Abstract
The aim of this study was to investigate functional reorganization of motor systems by probing connectivity between motor related areas in chronic stroke patients using functional magnetic resonance imaging (fMRI) in conjunction with a novel MR-compatible hand-induced, robotic device (MR_CHIROD). We evaluated data sets obtained from healthy volunteers and right-hand-dominant patients with first-ever left-sided stroke ≥6 months prior and mild to moderate hemiparesis affecting the right hand. We acquired T1-weighted echo planar and fluid attenuation inversion recovery MR images and multi-level fMRI data using parallel imaging by means of the GeneRalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) algorithm on a 3 T MR system. Participants underwent fMRI while performing a motor task with the MR_CHIROD in the MR scanner. Changes in effective connectivity among a network of primary motor cortex (M1), supplementary motor area (SMA) and cerebellum (Ce) were assessed using dynamic causal modeling. Relative to healthy controls, stroke patients exhibited decreased intrinsic neural coupling between M1 and Ce, which was consistent with a dysfunctional M1 to Ce connection. Stroke patients also showed increased SMA to M1 and SMA to cerebellum coupling, suggesting that changes in SMA and Ce connectivity may occur to compensate for a dysfunctional M1. The results demonstrate for the first time that connectivity alterations between motor areas may help counterbalance a functionally abnormal M1 in chronic stroke patients. Assessing changes in connectivity by means of fMRI and MR_CHIROD might be used in the future to further elucidate the neural network plasticity that underlies functional recovery in chronic stroke patients.
Keywords: Functional Magnetic Resonance Imaging (fMRI), brain, stroke
Introduction
Stroke is a major cause of morbidity and invalidity in modern society (Dobkin, 2005). In the United States alone, there are an estimated 731,000 incidents of stroke annually and 4 million stroke survivors, making stroke a major cause of long term disability (Broderick et al., 1998). Patients who survive a stroke usually recover at least some of the functionality compromised by the stroke within three months, however only 25% return to the level of daily physical functioning seen in community-matched persons who have not suffered a stroke (Lai et al., 2002). Thus, despite recent progress with thrombolysis (Hacke et al., 2008), recovery of motor function after stroke is usually incomplete.
In the past two decades, several investigators have attempted to understand the disturbances in the complicated cortical-subcortical network that occur following stroke as well as the mechanisms of stroke recovery. Since Chollet et al.’s demonstration of cortical reorganization using positron emission tomography (Chollet et al., 1991), subsequent studies using other imaging techniques, including integrated technologies, have extended our knowledge of the mechanisms that underlie functional and motor recovery in stroke patients (Cramer et al., 2000; Cramer et al., 2001; Calautti et al., 2003; Rossini et al., 2004; Tombari et al., 2004; Calautti et al., 2007; Loubinoux et al., 2007; Blickenstorfer et al., 2008; Dechaumont-Palacin et al., 2008). Recovery of motor function after stroke is thought to depend upon many factors, including resolution of edema and survival of the ischemic penumbra (Ward, 2007). Post-stroke brain plasticity includes change of function in existing synapses, synaptogenesis, cortical reorganization, and perhaps neurogenesis, and all these changes are stimulated by activity (Dietrichs, 2007).
While a causal link between cerebral reorganization and functional recovery has yet to be firmly established, brain imaging studies in chronic stroke patients have shown plastic changes, including enhanced bilateral activation of the sensorimotor cortex, increased activity in secondary or higher order sensorimotor areas, and recruitment of additional cortical areas during performance of a hand sensorimotor task (Weiller, 1998). Functional magnetic resonance imaging (fMRI) in particular has been described as a reliable method for assessing stroke patients (Kimberley et al., 2008). Nevertheless, the chronic stroke literature lacks functional integration studies examining functional reorganization of motor systems through assessment of altered connectivity between motor related areas.
Dynamic causal modeling (DCM) of fMRI data has been introduced as an approach to modeling the task-dependent influences that one area exerts over another, in order to infer connectivity strengths between activated areas within a distributed network (Friston et al., 1993; Friston et al., 2003). DCM, in contrast to other connectivity analysis methods such as structural equation modeling (SEM), accommodates the nonlinear and dynamic aspects of neuronal interactions by modeling neuronal activity explicitly using Buxton et al.’s balloon model (Buxton et al., 1998; Buxton et al., 2004). FMRI experiments have demonstrated strong temporal connections between spatially distinct but functionally related regions during rest in healthy brains (Biswal et al., 1995). Connectivity has also been described in patients with psychiatric disease (Schlosser et al., 2003; Schlosser et al., 2008). A recent DCM study indicated that hand motor disability following subacute stroke was associated with dysfunctional connectivity between ipsilesional and contralesional primary motor area (M1), and between ipsilesional supplementary motor area (SMA) and contralesional M1 (Grefkes et al., 2008). However, similar analysis has yet to be reported in patients with chronic stroke.
Here, we report an investigation of motor systems functional reorganization in which connectivity between motor related areas in chronic stroke patients was examined by combining fMRI with a novel MR-compatible hand-induced, robotic device (MR_CHIROD). We present findings suggesting that in chronic stroke the effective connectivity is altered due to neuroplasticity in the acute and subacute phases. Our findings are discussed in the contexts of compensation for a dysfunctional motor system and potential facilitation of rehabilitation using MR_CHIROD.
