Abstract
Plants contain several genes encoding thioredoxins (Trxs), small proteins involved in the regulation of the activity of many enzymes through dithiol-disulfide exchange. In addition to chloroplastic and cytoplasmic Trx systems, plant mitochondria contain a reduced nicotinamide adenine dinucleotide phosphate-dependent Trx reductase and a specific Trx o, and to date, there have been no reports of a gene encoding a plant nuclear Trx. We report here the presence in pea (Pisum sativum) mitochondria and nuclei of a Trx isoform (PsTrxo1) that seems to belong to the Trx o group, although it differs from this Trx type by its absence of introns in the genomic sequence. Western-blot analysis with isolated mitochondria and nuclei, immunogold labeling, and green fluorescent protein fusion constructs all indicated that PsTrxo1 is present in both cell compartments. Moreover, the identification by tandem mass spectrometry of the native mitochondrial Trx after gel filtration using the fast-protein liquid chromatography system of highly purified mitochondria and the in vitro uptake assay into isolated mitochondria also corroborated a mitochondrial location for this protein. The recombinant PsTrxo1 protein has been shown to be reduced more effectively by the Saccharomyces cerevisiae mitochondrial Trx reductase Trr2 than by the wheat (Triticum aestivum) cytoplasmic reduced nicotinamide adenine dinucleotide phosphate-dependent Trx reductase. PsTrxo1 was able to activate alternative oxidase, and it was shown to interact with a number of mitochondrial proteins, including peroxiredoxin and enzymes mainly involved in the photorespiratory process.
Thioredoxins (Trxs) are ubiquitous small proteins with a molecular mass around 12 to 14 kD involved in the reduction of disulfide bonds of other proteins. Trxs contain a conserved active site WCG/PPC and a low redox potential that confers reductive properties, being able to regulate specifically target proteins (Laloi et al., 2001). Animals contain only two types of Trxs, one cytoplasmic and one mitochondrial, both well studied and involved in numerous regulatory mechanisms, such as the reduction of peroxiredoxins (Prxs), activation of transcription factors, and signaling of apoptosis (Arner and Holmgren, 2000). Plants contain several nuclear genes encoding Trx proteins (Gelhaye et al., 2005). In Arabidopsis (Arabidopsis thaliana), at least 20 Trx genes have been reported (Meyer et al., 2002), classified into six families, Trx f, Trx h, Trx o, Trx m, Trx x, and Trx y, and a novel Trx s, recently described in Medicago truncatula that expands this family of proteins in legumes (Alkhalfioui et al., 2008). The subcellular location of the different Trxs showed that the chloroplast contains Trx f, m, x, and y and the atypical form CDSP32 (Collin et al., 2003). A Trx o was shown to encode a mitochondrial protein using western-blot and in vitro uptake assays (Laloi et al., 2001), and an additional mitochondrial h-type Trx was discovered in poplar (Populus trichocarpa; PtTrxh2; Gelhaye et al., 2004).
Chloroplastic Trxs are reduced by ferredoxin in a reaction catalyzed by the ferredoxin-Trx reductase, while the cytoplasmic and mitochondrial Trxs are reduced by NADPH Trx reductase (NADPH/TR; Schürman and Jacquot, 2000). The chloroplastic and cytoplasmic Trxs have been widely studied, being implicated in the regulation of the Benson-Calvin cycle enzymes (Trxs f), modulation of the NADP-malate dehydrogenase (Trxs m), and redox control of cell proliferation, response to pathogens, and oxidative stress among others (Trxs h; López Jaramillo et al., 1997; Reche et al., 1997; Gelhaye et al., 2005).
Our understanding of mitochondrial Trxs in plants is limited; they have been described to be involved in redox regulation, and the alternative oxidase has been shown to be a specific target (Gelhaye et al., 2004). Among possible functions, the detoxification of reactive oxygen species via a mitochondrial Prx has been proposed. PrxIIF has been identified in the genome of Arabidopsis as the only Prx that is targeted to the plant mitochondria (Finkemeier et al., 2005), the poplar and pea (Pisum sativum) mitochondrial PrxIIF have been characterized, and a redox-dependent interaction between pea PrxIIF and mitochondrial Trx was recently demonstrated (Gama et al., 2007; Barranco-Medina et al., 2008).
The presence of Trx in the nucleus has been described in animal systems, but only under certain stress conditions where a translocation of cytoplasmic Trx to the nuclear compartment occurred (Hirota et al., 1997; Arai et al., 2006). Some biological functions described for Trxs in this compartment included the supply of reducing equivalents to ribonucleotide reductases and Prx (Laurent et al., 1964; Chae et al., 1994), the regulation of activity of several transcription factors (Hirota et al., 1997), and the control of apoptosis signal-regulated kinase 1 activity (Saitoh et al., 1998). In plants, apart from its cytoplasmic location, a Trx h has been described to accumulate in the nucleus of aleurone and scutellum cells of wheat (Triticum aestivum) seeds, a location associated with the oxidative stress during germination (Serrato et al., 2001; Serrato and Cejudo, 2003). Unlike the nucleoredoxins, the animal and plant Trxs accumulate in the nucleus, do not have a signal peptide characteristic of this subcellular location, and the presence of Trx in the nucleus of leguminous plants has not been described.
This article presents the isolation from pea leaves of a cDNA encoding a novel mitochondrial Trx o protein. Activity assays, subcellular localizations using western-blot analysis, immunolocalization, in vitro import assays, and GFP tagging indicate a mitochondrial and nuclear localization. An affinity protein-protein interaction assay revealed interactions with a variety of mitochondrial proteins, and the ability to regulate mitochondrial alternative oxidase (AOX) is also shown.
RESULTS
Cloning of a PsTrxo1 and Analysis of Its Amino Acid Sequence
The cDNA sequence encoding the pea Trx, named PsTrxo1, comprised 546 bp with a predicted open reading frame of 181 amino acids (Fig. 1A), a calculated molecular mass of 20.1 kD, and a pI of 9.58. Subcellular prediction programs predict a mitochondrial targeting peptide of 69 amino acids to be cleaved upon import to produce a protein with a calculated mass of 12.6 kD and a pI of 6.3. This N-terminal domain is characteristic of mitochondrial targeting sequences particularly enriched in Ser (Glaser et al., 1998; Schneider et al., 1998). The mature protein contains the classic sequence WCGPC in the active site and displays high sequence similarity to Arabidopsis Trxo1 (60%; Laloi et al., 2001) compared with poplar Trxh2 (28%; Gelhaye et al., 2002; Fig. 1A).
Figure 1.
Amino acid sequence analysis of PsTrxo1. A, Sequence alignment of pea PsTrxo1, Arabidopsis AtTrxo1, and poplar PtTrxh2 using the ClustalW software. Catalytic sites are underlined. The transit peptide predicted by the MitoProt program in PsTrxo1 is shown in italics, and the anti-PsTrxo1 antibody produced against the peptide is shown in boldface. B, Phylogenetic tree of Arabidopsis Trxs and Trx o homologs from selected plant species (TreeView program). Protein sequences were obtained from the database or deduced from cDNA or EST sequences. The three described mitochondrial Trxs are circled.
An analysis of genomic DNA revealed the absence of introns in the sequence, and to our knowledge, all other Trx genes characterized in plants contain at least one intron. Comparison of protein sequences with all Arabidopsis Trxs and other Trxs o of different plants as described by Laloi et al. (2001) resulted in the protein being classified as a Trx o (Fig. 1B). Database searches revealed the existence of several ESTs with high similarity in the protein sequences (more than 80%), indicating a similar type of Trx in other legumes.
Activity of Recombinant PsTrxo1 in Vitro
Recombinant PsTrxo1 protein was able to reduce the insulin-disulfide bridges using dithiothreitol (DTT) as reductant. The in vivo electron donor for the cytoplasmic and mitochondrial Trxs is the NADPH-dependent Trx reductase (Pedrajas et al., 2000). To check if this system is operating for PsTrxo1, we analyzed the in vitro activity of PsTrxo1 protein using two Trx reductases (wheat cytoplasmic NADPH/TR and Saccharomyces mitochondrial Trr2) in the insulin reduction assay (Fig. 2). PsTrxo1 reduced insulin disulfide in a NADPH-dependent process in vitro with both reductases, but the mitochondrial Trr2 was more efficient, demonstrating that this protein can form an active Trx system in this organelle.
