Abstract
This study aimed to elucidate the role of L-type fatty acid-binding protein (L-FABP) in renal tubulointerstitial injury using a mouse adenine-induced renal injury model. C57BL/6 mice fed excess dietary adenine for 6 weeks showed a gradual increase in levels of blood urea nitrogen (BUN). They also showed severe tubulointerstitial pathological findings, such as fibrosis and macrophage infiltration without glomerular damage, which were attenuated by treatment with either allopurinol or Y-700, a new xanthine dehydroxygenase inhibitor. Because renal expression of L-FABP is defective in C57BL/6 mice, human L-FABP transgenic mice were fed an adenine-containing diet. Transgenic mice showed lower BUN levels and lower levels of pathological injury compared with wild-type mice. On the other hand, urinary levels and renal expression of L-FABP in the adenine group was significantly increased and attenuated by treatment with either allopurinol or Y-700. Urinary L-FABP was positively correlated with BUN levels and pathological damages in the tubulointerstitium. No increases in urinary protein, albumin, or N-acetyl-β-d-glucosaminidase levels were found for 6 weeks in any group. In conclusion, we demonstrated that urinary L-FABP levels can be used to monitor both dynamics and drug responses in a mouse adenine-induced tubulointerstitial injury model.
The prevalence of chronic kidney disease (CKD) is increasing all over the world and no treatment can completely reverse the progression of chronic kidney disease to end-stage renal disease, which requires an enormous amount of medical resources. Moreover, it is known that chronic kidney disease is a strong and independent risk factor for cardiovascular disease.1 Although urinary protein measurements are widely used for evaluation of chronic kidney disease progression, these markers mainly depend on the glomerular damages. It is reported that the most predictive factor for progression to end-stage renal disease is not the etiology of glomerular injury, but the degree of proteinuria and the tubulointerstitial damage such as fibrosis and inflammatory cell infiltration.2,3,4,5,6 New biomarkers that can evaluate tubulointerstitial damage will be useful for predicting the prognosis of chronic kidney disease patients and determining therapeutic strategy.
Fatty acid-binding protein (FABP) belongs to a superfamily of lipid-binding protein and is consisting of several different isoforms such as heart, brain, intestine, and liver-type. FABP carries fatty acids to peroxisome and mitochondria and regulates several gene expressions.7,8,9 L-type FABP (L-FABP) is predominantly expressed in human renal proximal tubules and can be detected in the urine only when the kidney is damaged.10 Mouse L-FABP in proximal tubules of C57BL/6 wild-type mice was virtually defective because of the silencing sequence localizing in the upstream of the promoter region. Therefore, we used human L-FABP transgenic mice, which are humanized by expressing higher protein levels of human L-FABP in proximal tubules that resemble the human kidney. We have reported that urinary L-FABP can be detected in human and animal kidney injury.11,12,13 In a cyclooxygenase inhibitor-induced renal injury model, a transient increase of urinary L-FABP can predict subsequent tubulointerstitial injury and decrease of peritubular capillary blood flow.11 Kamijo and colleagues14 recently reported that urinary L-FABP levels in chronic kidney disease patients were correlated significantly to the degree of proteinuria and the progression of chronic kidney disease measured by serum creatinine. However, it is still unclear whether the urinary L-FABP level can reflect tubulointerstitial injury independently from glomerular proteinuria. Expression of human L-FABP in the kidney can reduce acute kidney injury induced by ischemia reperfusion and cis-platinum injection.10,12,13 This study is aimed to demonstrate that urinary L-FABP can detect tubulointerstitial damage without any glomerular injury, monitor the drug response with a mouse tubulointerstitial injury model by administering adenine, and compare the deterioration of chronic tubulointerstitial injury between human L-FABP transgenic and wild-type mice.
Materials and Methods
Animal Experiments
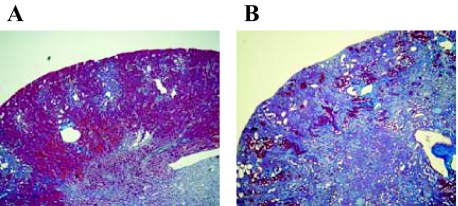
Male C57BL/6 wild-type and heterozygous human L-FABP transgenic mice were used in this experiment. The engineering of human L-FABP transgenic mice is detailed elsewhere.12 All mice were kept in specific pathogen-free conditions in our animal facility. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (U.S. Department of Health and Human Services Public Health Services, National Institutes of Health, NIH publication no. 86-23, 1985). They were allowed food and water ad libitum. A 0.5% w/w adenine-containing diet was used in several previous reports that showed the adenine-containing diet induced chronic renal injury in rats.15,16,17 In the preliminary study, we evaluated the different doses of the adenine diet (0.5, 0.2, 0.05% w/w) for inducing renal injury with male C57/BL6 wild-type mice. No mice in the 0.5% w/w diet group could survive more than 1 week. The animals fed a 0.05% w/w diet showed patchy and modest tubulointerstitial injury including tubular epithelial degradation and interstitial fibrosis. Blood urea nitrogen (BUN) in the 0.05% group did not increase. On the other hand, mice fed by a 0.2% w/w adenine diet showed a gradual increase of BUN and remarkable histological injury in the tubulointerstitium at 6 weeks (Table 1 and Figure 1). The survival rate of the 0.2% group was 100%. Therefore, the following experiments were performed with the 0.2% w/w adenine diet. The allopurinol group and the Y-700 group were fed a 0.2% w/w adenine-containing diet for 6 weeks and given allopurinol (25 mg/kg/day; Sigma, St. Louis, MO) or Y-700 (5 mg/kg/day; Mitsubishi Tanabe Pharma Corp., Osaka, Japan) in the drinking water from 2 weeks after the start of the adenine diet. The normal diet group was fed a normal diet and water. Blood and urine were collected at 0, 2, 4, and 6 weeks after the start of adenine diet. The mice were sacrificed at 6 weeks for collecting specimens.
