Abstract
Background
Trait negative affect has been implicated as a risk marker for cardiovascular disease, but the mechanisms underlying this association are uncertain.
Purpose
Our aim was to examine associations between trait measures of anger, hostility, depression, and anxiety with endothelial dysfunction via brachial artery flow-mediated dilation (FMD), an early indicator of cardiovascular disease.
Method
FMD was examined in 332 healthy older adults. Measures included Beck Anxiety and Depression Inventories, Cook–Medley Hostility Scale, and Spielberger State-Trait Anger Expression Inventory (Anger In, Anger Out, and Trait Anger).
Results
Mean age was 60.5±4.8 years; 83% of participants were Caucasian and 49% were female. FMD was greater in women compared to men (6.17% vs. 4.07%, p<0.001). Women reported significantly greater Anxiety (p<0.001), and men reported greater Hostility (p=0.004). In separate multivariable linear regression models controlling for cardiovascular risk factors, plus current hormone therapy for women, smaller FMD was associated with higher Anger In for women (β=− 0.222, p=0.04) and showed a trend with higher Hostility for men (β= −0.082, p=0.09).
Conclusion
Endothelial dysfunction, as indicated by less vasodilatation of the brachial artery, is positively associated with measures of hostility and anger suppression in healthy older adults. Thus, associations between negative affect and cardiovascular health may be apparent early in the disease process.
Keywords: Flow-mediated dilation, Endothelial dysfunction, Anger, Hostility, Depression, Anxiety
Introduction
There is strong prospective evidence linking negative affect and subsequent cardiovascular disease [1–11]. Chronic or dispositional anger and hostility [12], anxiety, and depression [13] may be likened to the effects of physiologic risk factors such as hypertension and obesity. Affective dispositions can be consider individually or as a group, showing both distinguishing factors and overlapping determinants [13, 14].
It is important to establish whether negative affect is related to early markers of subclinical disease prior to the onset of symptoms. One such marker is brachial artery flow-mediated dilation (FMD)—an easy to assess, non-invasive technique for evaluating endothelial dysfunction [15–17], a sign of cardiovascular disease found prior to the development of measurable atherosclerosis [18]. FMD is related to cardiovascular risk factors [19], provides a surrogate marker for health of the coronary arteries [20], and is an independent predictor of cardiac risk and events [21–23].
Studies examining associations between negative affect and FMD [24–29] have been limited by small sample sizes, the inclusion of a single gender, and/or use of a single predictor. Three small studies found impaired vasodilatation associated with concurrent clinical depression or a history of major depression [25–27] and another with high depressive symptoms in coronary heart disease patients [28]. A study looking at the effects of mental stress found an inverse association between hostility and FMD [29]. A study of postmenopausal women reported an inverse association between FMD and two psychosocial clusters, Anxiety/Depression and Type A/Anger [24].
Further investigation is warranted regarding associations between FMD and multiple measures of negative affect in large samples of healthy men and women, whose affective reports are unlikely to be biased by knowledge of poor health. Our study tested the hypothesis that depression, anxiety, anger, and hostility were associated with FMD in healthy older adults while controlling for traditional cardiovascular risk factors.
Methods
Participants were drawn from the Pittsburgh Healthy Heart Project, a 6-year observational study examining the bio-behavioral determinants of subclinical cardiovascular disease in healthy older adults [30–33]. Recruitment materials included advertisements and mass mailings for community dwelling adults age 50–70 years in the Pittsburgh metropolitan area (USA). After phone screening, 774 individuals completed an initial visit. Of these, 118 were excluded, including those on anti-hypertensive or lipid-lowering medication, those with a history of heart disease, stroke, diabetes requiring insulin, or other severe chronic disease (e.g., renal), those with cancer treatment in the past 6 months, pre/early peri-menopausal women (menses in past 6 months), and those with high systolic (SBP>180 mmHg) or diastolic blood pressure (> 110 mmHg) and/or excessive alcohol consumption (≥5 portions ≥3 times/week). Typical reasons for declining to participate (n=192) included inability to wear a portable blood pressure cuff for several days (e.g., body size, occupation) and time commitment of the study/protocol. We enrolled 464 adults from 1998 to 2000, of whom 332 completed a brachial artery scan. Participants provided informed written consent and were paid $100 for this portion. The Institutional Review Board at the University of Pittsburgh approved the study.