Methods
Study design and procedure
Twelve healthy volunteers and five chronic stroke patients provided written informed consent to participate in this cross-sectional study. All experiments were approved by the institutional review board at Massachusetts General Hospital and performed at the Athinoula A. Martinos Center for Biomedical Imaging. All participants used the MR_CHIROD during fMRI at approximately 45% of their maximum strength. Each subject’s maximum strength was determined a priori based on a dynamometer measurement of each participant’s maximum hand-grip strength. The fMRI protocol and data analysis methods employed are described below. The brain maps and connectivity strengths of stroke patients were compared to those of control subjects.
Patients
Patients (≥6 months after stroke) who agreed to be contacted for stroke recovery and rehabilitation studies were recruited through registries of stroke survivors maintained at Massachusetts General Hospital. Briefly, the inclusion criteria were first-ever left-sided ischemic subcortical middle cerebral artery (MCA) stroke confirmed by computed tomography (CT) or MRI ≥6 months prior; mild to moderate contralateral hemiparesis affecting the right hand; premorbid right hand dominance; ability to give written consent; and age 40–70 years. Exclusion criteria were defined by the NIH stroke scale questions (www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf) for decreased level of consciousness (questions 1a, 1b, 1c; each >0), aphasia (question 9; score >2), and neglect (question 11; score >2). No patient exhibited evidence of spasticity or joint stiffness while performing the motor task. Sensory modalities such as proprioception and ability to detect a pinprick and light touch were confirmed to be intact in the patients. No patient presented signs or history of somatosensory deficits of the right hand or other neurological or psychiatric disease, deafness and/or blindness, prior cerebrovascular disease, brainstem stroke, or multiple cerebral lesions.
Use of MR_CHIROD for MR neuroimaging
The design and testing of the first generation MR_CHIROD have been described in detail previously (Khanicheh et al., 2005; Khanicheh et al., 2006; Tzika et al., 2006), and the design of the second generation MR_CHIROD, was published recently (Khanicheh et al., 2008). Designs for two forms of the second-generation prototype were generated, one with rotary brakes and the other with linear brakes. The linear brake version was chosen for fabrication because of its relative simplicity and lower cost (Khanicheh et al., 2007). The assembled MR_CHIROD is shown in (Khanicheh et al., 2008). The MR_CHIROD consists of three major subsystems: a) an electro-rheological fluid (ERF) resistive element, b) handles, and c) two sensors, one of which is an optical encoder to measure patient-induced motion and the other of which is a force sensor. Each subsystem includes several components of varying complexity. All of the components were optimally designed with durability, safety, and MR-compatibility in mind and for the ability to withstand regular and high-stress testing.
The MR_CHIROD provides 200 N of resistive force and is controlled in real time (Khanicheh et al., 2008). It was empirically found that the maximum force of squeezing a dynamometer with fixed handles (Baseline® Hydraulic Hand Dynamometer, 200 lb, Best Priced Products, Inc.) is greater than the maximum force of dynamically squeezing the handles of the MR_CHIROD. Henceforth, maximum force of active squeezing has been estimated using the MR_CHIROD itself. Specifically, the MR_CHIROD was set at high values of resistive force and subjects were asked to squeeze. Maximum force was defined as the force at which subjects could barely complete one to three strokes of the MR_CHIROD. Patients’ maximum active squeezing force was 128 N ± 13 N (N = 5, male). For each patient, percent levels of applied force were calculated in Newtons using the patient’s own maximum force as a reference (100% of effort).
The MR_CHIROD was configured to securely attach to the scanner table next to the participant, who thus feels no weight. During testing, the MR_CHIROD’s power supply (Trek 609-C, ±4 kV / 20 mA) activating the ERF fluid (Fluidicon) and the DAQ unit (NI 6062-E) and laptop with control software (NI Labview) were located outside the RF-shielded MRI scanner room. The cables connecting the power supply and the sensors to MR_CHIROD were of appropriate length and impedance and properly shielded. The damper consisted of two electrodes and contained the ERF fluid. The piston (piston shaft drawn) moves through the ERF fluid with a controlled force of contraction provided by the voltage-controlled variable viscosity of the ERF fluid. A Faraday cage enclosed the device’s core, with an opening to allow unobstructed movement of the piston shaft. The negative electrode of the damper (connecting to the negative terminal of the power supply) and the Faraday cage were grounded to the penetration panel of the MR room. A low-pass filter (LPF) was attached to the penetration panel. Sensor readings (force, position) were transmitted through the penetration panel through grounded DSub-9 connectors. The sensor wires were coaxially shielded and all were properly grounded to the penetration panel. The sensor readings enabled real-time, closed-loop control of the ERF resistive element. The output from the control-loop regulated the voltage output of the power supply, in turn ensuring control of the ERF resistive element and force of contraction.
MR neuroimaging examination protocol
All studies were performed on a state-of-the-art 3-T MR system in order to obtain a high signal-to-noise ratio (SNR). We used a systematic approach to optimize our protocol with respect to SNR by varying the number of echoes, the echo time, the repetition time, the GeneRalized Autocalibrating Partially Parallel Acquisitions (GRAPPA) acceleration factor, the field of view (FOV), and the number of excitations (NEX). These factors were set such that the protocol could be completed in 30–45 min and a 12-channel Siemens Tim coil was used.
T1-weighted MR images
A high-resolution 3-dimensional T1-weighted, MP-RAGE (magnetization-prepared rapid gradient echo) image was acquired for anatomical reference and optimal gray-white matter contrast. Images were collected in a sagittal orientation at a 7° flip angle with the following parameters: TE = 4.73 ms; TR = 2530 ms; TI = 1100 ms; slice thickness = 1.00 mm; and matrix = 352 × 352 × 192.