Table 1.
BUN Changes Induced by Adenine-Containing Diet
| BUN (mg/dl) | ||||
|---|---|---|---|---|
| 0 week | 2 weeks | 4 weeks | 6 weeks | |
| 0.05% w/w | 20.2 ± 1.8 | 30.1 ± 8.3 | 30.2 ± 1.2 | 28.8 ± 2.1 |
| 0.2% w/w | 18.5 ± 0.7 | 74.1 ± 8.3 | 135.9 ± 4.1 | 222.6 ± 13.1 |
| 0.5% w/w | 24.4 ± 0.9 | N/A | N/A | N/A |
Data are means ± SEM; n = 6 ∼ 8 in each group. All the animals in the 0.5% group died within 1 week.
Figure 1.
Renal pathological injury induced by excess dietary adenine. Renal histological changes of the 0.05% (A) and 0.2% (B) adenine diet groups are shown (Masson’s trichrome staining). The animals were sacrificed at 6 weeks. Original magnifications, ×40.
BUN
BUN was measured using the urease-indophenol method with Urea N B (Wako Pure Chemical Industries Ltd., Osaka, Japan) as previously described.18
Urinary Biochemistry
The urinary levels of protein, albumin, creatinine, and N-acetyl-β-d-glucosaminidase (NAG) were measured using a protein assay (Bio-Rad Laboratories, Inc., Hercules, CA), Albuwell M kit (Exocell Inc., Philadelphia, PA), Nescoat VLII CRE (Alfresa Pharma Corp., Osaka, Japan), and N-acetyl-β-d-glucosaminidase test (Shionogi and Co. Ltd., Osaka, Japan), respectively. Urine protein, albumin, and N-acetyl-β-d-glucosaminidase levels were expressed as the ratio to the urinary creatinine concentration.
Enzyme-Linked Immunosorbent Assay for Urinary Human L-FABP
Urinary human L-FABP was measured using the sandwich enzyme-linked immunosorbent assay kit following the manufacturer’s protocol (CMIC Co. Ltd., Tokyo, Japan). The measurable range of this kit is between 4 and 400 ng/ml. Measurements were performed in duplicate. This assay system does not detect rodents’ L-FABP. Urinary h-L-FABP levels are expressed as the ratio of the urinary human L-FABP to the creatinine concentration.
Pathological Analysis
Kidneys were collected at 6 weeks after the adenine diet and fixed in 10% buffered formalin. Paraffin sections of 3 μm were stained with periodic acid-Schiff reagent and Masson’s trichrome. Immunohistochemical staining was performed using indirect immunohistochemical techniques. Biotin-free immunohistochemical staining using horseradish peroxidase-conjugated polymer system was conducted according to the manufacturer’s protocols (Histofine mouse stain kit and Histofine simple stain mouse MAX-PO (rat) kit; Nichirei Corp., Tokyo, Japan). The deparaffinized sections were preincubated with 3% hydrogen peroxide for 5 minutes and incubated with primary antibodies overnight at 4°C, followed by incubation with polymer-conjugated anti-mouse IgG. Protease K treatment was required for rat anti-mouse F4/80 macrophage antigen antibody (MCA497R; Serotec, Raleigh, NC); autoclaving in 10 mmol/L citrate-buffer (pH 6.0) was required for mouse anti-α-smooth muscle actin (SMA) monoclonal antibody (1A4; DakoCytomation Co. Ltd., Glostrup, Denmark). Diaminobenzidine tetrahydrochloride (Nichirei Corp.) was used for the substrate-chromogen reaction followed by counterstaining with hematoxylin. Immunohistochemistry for human L-FABP was also performed as previously described.10,12
The area of interstitial fibrosis was evaluated using a computer-aided evaluation program (AIS; Imaging Research Inc., St. Catharines, Ontario) as previously described.18 The area of α-smooth muscle actin-positive myofibroblast or F4/80-positive macrophage was also evaluated. Viewed at ×200 magnification, five randomly selected nonoverlapping fields in the kidney cortex were analyzed. The areas were depicted in digital images; then the percentage of the fibrotic area was calculated relative to the entire field area (percentage area).