Figure 2.
Activity of recombinant PsTrxo1 determined by the insulin-disulfide reduction assay. The reduction of insulin was measured by the increase in turbidity at 650 nm for 1 h at 30°C. Triangles, wheat NADPH/TR; circles, S. cerevisiae Trr2.
Subcellular Localization of PsTrxo1
Four sets of experiments were undertaken to determine the subcellular localization of PsTrxo1: western blot, immunogold labeling (Fig. 3), import assay of the precursor protein to mitochondria and chloroplasts, and GFP transient expression (Fig. 4). Additionally, using purified mitochondria, the partial purification of the native PsTrx protein was carried out by chromatography techniques (Fig. 5).
Figure 3.
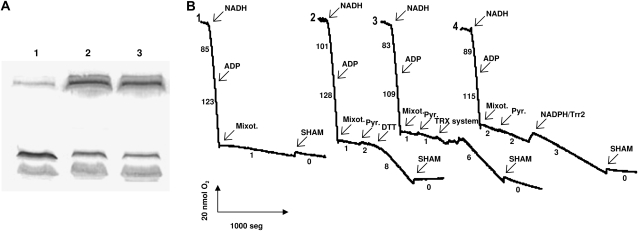
Subcellular location of PsTrxo1 in pea leaves. A, Western-blot analysis of recombinant PsTrxo1 (r), purified mitochondria (Mit.), nuclei (Nucl.), chloroplasts (Chl.), and cytoplasm (Cyt.) from pea leaves using the antibody generated against the peptide of the PsTrxo1 amino acid sequence indicated in Figure 1A. B, Immunolocalization of PsTrxo1 in pea leaves by transmission electronic microscopy. Left, Nonstained nucleus with detail below; right, mitochondria stained with lead citrate and uranyl acetate. The table shows the gold particles found in each cellular compartment. Eu., Euchromatin; Het., heterochomatin.
Figure 4.
Targeting of PsTrxo1 protein. A, Import of precursor PsTrxo1-35S into pea chloroplasts and potato mitochondria. Soybean AOX and pea small subunit of Rubisco (SSU) were used as controls. Lanes 1 and 4, Precursor protein alone; lane 2, precursor protein incubated with isolated chloroplasts; lane 3, as for lane 2 but with the addition of thermolysin after the import; lane 5, precursor protein incubated with isolated mitochondria; lane 6, as for lane 5 but with the addition of proteinase K after the import; lane 7, as for lane 5 but with the addition of valinomycin before the import; lane 8, as for lane 7 but with the addition of proteinase K after the import. Migration of the precursor (p) and mature (m) polypeptides is indicated. B, Subcellular location of PsTrxo1 protein in onion epidermal cells visualized by confocal microscopy. Epidermal onion layers were transiently transformed with the PsTrxo1 gene fused to GFP (panels 1–8) under the control of the 35S cauliflower mosaic virus promoter. After incubation for 24 h, cells were observed with a confocal microscope for emission spectrum of the GFP (panels 1 and 5). As a control for location, MitoTracker (panel 2) and DAPI staining (panel 6) as well as Nomarski observations (panels 4 and 8) were carried out. Panel 3 depicts the merged images from panels 1 and 2, and panel 7 depicts the merged images from panels 6 and 8. Arrows in panels 1 to 3 indicate mitochondria, and arrows in panels 5 to 7 indicate nuclei. As a control, the transformed cells with the reporter gene GFP (panels 9 and 10) are shown.
Figure 5.
Identification of the native mitochondrial PsTrxo1. A, Superdex 200 filtration chromatography of purified mitochondrial proteins. B and C, SDS-PAGE (B) and western-blot analysis (C) of recombinant PsTrxo1 (lane 1) and concentrated low molecular mass fractions indicated between arrows in A (lane 2). Molecular mass markers are shown in lane 3.
An antibody directed against a peptide of PsTrxo1, shown in Figure 1A, was used to analyze subcellular fractions of pea leaves. The anti-PsTrxo1 recognized the PsTrxo1 recombinant protein, and a band with an apparent molecular mass similar to that predicted (i.e. 12.6 kD) was detected in the mitochondrial fraction but not in the cytoplasmic and chloroplastic fractions (Fig. 3A). The absence of the 12.6-kD band in these latter fractions demonstrates that anti-PsTrxo1 antibody does not cross-react with cytoplasmic or chloroplastic Trxs. Nuclei were purified and analyzed by SDS-PAGE and tandem mass spectrometry (MS/MS) sequencing of the resulting proteins, finding as major proteins the histone H2A, histone H3, and histone H4 and some chloroplast proteins that represented minor contaminants (data not shown). In these nuclei, the antibody specifically detected a major band of an apparent molecular mass of 20.6 kD (Fig. 3A), corresponding to the PsTrxo1 protein translated from the cDNA outlined above (Fig. 1A), without any removal of the mitochondrial N-terminal targeting signal.
Immunogold Labeling Studies
To confirm the dual localization of PsTrxo1 in the pea leaves, an immunogold labeling study using anti-PsTrxo1 was performed. The results displayed high signal intensities in the nuclear heterochromatin and a signal of lower but high magnitude in mitochondria (Fig. 3B). A very low signal corresponding to chloroplasts and cytoplasm was also detected, with no signal detected from the cell wall or vacuole (data not shown). The intensities of the signals obtained from nuclei and mitochondria are in agreement with the western-blot analysis, where the intensity of the reaction with isolated nuclei was stronger. The quantification of this signal was carried out in 20 cells and is presented in the table in Figure 3B.
Import Assays
The mitochondrial nature of the protein was also verified by performing import assays to both pea chloroplasts and potato (Solanum tuberosum) mitochondria of PsTrxo1 precursor using Rubisco small subunit and AOX as chloroplastic and mitochondrial control proteins, respectively. When PsTrxo1 was translated in vitro, the product was expressed as a protein of 20.1 kD molecular mass (Fig. 4A). After incubation of PsTrxo1 precursor with mitochondria, a band of around 12.6 kD appeared, whereas no processed band was detected in the case of chloroplasts. This band is resistant to proteinase K, and the import was abolished by the addition of valinomycin, which dissipates the inner membrane potential. The mature PsTrxo1 protein obtained after in vitro import corresponds to the 12.6-kD protein immunodetected in pea mitochondria.
In Vivo Targeting Ability of PsTrxo1-GFP
To further analyze the targeting ability of PsTrxo1, a construct containing a translational fusion of its open reading frame to the GFP reporter gene under the control of the 35S promoter was used to bombard onion (Allium cepa) epidermal layers. As shown in Figure 4B, panels 1 and 5, the fluorescence emitted by the GFP reporter was targeted to the nucleus of bombarded cells and GFP signal was also detected in mitochondria, as shown by the merged fluorescences of GFP and MitoTracker (Fig. 4B3), further corroborating the presence of PsTrxo1 in both compartments. In contrast, the GFP fluorescence was distributed throughout the cells when the onion epidermal layers were bombarded with the GFP gene under the control of the 35S promoter (Fig. 4B9). DNA staining with 4′,6-diamidine-2-phenylindole (DAPI) was used as a control for nuclear location, and the corresponding blue fluorescence was detected in all cells with the exception of those where the presence of the PsTrxo1-GFP protein saturated the nucleus, making difficult the entrance of DAPI (Fig. 4B, panels 6 and 7).
Identification of Native PsTrxo1 in Pea Leaf Mitochondria
Detection of an organelle-specific protein requires the isolation of a great quantity of highly pure organelles, so intact functional mitochondria were obtained following a double gradient isolation method using optimum centrifugation techniques and washing cycles. The maximum obtained degree of chloroplastic, peroxisomal, and cytoplasmic contamination was about 0.01%, 0.07%, and 0.04%, as judged by NADP-dependent glyceraldehyde 3-phosphate dehydrogenase, catalase, and lactate dehydrogenase activities, respectively, and the intactness of the outer mitochondrial membrane was 85% to 90%.
