Abstract
Soil substrate membrane systems allow for microcultivation of fastidious soil bacteria as mixed microbial communities. We isolated established microcolonies from these membranes by using fluorescence viability staining and micromanipulation. This approach facilitated the recovery of diverse, novel isolates, including the recalcitrant bacterium Leifsonia xyli, a plant pathogen that has never been isolated outside the host.
The majority of bacterial species have never been recovered in the laboratory (1, 14, 19, 24). In the last decade, novel cultivation approaches have successfully been used to recover “unculturables” from a diverse range of divisions (23, 25, 29). Most strategies have targeted marine environments (4, 23, 25, 32), but soil offers the potential for the investigation of vast numbers of undescribed species (20, 29). Rapid advances have been made toward culturing soil bacteria by reformulating and diluting traditional media, extending incubation times, and using alternative gelling agents (8, 21, 29).
The soil substrate membrane system (SSMS) is a diffusion chamber approach that uses extracts from the soil of interest as the growth substrate, thereby mimicking the environment under investigation (12). The SSMS enriches for slow-growing oligophiles, a proportion of which are subsequently capable of growing on complex media (23, 25, 27, 30, 32). However, the SSMS results in mixed microbial communities, with the consequent difficulty in isolation of individual microcolonies for further characterization (10).
Micromanipulation has been widely used for the isolation of specific cell morphotypes for downstream applications in molecular diagnostics or proteomics (5, 15). This simple technology offers the opportunity to select established microcolonies of a specific morphotype from the SSMS when combined with fluorescence visualization (3, 11). Here, we have combined the SSMS, fluorescence viability staining, and advanced micromanipulation for targeted isolation of viable, microcolony-forming soil bacteria.
Microcultivation using the SSMS.
Soil (100 g) was collected 15 cm below the surface at Macquarie University and passed through a 2-mm-mesh sieve. Three replicates of the SSMS were set up as described previously (10, 12). The SSMS consists of a tissue culture insert that supplies nutrients in the form of soil extract to a polycarbonate membrane that contains the inoculum and also serves as a support for bacterial growth. After 7 days of incubation, mixed micro-CFUs (mCFUs) were visualized on a section of membrane by using SYBR green 11 staining and epifluorescence microscopy.
Immunofluorescent viability staining of microcolonies.
A LIVE/DEAD BacLight bacterial viability kit (L13152; Invitrogen, Mount Waverley, Victoria, Australia) was used as suggested by the manufacturer. This kit supplies applicator strips with SYTO9 and propidium iodide nucleic acid stains. For fluorescent staining, each membrane section was placed in an aliquot (100 μl) of a 1× concentration of BacLight staining solution for 10 min at room temperature. Membranes were washed twice by flotation on 200 μl 0.9% physiological saline for 5 min. Cells were visualized using a BX61 fluorescence microscope (Olympus, North Ryde, Australia) equipped with appropriate filters for SYTO9 and propidium iodide visualization. To confirm that there was no loss in cell viability after fluorescence staining, Escherichia coli cells were used as a control. An aliquot (200 μl) of E. coli cells (1 × 105/ml) was split, and one sample was stained with a 1× concentration of BacLight staining solution. Following two washes, stained and unstained E. coli cells (1 × 105/ml) were plated onto nutrient agar and colony formation was examined. No loss in E. coli viability was observed after BacLight staining.
Advanced micromanipulation and subcultivation of microcolonies.
Individual microcolonies of 10 μm in diameter, containing 50 to 1,000 cells, were targeted for isolation (Fig. 1). For isolation using an LMD6000 laser microdissection system (Leica, Sydney, NSW, Australia), a BacLight-stained membrane section was placed onto a glass slide. A section containing a live microcolony was isolated using a UV pulse laser. Excised regions fell into 0.5-ml Microfuge tubes containing 0.01× Ravan medium (12). For advanced micromanipulation, a BX51WIF epifluorescence microscope (Olympus, Sydney, Australia) equipped with a piezo-powered microdissector (Eppendorf, North Ryde, NSW, Australia) was used. A BacLight-stained membrane section was mounted onto 15 μl physiological saline on a glass slide. Aliquots of physiological saline (5 to 10 μl) were continually placed onto the edge of membranes to prevent them from drying out, with care being taken not to disturb the microcolonies. Live microcolonies were isolated using aspiration into sterile glass capillaries (TransferTips; Eppendorf) and transferred into a 96-well microtiter tray containing 200 μl 0.01× Ravan medium. Cultures were incubated at 22°C and subcultured every 7 days. After 3 subcultures, mCFUs were plated onto 0.01× Ravan agar. Pure colonies were obtained and identified using 16S rRNA gene PCR and sequencing (10).