Quantitative Polymerase Chain Reaction (PCR) Analysis for Transforming Growth Factor (TGF)-β1, Monocyte Chemoattractant Protein (MCP)-1, and L-FABP Expression
Real-time PCR assay was performed as described previously.18 Briefly, total RNA isolated from the harvested kidneys was reverse-transcribed to cDNA using MLV reverse transcriptase (High Capacity cDNA reverse transcription kit; Applied Biosystems, Foster City, CA) with random hexamers. Transcripts encoding Transforming Growth Factor-β1 and Monocyte Chemoattractant Protein-1 were measured using TaqMan real-time quantitative PCR with a sequence detection system (Prism 7000; Applied Biosystems) and TaqMan universal PCR master mix (Applied Biosystems). The TaqMan probes and primers for Transforming Growth Factor-β1 (assay identification number Mm00441724_m1), Monocyte Chemoattractant Protein-1 (Mm00441242_m1), and L-FABP (Hs00155026_m1) were assay-on-demand gene expression products (Applied Biosystems). To normalize for variance in loaded cDNA, 18S ribosomal RNA was amplified in a separate tube. Standard curves were prepared for each gene and the 18S ribosomal RNA in each experiment to normalize the relative expression of the genes of interest to the 18S ribosomal RNA control.
Western Blot Analysis for L-FABP in the Kidney
Harvested kidneys were homogenized on ice in a radioimmunoprecipitation assay buffer with protease inhibitors. The lysates were separated on a 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After transferring proteins from the gel to a polyvinylidene difluoride membrane (Amersham Biosciences Corp., Uppsala, Sweden), Western blot analysis was performed using 1:10 diluted horseradish peroxidase-labeled hL-FABP antibody (CMIC, Co. Ltd.) for 1 hour at room temperature. Subsequently, the chemiluminescent signal labeled using ECL Plus (Amersham Biosciences Corp., Arlington Heights, IL) was detected using a CCD camera system (LAS-4000mini; Fuji Photo Film Co. Ltd., Tokyo, Japan). The membrane was incubated at 50°C for 30 minutes in a stripping buffer to remove all of the probes and reprobed with the antibody to β-actin (Chemicon, Temecula, CA). Densitometric analysis of bands compared with the density of β-actin was performed using Multi Gauge, version 3.1 (Fuji Photo Film Co. Ltd.).
Statistical Analysis
The results are expressed as mean values ± SEM. Differences among experimental groups were determined using one-way analysis of variance with Fisher’s post hoc analysis. Differences for which P < 0.05 were considered significant. Correlations between two indicators were evaluated using the Spearman rank test.
Results
Evaluation on Adenine-Induced Mouse Tubulointerstitial Injury Model
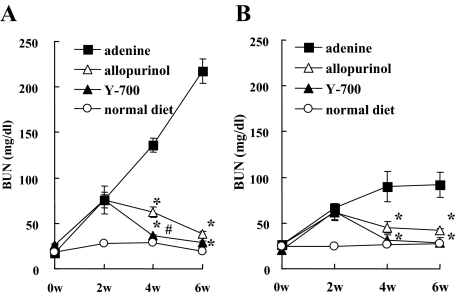
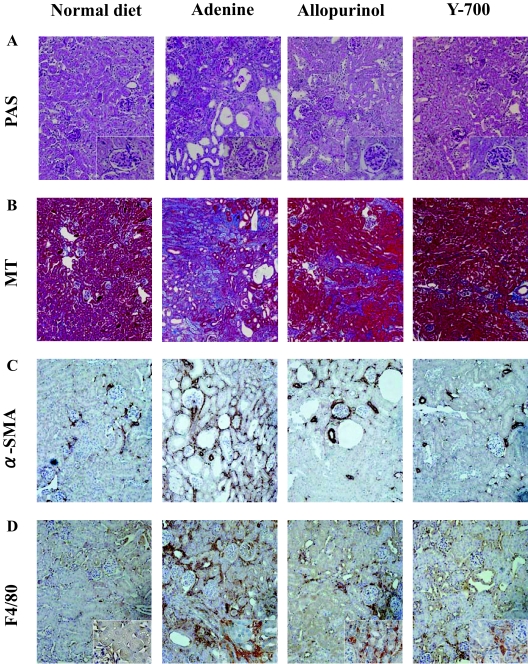
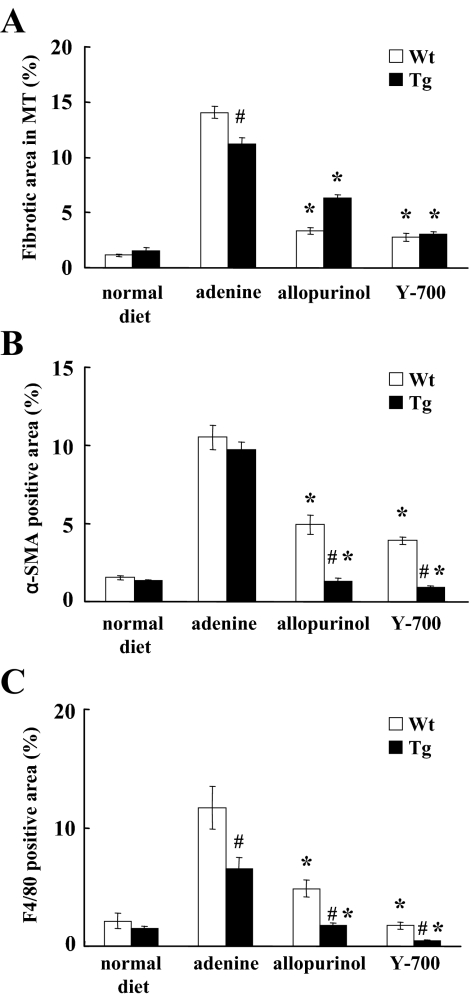
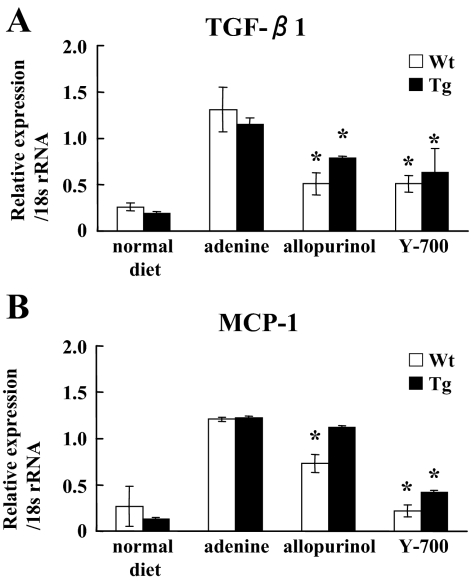