Fluid attenuation inversion recovery (FLAIR) MR images
A FLAIR image was acquired for each subject to provide anatomical localization of hyperintense regions and stroke lesions. FLAIR images were acquired with the following parameters: GRAPPA factor 2, 0.6 mm × 0.8 mm in plane resolution / 288 × 384 matrix, 5 mm slice thickness, and TR/TE/TI = 10000 ms / 71 ms / 2500 ms.
Multilevel fMRI
A high-resolution GRAPPA EPI sequence was used for whole-brain BOLD fMRI near optimal spatial resolution for BOLD detection. Typical parameters were used: 96 × 96 matrix (2 mm × 2 mm in-plane resolution), 50 slices / 3 mm slice thickness, GRAPPA factor 3, 85 auto-calibration lines, BW/px = 1.3KHz/px, TR/TE = 3000 ms / 30 ms. Sixty volumes (180 s) were used in fMRI processing.
Motor hand paradigm
As the study participant squeezed the device, we monitored the changing levels of force and compared precise measures of compression force with features of brain activation. Compression, because of its potential as a marker of motor function in stroke patients and because it can be performed by patients with motor deficits (Brunnstrom, 1970), has been used in several clinical brain-mapping studies (Honda et al., 1997; Schlosser et al., 1997; Lee et al., 1998; Cramer et al., 2001). Our experimental paradigm consisted of alternating action and rest periods, of 30 s each. During the action period, the participant compressed the robotic device and released continuously at a rate of 0.5 Hz. The palm of the participant’s right hand rested on the stationary handle of the device and his or her four fingers on the moving handle. The squeezing motion was performed by flexing the four fingers; the thumb was kept in a natural rest position such that it was not flexed to form a fist nor used to push against the handle.
All participants selected were right-handed and used only the paretic hand. The participant’s squeezing rate was guided by a visual ‘metronome’ cue circle oscillating radially at 0.5 Hz frequency, projected on a neutral-background screen. A fixation cross was projected during the rest periods. The stimulus was implemented using the PsychoPhysics Toolbox (www.psychtoolbox.org/). Previous experience has shown that head movement increases at rates above 1.5 Hz. Meanwhile movement below 1.5 Hz is generally associated with less fatigue, but also with decreased signal. A squeezing rate of 0.5 Hz was a well-justified compromise, especially given that the study was performed at 3 T which provides a high SNR. Moreover, It was confirmed empirically that this rate allows all subjects to complete full squeezing strokes of the device at all force levels.
The paradigm trial lasted 180 s, consisting of 3 action and 3 rest periods. The force of the paradigm was set at 45% of each individual subject’s maximum force to ensure that all subjects could perform the required motor task. It has been our consistent experience that patients can perform the protocol at this portion of their own maximum force. The use of percent levels compensates for performance confounds by constraining between-subjects performance to be approximately the same (Poldrack, 2000). Training took participants approximately 15 min and was performed before scanning. Patients performed the paradigm at each force level with the paretic hand. Motion artifacts were minimized by placing foam rubber pads and straps across the forehead and the arms. Participants’ arms were kept extended at their sides and extra foam padding was placed at the elbow to dampen vibratory couplings between the magnet bore wall and the participant’s arm as well as between the participant’s arm and body. The pads also served to minimize elbow flexion and additional reflexive motion, and to minimize translational and rotational head motion (typical translational head motion is well under 1 mm, typically ranging from 0.1 to 0.4 mm for most subjects). Care was taken to restrain inadvertent movement or squeezing of the non-task-performing hand.
FMRI data analysis
A standard processing stream was employed, using SPM (www.fil.ion.ucl.ac.uk/spm). Images were corrected for timing (time-slicing correction), aligned and normalized to MNI152 space. Additionally, a mask of each patient’s stroke lesion was drawn and incorporated into the normalization step for that patient (Brett et al., 2001). Images were then smoothed with a 4×(voxel dimensions) anisotropic Gaussian kernel. The 4× kernel was chosen to maximize sensitivity through use of large smoothing kernels (Friston et al., 1996) while retaining ample spatial specificity of the original functional images. A temporal 4-s FWHM Gaussian filter was applied to account for temporal autocorrelations in the time series. The fMRI time-series were corrected for confounds such as the global mean and low-frequency components. The voxel-wise activation threshold was set at P < 0.05, corrected for multiple comparisons. The force trace of the MR_CHIROD was used as a covariate and provided accurate timing information for the onset and duration of the squeezing blocks, correcting for possible timing errors in subject performance.
Our bilateral areas of interest were the primary motor cortex (M1), the supplementary motor area (SMA), and the cerebellum (Ce). SMA was defined as the medial wall of the hemisphere from the top of the brain to the depth of the cingulate sulcus, with the posterior boundary considered to be halfway between the extension of the central and precentral sulci onto the medial surface and the anterior boundary considered to be the vertical line through the anterior commissure (Dassonville et al., 1998). The location of activation in stroke patients was checked in normalized space using the Wake Forest University Pickatlas tool (Lancaster et al., 2000; Maldjian et al., 2003) and the Montreal Neurological Institute (MNI) Space Utility (MSU; www.fil.ion.ucl.ac.uk/spm/ext/) in SPM.
