenes (to afford predominantly benzylic alcohols), see: Doucet H, Fernandez E, Layzell TP, Brown JM. Chem. Eur. J. 1999;5:1320–1330.
- 5.(a) Laitar DS, Müller P, Sadighi JP. J. Am. Chem. Soc. 2005;127:17196–17197. doi: 10.1021/ja0566679. [DOI] [PubMed] [Google Scholar]; (b) Laitar DS, Tsui EY, Sadighi JP. Organometallics. 2006;25:2405–2408. [Google Scholar]
- 6.Mun S, Lee J-E, Yun J. Org. Lett. 2006;8:4887–4889. doi: 10.1021/ol061955a. [DOI] [PubMed] [Google Scholar]
- 7.NHC-Cu-catalyzed hydroboration of styrene with catecholborane has been reported (2.3–7.3:1 site-selectivity, favoring terminal C-B). See: Lillo V, Fructos MR, Ramírez J, Braga AAC, Maseras F, Díaz-Requejo MM, Pérez PJ, Fernández E. Chem., Eur. J. 2007;13:2614–2621. doi: 10.1002/chem.200601146.
- 8.For related DFT calculations, see: Dang L, Lin Z, Marder TB. Organometallics. 2008;27:4443–4454. and references cited therein.
- 9.Ito H, Ito S, Sasaki Y, Matsuura K, Sawamura M. J. Am. Chem. Soc. 2007;129:14856–14857. doi: 10.1021/ja076634o. [DOI] [PubMed] [Google Scholar]
- 10.Reactions can be performed in THF or toluene; see the Supporting Information for details
- 11.(a) Chaulagain MR, Sormunen GJ, Montgomery J. J. Am. Chem. Soc. 2007;129:9568–9669. doi: 10.1021/ja072992f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) ASAP. Lillo V, Prieto A, Bonet A, Díaz-Requejo MM, Ramírez J, Pérez PJ, Fernández E. Organometallics. 2009 [Google Scholar]
- 12.For example, see: Fernandez E, Maeda K, Hooper MW, Brown JM. Chem. Eur. J. 2000;6:1840–1846. doi: 10.1002/(sici)1521-3765(20000515)6:10<1840::aid-chem1840>3.0.co;2-6. ; Chen A, Ren L, Crudden CM. J. Org. Chem. 1999;64:9704–9710. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and spectral, analytical data for all products (PDF). This material is available on the web: http://www.pubs.acs.org