Blood urea nitrogen (BUN) levels in mice fed by the adenine-containing diet were continuously increased for 6 weeks. At 4 and 6 weeks, the BUN level of the adenine group was significantly higher than that of the normal diet group (Figure 2, A and B). Pathological evaluation showed irregular renal tubular dilatation, inflammatory cell infiltration, and remarkable interstitial fibrosis in the interstitium at 6 weeks (Figure 3, A and B). There was no remarkable glomerular lesion such as sclerosis and mesangial expansion. Interstitial fibrotic areas stained in blue by Masson’s trichrome were more prominent in the kidney of the adenine group than the normal diet group (Figure 3B). Immunohistochemical analysis with anti-α-SMA antibody was performed to detect interstitial myofibroblasts. Positive staining of α-smooth muscle actin was found at identical sites of the fibrotic area (Figure 3C). Macrophage infiltration was evaluated using immunohistochemistry for F4/80, a specific macrophage antigen.19 Infiltrated F4/80-positive staining cells were detected in the cortical peritubular capillary around fibrotic areas (Figure 3D). Quantitative analyses demonstrated that the fibrotic area, α-smooth muscle actin-positive, and F4/80-positive staining area in the adenine group were significantly larger than the normal diet group (Figure 4, A–C). Transforming growth factor (TGF)-β1 and Monocyte Chemoattractant Protein-1 expression in the kidney were examined using TaqMan real-time PCR assay. The expression levels of Transforming Growth Factor-β1 and Monocyte Chemoattractant Protein-1 were significantly increased in the adenine group compared with the normal diet group at 6 weeks (Figure 5, A and B).
Figure 2.
Time course of BUN levels in adenine-induced tubulointerstitial injury model. BUN was measured at 0, 2, 4, and 6 weeks in wild-type (A) and human L-FABP transgenic mice (B) (n = 5 in each group). *P < 0.05 versus adenine group, #P < 0.05 versus allopurinol group.
Figure 3.
Pathological findings at 6 weeks in C57BL/6 mice. Renal histological changes stained with periodic acid-Schiff (A) and Masson’s trichrome (B) and immunohistochemistry of α-smooth muscle actin (C), and F4/80 (D) are shown. Original magnifications: ×600 (A and D, insets); ×200 (A, C, and D); ×100 (B).
Figure 4.
Quantitative analyses of the pathological changes. Fibrotic area in Masson’s trichrome (A), positive staining of α-smooth muscle actin (B), and F4/80 (C) was quantified (n = 5 in each group). *P < 0.05 wild type (Wt) versus transgenic (Tg) mice, #P < 0.05 versus adenine group.
Figure 5.
Quantitative analyses of mRNA expression. Transforming Growth Factor-β1 (A) and Monocyte Chemoattractant Protein-1 (B) expressions were evaluated by real-time PCR (n = 5 in each group). *P < 0.05 versus adenine group.
Therapeutic Effect of Xanthine Dehydrogenase (XDH) Inhibitors on Adenine-Induced Mouse Tubulointerstitial Injury Model
Treatment with allopurinol and a new XDH inhibitor Y-700 was started 2 weeks after, when BUN levels in the adenine, allopurinol, and Y-700 group were almost similarly increased [adenine, 74.1 ± 8.3 mg/dl (n = 5); allopurinol, 75.7 ± 15.2 mg/dl (n = 5); Y-700, 75.9 ± 5.2 mg/dl (n = 5)]. Treatments of allopurinol and Y-700 significantly reduced BUN levels after starting administrations. Y-700 treatment appeared to be more effective than allopurinol especially at 4 weeks [allopurinol, 62.1 ± 5.9 mg/dl (n = 5) versus Y-700, 36.0 ± 1.5 mg/dl (n = 5); P < 0.05].