Since our simple motor task has been analyzed in many occasions in the past and the motor areas revealed here are well known, we conducted random effects analysis (2nd-level fMRI analysis) to pinpoint the exact location of these areas (M1, SMA, and Ce) in our groups in order to use them as building blocks of our DCM network. Our subsequent statistical comparison was performed not voxel-wise but for ROI-based results for which the two groups (control, patient) are sufficient.
Connectivity analysis
DCM (Friston et al., 2003; Mechelli et al., 2003), as implemented in the SPM2 software package, was employed. The aim of DCM is to estimate, and make inferences about the influence that one neural system exerts over another and how this is affected by the experimental context. For a combined hemodynamic and neuronal model, different priors are implemented to enable Bayesian parameter estimation. The estimated underlying neuronal activity is then used for the state equation (Friston et al., 2003).
The DCM model employed here included M1 and SMA contralateral to the affected hand and Ce ipsilateral to the affects hand (Figure 1). These regions were chosen because they have been reported to be activated during visually guided movements (Ellermann et al., 1998), and because they are considered important for rehabilitation therapy of patients after stroke to improve their motor abilities (Blickenstorfer et al., 2008). As expected, all three of these regions were activated in all subjects in this study.
Figure 1.

DCM used in this study. The model for intrinsic connections has links between primary motor area (M1), supplementary motor area (SMA), and cerebellum (Ce). All possible connections between these areas were allowed to account for plasticity changes in the stroke group.
Activated cortical areas were identified by examining the coordinates of the activated clusters in the MNI152 space (Montreal Neurological Institute MNI152 template, based on the stereotactic coordinate system of Talairach and (Lancaster et al., 2000) against published coordinates (Hanakawa et al., 2002; Solodkin et al., 2004), and was further aided by overlaying labeled masks of Brodmann Areas, according to the WFU Pickatlas tool (http://www.fmri.wfubmc.edu/; Lancaster et al., 2000; Maldjian et al., 2003; Maldjian et al., 2004) on the functional data. The SMA was identified at (-2, -4, 60) to (-4, -4, 60) in the vicinity of Brodmann area 6 (medial). The M1 was identified at (-38, -16, 56) to (-44, -10, 66) in Brodmann area 4 at the level of the Precentral Gyrus, by inspecting the activated cluster in the M1 area and the coordinates of the most activated voxels as reported by SPM2 and the boundaries of Precentral Gyrus and Brodmann Area 4 according to WFU Pickatlas. Peak voxels in the Ce were identified from (-22, -52, -18) to (-26, -62, -20).
Regions of interest (ROIs) were defined around these coordinates, including all voxels within a 6 mm radius. The DCM model was set up on these ROIs according to standard SPM specification. We allowed all possible connections between the brain areas (i.e. bi-directional coupling between SMA and Ce; between M1 and Ce; and between SMA and M1.) to account for plasticity changes in the stroke group. We also allowed the Mot to connect to the SMA, which is the only region in the model responsible for motor planning. The cognitive input encoding the motor task (Mot) was represented by a step function (Figure 1). The DCM analysis was then run on these VOIs and connectivity strengths and posterior probabilities were calculated (Buchel et al., 1997; Fletcher et al., 1999; Schlosser et al., 2003).
Statistical analysis
Normality of variances in activated voxels was tested using the Shapiro-Wilks test in R software (R 2.5.1), (P = 0.94). Comparisons between the control group and the patient group were carried out with an ANOVA design with group (patient or control) and connectivity pathway as fixed factors and patient or control identity as random factors. The design was run in the SPSS mixed program. Post-hoc comparisons between patients and controls for each connectivity pathway were carried out using a Bonferroni test with correction for multiple comparisons. A P value of 0.05 was used as the criterion for statistical significance for all comparisons.
Results
Healthy control subjects and stroke patients underwent online brain mapping with fMRI using our novel MR_CHIROD prototype. We used a simple model (Figure 1) that includes activation of brain regions seen both in healthy controls and stroke patients (Figures 2 and 3).
Figure 2.

Activated brain regions in representative healthy controls and chronic stroke patients using MR_CHIROD during fMRI, and regions (volumes) of interest used for DCM definition. The primary motor area (M1, green color), supplementary motor area (SMA, red color), and cerebellum (Ce, blue color) were identified and the coordinates of activated clusters verified by extracting the cluster coordinates. L and R denote left and right respectively.
Figure 3.
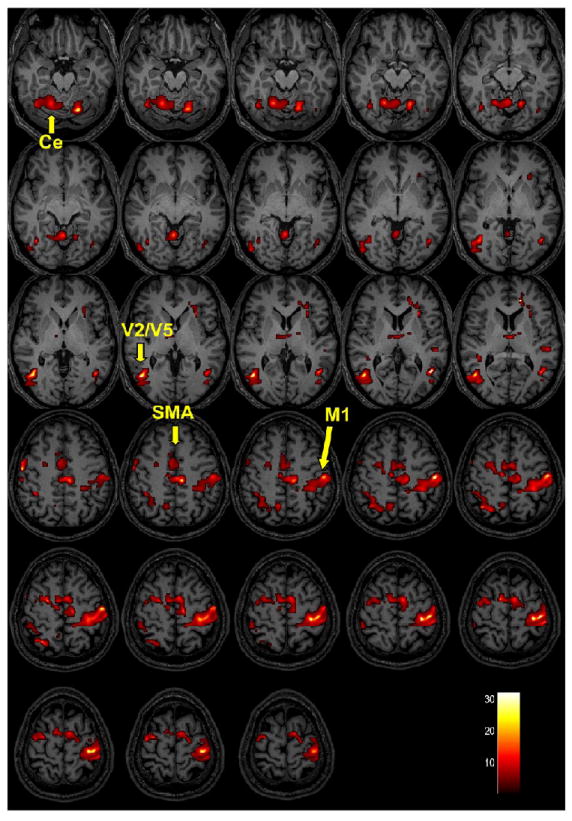

Group activations of brain regions in healthy controls and chronic stroke patients using MR_CHIROD during fMRI. Activation maps of healthy controls (Figure 3A) and chronic stroke patients (Figure 3B) are superimposed on T1-weighted anatomical image (group level, random effects analysis, activation threshold P < 0.01, uncorrected). The bar at the right bottom corner shows the t-score scale. Primary motor area (M1), supplementary motor area (SMA), cerebellum (Ce), visual areas (V2/V5). Note that M1 and Ce are less activated in stroke patients. The premotor cortex was not easily separated from M1 thus it is not labeled here.
