Abstract
Background and purpose:
Glucocorticoid-induced osteoporosis (GIO) is the leading cause of secondary osteoporosis. Clinical evidence suggests a role for genistein aglycone in the treatment of post-menopausal osteopenia although proof of efficacy in comparison with currently available treatments is still lacking. To clarify this issue, we investigated the effects of genistein on bone compared with alendronate in experimental GIO.
Experimental approach:
A total of 28 female Sprague-Dawley rats were used. GIO was induced by daily injections of methylprednisolone (MP; 30 mg·kg−1 s.c.) for 60 days. Sham GIO animals (Sham-MP) were injected daily with the MP vehicle. At the end of the osteoporosis development period, MP rats were randomized to receive: vehicle (n= 7), genistein aglycone (5 mg·kg−1 s.c.; n= 7) or alendronate (0.03 mg·kg−1 s.c.; n= 7). Treatment lasted 60 days. Sham-MP animals were treated with vehicle for an additional 60 days. At the beginning and at the end of treatments, animals were examined for bone mineral density and bone mineral content. Bone-alkaline phosphatase and carboxy-terminal collagen cross links were determined; femurs were removed and tested for breaking strength and histology.
Key results:
Genistein aglycone showed a greater increase in bone mineral density, bone mineral content and in breaking strength than alendronate and significantly increased bone-alkaline phosphatase (bone formation marker), reduced carboxy-terminal collagen cross links (bone resorption marker), compared with alendronate. Both treatments improved bone histology and the histological score.
Conclusion and implications:
The results strongly suggest that the genistein aglycone might be an alternative therapy for the management of secondary osteoporosis.
Keywords: genistein aglycone, glucocorticoid-induced osteoporosis, alendronate, b-ALP, CTX
Introduction
Bone loss arising from reduced oestrogen after menopause is responsible for the majority of reported cases of osteopenia and osteoporosis in women. The majority of bone loss occurs in the first 2 years after menopause (Lindsay and Cosman, 2003). As a consequence, vertebral fractures arise earlier in women compared with femoral fractures (Riggs and Melton, 1986). Osteoporosis is found in men, but develops at a lower rate with fractures occurring later in life compared with women (van Staa et al., 2005). There are also several secondary causes of osteoporosis, of which the most important is glucocorticoid-induced osteoporosis (GIO) (Devogelaer, 2006; van Staa, 2006; Woolf, 2007), a consequence of the widespread use of glucocorticoids for a variety of inflammatory conditions. Many studies have shown that glucocorticoids decrease bone mass and thereby increase the risk of fractures, particularly fractures of the ribs, spine and forearm. Studies have shown that 30–50% of all fractures occur in hospital settings, usually associated with administration of high doses of glucocorticoids (Adinoff and Hollister, 1983; Tsugeno et al., 2002; Kanis et al., 2005). The risk of hip, distal forearm and proximal humeral fractures are approximately double in rheumatoid arthritis patients treated with glucocorticoids, compared with patients without glucocorticoid therapy (Kung et al., 1999; Dolan et al., 2004). In addition, approximately 20% of patients on long-term glucocorticoid treatment in outpatient settings experience fragility fractures (Kotaniemi et al., 1996). A recent study identified 1.6 million oral glucocorticoid prescriptions over a 10 year period in the UK (Kung et al., 1999). The prevalence of oral glucocorticoid use was similar between men and women and was 0.9% of the total adult population, and use increased with age.
The susceptibility to bone loss may not be the same for all disorders for which glucocorticoids are used. Patients with end-stage chronic renal failure appear to be relatively resistant to skeletal effects of high-dose glucocorticoid therapy (Kanis et al., 2005), whereas younger patients, as well as transplant recipients, are highly susceptible to fracture (de Nijs et al., 2004). Finally, genetic variations of the enzyme 11β-hydroxysteroid dehydrogenase may modulate responsiveness to glucocorticoids and thus the risk of developing osteoporosis (Tomlinson et al., 2000; Cooper et al., 2002).
A wide variety of pharmacological interventions have been shown to decrease bone loss in GIO. Proposed treatments to help maintain or increase bone density include calcium supplementation, bisphosphonates, hormone replacement therapy, vitamin D in one of its many forms (cholecalciferol, calciferol, calcitriol, calcidiol, alfacalcidol), calcitonin, parathyroid hormone, fluoride, testosterone and anabolic steroids (Adachi et al., 1996; Ringe et al., 1999; Eastell et al., 2000; Boutsen et al., 2001; Crandall, 2002; Sambrook, 2007).
Several alternative therapeutic approaches have also been considered in recent years. For example, we previously have shown that treatment with pure genistein aglycone (54 mg·day−1) increased bone mineral density (BMD) at the lumbar spine and femoral neck in groups of post-menopausal women who were osteopenic or worse at baseline, with no clinically significant adverse effects on endometrium (Morabito et al., 2002; Crisafulli et al., 2004; Marini et al., 2007; Marini et al., 2008). In light of these observations, the present study aims to assess if genistein aglycone could be useful in the treatment of GIO and to assess how this compound compares in effectiveness with a commonly prescribed bisphosphonate, the therapeutic class now identified as the gold standard treatment for osteoporosis.