Mitochondria isolated from pea leaves exhibited Trx activity measured as the capacity of induction of the NADP-malate dehydrogenase (MDH; specific activity of about 30–35 units mg−1 protein). Twenty-five mitochondria isolation experiments of 50 g of pea leaves each were used for the partial purification of Trx to demonstrate its presence in this organelle. Three milligrams of mitochondrial proteins was subjected to FPLC on Superdex 200 (Fig. 5A). The detection of Trx signal by dot blot analysis of all the fractions eluted allowed us to concentrate the positive fractions (16.8–18.4 mL) of low molecular mass proteins. This fraction also presented Trx activity (450–475 units mg−1 protein) and was subjected to SDS-PAGE (Fig. 5B). The analysis by western blot of the concentrated low molecular mass fractions obtained after the FPLC fractionation showed the presence of a unique band recognized by the PsTrxo1 antibody (Fig. 5C) and allowed the identification of a protein band stained with PageBlue with a similar molecular mass to the recombinant PsTrxo1 (Fig. 5B, lane 2, arrow). MS/MS sequencing of the electrophoretic band resulted in two characteristic peptides, IDIDQEAIQDTLSR and KTDELIGADVAR, both contained in the sequence of the recombinant PsTrxo1 (Fig. 1A). This result indicated that mitochondria from pea leaves contain at least one Trx, being to our knowledge the first native Trx partially sequenced and identified by MS/MS from SDS-PAGE of plant mitochondria.
Target Proteins of the Mitochondrial PsTrxo1
The mutant PsTrxo1C37S was produced in Escherichia coli and used for the identification of the target mitochondrial proteins of PsTrxo1. Affinity chromatography on a His-binding coaffinity resin column grafted with a monocysteinic Trx mutant was used to identify the potential targets of PsTrxo1. When the less reactive Cys of the Trx active site is replaced by a Ser, the heterodisulfide formed by the most reactive Cys and the oxidized target is stabilized and the protein is trapped. MS was used to identify the major spots of soluble mitochondrial proteins trapped in this heterodisulfide form. In a set of experiments from SDS-PAGE of the target proteins in nonreducing and reducing conditions, four enzymes were found as targets of PsTrxo1: Gly dehydrogenase components P and T, the ATP synthase subunit α, and the short-chain alcohol dehydrogenase CPRD12. Two-dimensional electrophoresis of the target proteins in the same conditions led us to the identification of five possible new target proteins (Table I): thiosulfate sulfurtransferase (TST) or 3-mercaptopyruvate sulfurtransferase, elongation factor Tu, Ser hydroxymethyl transferase (SHM2), adenylate kinase, and Prx. The ATP synthase α-chain and component P of Gly dehydrogenase were identified again by MS/MS analysis. Apart from all of the described possible targets of PsTrxo1 and using this proteomic approach, homoserine dehydrogenase, catalase, and glycolate oxidase were found as contaminants of chloroplast/cytoplasm and peroxisomes in the mitochondrial fractions.
Table I.
Potential Trx target proteins of plant mitochondria identified by affinity chromatography using immobilized Trx resin
| Sequencea | Predicted Protein | EMBL Accession No. |
|---|---|---|
| VWVLDGGLPR | Thiosulfate sulfurtransferase (TST; Datisca glomerata) | AAD19957 |
| 3-Mercaptopyruvate sulfurtransferase (Arabidopsis thaliana) | CAB64716 | |
| VVDASWYMPNEQR | Unknown protein with a TST region (Picea sitchensis) | ABK24999 |
| VAIITGGASGIGEATAR | Short-chain alcohol dehydrogenase CPRD12, drought inducible (Vigna unguiculata) | BAA13541 |
| VNCVSPYLVATPLAK | ||
| HTAFFSNYRPQFYLR | Mitochondrial elongation factor Tu (Arabidopsis thaliana) | CAA61511 |
| ILDQGQAGDNVGLLLR | Unknown protein with an elongation factor Tu region (Vitis vivifera) | CAO22472 |
| ITNFYTNFQVDEIGR | Mitochondrial ATP synthase subunit α (Pisum sativum) | CAA28964 |
| EAFPGDVFYLHSR | ||
| VYGLNEIQAGELVEFASGVK | ||
| NTVPGDVSAMVPGGIR | Mitochondrial Ser hydroxymethyltransferase (Pisum sativum) | AAA33687 |
| GVNGTVAHEFIIDLR | Mitochondrial Gly dehydrogenase P-protein (Pisum sativum) | CAA38252 |
| ESPYLTHPIFNTYQTEHELLR | ||
| VGFISSGPPPR | Mitochondrial Gly dehydrogenase T-protein (Pisum sativum) | CAA52800 |
| RAEGGFLGADVILK | ||
| SLLALQGPLAAPVLQHLK | ||
| TGYTGEDGFEISVPSEHGVELAK | ||
| VVIFGLPGAYTGVCSSK | Mitochondrial peroxiredoxin (Pisum sativum) | CAG30523 |
| VLNFAIDDAILEER | Adenylate kinase (Vitis vinifera) | CAG30523 |
| HESTVGAGLPVIASLNR | Homoserine dehydrogenase (Glycine max) | AAZ98830 |
| IAVQSGAAGIIVSNHGAR | Glycolate oxidase (Glycine max) | BAG09382 |
| SIWVSYWSQADR | Catalase (Pisum sativum) | CAA42736 |
Obtained tryptic peptide sequences determined by collision-induced dissociation-MS/MS of doubly or triply charged ions generated by in-gel tryptic digestion of protein spots as specified in “Materials and Methods.”
AOX Reduction
AOX was analyzed by western blot as a mitochondrial potential target of PsTrxo1. The detection of oxidized AOX dimers and reduced monomers was performed using recombinant PsTrxo1-treated or untreated pea leaf mitochondria (Fig. 6A). PsTrxo1 reduced the inactive AOX dimers to active monomers (Fig. 6A, lane 1), suggesting that this mitochondrial Trx could be responsible for the activation of AOX. To test for activation of AOX, oxygen evolution was followed for measuring the AOX respiration pathway using previously oxidized pea and soybean (Glycine max) mitochondria, presenting around 0.95% and 8% of respiration state 3, respectively. In both of them, the activity of AOX was monitored in the presence and absence of the Trx system (NADPH/Trr2/PsTrxo1). A typical experiment of oxygen uptake by pea mitochondria is shown in Figure 6B (the uptake by soybean mitochondria is presented in Supplemental Fig. S1). Pea mitochondria were oxidized with 10 mm diamide, and respiration was followed in a Suc-depleted medium using NADH as a respiratory substrate. Addition of pyruvate (1 mm) produced a very low activation of the mixothiazol-insensitive respiration, and addition of the PsTrxo1, previously reduced by NADPH/Trr2, enhanced respiration by the alternative pathway (Fig. 6B3). A similar behavior was found for the soybean oxidase (Supplemental Fig. S1). However, the activation of pea AOX was higher (7.2-fold) than that of the soybean oxidase (1.3-fold), showing that AOX activity in vivo could be regulated by PsTrxo1. In soybean, the activation of the AOX by the Trx system was lower than with DTT (2.5-fold), but pea AOX activation by this system occurred at a higher rate than with DTT (4.8-fold; Fig. 6B2), corroborating again that the system NADPH/TR could be the natural reductant of PsTrxo1 in pea mitochondria. The addition of only NADPH/Trr2 (Fig. 6B4) did not enhance the AOX activity, indicating that the presence of PsTrxo1 is necessary for the regulation. The addition of PsTrxo1 alone did not modify the oxygen uptake (Supplemental Fig. S1, panel 5).
Figure 6.