The areas that were included in the DCM model specification were activated in both controls and patients when the subjects performed the task (Figure 2) and voxel-wise comparison (2-sample t-test) at the second (group) level did not show significant differences. Therefore, there is no potential problem in our analysis since the included of areas are activated in all subjects. DCM, while based on the fMRI analysis, is an intrinsically different analysis revealing different information, namely subtle effects relating to the coupling strength between activated areas. Therefore it is possible to see DCM differences even when voxel-wise comparison (2-sample t-test) at the second (group) level does not show differences between the activation patterns.
The DCM analysis of the specific impact of unimanual hand movement on the motor network is summarized in Table 1. These results show intrinsic neural coupling strengths for healthy controls and chronic stroke patients for our selected intrinsic model. Relative to controls, stroke patients exhibited reduced coupling between the cognitive input for the task, referred to as Mot, and motor planning in the SMA. M1 to SMA coupling was decreased in stroke patients, whereas SMA to M1 coupling was significantly increased (P = 0.09). Coupling of SMA to Ce was increased after stroke, while Ce to SMA coupling was significantly decreased (P = 0.04). Finally, both Ce to M1 and M1 to Ce coupling were decreased (P = 0.003 and 0.001, respectively).
Table 1.
Intrinsic neural coupling strengths in healthy controls and chronic stroke patients for the selected intrinsic model.
| Pathway | Controls (N = 11) | Patients (N = 5) | % change from controls b |
|---|---|---|---|
| Mot → SMA | 0.18 ± 0.03 a | 0.14 ± 0.01 | -22 |
| M1 → SMA | 0.64 ± 0.02 | 0.63 ± 0.08 | -2 |
| SMA → M1 | 0.17 ± 0.03 | 0.27 ± 0.07 | +59* |
| SMA → Ce | 0.12 ± 0.02 | 0.16 ± 0.02 | +33* |
| Ce → SMA | 0.41 ± 0.02 | 0.29 ± 0.05 | -29* |
| Ce → M1 | 0.40 ± 0.04 | 0.23 ± 0.02 | -43* |
| M1 → Ce | 0.55 ± 0.04 | 0.35 ± 0.06 | -36* |
values are means ± SE in Hz
values are percent (%) difference between patients and controls, while asterisks indicate statistical significance
Our DCM results are presented graphically in Figure 4. Note that all neural couplings between M1, SMA and Ce were affected by stroke. Of all the connection, the coupling between M1 and Ce was affected to a greater extent.
Figure 4.

Overview of pathways in which intrinsic neural coupling strengths in chronic stroke patients differed from those in healthy controls. Red arrows indicate an increase while blue arrows indicate a decrease.
Discussion
The present study demonstrates that fMRI using a novel hand device, MR_CHIROD, can detect functional connectivity disturbances in patients who have suffered a stroke, even when the stroke occurred six months or more prior. The study introduces a novel method of monitoring functional connectivity and confirms prior studies suggesting that recovery of function after stroke may be a consequence of central nervous system (CNS) reorganization and plasticity (Dietrichs, 2007). This method may be used to map functionally relevant adaptive changes in the human brain following CNS damage. It can thus lead to a greater understanding of how these changes are related to the recovery process, facilitate the development of novel therapeutic techniques designed to minimize CNS impairment, and assist in targeting these treatments to individual patients.
Our ability to control the resistivity and operability of ERF-controlled devices (Khanicheh et al., 2005; Khanicheh et al., 2006; Tzika et al., 2006; Khanicheh et al., 2007) may enable MR_CHIROD to assist in providing more sensitive, specific, and accurate brain mapping. Its MR-compatibility allows online brain function monitoring, which is an essential aspect of monitoring neurological disabilities associated with stroke recovery as well as recovery after brain tumor surgery, and monitoring of other CNS disorders including neurodegenerative disorders (e.g., Parkinson’s). Apart from patient monitoring, theoretically, it can also be used for monitoring muscle enhancement and augmentation in healthy individuals. The novelty of our device relies on its unconventional type of actuation, via electrorheological fluids (ERF), which change their viscosity in response to varying electric fields. This ERF-based device is unlike previously described devices (Siekierka et al., 2007; Tsekos et al., 2007) in that has been demonstrated to function in conjunction with fMRI for online brain mapping in chronic stroke patients. Furthermore, the MR_CHIROD readout provides information about the timing of the task (onset and duration of each squeezing cycle), as well as information about the amplitudes of each squeezing cycle, which enables MR_CHIROD information (force traces) to be used as covariates, resulting in much improved output (voxel-count) statistics at the group level. Finally, MR_CHIROD should also be a suitable form of robot-assisted rehabilitation for patients (Johnson et al., 2007).