Methods
Animals
All procedures were evaluated and approved by the Ethics committee of the University of Messina and complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, MD, USA). A total of 28 female Sprague-Dawley rats (Charles River, Italy), aged 8 months and weighing about 250–275 g were used during the experiment. During the experiment, animals were housed in the Animal Facility of the Department of Clinical and Experimental Medicine and Pharmacology, maintained under controlled environmental conditions (12 h light/dark cycle, temperature approximately 24°C) and provided with standard food for laboratory animals and water ad libitum.
Induction of osteoporosis, randomization and treatments
One group of animals (n= 21) was injected daily with methylprednisolone (MP) (30 mg·kg−1 in 0.9% NaCl solution), subcutaneously for 60 days to produce GIO while a control group (n= 7) was injected daily with the MP vehicle (1 mL·kg−1, subcutaneously of a 0.9 % NaCl solution) as shown in Figure 1. The latter group served as controls for the experiment (sham-MP). Following MP or vehicle administration and before randomization all animals underwent BMD and bone mineral content (BMC) evaluation to confirm the presence of GIO. MP animals were then divided into three groups of seven animals each, as summarized in Figure 1, and randomized to the following treatments for 60 days: vehicle (VEH; 1 mL·kg−1 subcutaneously of a 10% DMSO/in 0.9% NaCl solution; n= 7), genistein aglycone (5 mg·kg−1; n= 7) or alendronate (0.03 mg·kg−1; n= 7). The Sham-MP animals were also treated with vehicle for an additional 60 days.
Figure 1.
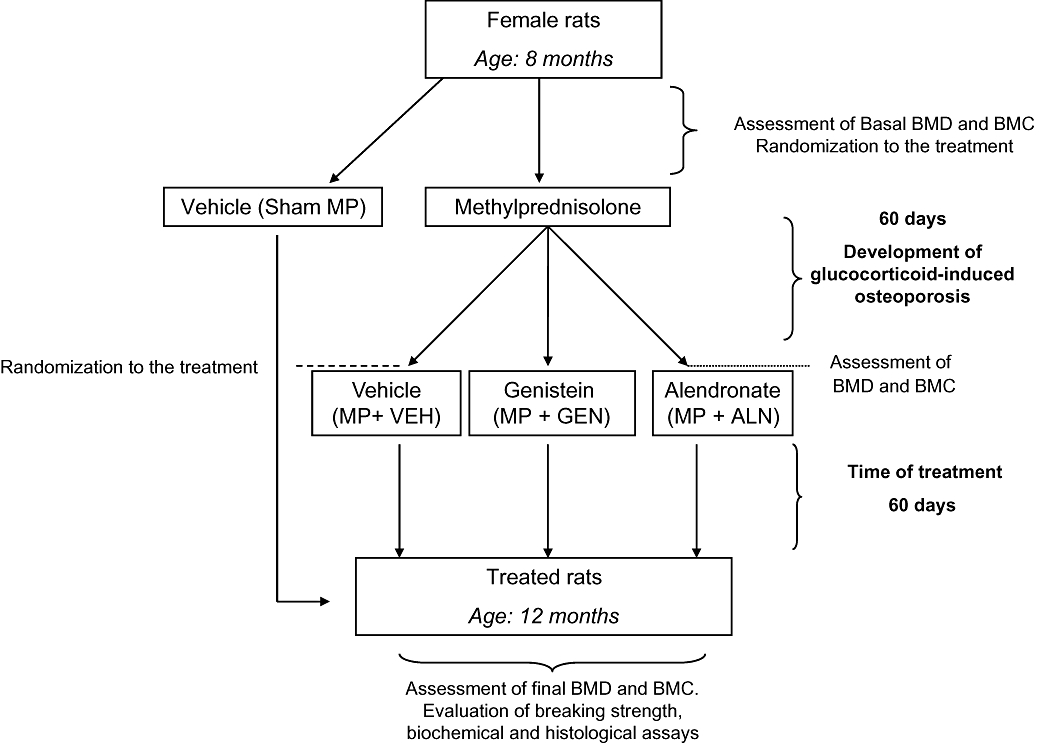
Flow chart of the experimental protocol. BMC, bone mineral content; BMD, bone mineral density; MP, methylprednisolone.
At the end of the treatment period, BMD and BMC measurements were taken. In addition, at the time of death, serum was collected to determine bone-alkaline phosphatase (b-ALP) and collagen C-telopeptides (CTX) levels. Right femurs were removed for histological examination, fixed in 10% neutral buffered formalin and stored. Left femurs were disarticulated and immediately tested for breaking strength assessment.
BMD and BMC
Bone mineral density and the relative BMC of the femurs were measured by using dual-energy X-ray absorptiometry (DEXA, Hologic QDR-4500A, Waltham, MA, USA). For basal and final measurements, animals were kept anaesthetized with sodium pentobarbital (50 mg·kg−1 i.p.). The rats were positioned in the middle of the measurement table and scans obtained in the high resolution mode. All animals were evaluated by the same technician and analysed by using the same method to minimize operational errors. Boxes were drawn to limit the area of interest and the BMD and BMC of proximal femurs obtained. During the analysis period, daily measurements were made for BMD and BMC following manufacturer's instructions, in order to assess the long-term reproducibility of the measured parameters (QC). A measured value of ±1.5% was taken as acceptable. Whenever two points obtained in succession were found outside the limits of the QC curve, the procedure was repeated. The coefficient of variation for femur BMD and BMC was 1.15% and 1.10% respectively. Moreover, accuracy of BMD and BMC final measurements were determined by duplicate scans of femurs.