Regulation of AOX by PsTrxo1. A, AOX reduction. Lane 1, Pea mitochondria were incubated with the NADPH/Trr2/PsTrxo1 system and subjected to SDS-PAGE; lane 2, as for lane 1 but without the addition of PsTrxo1; lane 3, control mitochondria. AOX dimers and monomers were immunodetected. B, AOX activation. Oxygen uptake by pea mitochondria previously treated with 10 mm diamide was measured in a Suc-depleted medium. Trace 1, NADH (2 mm) oxidation by purified mitochondria; traces 2 to 4, where indicated, different components were added to the oxygen electrode chamber. The Trx system was NADPH/Trr2/PsTrxo1 (trace 3). As controls, 5 mm DTT (trace 2) or NADPH/Trr2 (trace 4) was added. Numbers on the traces represent nmol O2 min−1 mg−1 protein from one of three experiments. Mixot., Mixothiazol (inhibitor of cytochrome c oxidase); Pyr., sodium pyruvate; SHAM, salicylhydroxamic acid (inhibitor of AOX).
DISCUSSION
Presence of PsTrxo1 in the Mitochondria
A gene encoding a novel Trx o has been identified and characterized in this study; it contained no introns, a novel feature for plant Trx genes, which have at least one intron (Meyer et al., 2002). Genome-wide analysis of intronless genes in rice (Oryza sativa) and Arabidopsis (Jain et al., 2008) identified genes encoding Trx reductases in both species and, only in rice, one Prx type 2 and one protein of the Trx family. Phylogenetic analysis grouped this pea Trx with the o type; thus, it is called PsTrxo1, indicating that although the gene structure is unique, it is a typical Trx protein (see below). The highest similarity found was 60% with AtTrxo1, indicating that Arabidopsis does not likely contain a Trx that is orthologous, suggesting that this gene has arisen recently in evolutionary terms. The lack of introns suggests that it may have arisen via retrotransposition, a well-described mechanism to explain gene duplication (Yu et al., 2007; O'Toole et al., 2008). Analysis of ESTs from a variety of plants identified cDNAs encoding proteins with higher similarity with PsTrxo1 compared with Trx genes from Arabidopsis, all of them belonging to the family Leguminosae (Fabaceae) that split from other angiosperms about 60 million years ago (Soltis et al., 2000). This suggests that this type of Trx gene may be restricted to legumes. The recombinant protein produced in E. coli verified Trx activity, being able to reduce insulin in an in vitro assay using two different Trx reductases and NADPH, suggesting that this Trx has the ability to play a role in the redox balance in mitochondria.
The analysis of the amino acid sequence of PsTrxo1 revealed the presence of a transit peptide that could direct the protein to mitochondria. A mitochondrial localization was confirmed by western-blot analysis, in vitro import assays, transient expression analysis of GFP fusions, and immunogold labeling. Although the presence of Trx and Trx reductase activities has been detected in plant mitochondria (Marcus et al., 1991; Konrad et al., 1996; Banze and Follmann, 2000), the native proteins responsible for these activities have not been identified and characterized. Molecular filtration chromatography by FPLC of solubilized mitochondrial proteins resulted in the identification and sequencing of peptides contained in the PsTrxo1 sequence, confirming that PsTrxo1 was responsible for the Trx activity detected with isolated pea mitochondria. This not only confirmed a mitochondrial localization for PsTrxo1 but also indicated that it was the only Trx identified in pea that coincided with the pea Trx activity.
Previous studies have identified potential mitochondrial targets but using cytoplasmic or chloroplastic forms of Trx (Balmer et al., 2004). In this study, we used the mitochondrial PsTrxo1C37S and identified nine potential mitochondrial Trx targets. An analysis of the proteins in mitochondria that are susceptible to disulfide bonding in Arabidopsis identified AOX, subunits of the ATP synthase, elongation factor Tu, and a range of dehydrogenases. Among the PsTrxo1-linked proteins, two proteins of the Gly decarboxylase complex, components P and T, and another important enzyme in photorespiration, SHMT, have been identified. The regulation of these two key enzymes implies the involvement of mitochondrial Trx in the regulation of the photorespiratory cycle in mitochondria. The activity of the Gly cleavage complex has been described to depend on the presence of DTT in the reaction medium, so Trx could be the natural reductant needed for the activity (Walker and Oliver, 1986). This enzyme involved in the photorespiratory pathway appears particularly susceptible to oxidative damage induced by drought and chilling (Taylor et al., 2002). Similarly, SHMT activity is a key enzyme in the photorespiratory event, and the shm1-1 mutant of Arabidopsis, defective in SHMT activity, has been shown to display a lethal photorespiratory phenotype when grown at ambient CO2 (Voll et al., 2006). In this context, it would be very interesting to analyze the role of PsTrxo1 in the photorespiratory events in response to oxidative damage situations such as those induced by NaCl and water stress.
Another Trx-linked protein found is the α-subunit of the mitochondrial ATP synthase, which links Trx with the control of ATP synthesis, in a similar manner to the control exerted by chloroplastic Trx f as the physiological reductant for the chloroplast ATP synthase (H+-translocating ATPase) from spinach (Spinacia oleracea; Schwarz et al., 1997). Other studies have shown the activation of chloroplastic ATP synthase by Trx (Malyan and Allison, 2002), which accelerated the DTT-dependent activation of CF1 (Stump et al., 1999). Another protein involved in the transformation of ATP into ADP is adenylate kinase, which has been shown to be a possible target of the mitochondrial PsTrxo1. This mitochondrial protein contains an intramolecular disulfide bond as described by Winger et al. (2007). The location of adenylate kinase in the intermembrane space of mitochondria and the presence of a disulfide relay system for the import of proteins into the mitochondria inner membrane (Bandlow et al., 1998) suggest that PsTrxo1, or another mitochondrial Trx, may be located in the intermembrane space and matrix and interact with intermembrane space proteins.
Another target of PsTrxo1, elongation factor Tu, promotes the GTP-dependent binding of aminoacyl-tRNA to the ribosome during the elongation of newly synthesized protein. As has been previously reported, the chloroplast elongation factor Tu was identified as a potential Trx target with chloroplasts (Balmer et al., 2004).
The binding between the pea mitochondrial proteins PrxIIF and Trx o was recently demonstrated using recombinant proteins and isolated mitochondria by isothermal titration calorimetry, molecular filtration, and coimmunoprecipitation (Barranco-Medina et al., 2008), and the mode of binding is characterized by extremely favorable enthalpy changes, demonstrating a role of pea Trx o as a physiological electron donor to PrxIIF.
AOX has been identified as a protein with an intramolecular disulfide bond in Arabidopsis (Winger et al., 2007), but it has not been identified as a Trx target using Trx-linked resins, although its regulation by Trx has been proposed (Gelhaye et al., 2004; Umbach et al., 2006). The fact that AOX was not identified as a target in these assays may be due to the low amount of native protein present in the pea mitochondria. As discussed below, the rate of AOX activity in pea is very low compared with that detected in other species such as soybean. We have shown the reduction of pea mitochondrial AOX homodimers by the recombinant PsTrxo1 in vitro, as was described for the poplar mitochondrial Trx PtTrxh2 (Gelhaye et al., 2004). Also, the activation of AOX, measured by oxygen consumption by this pathway, was performed by PsTrxo1 using a NADPH/TR system, showing the regulation of this enzyme by the mitochondrial Trx. In pea, the alternative respiration pathway has been shown to account for only a very low percentage of the total respiration, in contrast to the relatively higher rate in soybean. In this situation, PsTrxo1 without NADPH/TR is not able to reduce AOX and activate the alternative pathway, suggesting NADPH/TR as the possible in vivo reductant of the mitochondrial PsTrxo1. Another interesting difference between mitochondria of both species is the higher ability of PsTrxo1 to activate AOX in pea mitochondria compared with the soybean organelle, in which DTT is more efficient in the reduction than the Trx system. NADPH/TR/PsTrxo1, then, could be a highly effective system in the activation of the AOX pathway in pea. This activation had not been demonstrated for a Trx o, and the next step could be the characterization of their in vivo interaction by fluorescence resonance energy transfer. AOX plays an important role in decreasing reactive oxygen species (Maxwell et al., 1999; Yip and Vanlerberghe, 2001), and PsTrxo1 could play a major role in the activation of AOX and in the detoxification of reactive oxygen species in mitochondria in normal conditions and under stress situations.