Our parallel MRI (pMRI) approach to neuroimaging using GRAPPA is also novel (Pruessmann et al., 1999; Griswold et al., 2002; Heidemann et al., 2003). This method is not hindered by artifacts and is advantageous in high magnetic field conditions (Little et al., 2004; Pruessmann, 2004; Heidemann et al., 2006; Moeller et al., 2006) and we believe that our use of phased array coils further increased spatial and/or temporal resolution (Wald et al., 1995; Frederick et al., 1999; Mintzopoulos et al., 2007). Our results are in agreement with prior studies describing brain activation during visually guided movements (Deiber et al., 1991; Grafton et al., 1992; Clower et al., 1996; Kertzman et al., 1997; Ellermann et al., 1998). We demonstrate for the first time that an enhanced connectivity between M1 and SMA, and between SMA and Ce, underlies hand motor recovery following chronic stroke and this can be probably further enhanced by training. A dysfunctional coupling between M1 and Ce is a novel finding and is consistent with a prior study reporting decrease of bilateral cerebellar activation in recovering and clinically stable stroke patients (Tombari et al., 2004). On the other hand, cerebellar hyperactivity has been documented in Parkinson’s disease patients, where it was suggested to represent a compensatory mechanism for defective basal ganglia (Yu et al., 2007). Here, SMA seemed to recruit Ce, as indicated by an increased SMA to Ce coupling. This recruitment may compensate for reduced M1 to Ce coupling, which has a prominent role in visually guided movements (Glickstein et al., 1980; Stein et al., 1992; Ebner et al., 1997). Indeed, this may only be possible in patients with a preserved corticospinal system following a subcortical stroke (Ward et al., 2007). Furthermore, the adaptive activity within M1 and Ce over the course of task-specific therapy has been shown previously and supports our findings (Dong et al., 2007; Luft et al., 2008). Moreover, we have observed strengthened SMA to M1 and SMA to Ce couplings in chronic stroke patients following rehabilitation (unpublished observations). Hence it is our view that the strengthening of these couplings may help compensate for a dysfunctional M1.
Our findings extend a previous report on subacute stroke in which a dysfunctional M1 to SMA coupling was reported (Grefkes et al., 2008). Here in chronic stroke, an increased SMA to M1 coupling indicates recruitment of SMA to assist the M1 affected by stroke. This finding is in agreement with the hypothesis that there is reorganization within the SMA (Tombari et al., 2004). The SMA has been shown to be plastic under different circumstances. For example, SMA reorganization has been reported in healthy subjects trained with increasing force (Cramer et al., 2002) and in stroke patients asked to perform index-finger tapping (Cramer et al., 1997; Cramer et al., 2001). Indeed, enhancement of SMA activity, (i.e., by high-frequency transcranial magnetic or direct current stimulation) has been suggested as a potential means for ameliorating M1 dysfunction after stroke (Hummel et al., 2005a; Hummel et al., 2005b). Our findings suggest that spontaneous plasticity and rehabilitation after stroke in patients with minor corticospinal system damage can induce a similar effect, which might be enhanced by continuing rehabilitation in chronic stroke patients. Our study suggests that Ce may also be a target of rehabilitation since it is disconnected from M1. Strengthening the connectivity to SMA and/or M1 would be expected to benefit stroke patients. Of course this would require a preserved capacity for plasticity in chronic stroke patients.
Evidence of preserved capacity for plasticity in chronic stroke patients indicates that regional connectivity should be capable of modification and adjustment (Fasoli et al., 2003; Fasoli et al., 2004). Indeed, neuroimaging data in humans and corroborative neurophysiology studies in animals have shown considerable plasticity of M1 representations and cell properties related to motor skill learning and cognitive motor performance following pathological or traumatic changes (Sanes et al., 2000). The present findings support the notion of a plastic functional re-organization of SMA and M1 in adult mammals, which apparently results from broad connections and the capacity for activity-driven changes in synaptic strength (Sanes et al., 2000).
Conclusion
We propose that assessing effective connectivity by means of fMRI and DCM is useful for the evaluation of sensorimotor brain networks. Furthermore, investigating changes in connectivity caused by both brain injury and rehabilitative training will assist in monitoring and elucidating the physiology of functional recovery and neuroplasticity in patients who have suffered strokes. It is our view that brain fMRI using novel hand devices provides accurate monitoring and can be used in rehabilitation. We purport that this possibility is clinically relevant since it will allow caregivers to select the most appropriate rehabilitation approach for each patient and to fine-tune it based on brain maps obtained before and after a short trial of therapy.
Acknowledgments
This work was supported in part by a grant from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (Grant number R21 EB004665-01A2) to A. Aria Tzika. We thank Dr. Mavroidis the principal investigator of the subcontract to Northeastern University to build the MR_CHIROD. Finally we thank Ann Power Smith Ph.D. of Write Science Right for editorial assistance.
