Abstract
The effects of SB-772077-B [4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine], an aminofurazan-based Rho kinase inhibitor, on the pulmonary vascular bed and on monocrotaline-induced pulmonary hypertension were investigated in the rat. The intravenous injections of SB-772077-B decreased pulmonary and systemic arterial pressures and increased cardiac output. The decreases in pulmonary arterial pressure were enhanced when pulmonary vascular resistance was increased by U46619 [9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α], hypoxia, or Nω-nitro-l-arginine methyl ester. SB-772077-B was more potent than Y-27632 [trans-4-[(1R)-1-aminoethyl]-N-4-pyridinyl-cyclohexanecarboxamide dihydrochloride] or fasudil [5-(1,4-diazepane-1-sulfonyl)isoquinoline] in decreasing pulmonary and systemic arterial pressures. The results with SB-772077-B, fasudil, and Y-27632 suggest that Rho kinase is constitutively active and is involved in the regulation of baseline tone and vasoconstrictor responses. Chronic treatment with SB-772077-B attenuated the increase in pulmonary arterial pressure induced by monocrotaline. The intravenous injection of SB-772077-B decreased pulmonary and systemic arterial pressures in rats with monocrotaline-induced pulmonary hypertension. The decreases in pulmonary arterial pressure in response to SB-772077-B in monocrotaline-treated rats were smaller than responses in U46619-infused animals, and the analysis of responses suggests that approximately 60% of the pulmonary hypertensive response is mediated by a Rho kinase-sensitive mechanism. The observation that Rho kinase inhibitors decrease pulmonary arterial pressure when pulmonary vascular resistance is increased by interventions such as hypoxia, U46619, angiotensin II, nitric-oxide synthase inhibition, and Bay K 8644 [S-(-)-1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-[trifluoromethyl]phenyl)-3-pyridine carboxylic acid methyl ester] suggest that the vasodilatation is independent of the mechanisms used to increase intracellular calcium and promote vasoconstriction. The present results suggest that SB-772077-B would be beneficial in the treatment of pulmonary hypertensive disorders.
The small GTPase Rho A and its downstream effector, the Rho-associated coil-forming serine/threonine kinases (ROCKs) regulate a variety of physiologic functions, including vascular smooth muscle contraction (Amano et al., 2000; Riento and Ridley, 2003). ROCK-1 and ROCK-2 are two isoforms that are expressed in most cells and increase calcium sensitization by decreasing myosin phosphatase activity, leading to increased myosin light-chain phosphorylation and smooth muscle contraction (Leung et al., 1995; Amano et al., 1996; Ishizaki et al., 1996; Kimura et al., 1996; Matsui et al., 1996; Kureishi et al., 1997; Somlyo and Somlyo, 2000, 2003). Rho kinase activity is up-regulated in a number of cardiovascular diseases, including pulmonary hypertension, and Rho kinase inhibitors have a beneficial effect in the treatment of pulmonary hypertensive disorders (Uehata et al., 1997; Seko et al., 2003; Abe et al., 2004; Rikitake and Liao, 2005; Budzyn et al., 2006). In addition to the potential role of Rho kinase in pulmonary hypertension, it has been proposed recently that Rho kinase is involved in the regulation of baseline tone in the cardiovascular system and that ROCK inhibition can blunt vasoconstrictor responses independent of the method used to promote vasoconstriction (Dhaliwal et al., 2007; Badejo et al., 2008). The studies on the role of ROCK have been assisted by the development of selective Rho kinase inhibitors, and Y-27632 and fasudil are the prototypical agents that selectively target p1601 ROCK (Asano et al., 1987; Ishizaki et al., 1996; Leung et al., 1996; Uehata et al., 1997). Both agents have a beneficial effect in rodent models with pulmonary hypertension, and fasudil has been used in clinical studies (Nagaoka et al., 2004; Abe et al., 2004; Fukumoto et al., 2005; Ishikura et al., 2006). ROCK inhibitors with improved potency and selectivity have been developed. SB-772077-B is a novel aminofurazan-based ROCK inhibitor with anti-inflammatory activity and improved selectivity for some protein kinases but only 3- to 6-fold selectivity for mitogen- and stress-activated protein kinase 1 and ribosomal S6 kinase 1 (Doe et al., 2007). This agent has been shown to inhibit cytokine production by macrophages, relax precontracted aortic smooth muscle, and decrease systemic arterial pressure in spontaneously hypertensive rats and deoxycorticosterone acetate salt-sensitive hypertensive rats when given orally (Doe et al., 2007). Because of reported beneficial effects of Y-27632 and fasudil in pulmonary hypertension disorders, the present study was undertaken to investigate responses to a novel ROCK inhibitor in the pulmonary and systemic vascular beds in the rat and to evaluate the beneficial effect of this agent in the treatment of monocrotaline-induced pulmonary hypertension. The results of these studies show that SB-772077-B has potent vasodilator activity in the pulmonary and systemic vascular beds and has a beneficial effect in the treatment of monocrotaline-induced pulmonary hypertension in the rat. The present results indicate that this Rho kinase inhibitor with improved protein kinase selectivity and potency compared with Y-27632 and fasudil does not have selective pulmonary vasodilator activity and produces marked decreases in systemic arterial pressure in monocrotaline-treated rats.