The identification of the peroxisomal proteins catalase and glycolate oxidase as possible Trx targets raises the question of the presence of Trx and its role in this organelle, as described by Balmer et al. (2004) for catalase, possibly extending the role for Trx in the plant cell and consistent with the proposed role of PsTrxo1 in the photorespiratory process.
Presence of PsTrxo1 in the Nucleus
The transient expression of the PsTrxo1-GFP fusion protein in Allium epidermal cells allowed us to corroborate the presence of this Trx in the nucleus. The import of proteins into the nucleus is a highly selective process that depends on a nuclear location signal of short stretches of basic amino acids, which are not cleaved after import. However, this is not the case for the mammalian cytoplasmic Trxs, which lack an obvious nuclear location signal (Hirota et al., 2000). No clear nuclear location signal was found within the deduced amino acid sequence of PsTrxo1. The analysis of the common nuclear location signals has shown that about 50% of the nuclear proteins contain a bipartite motif comprising two basic amino acids, a spacer region of any 10 to 16 amino acids (nucleoplasmin and influenza virus BP1 protein, respectively, as examples) or even 37 amino acids (adenovirus DNA-binding protein), followed by a basic cluster in which three of the next five amino acids must be basic (Dingwall and Laskey, 1991). PsTrxo1 sequence presents in the C terminus one of these bipartite motifs (amino acids 157–181), which could be responsible for the direction of the protein to the nucleus. Using the transient expression assay, western-blot analysis, and immunogold labeling, clear evidence that the PsTrxo1 protein localizes to the nucleus was shown. In plants, Trxs h, typically located in cytoplasm, have been reported to accumulate in the nucleus of aleurone and scutellum cells during germination, a location associated with oxidative stress (Serrato and Cejudo, 2003), similar to that described for the nuclear mammalian Trxs (Hirota et al., 1999, 2000; Wei et al., 2000). However, in this study, PsTrxo1 is located in the nucleus under normal conditions, suggesting a role that may not be linked only to the stress response and extending the roles of these proteins in other processes in plants. Nuclear location is consistent with the large body of evidence indicating that members of the mammalian Trx family play key roles in transcriptional regulation; moreover, the presence of PsTrxo1 in the heterochromatin could be related to the described involvement of the mammalian mitochondrial/peroxisomal Prx PRDX5 in protecting the genome against oxidation and in the control of transcription of noncoding DNA (Kropotov et al., 2006).
CONCLUSION
A new Trx is present in leaves from pea plants. This Trx (PsTrxo1) has sequence similarity to the AtTrxo1, but as a different feature among Trxs, the PsTrxo1 gene is characterized by the absence of introns. Interestingly, PsTrxo1 is present not only in mitochondria but also in nuclei. The native mitochondrial Trx has been partially sequenced. The recombinant protein presented a higher capacity of insulin reduction with the S. cerevisiae mitochondrial Trr2 than with the wheat cytoplasmic NADPH/TR, suggesting that this system could be operating in vivo. PsTrxo1 was able to activate the AOX and presented a reduced number of mitochondrial target proteins, including PrxIIF and some photorespiratory enzymes, indicating that this Trx may have an important role in this process.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum) plants were cultured in a growth chamber, under optimum conditions, for 2 weeks as described by Jiménez et al. (1997).
cDNA Preparation and Genomic DNA Isolation
Total RNA was isolated from 100 mg of young pea leaves using the RNeasy Plant Mini Kit (Qiagen). cDNA was obtained by reverse transcription for PCR using SuperScript II reverse transcriptase (Invitrogen) with an oligo(dT)20 as a primer.
Two different methods were used in order to isolate genomic DNA, the Wizard sv Genomic DNA purification system (Promega) and the Nexttec clean-columns (Nexttec), using 1 g and 100 mg of young pea leaves, respectively.
Identification of a Gene Encoding a Trxo1
Two primers were constructed based on the sequence of pea (‘Lincoln’) mRNA for putative mitochondrial Trx (GenBank/EMBL database, accession no. AM235208): primer 1, 5′-ATGGTTGGAACCAGAAATTTGATC-3′, which encodes the peptide sequence MVGTRNLI; and primer 2, 5′-TTAGTCCTTCTTGAAGAGTTTCTC-3, used as the reverse primer, which encodes the peptide sequence EKLFKKD. PCR was performed at 56°C, and its product was gel purified, cloned in pGEM-T (Promega), and sequenced. The same experiment was performed using genomic DNA to obtain the number and positions of introns in the sequence.
Prediction of Subcellular Localization and Comparison of the Deduced Amino Acid Sequences of PsTrxo1 and Other Trxs
MitoProt (prediction of signal peptide; Claros and Vincens, 1996) and Predotar and TargetP (subcellular localization; Emanuelsson et al., 2000; Small et al., 2004), provided by the ExPASy server (http://us.expasy.org), were used for the analysis of the deduced amino acid sequences. The ClustalW program, provided by the European Bioinformatics Institute server (http://www.ebi.ac.uk), was used for alignment. TreeView (version 1.5.2) was employed to create an unrooted phylogenetic tree.
Expression and Purification of Recombinant and Mutant Trxs
A fragment of cDNA encoding the mature protein (amino acids 70–181), without the mitochondrial transit peptide, was obtained by reverse transcription-PCR. This fragment was cloned into the NcoI/BamHI sites of the pET3d expression vector (Novagen). The recombinant mutant pea Trx, PsTrxo1C37S, was prepared by replacing the second Cys of the active site of the PsTrxo1 as reported by Pérez-Pérez et al. (2006) and was cloned into the BamHI site of the pET15b vector, which adds a His tail. Escherichia coli BL21 (DE3) strains were transformed with the resulting constructions, and recombinant protein expression was induced by the addition of 1 mm isopropylthio-β-galactoside for 3 h at 37°C.
The purification of the recombinant PsTrxo1 was performed by FPLC of the bacterial lysate. The E. coli cells were broken with a French press, following by ammonium sulfate precipitation between 40% and 95% (w/v). The pellet was then suspended in buffer (25 mm Tris-HCl, pH 8.0, containing 150 mm NaCl) and chromatographed using a Superdex 75 column. The mutant PsTrxo1C37S was purified from cells that were broken with Alumin (Sigma) and sonication, using a Co2+ affinity chromatography system following the manufacturer's instructions (Talon Metal Affinity Resin; Clontech).
Preparation of Rabbit PsTrxo1 Antiserum
The polyclonal antibody against the C-terminal sequence ARLNHITEKLFKKD of mature PsTrxo1 was raised in New Zealand White rabbits by Sigma-Genosys.
Cell Fractionation
All steps were carried out at 4°C. Chloroplasts and cytoplasm from pea leaves were isolated as described by Gómez et al. (1999) and Hernández et al. (2000). For the isolation of mitochondria, two different methods were used consecutively. Mitochondria were first isolated from pea leaves as described by Day et al. (1985) and were subjected to a second round of Percoll gradient purification as described by Jiménez et al. (1997) to obtain a highly pure mitochondrial fraction. The integrity of the outer mitochondrial membrane was estimated from the succinate: cytochrome c oxidoreductase activity (Hernández et al., 1993) and different marker enzymes for other cell compartment were measured to check the organelle purification level (Jiménez et al., 1997). Around 25 mitochondrial isolation experiments (approximately 1.2 kg of pea leaves) were used for the Trx partial purification. For the isolation of highly pure nuclei, the Plant Nuclei Isolation Extraction Kit (CelLytic PN; Sigma) was used following the manufacturer's instructions.
Trx Activity
Two different methods were used to measure Trx activity. The insulin reduction method is based on the capacity of reduced Trx to reduce oxidized insulin, measuring the increase in turbidity at 650 nm for 1 h at 30°C as described by Martínez-Galisteo et al. (1993). Trx was reduced by DTT or the NADPH/TR system as described by Laloi et al. (2001) using the cytoplasmic NADPH/TR from wheat (Triticum aestivum) and the mitochondrial Trr2 from Saccharomyces cerevisiae. The second method was based in the induction of NADP-dependent MDH (EC 1.1.1.82; Scheibe et al., 1993). For this, the purification of a chloroplastic NADP-MDH from 500 g of pea leaves was carried out as described by Fickebser and Scheibe (1983) and Reche et al. (1997) using FPLC.