Biochemical analysis
At the end of the study, animals were killed by anaesthesia with chloral hydrate (400 mg·kg−1 i.p.) and blood collected by cardiac puncture. Blood was centrifuged and serum stored immediately at −20°C for analysis. Commercially available elisa kits for b-ALP (IDS Ltd., UK) and CTX (Nordic Bioscience Diagnostics, DK) were then used to evaluate duplicate sera of each animal for bone formation and resorption respectively.
Femur breaking strength
Immediately after death, the maximum load (breaking strength) tolerated by femurs, expressed in Newtons (N), was measured on coded samples by using a calibrated tensometer (Sans). Femurs were placed horizontally, connected at each end by a two-point sample holder (15 mm span) with the anterior aspect facing up. The load was placed at the centre of the bone at a rate of 10.0 mm·min−1 until the bone fractured.
Histology
Histological analysis was performed by an investigator unaware of the treatments. For bone tissue collection, the leg of each animal was disarticulated at the hip, knee and ankle. Femurs were then removed and immediately fixed in 10% neutral buffered formalin. The femur was cleaned of soft tissue, placed in decalcifying solution [8% hydrochloric acid (37% v: v) and 10% formic acid (89% v: v) in phosphate-buffered saline] for about 24 h at 37°C, dehydrated in 95% (v: v) ethanol and then embedded in paraffin. Three, 5 µm thick paraffin-embedded horizontal bone sections were cut from the proximal end of the diaphysis, stained with haematoxylin and eosin and studied by using light microscopy. Femoral heads, the area between hip-joint cartilage and metaphyseal cartilage, were scored for general bone quality and trabecular density according to the scoring system in Table 1, as already published (Bitto et al., 2008). For qualitative image analysis, the metaphyseal area proximal to the growth plate, and the cortical bone below the hip-joint cartilage were used for imaging analysis. The growth plate was generally very thin in osteoporotic bones and thicker in bones from treated animals. Cartilage integrity evaluation was considered as an additional index of bone quality, because osteoporosis is in the end also responsible for cartilage deterioration, due to an enhanced osteoclastic activity as in rheumatoid arthritis (Herrak et al., 2004) Thus a treatment that restores bone integrity is indirectly also able to preserve a good trophism of the cartilage. The analysis was carried out by three pathologists unaware of the treatments.
Table 1.
Histological scoring of osteoporotic changes
| Hip-joint cartilage integrity | Structure of trabecular bone | Quantity of trabecular bone (% of interest area) | |
|---|---|---|---|
| Score 0 | Cartilage complete | Normal | 90–100% |
| Score 1 | Cartilage complete | Partially reduced | 60–90% |
| Score 2 | Cartilage partially complete | Markedly reduced | 30–60% |
| Score 3 | Cartilage absent | Absent | 0–30% |
Statistical analysis
All data are expressed as means ± standard deviation (SD). The significance of difference in BMD femoral neck and BMC was assessed by a two-way repeated measures anova followed by Tukey's multiple comparison test. For all other data, comparisons between different treatments were analysed by one-way anova followed by Tukey's multiple comparison test. In all cases, a probability error of less than 0.05 was selected as the criterion for statistical significance. Graphs were drawn by using GraphPad Prism (version 4.0 for Windows).
Drugs
Genistein aglycone was a gift of Primus Pharmaceuticals Inc. Alendronate, and MP was purchased from Sigma Aldrich, Italy. All substances were prepared fresh daily and administered in a volume of 100 µL.
Results
Effect of the treatments on femoral BMD and BMC
Following MP administration for 60 days, glucocorticoid-treated animals showed significant decreases in femoral neck BMD (0.245 ± 0.004 g·cm−2) and BMC (0.420 ± 0.003 g) compared with that for the Sham-MP animals (BMD = 0.270 ± 0.002 g·cm−2; P < 0.01 and BMC = 0.436 ± 0.001 g; P < 0.01). MP rats were then randomized to different treatment groups (Figure 1).
At the end of the treatment period, both genistein aglycone and alendronate increased BMD (Figure 2A) and BMC (Figure 2B) in GIO rats to roughly the same level as in to the vehicle-treated group. There was no significant difference observed between two treatment groups (Figure 2A,B).
Figure 2.
