Abstract
Background and purpose:
Alogliptin, a highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor, enhances incretin action and pioglitazone enhances hepatic and peripheral insulin actions. Here, we have evaluated the effects of combining these agents in diabetic mice.
Experimental approach:
Effects of short-term treatment with alogliptin alone (0.01%–0.1% in diet), and chronic combination treatment with alogliptin (0.03% in diet) and pioglitazone (0.0075% in diet) were evaluated in db/db mice exhibiting early stages of diabetes.
Key results:
Alogliptin inhibited plasma DPP-4 activity up to 84% and increased plasma active glucagon-like peptide-1 by 4.4- to 4.9-fold. Unexpectedly, alogliptin alone lacked clear efficacy for improving glucose levels. However, alogliptin in combination with pioglitazone clearly enhanced the effects of pioglitazone alone. After 3–4 weeks of treatment, combination treatment increased plasma insulin by 3.8-fold, decreased plasma glucagon by 41%, both of which were greater than each drug alone, and increased plasma adiponectin by 2.4-fold. In addition, combination treatment decreased glycosylated haemoglobin by 2.2%, plasma glucose by 52%, plasma triglycerides by 77% and non-esterified fatty acids by 48%, all of which were greater than each drug alone. Combination treatment also increased expression of insulin and pancreatic and duodenal homeobox 1 (PDX1), maintained normal β-cell/α-cell distribution in islets and restored pancreatic insulin content to levels comparable to non-diabetic mice.
Conclusions and implications:
These results indicate that combination treatment with alogliptin and pioglitazone at an early stage of diabetes improved metabolic profiles and indices that measure β-cell function, and maintained islet structure in db/db mice, compared with either alogliptin or pioglitazone monotherapy.
Keywords: type 2 diabetes mellitus, alogliptin, pioglitazone, dipeptidyl peptidase-4, incretin, glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide, pancreatic islets, db/db mice
Introduction
Type 2 diabetes is a chronic, progressive disease that is associated with considerable co-morbidities and mortality (Scheen, 2007). The progressive nature of type 2 diabetes is largely due to the gradual loss of β-cell function and increase in insulin resistance over time (Giorgino et al., 2005; Rendell and Jovanovic, 2006). As chronic hyperglycaemia is a major contributing factor to the complications associated with type 2 diabetes (Ceriello, 2003; Giorgino et al., 2005), the primary goal for treating this disease is to restore normoglycaemia, both under fasting conditions and postprandially. However, due to disease progression, the effectiveness of oral anti-hyperglycaemic drugs decreases over time and a combination of several drugs is required to achieve and maintain adequate glycaemic control (Turner et al., 1999; Charpentier, 2002).
Dipeptidyl peptidase-4 (DPP-4) is an enzyme expressed on the endothelial lining of the vasculature and on the cell surfaces of a variety of organs; it is also present in soluble form in the circulation (Lambeir et al., 2003). DPP-4 degrades and inactivates the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (Lambeir et al., 2003; Drucker and Nauck, 2006). The incretins are released from gut endocrine cells in response to nutrient ingestion and play an important role in glucose homeostasis by stimulating glucose-dependent insulin secretion (Vilsboll and Holst, 2004; Drucker, 2007) and exhibiting trophic effects on pancreatic β-cells (Farilla et al., 2002; Rolin et al., 2002; Perfetti and Hui, 2004). Selective inhibitors of DPP-4 have thus recently emerged as a new class of agents to improve glycaemic control in patients with type 2 diabetes, having a mechanism of action distinct from that of other existing classes of oral anti-hyperglycaemic agents (Deacon, 2004; Drucker and Nauck, 2006). By increasing active forms of the incretin hormones, inhibitors of DPP-4 decrease both postprandial and fasting plasma glucose levels in patients with type 2 diabetes (Miller and St Onge, 2006; Ristic and Bates, 2006).
Alogliptin is an orally available, quinazolinone-based, non-covalent DPP-4 inhibitor under development as a once-daily treatment for type 2 diabetes (Feng et al., 2007). Alogliptin exhibits a IC50 value of approximately 6.9 nmol·L−1 for human recombinant DPP-4 compared with IC50 values of >100 000 nmol·L−1 for closely related serine proteases (DPP-2, DPP-8, DPP-9, fibroblast activation protein/seprase, prolyl endopeptidase and tryptase), indicating that it is a potent and highly selective inhibitor of DPP-4 (Feng et al., 2007; Lee et al., 2008). Alogliptin also improves glycaemic control in obese Wistar fatty rats and obese ob/ob mice (Feng et al., 2007; Moritoh et al., 2008)
Pioglitazone is a commercially available member of the thiazolidinedione (TZD) class of anti-hyperglycaemic agents (Ceriello et al., 2005; Waugh et al., 2006). It activates the nuclear receptor peroxisome proliferator-activated receptor-γ and improves glycaemic control in patients with type 2 diabetes by increasing insulin sensitivity in the liver, adipose tissue and skeletal muscle, increasing peripheral glucose uptake and decreasing hepatic glucose production (Yki-Jarvinen, 2004).