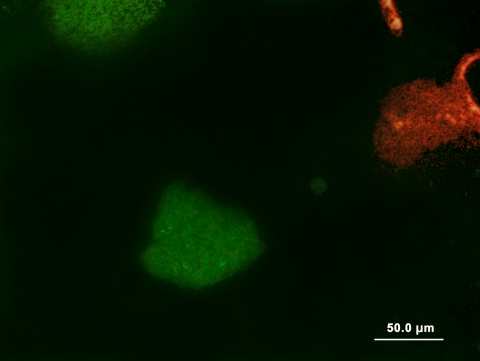
FIG. 1.
Fluorescence viability staining of microcolonies following microcultivation using the SSMS system. Live mCFUs (green) were easily differentiated from dead mCFUs (red) at a magnification of ×400. Differentiation of both live and dead cells enables specific morphotypes of mCFUs to be targeted for micromanipulation in growth membranes for subsequent cultivation in oligophilic media.
Isolation of live microcolony-forming soil bacteria from growth membranes.
After 7 days of incubation, live (green) and dead (red) mCFUs could be differentiated by epifluorescence microscopy (Fig. 1). We attempted to isolate live microcolonies, but the laser microdissection caused damage to the membranes, resulting in no cell viability. By comparison, no detrimental effects on viability were observed following fluorescence staining and micromanipulation in growth membranes. All isolated microcultures subsequently formed macroscopic colonies (Table 1).
TABLE 1.
Soil isolates recovered following fluorescence viability staining and advanced micromanipulation in the SSMS
| Isolate | Bacterial phylum (class) | Closest cultivated species (accession no.) | % 16S rRNA identity |
|---|---|---|---|
| mCFU17 | Proteobacteria (Alphaproteobacteria) | Novosphingobium subterraneum (AY752914.1) | 97 |
| mCFU23 | Actinobacteria | Lentzea waywayandensis (AF114813.1) | 99 |
| mCFU54 | Actinobacteria | “Arthrobacter boritolerans” (AB288059.1) | 98 |
| mCFU59 | Proteobacteria (Gammaproteobacteria) | Acinetobacter sp. (AB020207) | 97 |
| mCFU61 | Actinobacteria | Nocardioides jensenii (AF005006) | 97 |
| mCFU63 | Proteobacteria (Alphaproteobacteria) | Rhodopseudomonas palustris (EU882154.1) | 98 |
| mCFU65 | Actinobacteria | Leifsonia xyli (AJ717351.1) | 99 |
| mCFU68 | Proteobacteria (Alphaproteobacteria) | Rhodopseudomonas palustris (EU882154.1) | 98 |
Molecular analysis of pure cultures demonstrated that novel bacterial species were recovered, even within this pilot study (Table 1). 16S rRNA gene sequencing showed that four isolates exhibited high levels of similarity to bacteria from the Actinobacteria (97 to 99% similarity). While Actinobacteria are well represented by pure cultures, there is a great diversity of undescribed species within this phylum in Australian arid soils, warranting further investigation (18). Of particular interest was Leifsonia xyli (mCFU65), a slow-growing, small coryneform and gram-positive phytopathogen thought to be widespread in the environment but which had, until these experiments, never been directly isolated outside the host cell (6, 7, 31). The remaining isolates were most similar to bacteria within the Proteobacteria, including mCFU17, a putative novel species most similar to Novosphingobium subterraneum (Table 1).
Fluorescence has been widely applied to the differentiation of active and dead bacterial cells (2, 16, 17, 22). When combined with epifluorescence microscopy or flow cytometry, analysis of single cells can be performed (9, 16, 28). The BacLight fluorescence viability kit is a popular viability indicator, but the ongoing viability of bacterial cells following staining has not yet been reported. Here, the BacLight viability stain was used to successfully differentiate active and dead mCFUs, with no detrimental effects on downstream cell viability. It is important to note that the kit utilized here did not contain dimethyl sulfoxide as a cryoprotectant (13).