The pathological findings including the irregular renal tubular dilatation, inflammatory cell infiltration in the interstitium, and development of interstitial fibrosis were significantly attenuated in the allopurinol and Y-700 group (Figure 3, A and B). The increases of interstitial fibrotic areas, positive staining area of α-smooth muscle actin and F4/80 by adenine-containing diet were also significantly attenuated in the allopurinol and Y-700 group. Y-700 treatment showed better improvement in F4/80-positive cell infiltration than allopurinol, whereas no significant difference was found in fibrotic area and α-smooth muscle actin-positive area (Figure 4, A–C). The expression levels of Transforming Growth Factor-β1 and Monocyte Chemoattractant Protein-1 of the allopurinol and Y-700 group were significantly decreased than the adenine group. The expression of Monocyte Chemoattractant Protein-1 in Y-700 group was significantly lower than the allopurinol group (Figure 5, A and B).
Role of Renal L-FABP in Adenine-Induced Mouse Tubulointerstitial Injury Model
Urinary biomarkers in the present adenine-induced mouse tubulointerstitial injury model were examined with human L-FABP transgenic mice. In accordance with our previous reports,12,13 human L-FABP transgenic mice were resistant to renal injury induced by adenine diet. BUN levels at 6 weeks in human L-FABP transgenic mice (Tg) were lower than wild-type [wild-type adenine, 217.3 ± 19.6 mg/dl (n = 5) versus Tg adenine, 91.5 ± 5.9 mg/dl (n = 5); P < 0.05]. Allopurinol and Y-700 treatment significantly reduced BUN levels even in Tg mice fed by adenine-containing diet (Figure 2B). The fibrotic area and F4/80 but not α-smooth muscle actin-positive-staining area at 6 weeks in Tg were smaller than wild-type, and allopurinol and Y-700 treatment also decreased them in Tg mice (Figure 4, A–C). Allopurinol and Y-700 treatment decreased Transforming Growth Factor-β1 and/or Monocyte Chemoattractant Protein-1 expression in Tg mice, although there was no difference between untreated Tg and wild-type mice (Figure 5, A and B).
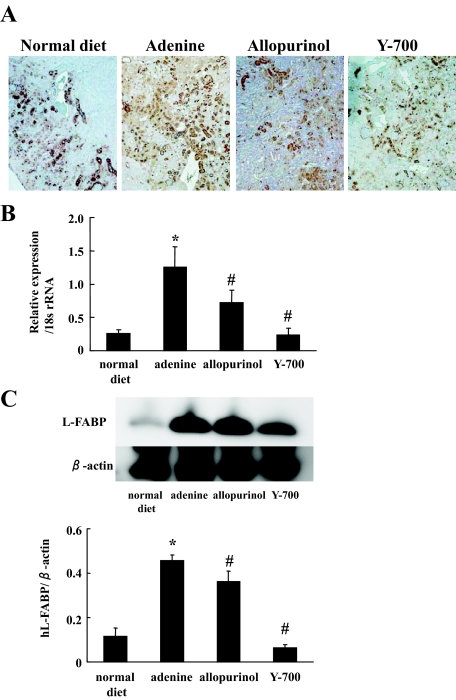
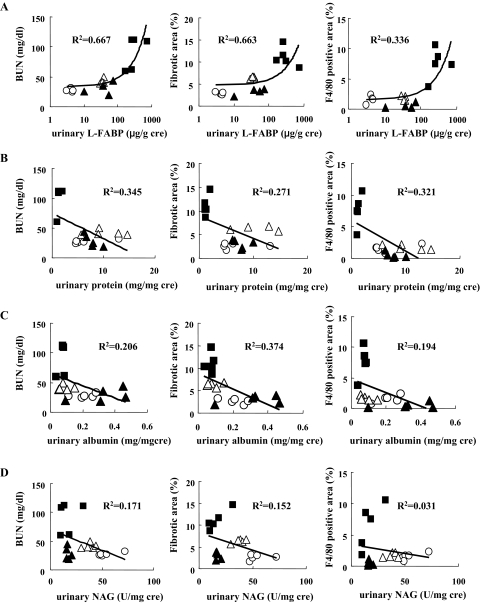
Quantitative PCR analysis, immunohistochemistry, and Western blot analysis revealed that the expression of L-FABP in the kidney was increased in this tubulointerstitial injury model. Allopurinol and Y-700 treatment decreased L-FABP expression in the kidney (Figure 6, A–C). We found good positive correlations between urinary L-FABP levels and the parameters of renal injury such as BUN, fibrotic area, and F4/80-positive staining area at 6 weeks (Figure 7A). Urinary L-FABP clearly distinguished nontreatment (adenine) group, treatment groups (allopurinol and Y-700), and the normal diet group. In contrast, urinary protein and albumin in the adenine group were lower than the normal diet group and showed negative correlation with the parameters of renal injury (Figure 7, B and C). Urinary N-acetyl-β-d-glucosaminidase did not show any significant correlation (Figure 7D).