Because of the complementary mechanisms of action of DPP-4 inhibitors and TZDs, combination therapy with these agents may provide additive or synergistic improvements in glycaemic control. Recently, DPP-4 inhibitors sitagliptin and vildagliptin in combination with pioglitazone therapy have shown beneficial effects in patients with type 2 diabetes that was inadequately controlled with pioglitazone alone (Rosenstock et al., 2006; Garber et al., 2007). Furthermore, initial treatment with vildagliptin plus pioglitazone in drug-naive patients with type 2 diabetes resulted in improved glycaemic control when compared with monotherapy (Rosenstock et al., 2007). However, the effects of combination treatment with DPP-4 inhibitors and TZDs on diabetic indices and on pancreatic β-cell function remain poorly understood.
Dipeptidyl peptidase-4 inhibitors have been shown to improve glucose intolerance in db/db mice with early-stage diabetes (6 weeks old) but not in the late-stage of the disease (23 weeks old) (Nagakura et al., 2003), suggesting that DPP-4 inhibitors are more effective in the presence of functional β-cells. In addition, because the mechanism of action of pioglitazone is dependent on the presence of insulin (Mizushige et al., 2002), its effects have been thought to be more pronounced in the early stages of diabetes where insulin secretion is preserved. The present study was thus designed to evaluate the effects of combination treatment with alogliptin and pioglitazone on glycaemic control, lipid and hormone profiles, and indices of β-cell function in db/db mice with early-stage type 2 diabetes.
Methods
Animals
The care and use of animals and the experimental protocols used in this research were approved by the Experimental Animal Care and Use Committee of Takeda Pharmaceutical Company, Ltd. (Osaka, Japan). Male Leprdb/Leprdb (db/db) mice and their non-diabetic Leprdb/+ (db/+) male littermates were obtained from Clea Japan (BKS.Cg –m+/+Leprdb/Jcl). All mice were housed in individual metal cages in a room with controlled temperature (23°C), humidity (55%) and lighting (lights on from 7:30 am to 7:30 pm) and were maintained on a laboratory chow diet (CE-2, Clea, Tokyo, Japan).
Assays for metabolic components
Glycosylated haemoglobin levels were analysed by an high performance liquid chromatography-based method using an automated analyser HLC-723 G7 (Tosoh, Tokyo, Japan). Plasma glucose, triglyceride and non-esterified fatty acid (NEFA) levels were measured using an autoanalyser 7080 (Hitachi, Tokyo, Japan). Plasma insulin (Rat Insulin RIA Kit; Millipore, MA, USA), glucagon (Glucagon Kit Daiichi; TFB, Tokyo, Japan) and adiponectin (Mouse Adiponectin RIA Kit; Millipore, MA, USA) levels were determined by radioimmunoassay (RIA). Plasma active GLP-1 levels [Glucagon-Like Peptide-1 (Active) ELISA Kit; Millipore, MA, USA] were determined by enzyme-linked immunosorbent assay.
Plasma DPP-4 assay
To measure DPP-4 activity, 10 µL of plasma was mixed with 40 µL of assay buffer containing 250 mmol·L−1 Tris-HCl (pH 7.5), 0.25% (wt·vol−1) bovine serum albumin (BSA) and 0.125% (wt·vol−1) 3-[(3-cholamidopropyl)dimethylammonio]propanesulphonic acid (CHAPS, Dojindo, Kumamoto, Japan) in 96-well microtiter plates. The samples were then mixed with 50 µL of 1 mmol·L−1 Gly-Pro-pNA·Tos (Peptide Institute, Osaka, Japan) to initiate the reaction and incubated at 30°C on a plate shaker. At 20 and 60 min after reaction initiation, DPP-4 activity was determined by monitoring the increase in absorbance at 405 nm using a microtiter plate reader (Dainippon Sumitomo Pharma, Osaka, Japan). Plasma DPP-4 activity of the treated mice was compared with that of the vehicle-treated db/db mice, which was set as 100%.