Protein concentration was measured according to the Bradford (1976) protocol.
PAGE and Western-Blot Analysis
Proteins were resolved using SDS-PAGE as described (Laemmli, 1970). Gels were stained with PageBlue (Fermentas). For western-blot analysis, proteins were transferred onto a nitrocellulose membrane using a semidry blotting apparatus (Bio-Rad). Immunoreaction was carried out using rabbit polyclonal antibody against PsTrxo1 diluted 1:2,000 in Tris-buffered saline (TBS) containing 1% (w/v) bovine serum albumin (BSA) and 0.1% (v/v) Tween 20. Goat anti-rabbit antibody conjugated to alkaline phosphatase (Boehringer Mannheim) was used as the secondary antibody. The antigen was detected by colorimetry using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche) following the manufacturer's protocol.
Partial Purification and Identification of the Native Mitochondrial Trx
Purified mitochondria were ruptured by three freeze-thaw cycles, resuspended in 10 mm HEPES-NaOH buffer, pH 7.2, containing 10% (v/v) glycerol, 1 mm MnCl2, 2 mm sodium ascorbate, 14 mm β-mercaptoethanol, 2 mm phenylmethylsulfonyl fluoride, and 0.1% (v/v) 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid (CHAPSO), and incubated on ice for 15 min followed by sonication for 1 min. The solution was concentrated using carboxymethyl cellulose. Three milligrams of the protein solution was then chromatographed on a Superdex 200 FPLC system (Pharmacia) equilibrated in 50 mm Tris-HCl buffer, pH 7.9, containing 150 mm NaCl and 10 mm DTT, at a flow rate of 0.8 mL min−1 and room temperature. Fractions were collected and stored at −80°C. Dot-blot assay on these fractions was performed using the antibody generated against a PsTrxo1 peptide. Once identified, the positive low molecular mass fractions were collected and concentrated using Amicon filters (YM3) followed by 10 mm DTT treatment for 15 min. SDS-PAGE and PageBlue protein staining together with western-blot analysis were performed in order to identify the band corresponding to Trx. Once identified, the band of interest was sequenced by MS/MS.
Proteomic Characterization
Proteins of interest were excised from gels and subjected to automated reduction, alkylation, and digestion with sequencing-grade bovine pancreas trypsin (Roche) using a ProGest digestor (Genomic Solutions) following the manufacturer's instructions. The tryptic peptide mixtures were analyzed with an Applied Biosystems Voyager-DE-Pro matrix-assisted laser-desorption ionization time of flight mass spectrometer. For peptide sequencing, the protein digest mixture was subjected to electrospray ionization mass spectrometric analysis (QTrap 2000 [Applied Biosystems] with a nanoelectrospray source [Protana]; Le Blanc et al., 2003). Collision-induced dissociation spectra were interpreted manually or using a licensed version of the MASCOT program (http://www.matrixscience.com) against the SwissProt/TrEMBL database (http://us.expasy.org/sprot/). MS/MS mass tolerance was set to ±0.6 D. Carbamidomethyl Cys and oxidation of Met were fixed and variable modifications, respectively. Amino acid sequence similarity searches were performed using BLAST (Altschul et al., 1997) at http://www.bork.embl.de/j/.
Electron Microscopy and Immunogold Labeling
Leaf samples were fixed and embedded in London Resin White acrylic resin. Ultrathin sections (60–70 nm) were placed on coated nickel grids. Grids were blocked in TBS plus 2.5% (w/v) BSA. They were then incubated for 2 h with anti-PsTrxo1 antibody diluted 1:200 in TBST buffer (TBS + 1% BSA + 0.05% Tween 20). After washing with TBST, the sections were incubated for 1.5 h with the secondary antibody goat anti-rabbit IgG, gold labeled (10 nm; British BioCell International), and diluted 1:50 with TBST. The sections were washed several times in TBST buffer, TBS, and water. Grids were observed with a Philips Tecnai 12 transmission electron microscope. Two different controls were used, one omitting the anti-PsTrxo1 antibody and the other using preimmune serum instead of anti-PsTrxo1.
Import of Radiolabeled Proteins into Isolated Mitochondria and Chloroplasts
Precursor proteins were synthesized from corresponding cDNA clones in pGEM-3Zf+ by an in vitro coupled transcription-translation system with the Promega TnT T7 or SP6 rabbit reticulocyte lysate system in the presence of [35S]Met according to the supplier's instructions. Intact chloroplasts were isolated from 10-d-old pea leaves as described by Bruce et al. (1994) and from potato (Solanum tuberosum) tuber mitochondria as described by Glaser et al. (1995). Chloroplast and mitochondria import assays were carried out as described by Waegemann and Soll (1995) and Glaser et al. (1995), respectively. The following precursor proteins were used as controls: soybean (Glycine max) AOX (Whelan et al., 1993) and, for pea, the small subunit of Rubisco (Anderson and Smith, 1986).
Location of the PsTrxo1-GFP Fusion Protein in Onion Epidermal Cells
To create the translational fusions of the PsTrxo1 gene to the GFP reporter gene, the corresponding complete cDNA including the transit peptide was PCR amplified using the following primers: forward oligo, 5′-CACCATGGTTGGAACCAGA-3′; reverse, 5′-GTCCTTCTTGAAGAGTTTC-3′. The fragment was cloned into the pENTR/D-TOPO vector (Invitrogen) and, following confirmation of sequence fidelity, was subcloned in-frame to the N-terminal GFP gene by a recombination reaction into the plasmid pMDC83 containing the cauliflower mosaic virus 35S promoter and a soluble modified green-shifted GFP as described by Curtis and Grossniklaus (2003). The resulting plasmid, pPsTrxo1-GFP, was sequenced to confirm that the site of fusion was in-frame. Primer design and the in vitro clonase recombination reactions were carried out according to the manufacturer's instructions (Invitrogen). As a control, pMDC83 without the PsTrxo1 insert was used.
Constructs were transferred to onion (Allium cepa) epidermal cells by biolistic bombardment. Inner epidermal layers of onion bulbs locally purchased were peeled and placed onto Murashige and Skoog agar medium diluted twice as described by Borrell et al. (2002). Particle bombardment was performed with a biolistic helium gun device (PSP-1000; DuPont) basically as described by Díaz et al. (2005). The GFP fluorescence was observed after 24 h of incubation at 22°C in the dark with a confocal ultraspectral microscope (TCS-Sp2-AOBS-UV; Leica). As a control for nuclear location, Nomarski observations were carried out and tissue samples were stained with DAPI (Serva). As a control for mitochondrial location, tissue samples were stained with MitoTracker Red 580 (Invitrogen).
Identification of Trx Target Proteins
Mitochondrial proteins were isolated as described above. For the identification of target mitochondrial proteins, we followed essentially the method described by Pérez-Pérez et al. (2006). Briefly, in each experiment, around 2.6 mg of recombinant protein PsTrxo1C37S was bound to 400 μL of His-binding coaffinity resin (Clontech) and incubated with 5 to 6 mg of mitochondrial proteins for 16 h at 4°C under gentle agitation. Washing and elution with imidazole of intact Trx target disulfide complexes were performed as described by the manufacturer. Two-dimensional nonreducing and reducing SDS-PAGE for optimal overall resolution of Trx and target proteins was performed. Parallel one-dimensional nonreducing and reducing SDS-PAGE was performed in order to increase the resolution of the protein bands to be sequenced as described above.
AOX Reduction and Activity
The reduction of pea AOX by PsTrxo1 was performed by incubation of purified pea mitochondria samples that had undergone three cycles of freeze/thaw with the NADPH/Trr2/PsTrxo1 system. Mitochondrial proteins were incubated for 45 min at 37°C with 12 μm PsTrxo1, 200 mm mitochondrial Trr2 from S. cerevisiae, and 2 mm NADPH before being subjected to nonreductive SDS-PAGE. AOX immunodetection was performed using a monoclonal antibody.