Figure 6.
Expression of human L-FABP in the kidney. Immunohistochemistry (A), quantitative RT-PCR (B), and Western blotting analysis (C) showed an increased expression of human L-FABP in the adenine group (n = 5 in each group). *P < 0.05 versus normal diet, #< 0.05 versus adenine group.
Figure 7.
Correlations between urinary markers [L-FABP (A), protein (B), albumin (C), and N-acetyl-β-d-glucosaminidase (D)] and BUN, fibrotic area, and F4/80 positively stained area. Urinary markers, BUN, and pathological changes were evaluated in human L-FABP transgenic mice of the adenine (filled square), allopurinol (open triangle), Y-700 (filled triangle), and normal diet (open circle) groups (n = 5 in each group).
Discussion
A mouse tubulointerstitial injury model by excess adenine diet showed a continuous increase of BUN and pathological injury with interstitial fibrosis and inflammatory cell infiltration. We demonstrated that urinary L-FABP levels can detect renal damage in the interstitium, whereas urinary protein and N-acetyl-β-d-glucosaminidase failed to show any positive correlation with interstitial injury. Urinary L-FABP can monitor the drug effects of allopurinol and a new XDH inhibitor Y-700 on this tubulointerstitial injury model. Moreover, overexpression of L-FABP in the kidney partly reduced tubulointerstitial injury.
It has been reported that the tubulointerstitial damage plays a key role in the progression of chronic kidney disease.3,4,5,6 However, there is no good monitoring measurement except renal biopsy to detect the damage in the tubulointerstitium accurately. In the present study, we found feeding an adenine-containing diet caused chronic renal tubulointerstitial injury in C57BL/6 mice and evaluated whether urinary biomarkers can detect tubulointerstitial damage independently from glomerular lesions. There have been several reports that showed the adenine-containing diet induced chronic renal injury in rats.15,16,17 Adenine is a substrate for XDH, which can oxidize adenine to 2,8-dihydroxyadenine (DHA) via an 8-hydroxyadenine intermediate.20 Because of the very low solubility of DHA, it is assumed that the deposition of DHA in renal tubules degenerates renal tubular epithelial cells and causes inflammatory injury with subsequent fibrotic changes.21 In the present study, we demonstrated the increased number of macrophage and myofibroblast in the interstitium and higher expression of Transforming Growth Factor-β1 and Monocyte Chemoattractant Protein-1 in the damaged kidney of our newly established model. We found modest CD3-positive T-cell infiltration but not tubulitis (data not shown). These findings were much more conspicuous in wild-type but rather lower in Tg mice.
Human adenine phosphoribosyltransferase (APRT) deficiency, an autosomal recessive disease, is characterized by kidney stones, crystalluria, hematuria, dysuria, and urinary tract infections.21 Some patients may progress to chronic renal failure, requiring dialysis or kidney transplantation.22 Renal pathological examination often finds crystalline precipitates in tubular lumens and epithelial cells accompanied with mononuclear cell infiltration, tubular atrophy, and interstitial fibrosis.23 APRT catalyzes the synthesis of adenosine monophosphate from adenine, whereas adenine is oxidized to DHA with APRT deficiency.20 Therefore, this mouse adenine-induced tubulointerstitial injury model appears to share the same mechanism of renal injury in human APRT deficiency. Clinically, APRT deficiency has been treated using allopurinol because allopurinol inhibits XDH and reduces DHA formation from adenine. Allopurinol is widely used for hyperuricemia or gout. Allopurinol is mainly eliminated from the kidney and sometimes causes severe side effects such as pancytopenia and exfoliative dermatitis especially in patients with renal insufficiency.24,25,26,27 Y-700 is one of the newly synthesized XDH inhibitors metabolized mainly through the liver, binds XDH with higher affinity, and has a longer half life than allopurinol.28 In the present study, we demonstrated the therapeutic efficacy of allopurinol and Y-700 on the adenine-induced mouse tubulointerstitial injury. In some parameters such as BUN at 4 weeks and Monocyte Chemoattractant Protein-1 expression in the kidney at 6 weeks, Y-700 showed more protective effects than allopurinol. There was no liver dysfunction evaluated by aspartate aminotransferase and alanine aminotransferase in the Y-700 group (data were not shown). Our data may suggest that Y-700 can be used more effectively and safely in the patients with renal dysfunction.