Materials and Methods
The experimental protocol used was approved by the Institutional Animal Care and Use Committee of the Tulane University School of Medicine. All experimental procedures were conducted in accordance with institutional guidelines. Two types of experiments were performed in this study. In the first type of experiments, hemodynamic measurements were made in control animals that were not treated with monocrotaline. In the second set of experiments, the rats were treated with monocrotaline (60 mg/kg i.v.), and hemodynamic measurements were made at later times. For all experiments, adult male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing 272 to 544 g were anesthetized with Inactin (thiobutabarbital sodium salt; 100 mg/kg i.p.) (Sigma-Aldrich, St. Louis, MO) and were placed in the supine position on an operating table. Supplemental doses of Inactin were administered intravenously to maintain a uniform level of anesthesia. Body temperature was maintained with a heating lamp. The trachea was cannulated with a short segment of PE-240 tubing to maintain a patent airway. The animals spontaneously breathed room air. In experiments with hypoxia, animals breathed a 10% O2 and 90% N2 gas mixture from a plastic hood placed over the end of the endotracheal tube. A femoral artery was catheterized with PE50 tubing for the measurement of systemic arterial pressure. The left jugular and femoral veins were catheterized with PE50 tubing for intravenous injections and infusions. For measurement of pulmonary arterial pressure, a 3F single lumen catheter with a curved tip and radio-opaque marker was passed form the right jugular vein into the pulmonary artery under fluoroscopic guidance (Picker-Surveyor Fluoroscope) as described previously (Dhaliwal et al., 2007; Badejo et al., 2008). In some experiments, a 3F catheter was passed into the left ventricle from the right carotid artery to measure left ventricular end-diastolic pressure as a measure of left atrial pressure. Vascular pressures, measured with Namic preceptor DT transducers, were digitized with a Biopac MP-100 data system and stored on a Dell personal computer. Cardiac output was measured by the thermodilution technique with a Cardiomax II (Columbus Instruments, Columbus, OH) computer. A known volume (0.2 ml) of room temperature 0.9% NaCl solution was injected into the jugular vein catheter with its tip near the right atrium. Changes in blood temperature were measured by a 1.5F thermistor microprobe (Columbus Instruments) positioned in the aortic arch from the left carotid artery.
Type 1 Experiments. In the first set of type 1 experiments, changes in pulmonary and systemic arterial pressure and cardiac output to intravenous injections of SB-772077-B (GlaxoSmithKline, Uxbridge, Middlesex, UK) dissolved in 0.9% NaCl were investigated under baseline conditions. The order of injection of the doses of SB-772077-B was randomized, and sufficient time (10–60 min) was permitted between injections for pressures to return toward baseline value.
In the second set of type 1 experiments, responses to intravenous injections of SB-772077-B were determined when pulmonary arterial pressure was increased to a high steady level by continuous intravenous infusion of the thromboxane (TP) receptor agonist U46619. U46619 (Cayman Chemical, Ann Arbor, MI) was dissolved in 95% ethyl alcohol and diluted with 0.9% NaCl solution. U46619 was infused with a Harvard infusion pump (Harvard Apparatus Inc., Holliston, MA). After starting the infusion at a high priming rate, the rate was adjusted to 240 to 400 ng/min to maintain pulmonary arterial pressure at approximately 30 mm Hg.
In the next set of type 1 experiments, the effects of treatment with the NOS inhibitor l-NAME on responses to SB-772077-B were investigated. The intravenous injections of the NOS inhibitor at doses of 5 to 25 mg/kg i.v. increased pulmonary and systemic arterial pressure and decreased cardiac output. After pulmonary and systemic arterial pressures had increased to a steady level after l-NAME injection, responses to intravenous injections of the Rho kinase inhibitor were determined.