Footnotes
Presented in part during the 5th Annual World Congress for Brain Mapping and Image Guided Therapy of IBMISPS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blickenstorfer A, Kleiser R, Keller T, Keisker B, Meyer M, Riener R, Kollias S. Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–21. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- Brunnstrom S. Movement therapy in hemiplegia. Harper & Row; New York: 1970. Motor behavior of adult patients with hemi-plegia. [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex. 1997;7:768–78. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–64. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34:1553–66. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34:322–31. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature. 1996;383:618–21. doi: 10.1038/383618a0. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Bastings EP. Mapping clinically relevant plasticity after stroke. Neuropharmacology. 2000;39:842–51. doi: 10.1016/s0028-3908(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Schaechter JD, Kaplan JD, Finklestein SP, Rosen BR. A functional MRI study of three motor tasks in the evaluation of stroke recovery. Neurorehabil Neural Repair. 2001;15:1–8. doi: 10.1177/154596830101500101. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. Effects of movement predictability on cortical motor activation. Neurosci Res. 1998;32:65–74. doi: 10.1016/s0168-0102(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Dechaumont-Palacin S, Marque P, De Boissezon X, Castel-Lacanal E, Carel C, Berry I, Pastor J, Albucher JF, Chollet F, Loubinoux I. Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: an FMRI study. Neurorehabil Neural Repair. 2008;22:154–65. doi: 10.1177/1545968307307118. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Dietrichs E. Brain plasticity after stroke--implications for post-stroke rehabilitation. Tidsskr Nor Laegeforen. 2007;127:1228–31. [PubMed] [Google Scholar]
- Dobkin BH. Clinical practice. Rehabilitation after stroke. N Engl J Med. 2005;352:1677–84. doi: 10.1056/NEJMcp043511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Winstein CJ, Albistegui-Dubois R, Dobkin BH. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair. 2007;21:412–28. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner TJ, Fu Q. What features of visually guided arm movements are encoded in the simple spike discharge of cerebellar Purkinje cells? Prog Brain Res. 1997;114:431–47. doi: 10.1016/s0079-6123(08)63379-8. [DOI] [PubMed] [Google Scholar]
- Ellermann JM, Siegal JD, Strupp JP, Ebner TJ, Ugurbil K. Activation of visuomotor systems during visually guided movements: a functional MRI study. J Magn Reson. 1998;131:272–85. doi: 10.1006/jmre.1998.1379. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. 2003;84:477–82. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- Fasoli SE, Krebs HI, Stein J, Frontera WR, Hughes R, Hogan N. Robotic therapy for chronic motor impairments after stroke: Follow-up results. Arch Phys Med Rehabil. 2004;85:1106–11. doi: 10.1016/j.apmr.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Buchel C, Josephs O, Friston K, Dolan R. Learning-related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cereb Cortex. 1999;9:168–78. doi: 10.1093/cercor/9.2.168. [DOI] [PubMed] [Google Scholar]
- Frederick Bd, Wald LL, Maas LC, 3rd, Renshaw PF. A phased array echoplanar imaging system for fMRI. Magn Reson Imaging. 1999;17:121–9. doi: 10.1016/s0730-725x(98)00157-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–35. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F. Corticopontine visual projections in macaque monkeys. J Comp Neurol. 1980;190:209–29. doi: 10.1002/cne.901900202. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Woods RP, Phelps ME. Human functional anatomy of visually guided finger movements. Brain. 1992;115(Pt 2):565–87. doi: 10.1093/brain/115.2.565. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, Fink GR. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63:236–46. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–10. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H. The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex. 2002;12:1157–70. doi: 10.1093/cercor/12.11.1157. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Ozsarlak O, Parizel PM, Michiels J, Kiefer B, Jellus V, Muller M, Breuer F, Blaimer M, Griswold MA, Jakob PM. A brief review of parallel magnetic resonance imaging. Eur Radiol. 2003;13:2323–37. doi: 10.1007/s00330-003-1992-7. [DOI] [PubMed] [Google Scholar]
- Heidemann RM, Seiberlich N, Griswold MA, Wohlfarth K, Krueger G, Jakob PM. Perspectives and limitations of parallel MR imaging at high field strengths. Neuroimaging Clin N Am. 2006;16:311–20. xi. doi: 10.1016/j.nic.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Honda M, Nagamine T, Fukuyama H, Yonekura Y, Kimura J, Shibasaki H. Movement-related cortical potentials and regional cerebral blood flow change in patients with stroke after motor recovery. J Neurol Sci. 1997;146:117–26. doi: 10.1016/s0022-510x(96)00291-2. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005a;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005b;19:14–9. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Johnson MJ, Feng X, Johnson LM, Winters JM. Potential of a suite of robot/computer-assisted motivating systems for personalized, home-based, stroke rehabilitation. J Neuroeng Rehabil. 2007;4:6. doi: 10.1186/1743-0003-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertzman C, Schwarz U, Zeffiro TA, Hallett M. The role of posterior parietal cortex in visually guided reaching movements in humans. Exp Brain Res. 1997;114:170–83. doi: 10.1007/pl00005617. [DOI] [PubMed] [Google Scholar]
- Khanicheh A, Mintzopoulos D, Weinberg B, Tzika AA, Mavroidis C. MR_CHIROD v.2: A fMRI Compatible Mechatronic Hand Rehabilitation device. Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics; Noodwijk, The Netherlands. 2007. pp. 883–889. [Google Scholar]