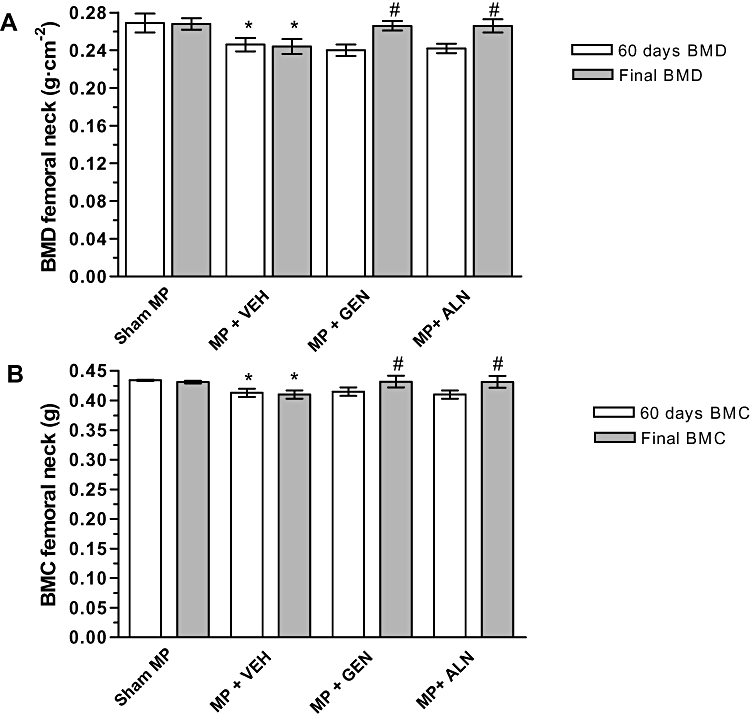
(A) Effects of alendronate (ALN) and genistein aglycone (GEN) on femoral bone mineral density (BMD) and (B) bone mineral content (BMC) in rats with glucocorticoid-induced osteoporosis (methylprednisolone, MP). Data are shown as the mean ± SD of seven animals. *P < 0.01 versus Sham-MP; #P < 0.005 versus MP + VEH (vehicle).
Effect of treatments on bone markers
At the end of the experiment, genistein aglycone significantly increased b-ALP (P < 0.001) compared with vehicle-and alendronate-treated groups (Figure 3A), suggesting a stimulation of osteoblast function. Serum b-ALP levels of the vehicle-treated group were found to be higher compared with the Sham-MP group (P < 0.05). Alendronate, known as an anti-resorptive compound, did not significantly affect serum b-ALP levels (Figure 3A).
Figure 3.
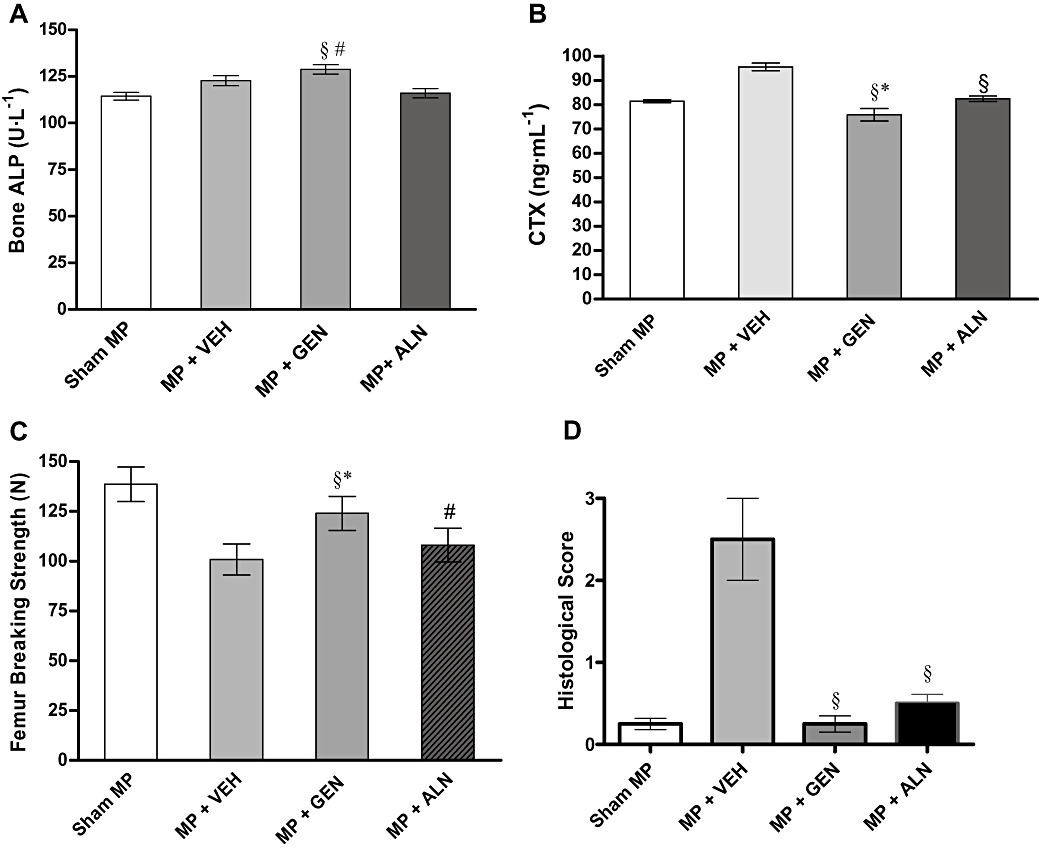
(A) Effects of alendronate (ALN) and genistein aglycone (GEN) on serum bone-alkaline phosphatase (b-ALP), (B) collagen C-telopeptides (CTX), (C) femur breaking strength and (D) histological score in rats with glucocorticoid-induced osteoporosis (methylprednisolone, MP). Data are shown as the mean ± SD of seven animals. b-ALP: §P < 0.05 versus MP + VEH (vehicle), #P < 0.001 versus MP + ALN. CTX: §P < 0.001 versus MP + VEH, *P < 0.05 versus MP + ALN. Femur breaking strength: §P < 0.001 versus MP + VEH, #P < 0.05 versus MP + VEH, *P < 0.05 versus MP + ALN. Histological score: §P < 0.001 versus MP + VEH.