The SSMS is a powerful approach for the cultivation of recalcitrant soil bacteria (10, 12, 26). However, the method has been limited by the difficulty of isolating pure microcolonies (10). While flow cytometry offers the opportunity for high-throughout isolation of single cells, advanced microdissection combined with fluorescence viability staining offers an alternative approach that facilitates the isolation of established intact microcolonies. Moreover, it offers the opportunity to select specific bacterial morphotypes of interest and is applicable to a wide range of microbial ecology studies.
Nucleotide sequence accession numbers.
Partial 16S rRNA gene sequences were deposited into the GenBank database under the accession numbers FJ362389 (mCFU17), FJ362390 (mCFU23), FJ362391 (mCFU54), FJ362392 (mCFU59), FJ362393 (mCFU61), FJ362394 (mCFU63), FJ362395 (mCFU65), and FJ362396 (mCFU68).
Acknowledgments
This research was funded by a Macquarie University Research Fellowship and a Macquarie University Research and Development Grant.
We thank Meredith Wallwork of the Adelaide Microscopy Unit at the University of Adelaide for help with laser cutting microdissection and Tristrom Winsley of UNSW for help with epifluorescence microscopy.
Footnotes
Published ahead of print on 20 March 2009.
REFERENCES
- 1.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in-situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berney, M., F. Hammes, F. Bosshard, H.-U. Weilenmann, and T. Egli. 2007. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73:3283-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binnerup, S., J. Bloem, B. Hansen, W. Wolters, M. Veninga, and M. Hansen. 2001. Ribosomal RNA content in microcolony forming soil bacteria measured by quantitative 16S rRNA hybridisation and image analysis. FEMS Microbiol. Ecol. 37:231-237. [Google Scholar]
- 4.Bollmann, A., K. Lewis, and S. S. Epstein. 2007. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl. Environ. Microbiol. 73:6386-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner, R. F., M. Emmert-Buck, K. Cole, T. Pohida, R. Chuaqui, S. Goldstein, and L. A. Liotta. 1997. Laser capture microdissection: molecular analysis of tissue. Science 5342:1481-1483. [DOI] [PubMed] [Google Scholar]
- 6.Brumbley, S. M., L. A. Petrasovits, R. G. Birch, and P. W. Taylor. 2002. Transformation and transposon mutagenesis of Leifsonia xyli subsp. xyli, causal organism of ratoon stunting disease of sugarcane. Mol. Plant-Microbe Interact. 15:262-268. [DOI] [PubMed] [Google Scholar]
- 7.Brumbley, S. M., L. A. Petrasovits, S. R. Hermann, A. J. Young, and B. J. Croft. 2006. Recent advances in the molecular biology of Leifsonia xyli subsp. xyli, causal organism of ratoon stunting disease. Australas. Plant Pathol. 35:681-689. [Google Scholar]
- 8.Connon, S. A., and S. J. Giovannoni. 2002. High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl. Environ. Microbiol. 68:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey, H. M., and D. B. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol. Rev. 60:641-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari, B. C., S. J. Binnerup, and M. Gillings. 2005. Microcolony cultivation on a soil substrate membrane system selects for previously uncultured soil bacteria. Appl. Environ. Microbiol. 71:8714-8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari, B. C., N. Tujula, K. Stoner, and S. Kjelleberg. 2006. Catalyzed reporter deposition-fluorescence in situ hybridization allows for enrichment-independent detection of microcolony-forming soil bacteria. Appl. Environ. Microbiol. 72:918-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari, B. C., T. Winsley, M. Gillings, and S. Binnerup. 2008. Cultivating previously uncultured soil bacteria using a soil substrate membrane system. Nat. Protoc. 3:1261-1269. [DOI] [PubMed] [Google Scholar]
- 13.Glindemann, D., J. Novak, and J. Witherspoon. 2006. Dimethyl sulfoxide (DMSO) waste residues and municipal waste water odor by dimethyl sulfide (DMS): the north-east WPCP plant of Philadelphia. Environ. Sci. Technol. 40:202-207. [DOI] [PubMed] [Google Scholar]