To measure the AOX activity, freshly purified pea and soybean mitochondria isolated as described by Jiménez et al. (1997) and Day et al. (1985), respectively, were incubated with 10 mm diamide for 30 min at 4°C and washed twice. Activation of AOX was measured after incubation of the freshly purified mitochondria with 16 μm PsTrxo1 previously reduced for 45 min in the presence of 16 mm NADPH and 1.6 μm Trr2. The oxygen uptake was measured with a Clark-type O2 electrode (Rank Brothers) in a Suc-depleted respiration medium (5 mm KH2PO4, 10 mm N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid, pH 7.2, 10 mm KCl, and 2 mm Mg2SO4) at 25°C using 5 μm mixothiazol and 1 mm salicylhydroxamic acid as inhibitors of the cytochrome and AOX respiration pathways, respectively. Subsequent additions were made as described in Figure 6B.
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: for Arabidopsis thaliana, Ath2 (S58123), Ath3 (S58118), Ath4 (S58119), Ath5 (S58120), Ath7 (AAD39316), Ath8 (AAG52561), Ath9 (AAG51342), Atf1 (Q9XFH8), Atf2 (Q9XFH9), Atm1 (O48737), Atm2 (AAF15949), Atm3 (AAF15950), Atm4 (Q9SEU6), Aty1 (Q6NPF9), Aty2 (Q8L7S9), Ato1 (AAC12840), Ato2 (AF396650), Atx (AAF15952), AtCXXS1 (Q8GXV2), and TDX (Q7XJ63); for Populus trichocarpa, Pth2 (AF483266). Accession numbers for EST contigs are as follows: Trifolium pratense, Tpo1 (BB932971); Glycine max, Gmo1 (CO982355); Vigna unguiculata, Vuo1 (FG912242); Phaseolus vulgaris, Pvo1 (CV538300); Gossypium hirsutum, Gho1 (AI725806 and AI729485); Solanum lycopersicum, Lso1 (BE462179, AW037483, AW037392, and BE433326); and Oryza sativa, Oso1 (C27892 and AU100897).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure S1. Soybean AOX activation by PsTrxo1.
Supplementary Material
Acknowledgments
We are grateful to Dr. J.M. Gómez (Centro de Edafología y Biología Aplicada del Segura, Consejo Superior de Investigaciones Científicas, Murcia, Spain) for the purified malate dehydrogenase, Dr. M. Ribas-Carbó (University of Illes Balears, Spain) for AOX antibody, and Dr. Cejudo (Institute of Plant Biochemistry and Photosynthesis, Consejo Superior de Investigaciones Científicas, Sevilla, Spain) and Dr. Pedrajas (University of Jaen, Spain) for giving us TRs.
This work was supported by the DGI-FEDER (grant nos. BFU2005–02051/BFI and BFU2007–61563) and the SÉNECA Foundation, Murcia (grant no. 07743/GERM/07 and support for M.C.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ana Jiménez (ajimenez@cebas.csic.es).
The online version of this article contains Web-only data.
References
- Alkhalfioui F, Renard M, Frendo P, Keichinger C, Meyer Y, Gelhaye E, Hirasawa M, Knaff DB, Ritzenthaler C, Montrichard F (2008) A novel type of thioredoxin dedicated to symbiosis in legumes. Plant Physiol 148 424–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Smith SM (1986) Synthesis of the small subunit of ribulose-bisphosphate carboxylase from genes cloned into plasmids containing the sp6 promoter. Biochem J 240 709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai RJ, Masutani H, Yodoi J, Debbas V, Laurindo FR, Stern A, Monteiro HP (2006) Nitric oxide induces thioredoxin-1 nuclear translocation: possible association with the p21Ras survival pathway. Biochem Biophys Res Commun 348 1254–1260 [DOI] [PubMed] [Google Scholar]
- Arner ESJ, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267 6102–6109 [DOI] [PubMed] [Google Scholar]
- Balmer Y, Vensel WH, Tanaka CK, Hurkman WJ, Gelhaye E, Rouhier N, Jacquot J, Manieri W, Schürmann P, Droux M, et al (2004) Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proc Natl Acad Sci USA 101 2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandlow W, Strobel G, Schricker R (1998) Influence of N-terminal sequence variation on the sorting of major adenylate kinase to the mitochondrial intermembrane space in yeast. Biochem J 15 359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banze M, Follmann H (2000) Organelle-specific NADPH thioredoxin reductase in plant mitochondria. J Plant Physiol 156 126–129 [Google Scholar]
- Barranco-Medina S, Krell T, Bernier-Villamor L, Sevilla F, Lázaro JJ, Dietz KJ (2008) Hexameric oligomerization of mitochondrial peroxiredoxin PrxIIF and formation of ultrahigh affinity complex with its electron donor thioredoxin Trx-o. J Exp Bot 59 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell A, Cutanda MC, Lumbreras V, Pujal J, Goday A, Culianez-Macia FA, Pages M (2002) Arabidopsis thaliana Atrab28: a nuclear targeted protein related to germination and toxic cation tolerance. Plant Mol Biol 50 249–259 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Bruce BD, Perry S, Froelich J, Keegstra K (1994) In vitro import of proteins into chloroplasts. In SB Gelvin, RA Schilferoot, eds, Plant Molecular Biology Manual, Ed 2. Kluwer Academic, Dordrecht, The Netherlands, pp J1–J15
- Chae HZ, Chung SJ, Rhee SG (1994) Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem 269 27670–27678 [PubMed] [Google Scholar]
- Claros MG, Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241 779–786 [DOI] [PubMed] [Google Scholar]
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin JM, Knaff DB, Miginiac-Maslow M (2003) The Arabidopsis plastidial thioredoxins: new functions and new insight into specificity. J Biol Chem 278 23747–23752 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A Gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Neuberger M, Douce R (1985) Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 12 219–228 [Google Scholar]
- Díaz I, Martínez M, La Moneda I, Rubio-Somoza I, Carbonero P (2005) The DOF protein, SAD, interacts with GAMYB in plant nuclei and activates transcription of endosperm-specific genes during barley seed development. Plant J 42 652–662 [DOI] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA (1991) Nuclear targeting sequences: a consensus? Trends Biochem Sci 16 478–481 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Fickebser K, Scheibe R (1983) Purification and properties of NADP-dependent malate dehydrogenase from pea leaves. Biochim Biophys Acta 749 249–254 [Google Scholar]
- Finkemeier I, Goodman M, Lamkemeyer P, Kandlbinder A, Sweetlove LJ, Dietz KJ (2005) The mitochondrial type II peroxiredoxin F is essential for redox homeostasis and root growth of Arabidopsis thaliana under stress. J Biol Chem 280 12168–12180 [DOI] [PubMed] [Google Scholar]
- Gama F, Keech O, Eymery F, Finkemeier I, Gelhaye E, Gardestrom P, Dietz KJ, Rey P, Jacquot JP, Rouhier N (2007) The mitochondrial type II peroxiredoxin from poplar. Physiol Plant 129 196–206 [Google Scholar]
- Gelhaye E, Rouhier N, Gerard J, Jolivet Y, Gualberto J, Navrot N, Ohlsson P, Wingsle G, Hirasawa M, Knaff DB, et al (2004) A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc Natl Acad Sci USA 101 14545–14550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelhaye E, Rouhier N, Laurent P, Sautiere PE, Martin F, Jacquot JP (2002) Isolation and characterization of an extended thioredoxin h from poplar. Physiol Plant 11 165–171 [DOI] [PubMed] [Google Scholar]
- Gelhaye E, Rouhier N, Navrot N, Jacquot JP (2005) The plant thioredoxin system. Cell Mol Life Sci 62 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser E, Corp C, Hugosson M, von Stedingk E (1995) Macromolecular movement into mitochondria. Methods Cell Biol 50 269–281 [DOI] [PubMed] [Google Scholar]
- Glaser E, Sjoling S, Tanudji M, Whelan J (1998) Mitochondrial protein import in plants: signals, sorting, targeting, processing and regulation. Plant Mol Biol 38 311–338 [DOI] [PubMed] [Google Scholar]
- Gómez JM, Hernández JA, Jiménez A, del Río LA, Sevilla F (1999) Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic Res 31 S11–S18 [DOI] [PubMed] [Google Scholar]
- Hernández JA, Corpas FJ, Gómez JM, del Río LA, Sevilla F (1993) Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89 103–110 [Google Scholar]