Levels of plasma CTX were significantly higher in the vehicle-treated group compared with the Sham-MP group (P < 0.001). Both genistein aglycone and alendronate significantly reduced CTX plasma levels (P < 0.001 vs. MP + VEH). However, anova analysis showed that genistein aglycone lowered CTX levels more when compared with alendronate (P < 0.05; Figure 3B).
Effect of the treatments on the mechanical properties of the femur and on bone histology
In femoral strength tests, the vehicle-treated group had a significantly reduced breaking strength compared with Sham-MP rats (P < 0.001) (Figure 3C). Both genistein aglycone and alendronate improved the breaking strength of the femur. Genistein aglycone-treated animals, however, showed statistically improved femur strength compared with alendronate-treated animals (P < 0.05; Figure 3C).
After staining thin sections of the femoral heads, histological scoring showed that genistein aglycone reversed GIO more effectively than alendronate (Figure 3D) although both treatments were dramatically better than the vehicle control. Histological analysis revealed that the femoral heads collected from both genistein aglycone-and alendronate-treated rats had restored architecture of the cortical and trabecular structure with well-organized bone matrix (Figure 4). Histological staining and scoring both showed that genistein aglycone showed greater effects on bone structure compared with alendronate and correlated well with enhanced breaking strength of femurs subjected to a constant load.
Figure 4.
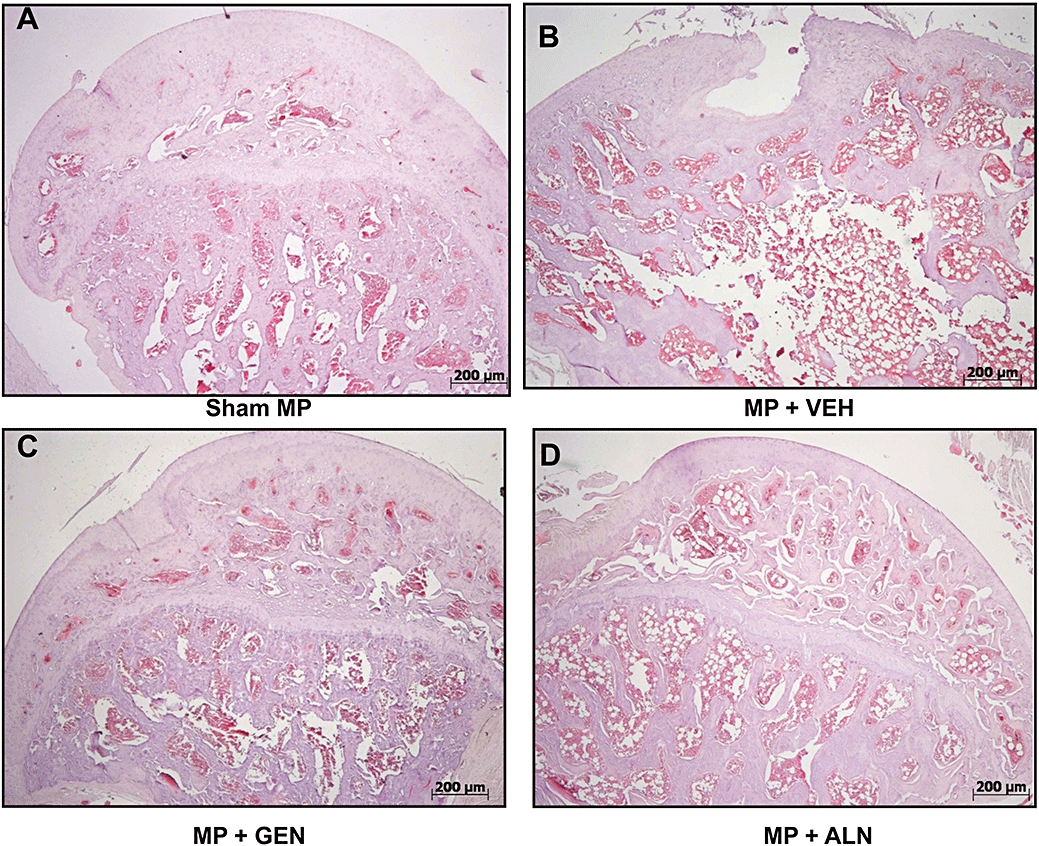
Light microscopy of the bone structure of the femur head taken from the different treatment groups. (haematoxylin and eosin stain; original magnification 5×). ALN, alendronate; GEN, genistein aglycone; MP, methylprednisolone; VEH, vehicle.
Discussion
An increasing amount of clinical evidence suggests a role for the isoflavone genistein aglycone in the treatment of post-menopausal bone loss (Albertazzi, 2002; Morabito et al., 2002; Crisafulli et al., 2004; Marini et al., 2007; Bitto et al., 2008; Marini et al., 2008). Proof of efficacy, however, in the treatment of GIO in comparison with currently available treatments is still lacking. In order to clarify this issue, we investigated the effects of genistein aglycone compared with alendronate in an experimental animal model of GIO.