In the next set of type 1 experiments, responses to intravenous injections of SB-772077-B were investigated when pulmonary arterial pressure was increased by ventilation with a hypoxic gas mixture (10% O2 and 90% N2). The intravenous injections of SB-772077-B were administered when pulmonary arterial pressure had reached a plateau (5–8 min). In pretreatment experiments, the effect of an intravenous injection of SB-772077-B, 5 min before the onset of ventilation with the 10% O2 and 90% N2 gas mixture, was initiated was also determined. Arterial PO2, PCO2, and pH were measured in a blood sample (0.2 ml) from the femoral artery with a Radiometer NPT analyzer.
The effect of intravenous injection of SB-772077-B on increases in pulmonary arterial pressure in response to intravenous injection of angiotensin II, U46619, and Bay K 8644 was investigated in the next set of type 1 experiments. In these experiments, responses to the vasoconstrictor agents were compared before and 5 min after intravenous injection of 300 μg/kg SB-772077-B.
Type 2 Experiments. In the first set of type 2 experiments, responses to intravenous injections of SB-772077-B were investigated in monocrotaline-treated rats. Monocrotaline (S. B. Penick and Company, Pennsville, NJ) was injected into the tail vein in a dose of 60 mg/kg. The hemodynamic parameters were assessed 21 and 28 days after treatment with monocrotaline in anesthetized rats, and responses to intravenous injections of the Rho kinase inhibitor were determined. The effects of treatment with SB-772077-B starting on day 15 after administration of monocrotaline were also investigated, and pulmonary and arterial pressures and cardiac output were measured on day 36.
The hemodynamic data are expressed as mean ± S.E. Pulmonary vascular resistance was calculated by dividing mean pulmonary arterial pressure by cardiac output after determining that left ventricular end-diastolic pressure was not changed by the Rho kinase inhibitor. Systemic vascular resistance was calculated by dividing mean systemic arterial pressure by the cardiac output. The data were analyzed using paired and grouped Student's t tests and analysis of variance with a post hoc test. The criteria for significance was p < 0.05.
Results
Cardiopulmonary Responses to SB-772077-B. Cardiopulmonary responses to SB-772077-B were investigated in the anesthetized rat, and these data are summarized in Fig. 1. The intravenous injection of the Rho kinase inhibitor in doses of 10 to 300 μg/kg caused small decreases in pulmonary arterial pressure, larger dose-dependent decreases in systemic arterial pressures, and increases in cardiac output (Fig. 1A).
Fig. 1.
Bar graphs comparing changes in pulmonary and systemic arterial pressures and cardiac output in response to intravenous injections of SB-772077-B (10–300 μg/kg) under control baseline conditions (A) and during continuous intravenous infusion of the TP receptor agonist U46619 to increase pulmonary arterial pressure to approximately 30 mm Hg (B). n, number of experiments. *, p < 0.05 compared with baseline value.
Responses to SB-772077-B were investigated when pulmonary arterial pressure was increased by intravenous infusion of the TP receptor agonist U46619 (Table 1). When pulmonary arterial pressure was increased to approximately 30 mm Hg with U46619, the intravenous injections of the Rho kinase inhibitor in doses of 10 to 300 μg/kg produced larger dose-dependent decreases in pulmonary arterial pressure, similar dose-dependent decreases in systemic arterial pressure, and increases in cardiac output (Fig. 1B). Inasmuch as cardiac output was increased, and left ventricular end-diastolic pressure was unchanged, the decreases in pulmonary and systemic arterial pressures indicate that pulmonary and systemic vascular resistances are decreased by the Rho kinase inhibitor.
TABLE 1.
Effect of U46619 infusion on systemic and pulmonary arterial pressure and on cardiac output Values are mean ± S.E.
| Systemic Arterial Pressure | Pulmonary Arterial Pressure | Cardiac Output | |
|---|---|---|---|
| mm Hg | ml/min | ||
| Control | 90 ± 7 | 18 ± 1 | 108 ± 5 |
| U46619 infusion | 87 ± 4 | 32 ± 1* | 92 ± 8* |
| n = 9–17 | |||
p < 0.05 compared with control
Comparison of Responses with Y-27632 and Fasudil. Responses to SB-772077-B were compared with responses to the prototypical Rho kinase inhibitors Y-27632 and fasudil, and these data are summarized in Fig. 2. In terms of relative potency, the dose-response curves for the decreases in systemic and pulmonary arterial pressures in response to intravenous injections of the three Rho kinase inhibitors when pulmonary arterial pressure was increased to similar values with U46619 were parallel (Fig. 2). The dose-response curves for SB-772077-B were 1 half-log unit to the left of the curves for Y-27632 and 1 log unit to the left of the curves for fasudil when doses are expressed on a micromole per kilogram basis (Fig. 2).
Fig. 2.
Dose-response curves comparing relative potency of SB-772077-B, Y-27632, and fasudil in decreasing pulmonary and systemic arterial pressures in U-44619-infused animals. n, number of experiments.