A nurse obtained medical history, anthropometric measures, and 12-h fasting blood draw 3 months prior to the brachial scan. Standard enzymatic assays used to measure total serum cholesterol, high-density lipoproteins, triglycerides, and glucose were completed at the Department of Epidemiology Nutrition Laboratory. Low-density lipoproteins were estimated by the Friedewald equation. Blood pressure was obtained after participants were seated for 30 min and calculated as the average of the second and third measurement. Height and weight were used to calculate body mass index (BMI: kg/m2), and waist circumference was measured at the narrowest width or at the level of the umbilicus (navel). Smoking was categorized as current, ever, and never.
Brachial arteries were examined using an ultrasound scanner (Toshiba SSA-270A and SAA-140A, Tustin, CA, USA) equipped with a 7.5- or 5.0-MHz linear array imaging probe. Scanning was completed by trained ultrasound technicians in a dimly lit room with participant in the supine position. There were no pre-scan restrictions (e.g., smoking, fasting) for participants. After 10 min of rest, a blood pressure cuff was placed distal to the right elbow with the arm extended perpendicular to the body. A single baseline brachial lumen diameter was computed as the distance between the lumen–intima interfaces 1–2 cm proximal to the antecubital fossa. The cuff was then inflated 30 mmHg above the participant’s SBP for 4 min. After cuff release, brachial artery images were obtained over 3 min and digitized for later scoring. At 30-s intervals, end-diastolic images from three separate cardiac cycles were averaged from 140 pixel-width measurements taken across 1 cm for 3 min. Absolute change in diameter was calculated as the peak (widest) diameter measure minus the baseline diameter, which was achieved 60 or 90 s after cuff release for 53% of subjects. FMD, the percentage change in brachial diameter, was calculated as the absolute difference divided by the baseline diameter times 100. An intra-class correlation of 0.72 evidenced good reproducibility of the equipment and technicians in our lab (for 20 women with a second scan within 7 months by technicians blinded to the prior scan) [24].
Negative affect was assessed by computer administration, using a number of self-report scales during several assessment sessions, occurring an average of 2 months before and after the measurement of FMD (range= 11 months prior to FMD to 9 months after FMD). The following scales were used: Beck Anxiety Inventory (21 items, severity of anxiety symptoms); [34] Beck Depression Inventory-II (21 items, symptoms of depression within the past week (rather than past 2 weeks as per scale protocol)); [35] Cook–Medley Hostility Scale (50 true/false items, trait cynicism and mistrust (where one item, “I commonly wonder what hidden reason another person may have for doing something nice for me,” was imputed from the other 49 for all participants because of an omission error on the computer presentation)); [36] and Spielberger State-Trait Anger Expression Inventory (44 items, angry temperament) [37] subscales of Anger In (extent to which feelings of anger are suppressed), Anger Out (extent to which feelings of anger are expressed), and Trait Anger (general feelings of anger/predisposition to find a large range of situations annoying). Items were summed within each scale such that a higher score indicated more frequent or intense negative affect. There were high positive correlations between the measures of negative affect [33]. For analyses, natural logarithm transformation was used for Anxiety, Depression and Trait Anger, which were positively skewed.
All analyses were imp lemented using SAS version 9.1 (SAS Institute, Cary, NC, USA). Baseline characteristics and univariate associations were evaluated via standard statistical methods.
Using general linear regression models, associations between negative affect, and FMD independent of traditional cardiovascular risk factors were examined. Models were stratified by sex because of gender differences in baseline diameter size, potential influences of hormone therapy on FMD [38], and to avoid constraining model associations by gender. Cardiovascular risk factors, chosen a priori based on FMD literature, were age, SBP, BMI and current smoking, and current hormone therapy use for women [15, 19, 22, 24, 38]. Each measure of negative affect was individually added as the independent variable to either the model for men or for women. We examined the change in amount of variance in data that was explained by the model and whether psychosocial measures were significantly associated with FMD. To verify results, regression analyses were evaluated with scatter plots, quadratic terms, model residuals, and influence diagnostics [39, 40].