The manner in which glucocorticoids induce bone loss is complex and incompletely understood (Patschan et al., 2001; Canalis et al., 2007), in part because there are no animal models absolutely comparable to humans. A major effect on the skeleton is a decrease in bone formation and unchanged or enhanced bone resorption (Lane, 2001). Glucocorticoid therapy results in rapid loss of BMD, which is greatest in the first year of therapy and may be as high as 30% or more in the first 3–6 months depending on dose (Eastell et al., 2000; Adachi et al., 2001). There is some evidence that these effects are partially reversible upon cessation of glucocorticoid treatments suggesting that bone retains the capacity to rebuild itself by virtue of having some residual osteoblast activity (Jia et al., 2006). Glucocorticoids are thought to directly affect the differentiation, activity and lifespan of osteoblasts and osteocytes (O'Brien et al., 2004). Glucocorticoids inhibit expression of genes important for bone formation including those responsible for the production of collagen A1, transforming growth factor-β, fibronectin-and insulin-like growth factor-1 (Iu et al., 2005). The exact reason for increased bone resorption is unclear, but might include relative immobility of people sufficiently ill to require glucocorticoid therapy, intestinal malabsorption of calcium and gonadal hormone deficiency. The mechanisms for bone resorption in glucocorticoid therapy have not been fully established, but include activation of important and relevant kinase systems (Horsch et al., 2007; Soares-Schanoski et al., 2007), increased production of receptor activator of nuclear factor-κB ligand (RANKL) and reduced production of osteoprotegerin, resulting in increased osteoclast recruitment and survival (Weinstein et al., 2002).
Histomorphometric analysis of biopsies from glucocorticoid-treated individuals has shown a reduction in bone formation at the cellular and tissue level, resulting in reduced bone volume and trabecular thickness (Dalle Carbonare et al., 2005) and a decrease in the number of viable osteocytes (O'Brien et al., 2004). There is also some evidence that glucocorticoids cause thinning of trabecular elements, in contrast to post-menopausal osteoporosis, where loss of trabeculae is more characteristic (Natsui et al., 2006). Higher doses of glucocorticoids are associated with an increase in bone turnover and resorption, leading to greater bone loss and disruption of cancellous bone architecture (Tomlinson et al., 2000; Cooper et al., 2002). Glucocorticoids also affect many other target tissues that in turn may have an impact on skeletal metabolism. These include reduced intestinal absorption and increased renal excretion of calcium (Reid and Ibbertson, 1987; Morris et al., 1990). Despite the complex pathophysiology associated with glucocorticoid treatment, the ultimate effect on bone is similar in many respects to post-menopausal osteoporosis. There is an imbalance between the amount of bone resorbed and that formed during each bone remodelling cycle and, in patients who are relatively immobilized, bone turnover is also increased.
The risk of fracture following use of glucocorticoids may not be related only to loss of bone tissue and the underlying disorder for which they are prescribed. Interestingly, the risk of fracture appears to increase rapidly upon exposure to glucocorticoids (van Staa et al., 2005; Devogelaer, 2006; Woolf, 2007). Risk of fracture, however, also remains elevated after stopping treatment (Morris et al., 1990). Similarly, we found in our experimental animal model of GIO that the cessation of glucocorticoid treatment was not sufficient to fully recover BMD nor to restore proper histological structure or bone resistance to rupture.
In our study, both the isoflavone, genistein aglycone, and the bisphosphonate, alendronate, succeeded in treating GIO in this experimental rat model: both increased BMD and bone breaking strength and ameliorated the histological damage caused by MP. Surprisingly, however, alendronate, a very useful drug for the prevention of fractures in post-menopausal women with osteoporosis, had a lesser effect on restoring bone quality compared with genistein aglycone. In addition, genistein aglycone induced a significant increase in serum b-ALP confirming its role as a bone forming agent. Genistein aglycone may act on de novo protein synthesis and on amplification of the interaction between the oestrogen receptor (ER) complex and nuclear DNA in osteoblasts. These cells express both ER-β and ER-α, but during the bone mineralization phase, ER-β is up-regulated significantly in bone tissue (Arts et al., 1997). Genistein aglycone may act specifically on trabecular bone by a mechanism involving ER-β during the bone mineralization phase (Kuiper et al., 1998).
The dose of genistein aglycone (5 mg·kg−1) administered to our experimental animals represents the approximate human equivalent dose of 54 mg·day−1, the same as was used in our recently reported clinical trial that showed that genistein aglycone is able to increase BMD and to promote bone formation also through the stimulation of the osteoprotegerin/sRANKL system in osteopenic, post-menopausal women (Marini et al., 2008). Genistein aglycone shows positive histological evidence of reducing bone and cartilage erosion. It has been reported that there is a correlation between subchondral bone loss and cartilage degradation (Lajeunesse and Reboul, 2003). Enhanced femoral breaking strength in GIO animals given genistein aglycone is also further proof that genistein acts to rebuild bone via a stimulation of osteoblast function. Indeed, although all pharmacological treatments succeeded in improving the breaking strength of the femur, only genistein aglycone caused a statistically significant increase in serum b-ALP, a crucial measure of bone reconstruction. In the MP + VEH group, replacing administration of MP with subsequent vehicle administration demonstrated that there was residual osteoblast activity demonstrated by a small increase in b-ALP. No such increase was observed in alendronate-treated animals suggesting a suppression of any remaining osteoblastic activity.