- Hernández JA, Jiménez A, Mullineaux PM, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ 23 853–862 [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J (1997) AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci USA 94 3633–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Murata M, Takashima Y, Chen FS, Itoh T, Fukuda K, Yodoi J (2000) Nucleoredoxin, glutaredoxin, and thioredoxin differentially regulate NF-kB, AP-1, and CREB activation in HEK293 cells. Biochem Biophys Res Commun 21 177–182 [DOI] [PubMed] [Google Scholar]
- Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J (1999) Distinct roles of thioredoxin in the cytoplasm and in the nucleus: a two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem 24 27891–27897 [DOI] [PubMed] [Google Scholar]
- Jain M, Khurana P, Tyagi AK, Khurana JP (2008) Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct Integr Genomics 8 69–78 [DOI] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F (1997) Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol 114 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Banze M, Follmann F (1996) Mitochondria of plant leaves contain two thioredoxins: completion of the thioredoxin profile of higher plants. J Plant Physiol 149 317–321 [Google Scholar]
- Kropotov A, Serikov V, Suh J, Smirnova A, Bashkirov V, Zhivotovsky B, Tomilin N (2006) Constitutive expression of the human peroxiredoxin V gene contributes to protection of the genome from oxidative DNA lesions and to suppression of transcription of noncoding DNA. FEBS J 273 2607–2617 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Laloi C, Rayapuram N, Chartier Y, Grienenberger JM, Bonnard G, Meyer Y (2001) Identification and characterization of a mitochondrial thioredoxin system in plants. Proc Natl Acad Sci USA 98 14144–14149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent TC, Moore EC, Reichard P (1964) Enzymatic synthesis of deoxyribonucleotides. 4. Isolation and characterization of thioredoxin hydrogen donor from Escherichia coli. J Biol Chem 239 3436–3444 [PubMed] [Google Scholar]
- Le Blanc JC, Hager JW, Ilisiu AM, Hunter C, Zhong F, Chu I (2003) Unique scanning capabilities of a new hybrid linear ion trap mass spectrometer (Q TRAP) used for high sensitivity proteomics applications. Proteomics 3 859–869 [DOI] [PubMed] [Google Scholar]
- López Jaramillo J, Chueca A, Jacquot JP, Hermoso R, Lázaro JJ, Sahrawy M, López Gorgé J (1997) High-yield expression of pea thioredoxin m and assessment of its efficiency in chloroplast fructose-1,6-bisphosphatase activation. Plant Physiol 114 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyan AN, Allison WS (2002) Properties of noncatalytic sites of thioredoxin-activated chloroplast coupling factor 1. Biochim Biophys Acta 1554 153–158 [DOI] [PubMed] [Google Scholar]
- Marcus F, Chamberlain SH, Chu C, Masiarz FR, Shin S, Yee BC, Buchanan BB (1991) Plant thioredoxin-h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys 287 195–198 [DOI] [PubMed] [Google Scholar]
- Martínez-Galisteo E, Padilla CA, García-Alfonso C, López-Barea J, Bárcena JA (1993) Purification and properties of bovine thioredoxin system. Biochimie 75 803–809 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y, Vignols F, Reichheld JP (2002) Classification of plant thioredoxins by sequence similarity and intron position. Methods Enzymol 347 394–402 [DOI] [PubMed] [Google Scholar]
- O'Toole N, Hattori M, Andres C, Iida K, Lurin C, Schmitz-Linneweber C, Sugita M, Small I (2008) On the expansion of the pentatricopeptide repeat gene family in plants. Mol Biol Evol 25 1120–1128 [DOI] [PubMed] [Google Scholar]
- Pedrajas JF, Miranda-Vizuete A, Javanmardy N, Gustafsson JA, Spyrou G (2000) Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J Biol Chem 275 16296–16301 [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez ME, Florencio FJ, Lindahl AM (2006) Selecting thioredoxins for disulphide proteomics: target proteomes of three thioredoxins from the cyanobacterium Synechocystis. Proteomics 6 S186–S195 [DOI] [PubMed] [Google Scholar]
- Reche A, Lázaro JJ, Hermoso R, Chueca A, López-Gorgé J (1997) Binding and activation of pea chloroplast fructose-1,6-bisphosphatase by homologous thioredoxins m and f. Physiol Plant 101 463–470 [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Geissler A, Rother T (1993) Analysis of biophysical differences between oxidized and reduced chloroplast NADP malate-dehydrogenase. Arch Biochem Biophys 300 635–640 [DOI] [PubMed] [Google Scholar]
- Schneider G, Sjöling S, Wallin E, Wrede P, Glaser E, von Heijne G (1998) Feature-extraction from endopeptidase cleavage sites in mitochondrial targeting peptides. Proteins 30 49–60 [PubMed] [Google Scholar]
- Schürman P, Jacquot JP (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol 51 371–400 [DOI] [PubMed] [Google Scholar]
- Schwarz O, Schürmann P, Strotmann H (1997) Kinetics and thioredoxin specificity of thiol modulation of the chloroplast H+-ATPase. J Biol Chem 272 16924–16927 [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Cejudo FJ (2003) Type-H thioredoxins accumulate in the nucleus of developing wheat seed tissues suffering oxidative stress. Planta 217 392–399 [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Crespo JL, Florencio FJ, Cejudo FJ (2001) Characterization of two thioredoxins h with predominant localisation in the nucleus of aleurone and scutellum cells of germinating wheat seeds. Plant Mol Biol 46 361–371 [DOI] [PubMed] [Google Scholar]
- Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4 1581–1590 [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, Savolainen V, Hahn WH, Hoot SB, Fay MF, et al (2000) Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot J Linn Soc 133 381–461 [Google Scholar]
- Stump MP, Motohashi K, Hisabori T (1999) Chloroplast thioredoxin mutants without active-site cysteines facilitate the reduction of the regulatory disulphide bridge on the α-subunit of chloroplast ATP synthase. Biochem J 341 157–163 [PMC free article] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Millar AH (2002) Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 277 42663–42668 [DOI] [PubMed] [Google Scholar]
- Umbach AL, Ng VS, Siedow JN (2006) Regulation of plant alternative oxidase activity: a tale of two cysteines. Biochim Biophys Acta 1757 135–142 [DOI] [PubMed] [Google Scholar]
- Voll LM, Jamai A, Renne P, Voll H, McClung CR, Weber PM (2006) The photorespiratory Arabidopsis shm1 mutant is deficient in SHM1. Plant Physiol 140 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waegemann K, Soll J (1995) Characterization and isolation of the chloroplast protein import machinery. Methods Cell Biol 50 255–267 [DOI] [PubMed] [Google Scholar]
- Walker JL, Oliver DJ (1986) Glycine decarboxylase multienzyme complex: purification and partial characterization from pea leaf mitochondria. J Biol Chem 261 2214–2221 [PubMed] [Google Scholar]
- Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, Spitz DR, Goswami PC, Yodoi J, Gius D (2000) Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res 60 6688–6695 [PubMed] [Google Scholar]
- Whelan J, McIntosh L, Day DA (1993) Sequencing of a soybean alternative oxidase cDNA clone. Plant Physiol 103 1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger AM, Taylor NL, Heazlewood JL, Day DA, Millar AH (2007) Identification of intra- and intermolecular disulphide bonding in the plant mitochondrial proteome by diagonal gel electrophoresis. Proteomics 7 4158–4170 [DOI] [PubMed] [Google Scholar]
- Yip JYH, Vanlerberghe GC (2001) Mitochondrial alternative oxidase acts to dampen the generation of active oxygen species during a period of rapid respiration induced to support a high rate of nutrient uptake. Physiol Plant 112 327–337 [DOI] [PubMed] [Google Scholar]
- Yu Z, Morais D, Ivanga M, Harrison PM (2007) Analysis of the role of retrotransposition in gene evolution in vertebrates. BMC Bioinformatics 8 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.