We have recently demonstrated that urinary L-FABP is a useful biomarker in the mouse and human kidney injury.10,11,12,13 Urinary L-FABP can also detect human chronic kidney disease by chronic glomerulonephritis and diabetic nephropathy and shows a good correlation with urinary protein.14,29,30 chronic kidney disease is defined by decreased glomerular filtration rate31 and the most important predicting factors of progression of chronic kidney disease are urinary protein and interstitial damage independently from the type of glomerular injury. Urinary protein and albumin can detect the early changes of glomerular injury in chronic kidney disease and predict cardiovascular events even in the patients without obvious kidney disease.32 In the present study, although our mouse adenine-induced tubulointerstitial injury model showed severe renal interstitial damage, there was no glomerular injury and urinary levels of protein, albumin, and even N-acetyl-β-d-glucosaminidase were not increased. In contrast, urinary L-FABP was drastically increased and showed a positive correlation with pathological injury including fibrosis and macrophage infiltration. Urinary protein and albumin in the adenine group was lower than the normal diet group. Urinary protein in the human chronic kidney disease should be consisting of increased glomerular protein leak (albumin) and tubular excretion (Tamm-Horsfall protein). In the present animal model, lower urinary protein in the adenine group may indicate decreased tubular protein excretion without increase of glomerular protein leakage. Urinary albumin was also mildly decreased. It may be possible that severe tubulointerstitial injury causes atubular glomeruli that cannot filtrate any solute from the blood. Decreased physiological albumin leak because of atubular glomeruli may explain the lower than normal urinary albumin levels in this model. It may be possible that glomerular damage evaluated by urinary protein and albumin can be underestimated because of tubulointerstitial injury. Urinary biomarker panel including glomerular (albumin) and tubulointerstitial (L-FABP) markers will provide more precise prediction on chronic kidney disease progression. Urinary L-FABP can reflect the improvement by the therapeutic intervention by XDH inhibitors. Monitoring the drug effects is one of the most important factors in biomarker development.33 Taken together, urinary L-FABP was more sensitive and reliable biomarker than other conventional urinary markers such as albumin, protein, and N-acetyl-β-d-glucosaminidase in the present adenine-induced tubulointerstitial injury model, and presumably to human chronic renal interstitial injury. Although N-acetyl-β-d-glucosaminidase is a lysosomal enzyme found mainly in proximal tubular cells and has been shown to be a sensitive proximal tubular injury marker in the setting of a wide variety of acute kidney injuries,34 it failed to detect tubular injury in the present study. It is reported that combining several renal markers increased sensitivity and specificity of diagnosis in acute kidney injury.35 Comparison of L-FABP with other newly developed tubular injury markers such as neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) and application of renal biomarker panel will be necessary to improve the monitoring of renal damage in chronic kidney disease.
We also reported that human L-FABP overexpression has protective effects against acute kidney injury in some animal models possibly because of reducing oxidative stress.12,36,37 Here, we found the same tendency that human L-FABP mice showed lower BUN levels and less severe pathological injury by adenine-containing diet than wild-type mice. Human L-FABP expression in the kidney improved renal function (BUN), inflammatory change (F4/80-positive cell infiltration), and fibrotic change (Masson’s trichrome staining), however, there was no significant difference between human L-FABP transgenic mice and wild-type in terms of cytokine levels (TGF-β and Monocyte Chemoattractant Protein-1) and early marker of fibrosis development (myofibroblast stained by anti-α-smooth muscle actin antibody). This is possibly because we evaluated tubulointerstitial injury after 6 weeks adenine administration and might not be able to detect early changes of interstitial fibrosis development.
In this model, peritubular capillary loss and decreased blood flow in peritubular capillary were observed in fibrotic area (data not shown). Chronic hypoxia caused by decreased peritubular capillary blood flow may up-regulate the expression of L-FABP because the promoter of L-FABP gene contains the hypoxia-responding element and hypoxia can increase L-FABP expression in acute kidney injury.10,12 Under ischemic conditions or nephrotoxic insults, lipid peroxidation products will accumulate in tubular cells and damage them. L-FABP is presumably capable of binding these noxious lipid peroxidative products and transferring them to urinary spaces with cytotoxic lipids. Further investigations are needed to figure out the mechanism of L-FABP for protecting interstitial damage in this model.
In conclusion, we found feeding an adenine-containing diet caused chronic renal tubulointerstitial injury with a continuous increase of BUN and remarkable pathological damages only in the renal interstitium, which were improved by the XDH inhibitors, allopurinol or Y-700. Urinary L-FABP showed positive correlations with BUN and pathological changes, whereas other conventional urinary markers failed to detect the interstitial damages. Urinary L-FABP can monitor the improvement by XDH inhibitor treatment. Moreover, the expression of human L-FABP in renal proximal tubules reduced tubulointerstitial injury. Therefore, urinary L-FABP will be a novel biomarker to detect the progression of renal interstitial injury.
Acknowledgments
We thank Ms. Mami Haba and Mr. Masakazu Miura for their skilled assistance.
Footnotes
Address reprint requests to Dr. Eisei Noiri, Department of Nephrology 107 Lab., University Hospital, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8655, Japan. E-mail: noiri-tky@umin.ac.jp.
Supported by The BioBank Japan Project on the Implementation of Personalized Medicine (grant 3023168, MEXT, Japan, to E.N.; KAKEN-HI grant 19590935, MEXT, Japan, to E.N. and T.S.; Special Coordination Funds for Promoting Science and Technologies grant 1200015, MEXT, Japan, to E.N.); the National Institutes of Health/The National Institute of Diabetes and Digestive and Kidney Diseases (grant RO1-DK075976 to D.P.); and the Veterans Administration (merit award to D.P.).