Genistein aglycone-treated animals showed restored and well-organized architecture of both cortical and trabecular bone matrix in femur head of osteoporotic rats. This correlates well with decreases in a critical bone resorption marker (CTX), increases in a bone formation marker (b-ALP) and enhanced resistance to fracture observed in femurs subjected to a constant load. Collectively, our results strongly suggest that the isoflavone genistein aglycone might be a new potential therapy for the management of GIO.
Usually, drugs used in management of osteoporosis have been classified as predominantly ‘anti-resorptive agents’ or as ‘bone-forming agents’. On the basis of the present results, however, genistein aglycone might represent a therapy with bone-forming as well as an anti-resorptive activity. The only other compound shown with such activity is strontium ranelate (Seeman et al., 2008). Strontium ranelate, which is only available in Europe, reduces the risk of vertebral fractures in patients with osteopenia. A recent publication, however, showed that strontium ranelate did not stimulate bone formation in ovariectomized rats (Fuchs et al., 2008). Clinical studies in humans, however, are needed to truly assess the efficacy of genistein aglycone in preventing or mitigating GIO.
Statement of conflicts of interests
B Burnett, R Levy, MA Armbruster are employees of Primus Pharmaceuticals, Inc., Scottsdale, Arizona, USA.
Glossary
Abbreviations:
- b-ALP
bone-alkaline phosphatase
- BMC
bone mineral content
- BMD
bone mineral density
- CTX
collagen C-telopeptides
- ER
oestrogen receptor
- MP
methylprednisolone
- sRANKL
soluble receptor activator of nuclear factor-κB ligand
References
- Adachi JD, Bensen WG, Bianchi F, Cividino A, Pillersdorf S, Sebaldt RJ, et al. Vitamin D and calcium in the prevention of corticosteroid induced osteoporosis: a 3 year follow up. J Rheumatol. 1996;23:995–1000. [PubMed] [Google Scholar]
- Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Adinoff AD, Hollister JR. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983;309:265–258. doi: 10.1056/NEJM198308043090502. [DOI] [PubMed] [Google Scholar]
- Albertazzi P. Purified phytoestrogens in postmenopausal bone health: is there a role for genistein? Climacteric. 2002;5:190–196. [PubMed] [Google Scholar]
- Arts J, Kuiper GG, Janssen JM, Gustafsson JA, Lowik CW, Pols HA, et al. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology. 1997;138:5067–5070. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- Bitto A, Burnett BP, Polito F, Marini H, Levy RM, Armbruster MA, et al. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and oestradiol. Br J Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutsen Y, Jamart J, Esselinckx W, Devogelaer JP. Primary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. J Bone Miner Res. 2001;16:104–112. doi: 10.1359/jbmr.2001.16.1.104. [DOI] [PubMed] [Google Scholar]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Rabbitt EH, Goddard PE, Bartlett WA, Hewison M, Stewart PM. Autocrine activation of glucocorticoids in osteoblasts increase with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- Crandall C. Parathyroid hormone for treatment of osteoporosis. Arch Intern Med. 2002;162:2297–309. doi: 10.1001/archinte.162.20.2297. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Altavilla D, Squadrito G, Romeo A, Adamo EB, Marini R, et al. Effects of the phytoestrogen genistein on the circulating soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin system in early postmenopausal women. J Clin Endocrinol Metab. 2004;89:188–192. doi: 10.1210/jc.2003-030891. [DOI] [PubMed] [Google Scholar]
- Dalle Carbonare L, Bertoldo F, Valenti MT, Zenari S, Zanatta M, Sella S, et al. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron. 2005;35:645–652. doi: 10.1016/j.micron.2005.07.009. Review. [DOI] [PubMed] [Google Scholar]
- Devogelaer JP. Glucocorticoid-induced osteoporosis: mechanisms and therapeutic approach. Rheum Dis Clin North Am. 2006;32:733–757. doi: 10.1016/j.rdc.2006.09.001. Review. [DOI] [PubMed] [Google Scholar]
- Dolan AL, Koshy E, Waker M, Goble CM. Access to bone densitometry increases general practitioners' prescribing for osteoporosis in steroid treated patients. Ann Rheum Dis. 2004;63:183–186. doi: 10.1136/ard.2003.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R, Devogelaer JP, Peel NF, Chines AA, Bax DE, Sacco-Gibson N, et al. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int. 2000;11:331–337. doi: 10.1007/s001980070122. [DOI] [PubMed] [Google Scholar]
- Fuchs RK, Allen MR, Condon KW, Reinwald S, Miller LM, McClenathan D, et al. Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporos Int. 2008;19:1331–1341. doi: 10.1007/s00198-008-0602-6. [DOI] [PubMed] [Google Scholar]
- Herrak P, Görtz B, Hayer S, Redlich K, Reiter E, Gasser J, et al. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004;50:2327–2337. doi: 10.1002/art.20384. [DOI] [PubMed] [Google Scholar]