- 14.Greene, K. 2002. Microbiology. New method for culturing bacteria. Science 296:1000. [DOI] [PubMed] [Google Scholar]
- 15.Harsch, M., K. Bendrat, G. Hofmeier, D. Branscheid, and A. Niendorf. 2001. A new method for histological microdissection utilizing an ultrasonically oscillating needle: demonstrated by differential mRNA expression in human lung carcinoma tissue. Am. J. Pathol. 158:1985-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewitt, C. J., and G. Nebe-Von-Caron. 2004. The application of multi-parameter flow cytometry to monitor individual microbial cell physiological state. Adv. Biochem. Eng. Biotechnol. 89:197-223. [DOI] [PubMed] [Google Scholar]
- 17.Hoefel, D., W. L. Grooby, P. T. Monis, S. Andrews, and C. P. Saint. 2003. Enumeration of water-borne bacteria using viability assays and flow cytometry: a comparison to culture-based techniques. J. Microbiol. Methods 55:585-597. [DOI] [PubMed] [Google Scholar]
- 18.Holmes, A. J., J. Bowyer, M. P. Holley, M. O'Donoghue, M. Montgomery, and M. R. Gillings. 2000. Diverse, yet-to-be-cultured members of the Rubrobacter subdivision of the Actinobacteria are widespread in Australian arid soils. FEMS Microbiol. Ecol. 33:111-120. [DOI] [PubMed] [Google Scholar]
- 19.Hugenholtz, P., B. M. Goebel, and N. R. Pace. 1998. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J. Bacteriol. 180:4765-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugenholtz, P., and G. W. Tyson. 2008. Microbiology: metagenomics. Nature 455:481-483. [DOI] [PubMed] [Google Scholar]
- 21.Janssen, P. H., P. S. Yates, B. E. Grinton, P. M. Taylor, and M. Sait. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl. Environ. Microbiol. 68:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joux, F., and P. Lebaron. 2000. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2:1523-1535. [DOI] [PubMed] [Google Scholar]
- 23.Kaeberlein, T., K. Lewis, and S. S. Epstein. 2002. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 296:1127-1129. [DOI] [PubMed] [Google Scholar]
- 24.Leadbetter, J. R. 2003. Cultivation of recalcitrant microbes: cells are alive, well and revealing their secrets in the 21st century laboratory. Curr. Opin. Microbiol. 6:274-281. [DOI] [PubMed] [Google Scholar]
- 25.Rappe, M. S., S. A. Connon, K. L. Vergin, and S. L. Giovannoni. 2002. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418:630-633. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen, L. D., C. Zawadsky, S. J. Binnerup, G. Øregaard, S. J. Sørensen, and N. Kroer. 2008. Cultivation of hard-to-culture subsurface mercury-resistant bacteria and discovery of new merA gene sequences. Appl. Environ. Microbiol. 74:3795-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simu, K., and Å. Hagström. 2004. Oligotrophic bacterioplankton with a novel single-cell life strategy. Appl. Environ. Microbiol. 70:2445-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stocks, S. M. 2004. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A 61:189-195. [DOI] [PubMed] [Google Scholar]
- 29.Stott, M. B., M. A. Crowe, B. W. Mountain, A. V. Smirnova, S. Hou, M. Alam, and P. F. Dunfield. 2008. Isolation of novel bacteria, including a candidate division, from geothermal soils in New Zealand. Environ. Microbiol. 10:2030-2041. [DOI] [PubMed] [Google Scholar]
- 30.Watve, M., V. Shejval, C. Sonawane, M. Rahalkar, A. Matapurkar, Y. Shouche, M. Patole, N. Phadnis, A. Champhenkar, K. Damle, S. Karandikar, V. Kshirsagar, and M. Jog. 2000. The ‘K’ selected oligophilic bacteria: a key to uncultured diversity? Curr. Sci. 78:1535-1542. [Google Scholar]
- 31.Young, A. J., L. A. Petrasovits, B. J. Croft, M. Gillings, and S. M. Brumbley. 2006. Genetic uniformity of international isolates of Leifsonia xyli subsp. xyli, causal agent of ratoon stunting disease of sugarcane. Australas. Plant Pathol. 35:503-511. [Google Scholar]
- 32.Zengler, K., G. Toledo, M. Rappe, J. Elkins, E. J. Mathur, J. M. Short, and M. Keller. 2002. Cultivating the uncultured. Proc. Natl. Acad. Sci. USA 99:15681-15686. [DOI] [PMC free article] [PubMed] [Google Scholar]