Secondary analyses included examining interactions of negative affect with sex and hormone therapy separately and consideration of aggregate measures of negative affect. For presentation purposes, sex-specific tertiles of negative affect (low/medium/high) were examined in relation to FMD.
Results
Participants were 83% Caucasian, 49% female, and 76% married, and 76% had greater than a high school education. Fifty-two percent of the women were taking hormone therapy, and 7% of all participants were current smokers. In general, participants had normal lipids and blood pressure and reported low levels of negative affect with moderate variance (Table 1). FMD scores range from − 3.6% to 31.7%.
Table 1.
Participant characteristics
| All (N=332) | Men (n=168) | Women (n=164) | |
|---|---|---|---|
| Age (years) | 60±5 | 60±5 | 60±4 |
| Systolic blood pressure (mmHg) | 130±15 | 130±15 | 130±15 |
| Diastolic blood pressure (mmHg) | 80±9 | 82±9 | 79±9a |
| Total cholesterol (mg/dL) | 214±36 | 208±31 | 220±39b |
| High-density lipoproteins (mg/dL) | 54±16 | 47±12 | 61±16c |
| Low-density lipoproteins (mg/dL) | 132±33 | 131±28 | 133±38 |
| Triglycerides (mg/dL) | 119 [84, 170] | 125 [91, 189] | 113 [79, 158]a |
| Glucose (mg/dL) | 90 [86, 95] | 92 [87, 98] | 89 [84, 94]c |
| Waist circumference (cm) | 92±12 | 98±9 | 86±12c |
| Body mass index (kg/m2) | 27.9±4.7 | 28.0±3.4 | 27.8±5.8 |
| Current/former/never smokers (%) | 7/43/51 | 7/49/44 | 6/37/57 |
| Beck Anxiety Inventory (0–63) | 4 (0–33) | 3 [1, 5] | 5 [2, 8]c |
| Beck Depression Inventory II (0–63) | 3 (0–19) | 3 [1, 6] | 3 [1, 6] |
| Cook–Medley Hostility Scale (0–50) | 12 (2–35) | 13 [9, 18] | 11 [7, 15]b |
| Spielberger: Anger In (8–32) | 14 (8–29) | 14 [13, 16] | 14 [12, 17] |
| Spielberger: Anger Out (8–32) | 13 (8–23) | 13 [11, 15] | 13 [11, 15] |
| Spielberger: Trait Anger (10–40) | 15 (10–34) | 15 [13, 17] | 14 [13, 16] |
| Baseline brachial diameter (mm)d | 3.54±0.67 | 3.97±0.50 | 3.09±0.42c |
| Absolute change in diameter (mm) | 0.17±0.15 | 0.16±0.15 | 0.18±0.14 |
| Flow-mediated dilatation (%) | 5.11±4.82 | 4.07±3.96 | 6.17±5.38c |
Data are reported as mean ± standard deviation, median [inter-quartile range], median (minimum–maximum), or percentage
p<0.05
p<0.01
p<0.001 comparisons between men and women
Adjusted for height
Measures of blood pressure, lipids, glucose, body size, and ever smoking were not significantly associated with FMD in overall or sex-specific correlations. For men, current smokers had smaller vasodilatation (1.76%) than non-smokers (4.25%, p=0.004). For women, age showed a significant negative association with FMD (r=−0.25, p=0.001), and those on hormone therapy had greater vasodilatation (6.74%) than those not currently taking hormones (5.35%, p=0.095). There were negative correlation coefficients between FMD and Anger In (Spearman’s r = −0.18, p=0.02) for women and with Hostility (r = −0.14, p=0.06) and Trait Anger (r = −0.14, p=0.08) for men and positive associations between FMD and Anxiety (r=0.17, p=0.03) and Depression (r=0.13, p=0.08) for women. Remaining measures of negative affect showed no association in bivariate analyses.
In men, the base model (i.e., age, SBP, BMI, and current smoking) explained 3.0% variation in the data (with an Adjusted R2 of 0.005 or 0.5%; Table 2). Current smoking was inversely associated with FMD (β= −2.629, p=0.03), while other cardiovascular risk factors did not show a significant association (p>0.15). When measures of negative affect were added to this multivariable model in individual analyses, Hostility increased the Adjusted R2 to 0.017, hence explaining an additional 1.2% of the variance beyond the base model. Men with higher Hostility showed a trend for smaller FMD (β= −0.082, p=0.09). As estimated by the beta coefficient, a one-unit increase in Hostility was associated with a 0.08% unit decrease in brachial artery vasodilatation.