- Horsch K, de Wet H, Schuurmans MM, Allie-Reid F, Cato AC, Cunningham J, et al. Mitogen activated protein kinase phosphatase 1/dual specificity phosphatase 1 mediates glucocorticoid inhibition of osteoblast proliferation. Mol Endocrin. 2007;21:2929–2940. doi: 10.1210/me.2007-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iu MF, Kaji H, Sowa H, Naito J, Sugimoto T, Chihara K. Dexamethasone suppresses Smad3 pathway in osteoblastic cells. J Endocrinol. 2005;185:131–138. doi: 10.1677/joe.1.05962. [DOI] [PubMed] [Google Scholar]
- Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. Review. [DOI] [PubMed] [Google Scholar]
- Kotaniemi A, Piirainen H, Paimela L, Leirisalo-Repo M, Uoti-Reilama K, Lahdentausta P, et al. Is continuous intranasal salmon calcitonin effective in treating axial bone loss in patients with active rheumatoid arthritis receiving low dose glucocorticoid therapy? J Rheumatol. 1996;23:1875–1879. [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kung AW, Chan TM, Lau CS, Wong RW, Yeung SS. Osteopenia in young hypogonadal women with systemic lupus erythematosus receiving chronic steroid therapy: a randomized controlled trial comparing calcitriol and hormonal replacement therapy. Rheumatology. 1999;38:1239–1244. doi: 10.1093/rheumatology/38.12.1239. [DOI] [PubMed] [Google Scholar]
- Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol. 2003;15:628–633. doi: 10.1097/00002281-200309000-00018. Review. [DOI] [PubMed] [Google Scholar]
- Lane NE. An update on glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am. 2001;27:235–253. doi: 10.1016/s0889-857x(05)70196-4. Review. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Cosman F. In: Atlas of Clinical Endocrinology: Osteoporosis. Stanley Korenman, Eric S Orwoll., editors. Philadelphia: Current Medicine Group LLC; 2003. Edited by. [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–847. doi: 10.7326/0003-4819-146-12-200706190-00005. [DOI] [PubMed] [Google Scholar]
- Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res. 2008;23:715–720. doi: 10.1359/jbmr.080201. [DOI] [PubMed] [Google Scholar]
- Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, et al. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- Morris HA, Need AG, O'Loughlin PD, Horowitz M, Bridges A, Nordin BEC. Malabsorption of calcium in corticosteroid-induced osteoporosis. Calcif Tissue Int. 1990;46:305–308. doi: 10.1007/BF02563820. [DOI] [PubMed] [Google Scholar]
- Natsui K, Tanaka K, Suda M, Yasoda A, Sakuma Y, Ozasa A, et al. High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int. 2006;17:105–108. doi: 10.1007/s00198-005-1923-3. [DOI] [PubMed] [Google Scholar]
- de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW. Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int. 2004;15:589–602. doi: 10.1007/s00198-004-1614-5. Review. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Patschan D, Loddenkemper K, Buttgereit F. Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone. 2001;29:498–505. doi: 10.1016/s8756-3282(01)00610-x. [DOI] [PubMed] [Google Scholar]
- Reid IR, Ibbertson HK. Evidence for decreased tubular reabsorption of calcium in glucocorticoid treated asthmatics. Horm Metab Res. 1987;27:200–204. doi: 10.1159/000180820. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., III Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Ringe JD, Coster A, Meng T, Schacht E, Umbach R. Treatment of glucocorticoid-induced osteoporosis with alfacalcidol/calcium versus vitamin D/calcium. Calcif Tissue Int. 1999;65:337–340. doi: 10.1007/s002239900708. [DOI] [PubMed] [Google Scholar]
- Sambrook PN. Anabolic therapy in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357:2084–2086. doi: 10.1056/NEJMe0706770. [DOI] [PubMed] [Google Scholar]
- Seeman E, Devogelaer JP, Lorenc R, Spector T, Brixen K, Balogh A, et al. Strontium ranelate reduces the risk of vertebral fractures in patients with osteopenia. J Bone Miner Res. 2008;23:433–438. doi: 10.1359/jbmr.071105. [DOI] [PubMed] [Google Scholar]
- Soares-Schanoski A, Gómez-Piña V, del Fresno C, Rodríguez-Rojas A, García F, Glaría A, et al. 6-Methylpresisolone down regulates IRAK-M in human and murine osteoclasts and boost bone resorbing activity: a putative mechanism for corticoid induced osteoporosis. J Leukoc Biol. 2007;82:700–709. doi: 10.1189/jlb.1106673. [DOI] [PubMed] [Google Scholar]
- van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2006;79:129–137. doi: 10.1007/s00223-006-0019-1. Review. [DOI] [PubMed] [Google Scholar]
- van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Bujalska I, Stewart PM, Cooper MS. The role of 11 beta-hydroxysteroid dehydrogenase in central obesity and osteoporosis. Endocr Res. 2000;26:711–722. doi: 10.3109/07435800009048591. [DOI] [PubMed] [Google Scholar]
- Tsugeno H, Tsugeno H, Fujita T, Goto B, Sugishita T, Hosaki Y, et al. Vertebral fracture and cortical bone changes in corticosteroid-induced osteoporosis. Osteoporos Int. 2002;13:650–656. doi: 10.1007/s001980200088. [DOI] [PubMed] [Google Scholar]
- Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, et al. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AD. An update on glucocorticoid-induced osteoporosis. Curr Opin Rheumatol. 2007;19:370–375. doi: 10.1097/BOR.0b013e328133f5c7. Review. [DOI] [PubMed] [Google Scholar]