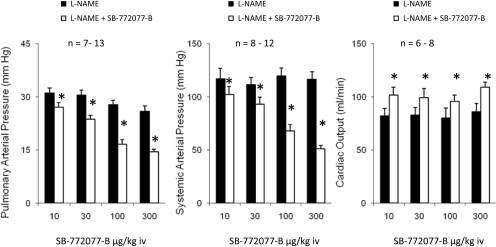
Responses in l-NAME-Treated Animals. Responses to SB-772077-B were investigated in l-NAME-treated animals, and these data are summarized in Fig. 3. The intravenous injection of l-NAME in doses of 5 to 25 mg/kg increased pulmonary and systemic arterial pressures and decreased cardiac output (Table 2). The intravenous injection of SB-772077-B produced significant dose-related decreases in pulmonary and systemic arterial pressures and increases in cardiac output, indicating that the Rho kinase inhibitor had potent pulmonary and systemic vasodilator activity in animals in which NOS was inhibited, and endothelial function was impaired (Fig. 3).
Fig. 3.
Bar graphs comparing decreases in pulmonary and systemic arterial pressure and increases in cardiac output in response to intravenous injections of SB-772077-B in l-NAME-treated animals. The intravenous injections of l-NAME in doses of 5 to 25 mg/kg i.v. produced a significant increase in pulmonary and systemic arterial pressures. n, number of experiments. *, significantly different from baseline.
TABLE 2.
Effect of l-NAME on systemic and pulmonary arterial pressure and on cardiac output Values are mean ± S.E.
| Systemic Arterial Pressure | Pulmonary Arterial Pressure | Cardiac Output | |
|---|---|---|---|
| mm Hg | ml/min | ||
| Control | 95 ± 4 | 17 ± 1 | 120 ± 3 |
| l-NAME (5–25 mg/kg) | 128 ± 5* | 30 ± 1* | 78 ± 5* |
| n = 12–16 | |||
p < 0.05 compared with control
Effects on the Hypoxic Pulmonary Vasoconstrictor Response. Ventilation with a 10% O2-90% N2 gas mixture decreased arterial PO2 from 80 to 32 mm Hg and increased pulmonary arterial pressure. When pulmonary arterial pressure was increased by ventilation with the 10% O2 and 90% N2 gas mixture, the intravenous injections of SB-772077-B decreased pulmonary arterial pressure in a dose-related manner (Fig. 4A). The injection of SB-772077-B in a dose of 300 μg/kg i.v. completely reversed the hypoxic pulmonary vasoconstrictor response (Fig. 4B). The administration of 300 μg/kg i.v. SB-772077-B 5 min before ventilation with the hypoxic gas mixture prevented the increase in pulmonary arterial pressure response to hypoxia (Fig. 4C).
Fig. 4.
Bar graphs showing the decreases in pulmonary arterial pressure in response to SB-772077-B when pulmonary arterial pressure was increased by ventilation with the 10% O2/90% N2 gas mixture. The Rho kinase inhibitor was injected when the increase in pulmonary arterial pressure reached a peak in response to ventilation with the hypoxic gas mixture (A). The bar graph in B shows that the hypoxic pulmonary vasoconstrictor response is completely reversed by the intravenous injection of SB-772077-B (300 μg/kg) injected at the peak of the response to hypoxia. Bar graph in C shows that the hypoxic pulmonary vasoconstrictor response is prevented by intravenous injections of SB-772077-B (300 μg/kg) 5 min before the hypoxic response was initiated. n, number of experiments. *, response is significantly different from control.
Effect of SB-772077-B on Responses to Vasoconstrictor Agents. The effects of the Rho kinase inhibitor on responses to the vasoconstrictor agents are summarized in Fig. 5. The intravenous injections of angiotensin II, Bay K 8644, and U46619 increased pulmonary arterial pressure, and the increases in pulmonary arterial pressure were reduced significantly by intravenous injections of 300 μg/kg i.v. SB-772077-B 5 min before intravenous injection of the vasoconstrictor agents (Fig. 5).
Fig. 5.
Effect of SB-772077-B on increases in pulmonary arterial pressure in response to bolus intravenous injections of angiotensin II, U46619, and Bay K 8644. The vasoconstrictor agonists were injected before and 5 min after intravenous injection of SB-772077-B (300 μg/kg). n, number of experiments. *, significantly different from control.