- Khanicheh A, Mintzopoulos D, Weinberg B, Tzika AA, Mavroidis C. MR_CHIROD v.2: magnetic resonance compatible smart hand rehabilitation device for brain imaging. IEEE Trans Neural Syst Rehabil Eng. 2008;16:91–8. doi: 10.1109/TNSRE.2007.910286. [DOI] [PubMed] [Google Scholar]
- Khanicheh A, Muto A, Triantafyllou C, Astrakas LG, Mavroidis C, Tzika A. MR Compatible ERF-Based Robotic Device for Hand Rehabilitation After Stroke. Proc Intl Soc Mag Reson Med. 2005;13:1110. [Google Scholar]
- Khanicheh A, Muto A, Triantafyllou C, Weinberg B, Astrakas L, Tzika A, Mavroidis C. fMRI-compatible rehabilitation hand device. J Neuroengineering Rehabl. 2006;3:24–35. doi: 10.1186/1743-0003-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberley TJ, Khandekar G, Borich M. fMRI reliability in subjects with stroke. Exp Brain Res. 2008;186:183–90. doi: 10.1007/s00221-007-1221-8. [DOI] [PubMed] [Google Scholar]
- Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33:1840–4. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Jack CR, Jr, Riederer SJ. Mapping of the central sulcus with functional MR: active versus passive activation tasks. AJNR Am J Neuroradiol. 1998;19:847–52. [PMC free article] [PubMed] [Google Scholar]
- Little MW, Papadaki A, McRobbie DW. Proc Intl Soc Mag Reson Med. Kyoto; Japan: 2004. An investigation of GRAPPA in conjuction with fMRI of the occipital cortex at 3T; p. 1025. [Google Scholar]
- Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F. Prognostic Value of fMRI in Recovery of Hand Function in Subcortical Stroke Patients. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, Whitall J, McCombe-Waller S, Katzel L, Goldberg AP, Hanley DF. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke. 2008;39:3341–50. doi: 10.1161/STROKEAHA.108.527531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke H, Merboldt KD, Frahm J. The cost of parallel imaging in functional MRI of the human brain. Magn Reson Imaging. 2006;24:1–5. doi: 10.1016/j.mri.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: bottom-up or top-down mediation? J Cogn Neurosci. 2003;15:925–34. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mintzopoulos D, Astrakas LG, Zurakowski D, Wiggins GC, Wald LL, Rosen BR, Tzika AA. Optimized fMRI of Hand-Squeezing at 3T. Proc Intl Soc Mag Reson Med. 2007;15:3331. [Google Scholar]
- Moeller S, Van de Moortele PF, Goerke U, Adriany G, Ugurbil K. Application of parallel imaging to fMRI at 7 Tesla utilizing a high 1D reduction factor. Magn Reson Med. 2006;56:118–29. doi: 10.1002/mrm.20934. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues--a theoretical review. Neuroimage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP. Parallel imaging at high field strength: synergies and joint potential. Top Magn Reson Imaging. 2004;15:237–44. doi: 10.1097/01.rmr.0000139297.66742.4e. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–62. [PubMed] [Google Scholar]
- Rossini PM, Dal Forno G. Integrated technology for evaluation of brain function and neural plasticity. Phys Med Rehabil Clin N Am. 2004;15:263–306. doi: 10.1016/s1047-9651(03)00124-4. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schlosser MJ, McCarthy G, Fulbright RK, Gore JC, Awad IA. Cerebral vascular malformations adjacent to sensorimotor and visual cortex. Functional magnetic resonance imaging studies before and after therapeutic intervention. Stroke. 1997;28:1130–7. doi: 10.1161/01.str.28.6.1130. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–63. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schlosser RG, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage. 2008;43:645–55. doi: 10.1016/j.neuroimage.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Siekierka EM, Eng K, Bassetti C, Blickenstorfer A, Cameirao MS, Dietz V, Duff A, Erol F, Ettlin T, Hermann DM, Keller T, Keisker B, Kesselring J, Kleiser R, Kollias S, Kool JP, Kurre A, Mangold S, Nef T, Pyk P, Riener R, Schuster C, Tosi F, Verschure PF, Zimmerli L. New technologies and concepts for rehabilitation in the acute phase of stroke: a collaborative matrix. Neurodegener Dis. 2007;4:57–69. doi: 10.1159/000100360. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb Cortex. 2004;14:1246–55. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Stein JF, Glickstein M. Role of the cerebellum in visual guidance of movement. Physiol Rev. 1992;72:967–1017. doi: 10.1152/physrev.1992.72.4.967. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–39. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Tsekos N, Khanicheh A, Christoforou E, Mavroidis C. Magnetic Resonance-Compatible Robotic and Mechatronics Systems for Image-Guided Interventions and Rehabilitation: A Review Study. Annu Rev Biomed Eng. 2007;9:351–387. doi: 10.1146/annurev.bioeng.9.121806.160642. [DOI] [PubMed] [Google Scholar]
- Tzika A, Khanicheh A, Muto A, Triantafyllou C, Astrakas LG, Mavroidis C. Novel rehabilitation hand robots and fMRI in Stroke [Abstract] European Radiology. 2006;16(Supplement1):183. [Google Scholar]
- Wald LL, Carvajal L, Moyher SE, Nelson SJ, Grant PE, Barkovich AJ, Vigneron DB. Phased array detectors and an automated intensity-correction algorithm for high-resolution MR imaging of the human brain. Magn Reson Med. 1995;34:433–9. doi: 10.1002/mrm.1910340321. [DOI] [PubMed] [Google Scholar]
- Ward NS. Future perspectives in functional neuroimaging in stroke recovery. Eura Medicophys. 2007;43:285–94. [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Frackowiak RS, Thompson AJ, Greenwood RJ, Rothwell JC. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–73. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C. Imaging recovery from stroke. Exp Brain Res. 1998;123:13–7. doi: 10.1007/s002210050539. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]