Table 2.
Multivariable generalized linear regression models of FMD for each negative affect measure by sex
| Individual models | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| β | CI | p value | R2 | β | CI | p value | R2 | |
| Base modela | 0.005 | 0.081 | ||||||
| Base+Anxietyb | −0.46 | −1.20, 0.29 | 0.23 | 0.008 | 0.67 | −0.53, 1.87 | 0.27 | 0.083 |
| Base+Depressionb | −0.21 | −0.96, 0.54 | 0.58 | 0.001 | 0.51 | −0.44, 1.45 | 0.29 | 0.081 |
| Base+Hostility | −0.08 | −0.18, 0.01 | 0.09 | 0.017 | 0.05 | −0.09, 0.19 | 0.52 | 0.077 |
| Base+Anger In | −0.09 | −0.27, 0.10 | 0.35 | 0.004 | −0.22 | −0.44, −0.01 | 0.04 | 0.119 |
| Base+Anger Out | −0.12 | −0.35, 0.12 | 0.33 | 0.004 | 0.12 | −0.17, 0.40 | 0.41 | 0.098 |
| Base+Trait Angerb | −1.89 | −4.84, 1.06 | 0.21 | 0.008 | 1.06 | −3.03, 5.15 | 0.61 | 0.095 |
β: parameter estimate (non-standardized), represents the change in FMD associated with a one-unit change in the measure of negative affect when controlling for factors in the base model; for those models in which the negative affect measure is transformed (i.e., Anxiety, Depression, Trait Anger), β represents the change in FMD for an unit increase in negative affect on the natural log scale. p value indicates whether the measure of negative affect was statistically significant after accounting for the base model covariates. R2: Adjusted R2, represents the variance explained by the whole model (base model+measure of negative affect)
FMD flow-mediated dilation, CI 95% confidence interval
Base model=age, SBP, BMI, and current smoker (p=0.03) for men, plus current hormone therapy in models for women (age, p<0.001); all other covariates p>0.15
Natural logarithm transformation (i.e., base e)
In women, the base model (including hormone therapy) explained 11.0% variation in the data (with an Adjusted R2 of 0.081 or 8.1%). Age was inversely associated with FMD (β= −0.358, p=0.0002), while other factors did not show a significant association. When measures of negative affect were individually added to this multivariable model, Anger In increased the Adjusted R2 to 0.119, hence explaining 3.8% of the variance beyond the base model. Women with higher Anger In had significantly smaller FMD (β= −0.222, p=0.04). As estimated by the beta coefficient, a one-unit increase in Anger In was associated with a 0.22% unit decrease in brachial artery vasodilatation.
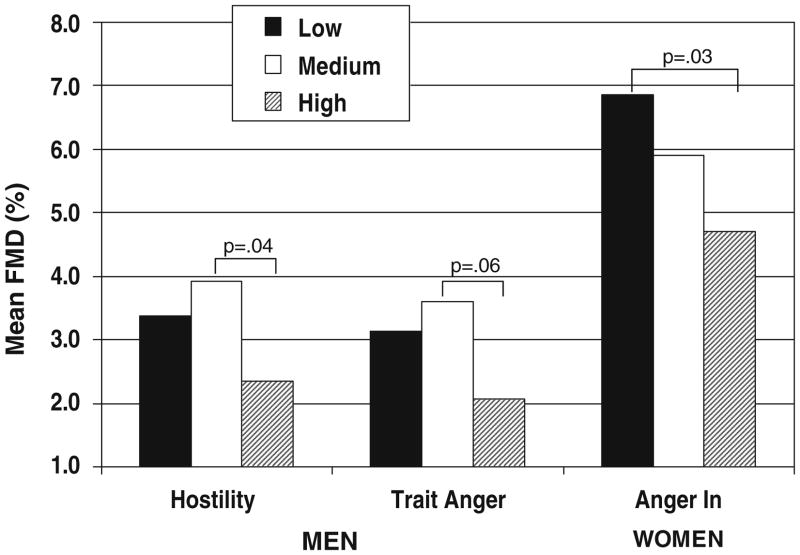
Secondary analyses showed no significant interaction effects between sex and negative affect. There were positive interactions between hormone therapy with Anxiety (p=0.04) and Anger In (p=0.099). Specifically, women not on hormone therapy showed a positive association between Anxiety and FMD (β=2.055, p=0.004), while women on hormone therapy showed an inverse association between Anger In and FMD (β= −0.409, p=0.02). Attempts to create aggregate measures of negative affect did not produce stronger associations. Figure 1 provides a graphical representation of the results, whereby vasodilatation decreases for specific measures of negative affect in men and women.