Effect of SB-772077-B in Monocrotaline-Treated Animals. The intravenous injection of monocrotaline increases pulmonary arterial pressure in the rat, and the pulmonary hypertensive response develops over a period of weeks (Table 3). The effect of chronic treatment with SB-772077-B on the development of pulmonary hypertension in the monocrotaline-treated rat was investigated, and these data are summarized in Fig. 6. In animals treated with monocrotaline, mean pulmonary arterial pressure averaged 46 ± 4 mm Hg when the animals were catheterized, and right heart pressures were measured 28 days after the administration of the plant alkaloid (Table 3). When monocrotaline-treated animals were injected with 3 or 6 mg/kg i.p. SB-772077-B starting on days 15 through 35, pulmonary arterial pressure averaged 28 ± 2 mm Hg on day 36, when right heart pressures were measured (Fig. 6). Systemic arterial pressure was not changed significantly compared in monocrotaline-treated and monocrotaline and SB-772077-B-treated animals on days 29 and 36 after intravenous injection of the plant alkaloid (Table 3).
TABLE 3.
Effect of monocrotaline and treatment with SB-772077-B on systemic and pulmonary arterial pressure and on cardiac output Values are mean ± S.E.
| n | Systemic Arterial Pressure | Pulmonary Arterial Pressure | Cardiac Output | |
|---|---|---|---|---|
| mm/Hg | ml/min | |||
| Control | 7–11 | 95 ± 5 | 17 ± 1 | 110 ± 8 |
| Monocrotaline (28 days) | 9–10 | 90 ± 7 | 46 ± 4* | 91 ± 9 |
| Monocrotaline + SB-772077-B (35 days) | 8 | 96 ± 9 | 28 ± 2** | 102 ± 8 |
p < 0.05 compared with control
p < 0.05 when compared with monocrotaline
Fig. 6.
Effect of intravenous injections of SB-772077-B on pulmonary and systemic arterial pressures and cardiac output in monocrotaline-treated rats. The responses to SB-772077-B were measured on day 29 after treatment with the plant alkaloid in a dose of 60 mg/kg i.v.
In addition to attenuating monocrotaline-induced pulmonary hypertension, it has been suggested that Rho kinase inhibitors have a selective vasodilator effect in the pulmonary vascular bed. To determine whether SB-772077-B has a selective effect, decreases in pulmonary and systemic arterial pressure response to intravenous injections of the Rho kinase inhibitor were evaluated in monocrotaline-treated rats. The intravenous injection of SB-772077-B in monocrotaline-treated rats produced significant decreases in pulmonary and systemic arterial pressures, and the percentage decreases in pulmonary arterial pressure were not greater than percent decreases in systemic arterial pressure (Fig. 6).
The intravenous injection of the 300 μg/kg dose of SB-772077-B decreased pulmonary arterial pressure to 23 ± 4 mm Hg in monocrotaline-treated rats and to 13 ± 1 in control untreated rats. The difference in the lowest value or nadir in pulmonary arterial pressure in response to SB-772077-B injection in control and in monocrotaline-treated animals may provide an estimate of fixed and reversible vascular resistance in the pulmonary vascular bed in monocrotaline-treated animals. The comparison of decreases in pulmonary arterial pressure in response to intravenous injection of SB-772070-B 300 μg/kg in control and in monocrotaline-treated animals suggests that 60% of the increase in resistance is reversible, and 40% is fixed (Fig. 7).
Fig. 7.
Comparison of decreases in pulmonary arterial pressure in response to intravenous injection of SB-772077-B (300 μg/kg) in control animals and in animals with monocrotaline-induced pulmonary hypertension. The decrease in pulmonary arterial pressure in response to the Rho kinase inhibitor provides an estimate of the amount of vasoconstrictor tone in the pulmonary vascular bed in control animals under baseline conditions. The decrease in pulmonary arterial pressure in response to intravenous injection of SB-772077-B in monocrotaline-treated animals provides an estimate of the amount of SB-772077-B reversible vasoconstrictor tone in rats with monocrotaline-induced pulmonary hypertension. The difference between the baseline value of tone or nadir in the control animals and in the monocrotaline-treated animals provides an estimate of the amount of SB-772077-B nonreversible (or fixed) tone. These data suggest that 60% of the vasoconstrictor tone in the monocrotaline-treated animals is reversible tone, and 40% of the tone is fixed due to structural alterations in the pulmonary vascular bed. n, number of animals. *, p < 0.05 compared with initial value.
Discussion
Results of the present study show that SB-772077-B has potent vasodilator activity in the pulmonary and systemic vascular beds in the rat and was more potent than Y-27632 or fasudil in U46619-infused animals. This novel aminofurazan-based Rho kinase inhibitor had a beneficial effect in monocrotaline-induced pulmonary hypertension, and animals treated with SB-772077-B for 21 days had significantly lower pulmonary arterial pressures compared with untreated control rats. The comparison of responses to SB-772077-B in control and monocrotaline-treated animals can provide an estimate of the amount of reversible and fixed vasoconstrictor tone in the pulmonary vascular bed in animals with pulmonary hypertension.