References
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol. 1981;15:167–171. [PubMed] [Google Scholar]
- Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- Veerkamp JH, Peeters RA, Maatman RG. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta. 1991;1081:1–24. doi: 10.1016/0005-2760(91)90244-c. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59:1096–1116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunerland NH, Spener F. Fatty acid-binding proteins—insights from genetic manipulations. Prog Lipid Res. 2004;43:328–349. doi: 10.1016/j.plipres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Noiri E, Yamamoto T, Sugaya T, Negishi K, Maeda R, Nakamura K, Portilla D, Goto M, Fujita T. Urinary human L-FABP is a potential biomarker to predict COX-inhibitor-induced renal injury. Nephron Exp Nephrol. 2008;108:e19–e26. doi: 10.1159/000112912. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid-binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Sugaya T, Li S, Megyesi J, Nagothu K, Portilla D. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 2007;72:348–358. doi: 10.1038/sj.ki.5002304. [DOI] [PubMed] [Google Scholar]
- Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143:23–30. doi: 10.1016/j.lab.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Nagano N, Miyata S, Abe M, Kobayashi N, Wakita S, Yamashita T, Wada M. Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Int. 2006;69:531–537. doi: 10.1038/sj.ki.5000020. [DOI] [PubMed] [Google Scholar]
- Ataka K, Maruyama H, Neichi T, Miyazaki J, Gejyo F. Effects of erythropoietin-gene electrotransfer in rats with adenine-induced renal failure. Am J Nephrol. 2003;23:315–323. doi: 10.1159/000072913. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44:230–234. doi: 10.1159/000183992. [DOI] [PubMed] [Google Scholar]
- Doi K, Okamoto K, Negishi K, Suzuki Y, Nakao A, Fujita T, Toda A, Yokomizo T, Kita Y, Kihara Y, Ishii S, Shimizu T, Noiri E. Attenuation of folic acid-induced renal inflammatory injury in platelet-activating factor receptor-deficient mice. Am J Pathol. 2006;168:1413–1424. doi: 10.2353/ajpath.2006.050634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;67:2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Wyngaarden JB, Dunn JT. 8-Hydroxyadenine as the intermediate in the oxidation of adenine to 2,8-dihydroxyadenine by xanthine oxidase. Arch Biochem Biophys. 1957;70:150–156. doi: 10.1016/0003-9861(57)90088-7. [DOI] [PubMed] [Google Scholar]
- de Vries A, Sperling O. Implications of disorders of purine metabolism for the kidney and the urinary tract. Ciba Found Symp. 1977;48:179–206. doi: 10.1002/9780470720301.ch12. [DOI] [PubMed] [Google Scholar]
- Fye KH, Sahota A, Hancock DC, Gelb AB, Chen J, Sparks JW, Sibley RK, Tischfield JA. Adenine phosphoribosyltransferase deficiency with renal deposition of 2,8-dihydroxyadenine leading to nephrolithiasis and chronic renal failure. Arch Intern Med. 1993;153:767–770. [PubMed] [Google Scholar]
- Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T. Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis. 2001;38:473–480. doi: 10.1053/ajkd.2001.26826. [DOI] [PubMed] [Google Scholar]
- Fam AG. Gout in the elderly: clinical presentation and treatment. Drugs Aging. 1998;13:229–243. doi: 10.2165/00002512-199813030-00006. [DOI] [PubMed] [Google Scholar]
- Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925–934. [PubMed] [Google Scholar]
- Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs. 2004;64:2399–2416. doi: 10.2165/00003495-200464210-00003. [DOI] [PubMed] [Google Scholar]
- Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–1655. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- Fukunari A, Okamoto K, Nishino T, Eger BT, Pai EF, Kamezawa M, Yamada I, Kato N. Y-700 [1-[3-Cyano-4-(2,2-dimethylpropoxy)phenyl]-1H-pyrazole-4-carboxylic acid]: a potent xanthine oxidoreductase inhibitor with hepatic excretion. J Pharmacol Exp Ther. 2004;311:519–528. doi: 10.1124/jpet.104.070433. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Koide H. Effect of pioglitazone on urinary liver-type fatty acid-binding protein concentrations in diabetes patients with microalbuminuria. Diabetes Metab Res Rev. 2006;22:385–389. doi: 10.1002/dmrr.633. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Effect of pitavastatin on urinary liver-type fatty acid-binding protein levels in patients with early diabetic nephropathy. Diabetes Care. 2005;28:2728–2732. doi: 10.2337/diacare.28.11.2728. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- Tolkoff-Rubin NE, Rubin RH, Bonventre JV. Noninvasive renal diagnostic studies. Clin Lab Med. 1988;8:507–526. [PubMed] [Google Scholar]
- Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:904–912. doi: 10.1681/ASN.2006030221. [DOI] [PubMed] [Google Scholar]
- Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, Burczynski FJ. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- Kamijo-Ikemori A, Sugaya T, Obama A, Hiroi J, Miura H, Watanabe M, Kumai T, Ohtani-Kaneko R, Hirata K, Kimura K. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol. 2006;169:1107–1117. doi: 10.2353/ajpath.2006.060131. [DOI] [PMC free article] [PubMed] [Google Scholar]