Fig. 1.
Mean flow-mediated dilation for select measure of negative affect. Mean percent of FMD adjusted for age, SBP, BMI, and current smoking, plus current hormone therapy for women displayed by tertiles of negative affect: men—Hostility score low = 2–10, medium = 11–16, high = 17–35; Trait Anger score low = 10–13, medium = 14–16, high = 17–27; Women Anger In score low = 8–13, medium = 14–16, high = 17–26
Discussion
After controlling for traditional cardiovascular risk factors in healthy older adults, we found smaller brachial artery vasodilatation in men with higher hostility and in women with higher anger suppression. Our research bolsters evidence of an inverse association between negative affect and cardiovascular health and implies that these links may be evident early in the disease continuum. We used a larger sample of both men and women than previous studies of negative affect and FMD, and our results are consistent with the possibility that negative affect may influence endothelial function in both men and women.
Our study supports prior evidence of a link between anger/hostility and subclinical cardiovascular disease. In postmenopausal women, Harris et al. found an inverse association between vasodilatation and an aggregate Type A/Anger measure, including individual associations with Trait Anger and Type A personality [24]. A small study in men and women found an inverse association between hostile affect and FMD [29]. Similarly, we found women with higher Anger In and men with higher Hostility showed less vasodilation. In previous work, hostility and anger show positive associations with other subclinical measures (e.g., carotid artery intima–media thickness (IMT) [5, 6], IMT progression [8, 9], coronary calcification [10]), and cardiovascular risk factors, events, and mortality [3, 4].
In contrast, we did not find an inverse association between FMD and Depression or Anxiety, as has been reported by others [24–28]. Three of these studies [25–27] looked at current or history of major depression, which was not evaluated in our sample, and one examined high depressive symptoms [28], thus possibly more indicative of chronic depression. In contrast, depression and anxiety in our sample was skewed toward lower scores, and unadjusted associations were in the opposite direction for women. Interestingly, in the large FMD study of healthy women [24], the individual scales of Trait Anxiety and Anger In, and not Depression or Social Support, were the measures of the Anxiety/Depression cluster that were specifically associated with smaller diameter change. Other studies of subclinical cardiovascular disease have reported a link with a history of two or more major depression episodes [11], but not with depressive symptoms [32]. Similarly, sustained anxiety was positively associated with IMT progression [7], but trait and general anxiety were not associated with IMT or IMT progression in previous research [9, 33]. Thus in early endothelial dysfunction and atherosclerosis, chronic anxiety/depressive symptomatology may be more influential than anxiety or depressive symptoms.
It may also be that anxiety/depressive symptomatology is linked with future morbidity or disease progression rather than concurrently measured subclinical cardiovascular disease [33]. Depression and anxiety are consistently noted following cardiac surgery [41], and associations between high depressive symptoms and FMD have been found in patients with established cardiovascular disease [28]. Notably, in our sample, we previously reported higher depressive symptoms related to 3-year change in carotid IMT [33], but not baseline IMT [32]. In the Harris et al. study [24], where an inverse association was found between anxiety/depression factor and FMD, scales were administered an average of 14 and 1.5 years prior to FMD measurement.
While overlap exists in negative affect constructs [13, 14, 42], we did not find a relationship between FMD and all measures. Attempts to create aggregate measures did not produce stronger associations. It is feasible that some of these scales, for example, the hostility/anger measures are more sensitive to the detection of mild symptoms. Levels of depression and anxiety in our participants were lower than other healthy groups, while anger measures were comparable.