The intravenous injection of 300 μg/kg SB-772077-B reversed the pulmonary hypertensive response to U46619 infusion, l-NAME treatment, and ventilatory hypoxia, whereas in monocrotaline-treated animals, the intravenous injection of the Rho kinase inhibitor decreased pulmonary arterial pressure to 28 ± 2 mm Hg. These data suggest that approximately 60% of the vasoconstrictor tone is mediated by a SB-772077-B reversible mechanism in the monocrotaline-treated animal, and approximately 40% of the vasoconstrictor tone may represent fixed resistance, consistent with studies with fasudil in which right ventricular systolic pressure was measured (Oka et al., 2007).
Recent studies have provided evidence that Rho kinase-mediated calcium sensitization plays an important role in the regulation of vasoconstrictor tone in the pulmonary vascular bed (Dhaliwal et al., 2007; Badejo et al., 2008). In addition to reversing pulmonary hypertensive responses to U46619, l-NAME, and ventilatory hypoxia, SB-772077-B decreased pulmonary arterial pressure in monocrotaline-treated rats with pulmonary hypertension in which endothelial function is impaired (Baber et al., 2007). These data are consistent with the hypothesis that the Rho kinase inhibitor can reverse pulmonary hypertension independent of the mechanism used to promote vasoconstriction, including l-NAME-induced endothelial dysfunction. The observation that increases in pulmonary arterial pressure in response to Bay K 8644 are attenuated by the Rho kinase inhibitor suggest that vasoconstrictor responses can be suppressed independent of the mechanism used to increase intracellular calcium levels.
The intravenous injections of SB-772077-B in animals with monocrotaline-induced pulmonary hypertension decreased pulmonary and systemic arterial pressures. The comparison of decreases in pulmonary and systemic arterial pressure in the pulmonary hypertensive animals indicates that SB-772077-B does not have selective pulmonary vasodilator activity.
Studies on the role of Rho kinase in the regulation of vasoconstrictor tone have been aided by the development of selective Rho kinase inhibitors. Results with the prototypical inhibitors, fasudil and Y-27632, have provided support for the concept that Rho kinase plays an important role in the regulation of baseline tone and vasoconstrictor responses in the pulmonary vascular bed (Nagaoka et al., 2004; Dhaliwal et al., 2007; Badejo et al., 2008). SB-772077-B is a member of a novel class of aminofurazan-based inhibitors with anti-inflammatory activity and enhanced potency and selectivity for ROCK 1 and 2 (Doe et al., 2007). The intravenous injections of SB-772077-B decreased pulmonary and systemic arterial pressures and increased cardiac output. Inasmuch as left ventricular end-diastolic pressure is not changed, these data indicate that SB-772077-B has significant vasodilator activity in the pulmonary and systemic vascular beds. The decreases in pulmonary arterial pressure were modest under baseline conditions when vasoconstrictor tone was low but were enhanced when pulmonary vascular resistance was increased, and these data are similar to studies with PGI2 and other vasodilator agents in the pulmonary vascular bed (Hyman and Kadowitz, 1979; Casey et al., 2008). Under elevated tone conditions, SB-772077-B was more potent than the prototypical inhibitors in decreasing pulmonary and systemic arterial pressure. Although dose-response curves for the novel aminofurazan inhibitor were 1 half-log unit and 1 log unit to the left of curves for Y-27632 and fasudil, the three agents decreased pulmonary and systemic arterial pressure in a similar nonselective manner and had similar efficacy.
Hypoxic pulmonary vasoconstriction is an important mechanism for the matching of ventilation with perfusion in the lung (von Euler and Liljestrand, 1946). Although the mechanism by which ventilatory hypoxia increases pulmonary arterial pressure is uncertain, most studies show that intracellular calcium concentration is increased in small intrapulmonary arteries (Evans and Dipp, 2002; Moudgil et al., 2005; Gupte and Wolin, 2008). It also has been hypothesized that Rho kinase-mediated calcium sensitization is involved in the hypoxic pulmonary vasoconstrictor response (Robertson et al., 2000; Aaronson et al., 2006). The results of the present study showing that SB-772077-B prevents and reverses the acute hypoxic pulmonary vasoconstrictor response is consistent with this hypothesis (Robertson et al., 2000; Aaronson et al., 2006). However, this is not a specific effect, and the doses of SB-772077-B that attenuate the response to hypoxia inhibit responses to other vasoconstrictor interventions in the pulmonary vascular bed, including Bay K 8644, an agent that promotes calcium entry in vascular smooth muscle cells. The present results and previous studies with fasudil and isradipine, an L-type calcium entry-blocking agent, suggest that inhibition of calcium entry or inhibition of Rho kinase have similar effects in preventing or reversing hypoxic pulmonary vasoconstriction in the intact rat (Badejo et al., 2008).