Attitudinal or cognitive measures of hostility and anger might be more important correlates of endothelial dysfunction. Research examining trait anger scales via factor analyses showed Speilberger Anger In and components of the Cook–Medley scale loaded on the cynical cognition factor rather than behavioral aggression or angry affect factors [14]. People who score high on measures of Anger In and Hostility tend to ruminate a lot about others, but are not necessarily more physically aggressive or violent than others in the general population, and it may be this particular trait that is most congruent with FMD.
We reported gender variations in scales associated with FMD. This may have been due to the influence of hormone therapy [24, 38] or age on FMD in women, to the wider variance in respective scales for men and women, to the differential prevalence of endothelial dysfunction by gender, or to measurement “noise” in the construct of anger/hostility. We did not find a sex by negative affect interaction although our data indicate that hormone therapy may influence associations with FMD in women.
Psychosocial traits may influence cardiovascular disease via physiologic and behavioral pathways. Physiologic mechanisms include excessive sympathetic nervous system and hypothalamic–pituitary–adrenal axis activity [43, 44], potentially contributing to greater platelet activation [45], exaggerated cardiovascular reactivity [44], and systemic inflammation [46]. Behavioral pathways include links between negative affect and adverse health habits including increased smoking, alcohol use, sedentary behavior, and non-adherence to medical interventions [47–49]. Genetic influences may also play a role, predisposing both psychological factors and physiological vulnerabilities [42, 50].
FMD is a useful measure for evaluating the influences of negative affect on endothelial dysfunction. The vascular endothelium is critical in vascular growth, vasoprotection, and vasoregulation. FMD is thought to reflect nitric oxide bioavailability and the effects of shear stress on the endothelium [51]. FMD is impaired in patients with coronary artery disease [15] and improves with treatment [52]. A number of cardiovascular risk factors that have been associated with FMD in previous research [15, 19, 22] were not shown to be related in this study, a fact that may be attributed, in part, to the overall health of the sample.
Given the cross-sectional nature of our analyses, a causal relationship between vascular system dysfunction and psychosocial traits cannot be established. Furthermore, etiologic and behavioral predispositions not captured here underlie both vascular health and negative affect. Nonetheless, by evaluating healthy individuals, our study reduced some of the confounding influences that may impact such associations. In showing that the influence of negative affect on cardiovascular health is not limited to individuals with established cardiovascular disease, this study suggests that such associations may develop early in the disease process. Hostility, anger, depression, and anxiety are enduring and relatively stable psychosocial characteristics and as such are appropriate for examining influences on subclinical cardiovascular disease.
In conclusion, hostile, mistrustful attitudes, and a tendency to harbor unexpressed anger were shown to be associated with impaired vasodilatation of the brachial artery in healthy older adults. This study showed inverse associations between negative affect and cardiovascular health using FMD, confirms that such associations may be present for both men and women, and suggests that this connection may be apparent early in the disease process.
Acknowledgments
This research was funded by the National Heart Lung and Blood Institute (HL56346 and supplements) and the American Heart Association. We gratefully acknowledge the important contributions of the staff and study participants in time, data collection, and dedication to research.
Contributor Information
Laura L. Schott, Email: schottll@upmc.edu, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, A546 Crabtree Hall, Pittsburgh, PA 15261, USA
Thomas W. Kamarck, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA
Karen A. Matthews, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, A546 Crabtree Hall, Pittsburgh, PA 15261, USA, Department of Psychology, University of Pittsburgh, Pittsburgh, PA, USA, Department of Psychiatry, University of Pittsburgh/University of Pittsburgh Medical Center, Pittsburgh, PA, USA
Sarah E. Brockwell, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, A546 Crabtree Hall, Pittsburgh, PA 15261, USA
Kim Sutton-Tyrrell, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, A546 Crabtree Hall, Pittsburgh, PA 15261, USA.
References
- 1.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 2.Smith TW, Ruiz JM. Psychosocial influences on the development and course of coronary heart disease: current status and implications for research and practice. J Consult Clin Psychol. 2002;70:548–68. doi: 10.1037//0022-006x.70.3.548. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Gump BB, Harris KF, Haney TL, Barefoot JC. Hostile behaviors predict cardiovascular mortality among men enrolled in the Multiple Risk Factor Intervention Trial. Circulation. 2004;109:66–70. doi: 10.1161/01.CIR.0000105766.33142.13. [DOI] [PubMed] [Google Scholar]
- 4.Williams JE, Nieto FJ, Sanford CP, Couper DJ, Tyroler HA. The association between trait anger and incident stroke risk: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2002;33:13–9. doi: 10.1161/hs0102.101625. [DOI] [PubMed] [Google Scholar]
- 5.Bleil ME, McCaffery JM, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Anger-related personality traits and carotid artery atherosclerosis in untreated hypertensive men. Psychosom Med. 2004;66:633–9. doi: 10.1097/01.psy.0000138128.68838.50. [DOI] [PubMed] [Google Scholar]
- 6.Everson-Rose SA, Lewis TT, Karavolos K, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: the Study of Women’s Health Across the Nation (SWAN) Heart Study. Am Heart J. 2006;152:982–13. doi: 10.1016/j.ahj.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Paterniti S, Zureik M, Ducimetiere P, Touboul PJ, Feve JM, Alperovitch A. Sustained anxiety and 4-year progression of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:136–41. doi: 10.1161/01.atv.21.1.136. [DOI] [PubMed] [Google Scholar]
- 8.Raikkonen K, Matthews KA, Sutton-Tyrrell K, Kuller LH. Trait anger and the metabolic syndrome predict progression of carotid atherosclerosis in healthy middle-aged women. Psychosom Med. 2004;66:903–8. doi: 10.1097/01.psy.0000143638.31297.11. [DOI] [PubMed] [Google Scholar]
- 9.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Jansen-McWilliams L. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosom Med. 1998;60:633–8. doi: 10.1097/00006842-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Iribarren C, Sidney S, Bild DE, Liu K, Markovitz JH, Roseman JM, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. J Am Med Assoc. 2000;283:2546–51. doi: 10.1001/jama.283.19.2546. [DOI] [PubMed] [Google Scholar]
- 11.Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165:1229–36. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- 12.Kop WJ. Chronic and acute psychological risk factors for clinical manifestations of coronary artery disease. Psychosom Med. 1999;61:476–87. doi: 10.1097/00006842-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 14.Martin R, Watson D, Wan CK. A three-factor model of trait anger: dimensions of affect, behavior, and cognition. J Pers. 2000;68:869–97. doi: 10.1111/1467-6494.00119. [DOI] [PubMed] [Google Scholar]
- 15.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 16.Herrington DM, Fan L, Drum M, Riley WA, Pusser BE, Crouse JR, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–28. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 18.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–43. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–41. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 21.Gokce N, Keaney JFJ, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 22.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–97. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 23.Yeboah J, Sutton-Tyrrell K, McBurnie MA, Burke GL, Herrington DM, Crouse JR. Association between brachial artery reactivity and cardiovascular disease status in an elderly cohort: the cardiovascular health study. Atherosclerosis. 2008;197:768–76. doi: 10.1016/j.atherosclerosis.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris KF, Matthews KA, Sutton-Tyrrell K, Kuller LH. Associations between psychological traits and endothelial function in postmenopausal women. Psychosom Med. 2003;65:402–9. doi: 10.1097/01.psy.0000035720.08842.9f. [DOI] [PubMed] [Google Scholar]
- 25.Broadley AJM, Korszun A, Jones CJH, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–3. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am J Cardiol. 2001;88:196–8. doi: 10.1016/s0002-9149(01)01623-x. Erratum appears in Am J Cardiol 2001 Sep 15;88(6):722. [DOI] [PubMed] [Google Scholar]
- 27.Wagner JA, Tennen H, Mansoor GA, Abbott G. History of major depressive disorder and endothelial function in postmenopausal women. Psychosom Med. 2006;68:80–6. doi: 10.1097/01.psy.0000195868.68122.9e. [DOI] [PubMed] [Google Scholar]