The results of the present study show that chronic treatment with SB-772077-B had a beneficial effect in attenuating the increase in pulmonary arterial pressure in response to monocrotaline. In addition, systemic arterial pressure and cardiac output were preserved in SB-772077-B-treated animals. The observation that systemic arterial pressure is normal at the same time the pulmonary hypertensive response was attenuated suggests that the chronic treatment with SB-772077-B was effective. The mechanism by which Rho kinase inhibitors produce a beneficial effect is unknown and is under investigation in many laboratories. In addition, these studies show that the Rho kinase inhibitor does not have a selective pulmonary vasodilator effect in animals with monocrotaline-induced pulmonary hypertension. These results are different from results with fasudil in monocrotaline-treated animals (Jiang et al., 2007). In the studies with fasudil, the oral administration of the Rho kinase inhibitor was reported to decrease pulmonary arterial pressure in a dose that did not significantly decrease systemic arterial pressure (Jiang et al., 2007). The reason for the difference in results is uncertain; however, in those studies fasudil was administered by oral gavage in conscious rats (Jiang et al., 2007). In future studies, we will investigate the effects of SB-772077-B when administered by oral gavage to determine whether route of administration can have an influence on relative vasodilator responses in the pulmonary and systemic vascular beds.
The mechanism by which Rho kinase inhibitors produce a beneficial effect in monocrotaline-induced pulmonary hypertension has not been established. SB-772077-B and Y-27632 have been shown to inhibit LPS-induced release of IL-6 and TNF-α from macrophages (Doe et al., 2007). The disruption of actin stress fiber formation and the inhibition of inflammatory cytokine release may have a role in the beneficial effect of the Rho kinase inhibitors in reducing the remodeling that occurs in the pulmonary vascular bed in monocrotaline-treated animals (Riento and Ridley, 2003; Abe et al., 2004; Xing et al., 2006; Oka et al., 2007). The results of the present study show that SB-772077-B treatment starting 14 days after administration of monocrotaline and continued for 3 weeks markedly reduced the pulmonary hypertensive response to monocrotaline. The mechanism by which the Rho kinase inhibitors produce their beneficial effect is under study. It has been reported that fasudil treatment has a beneficial effect in the treatment of pulmonary hypertension in two small clinical studies (Fukumoto et al., 2005; Ishikura et al., 2006). In future studies, we will investigate the effect of oral administration and intraperitoneal injection of SB-772077-B on mortality in monocrotaline-treated rats.
In summary, the results of the present study show that chronic administration of SB-772077-B has a beneficial effect in the treatment of monocrotaline-induced pulmonary hypertension. In addition, this novel Rho kinase inhibitor had potent vasodilator activity in the pulmonary and systemic vascular beds and decreased pulmonary arterial pressure in monocrotaline-treated animals in a nonselective manner. The present data show that SB-772077-B attenuates pulmonary vasoconstrictor responses mediated by diverse mechanisms, including G-coupled receptor activation, enhanced calcium entry, hypoxia, and NOS inhibition. Although SB-772077-B was more potent than the prototypical Rho kinase inhibitors, fasudil and Y-27632, it was similar to these agents in that it does not have selective vasodilator effect in the pulmonary vascular bed. The experiments with SB-772077-B indicate that approximately 60% of the pulmonary hypertensive response to monocrotaline can be reversed by the Rho kinase inhibitor and that this represents the reversible component of pulmonary hypertension in the monocrotaline-treated rat. The present data suggest that chronic administration of SB-772077-B would be useful in the treatment of pulmonary hypertensive disorders, although this agent does not have selective pulmonary vasodilator activity.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL62000, HL77421, F31-HL091722–01].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.151449.
ABBREVIATIONS: ROCK, Rho-associated coil-forming serine/threonine kinase; Y-27632, trans-4-[(1R)-1-aminoethyl]-N-4-pyridinyl-cyclohexanecarboxamide dihydrochloride; fasudil, 5-(1,4-diazepane-1-sulfonyl)isoquinoline; SB-772077-B, 4-(7-((3-amino-1-pyrrolidinyl)carbonyl)-1-ethyl-1H-imidazo(4,5-c)pyridin-2-yl)-1,2,5-oxadiazol-3-amine; TP, thromboxane; NOS, nitric-oxide synthase; l-NAME, Nω-nitro-l-arginine methyl ester; Bay K 8644, S-(-)-1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-[trifluoromethyl]phenyl)-3-pyridine carboxylic acid methyl ester; U46619, 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α.