- 28.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol. 2005;46:656–9. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercho-lesterolemia in subjects without cardiovascular disease. Am J Cardiol. 2003;92:687–91. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 30.Janicki DL, Kamarck TW, Shiffman S, Sutton-Tyrrell K, Gwaltney CJ. Frequency of spousal interaction and 3-year progression of carotid artery intima medial thickness: the Pittsburgh Healthy Heart Project. Psychosom Med. 2005;67:889–96. doi: 10.1097/01.psy.0000188476.87869.88. [DOI] [PubMed] [Google Scholar]
- 31.Kamarck TW, Janicki DL, Shiffman S, Polk DE, Muldoon MF, Liebenauer LL, et al. Psychosocial demands and ambulatory blood pressure: a field assessment approach. Physiol Behav. 2002;77:699–704. doi: 10.1016/s0031-9384(02)00921-6. [DOI] [PubMed] [Google Scholar]
- 32.Kamarck TW, Muldoon MF, Shiffman S, Sutton-Tyrrell K, Gwaltney C, Janicki DL. Experiences of demand and control in daily life as correlates of subclinical carotid atherosclerosis in a healthy older sample. Health Psychol. 2004;23:24–32. doi: 10.1037/0278-6133.23.1.24. [DOI] [PubMed] [Google Scholar]
- 33.Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–33. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 35.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio: Psychological; 1996. [Google Scholar]
- 36.Cook WW. Proposed hostility and Pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–8. [Google Scholar]
- 37.Spielberger CD, Jacobs GA, Russell SF, Crane RS. Assessment of anger: the State-Trait Anger Scale. In: Butcher JN, Spielberger CD, editors. Advances in personality assessment. Vol. 2. Hillsdale: LEA; 1983. [Google Scholar]
- 38.Herrington DM, Espeland MA, Crouse JR, III, Robertson J, Riley WA, McBurnie MA, et al. Estrogen replacement and brachial artery flow-mediated vasodilation in older women. Arterioscler Thromb Vasc Biol. 2001;21:1955–61. doi: 10.1161/hq1201.100241. [DOI] [PubMed] [Google Scholar]
- 39.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics. New York: Wiley; 1980. [Google Scholar]
- 40.Cook RD, Weisberg S. Residuals and influence in regression. London: Chapman and Hall; 1982. [Google Scholar]
- 41.Rymaszewska J, Kiejna A, Hadry T. Depression and anxiety in coronary artery bypass grafting patients. European Psychiatry: the Journal of the Association of European Psychiatrists. 2003;18:155–60. doi: 10.1016/s0924-9338(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 42.Raynor DA, Pogue-Geile MF, Kamarck TW, McCaffery JM, Manuck SB. Covariation of psychosocial characteristics associated with cardiovascular disease: genetic and environmental influences. Psychosom Med. 2002;64:191–203. doi: 10.1097/00006842-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Sherwood A, Hughes JW, Kuhn C, Hinderliter AL. Hostility is related to blunted beta-adrenergic receptor responsiveness among middle-aged women. Psychosom Med. 2004;66:507–13. doi: 10.1097/01.psy.0000132876.95620.04. [DOI] [PubMed] [Google Scholar]
- 44.Suarez EC, Kuhn CM, Schanberg SM, Williams RBJ, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosom Med. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Markovitz JH, Matthews KA, Kiss J, Smitherman TC. Effects of hostility on platelet reactivity to psychological stress in coronary heart disease patients and in healthy controls. Psychosom Med. 1996;58:143–9. doi: 10.1097/00006842-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, Sowers MF. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom Med. 2007;69:124–30. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- 47.Brummett BH, Babyak MA, Siegler IC, Mark DB, Williams RB, Barefoot JC. Effect of smoking and sedentary behavior on the association between depressive symptoms and mortality from coronary heart disease. Am J Cardiol. 2003;92:529–32. doi: 10.1016/s0002-9149(03)00719-7. [DOI] [PubMed] [Google Scholar]
- 48.Pulkki L, Kivimaki M, Elovainio M, Viikari J, Keltikangas-Jarvinen L. Contribution of socioeconomic status to the association between hostility and cardiovascular risk behaviors: a prospective cohort study. Am J Epidemiol. 2003;158:736–42. doi: 10.1093/aje/kwg204. [DOI] [PubMed] [Google Scholar]
- 49.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 50.Weidner G, Rice T, Knox SS, Ellison RC, Province MA, Rao DC, et al. Familial resemblance for hostility: the National Heart, Lung, and Blood Institute Family Heart Study. Psychosom Med. 2000;62:197–204. doi: 10.1097/00006842-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive post-menopausal women. J Am Coll Cardiol. 2002;40:505–10. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]