References
- Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, and Ward JPT (2006) Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol 570 53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, et al. (2004) Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94 385-393. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y, and Kaibuchi K (2000) Regulation and functions of Rho-associated kinase. Exp Cell Res 261 44-51. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, and Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271 20246-20249. [DOI] [PubMed] [Google Scholar]
- Asano T, Ikegaki I, Satoh S, Suzuki Y, Shibuya M, Takayasu M, and Hidaka H (1987) Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther 241 1033-1040. [PubMed] [Google Scholar]
- Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, Hyman AL, and Kadowitz PJ (2007) Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol 292 H1120-H1128. [DOI] [PubMed] [Google Scholar]
- Badejo AM Jr, Dhaliwal JS, Casey DB, Gallen TB, Greco AJ, and Kadowitz PJ (2008) Analysis of pulmonary vasodilator responses to the Rho-kinase inhibitor fasudil in the anesthetized rat. Am J Physiol Lung Cell Mol Physiol 295 L828-L836. [DOI] [PubMed] [Google Scholar]
- Budzyn K, Marley PD, and Sobey CG (2006) Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci 27 97-104. [DOI] [PubMed] [Google Scholar]
- Casey DB, Badejo AM Jr, Dhaliwal JS, Murthy SN, Hyman AL, Nossaman BD, and Kadowitz PJ (2009) Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol sensitive mechanism in the rat. Am J Physiol Heart Circ Physiol 296 H524-H533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal JS, Casey DB, Greco AJ, Badejo AM Jr, Gallen TB, Murthy SN, Nossaman BD, Hyman AL, and Kadowitz PJ (2007) Rho-kinase and Ca++ entry mediate increased pulmonary and systemic vascular resistance in L-NAME treated rats. Am J Physiol Lung Cell Mol Physiol 293 L1306-L1313. [DOI] [PubMed] [Google Scholar]
- Doe C, Bentley R, Behm DJ, Lafferty R, Stavenger R, Jung D, Bamford M, Panchal T, Grygielko E, Wright LL, et al. (2007) Novel Rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther 320 89-98. [DOI] [PubMed] [Google Scholar]
- Evans AM and Dipp M (2002) Hypoxic pulmonary vasoconstriction: cyclic adenosine diphosphate-ribose, smooth muscle Ca(2+) stores and the endothelium. Respir Physiol Neurobiol 132 3-15. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, and Shimokawa H (2005) Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 91 391-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte SA and Wolin MS (2008) Oxidant and redox signaling in vascular oxygen sensing: implications for systemic and pulmonary hypertension. Antioxid Redox Signal 10 1137-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AL and Kadowitz PJ (1979) Pulmonary vasodilator activity of prostacyclin (PGI2) in the cat. Circ Res 45 404-409. [DOI] [PubMed] [Google Scholar]
- Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, and Nakano T (2006) Beneficial acute effects of Rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J 70 174-178. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, et al. (1996) The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 15 1885-1893. [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, and Shimokawa H (2007) Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol 49 85-89. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273 245-248. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, and Ito M (1997) Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272 12257-12260. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, and Lim L (1996) The p160 Rho-A binding kinase ROCKa is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16 5313-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, and Lim L (1995) A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem 270 29051-29054. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, and Kaibuchi K (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15 2208-2216. [PMC free article] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, and Archer SL (2005) Hypoxic pulmonary vasoconstriction. J Appl Physiol 98 390-403. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, and Oka M (2004) Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 287 L665-L672. [DOI] [PubMed] [Google Scholar]
- Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, and McMurtry IF (2007) Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100 923-929. [DOI] [PubMed] [Google Scholar]
- Riento K and Ridley AJ (2003) ROCKs: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4 446-456. [DOI] [PubMed] [Google Scholar]
- Rikitake Y and Liao JK (2005) ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther 3 441-451. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson TP, Dipp M, Ward JP, Aaronson PI, and Evans AM (2000) Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol 131 5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, and Nakano T (2003) Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 92 411-418. [DOI] [PubMed] [Google Scholar]
- Somlyo AP and Somlyo AV (2000) Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522 177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP and Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83 1325-1358. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389 990-994. [DOI] [PubMed] [Google Scholar]
- von Euler US and Liljestrand G (1946) Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand 12 310-320. [Google Scholar]
- Xing XQ, Gan Y, Wu SJ, Chen P, Zhou R, and Xiang XD (2006) Rho-kinase as a potential therapeutic target for the treatment of pulmonary hypertension. Drug News Perspect 19 517-522. [DOI] [PubMed] [Google Scholar]