Short-term study in db/db mice
After an acclimation period of 12 days, 8 week old db/db mice were divided into four groups (eight mice per group) based on body weight and food consumption and fed a powder CE-2 diet containing 0.01%, 0.03% or 0.1% of alogliptin for 2 days. Control db/db and db/+ mice (eight and five mice respectively) were fed a drug-free CE-2 diet (vehicle). After 2 days of treatment, blood samples were collected, and plasma active GLP-1 levels and plasma DPP-4 activity were determined.
Chronic study in db/db mice
After an acclimation period of 6 days, 6 week old db/db mice were divided into four groups (eight mice per group) based on glycosylated haemoglobin, plasma glucose, plasma insulin and body weight. The mice were fed a powder CE-2 diet containing 0.03% alogliptin (equivalent to 76.4 mg·kg−1·day−1) alone, 0.0075% pioglitazone (15.4 mg·kg−1·day−1) alone or 0.03% alogliptin (56.5 mg·kg−1·day−1) and 0.0075% pioglitazone (14.1 mg·kg−1·day−1) combined. Control db/db and db/+ mice (eight and four mice respectively) were fed a drug-free CE-2 diet (vehicle). After 14 and 21 days of treatment, blood samples were collected from the orbital veins under feeding conditions and glycosylated haemoglobin, plasma glucose, triglyceride, NEFA and insulin levels were determined. Plasma DPP-4 activity was determined after 21 days and plasma glucagon and adiponectin levels were determined after 23 days of treatment. After 25 days of treatment, the mice were fasted for 17 h and received an oral glucose tolerance test (OGTT) followed by isolation of the pancreas (after the 26 day study period). Body weight and food consumption were recorded at regular intervals. The animals were 8 weeks of age after 14 days of treatment, approximately 9 weeks of age after 21 and 23, and approximately 10 weeks of age after the 26 day study period.
OGTT
After 25 days of treatment, the mice were fasted for 17 h and then given an oral glucose load (0.5 g·kg−1). Blood samples were collected at specified time points of 0 (pre-glucose/fasting glucose levels), 15, 30, 60 and 120 min post glucose for the determination of plasma glucose levels. The total area under the glucose curve was determined from time 0 to 120 min (AUC0–120 min) after glucose administration. Fasting plasma triglyceride levels were determined using the samples obtained at 0 min.
Isolation of the pancreas and measurement of insulin and glucagon content
Mice were killed by carbon dioxide inhalation after an overnight fast. Pancreata were isolated and cut into two sections. One section was homogenized in acid-ethanol containing 74% ethanol with 0.15 mol·L−1 HCl for the determination of insulin and glucagon concentrations, and the other section was placed in Bouin's fixative solution (Polysciences; Warrington, PA, USA) for immunohistochemical analysis. The homogenized tissues were extracted overnight at 4°C and centrifuged at 12 000×g for 10 min. The resultant supernatants were then diluted with PBS containing 0.1% BSA, and the insulin and glucagon levels in the supernatants were determined by rat insulin RIA (Millipore, MA, USA) and glucagon RIA (TFB, Tokyo, Japan).
Histological analysis
After the overnight fixation, tissue samples were washed and placed in 10% buffered formalin, and subsequently embedded in paraffin. The paraffin sections (4 µm in thickness) were dried on slides overnight at 37°C, and were deparaffinized and rehydrated at room temperature. For antigen retrieval, the sections were heated for 15 min at 90°C in a microwave oven to stain for glucagon and the transcription factor pancreatic and duodenal homeobox 1 (PDX1), and the endogenous peroxidase was then blocked with 80% methanol containing 0.6% hydrogen peroxide for 15 min. The sections were rinsed with distilled water and placed in 3% hydrogen peroxide for 15 min. Next, the sections were rinsed with distilled water and washed in Tris-buffered saline with Tween buffer (50 mmol·L−1 Tris-HCl, 150 mmol·L−1 NaCl, pH 7.6, 0.05% Tween-20) for 5 min. The sections were then reacted with ready-to-use guinea pig anti-insulin antibody (Dako, Tokyo, Japan) or ready-to-use rabbit anti-glucagon antibody (Dako, Tokyo, Japan) for 60 min at room temperature, or with rabbit anti-PDX1 antibody (Transgenic, Kumamoto, Japan) at a concentration of 5 µg·mL−1 overnight at 4°C. The sections were washed with Tris-buffered saline with Tween, and bound antibody was detected using a ready-to-use polymer-labelled Envision+ system (Dako, Tokyo, Japan) for 30 min. The sections were rinsed with Tris-buffered saline with Tween and developed for 1 min using 3,3′-diaminobenzidine tetrahydrochloride substrate (DAB). Finally, the slides were washed with distilled water, counterstained with haematoxylin and mounted.
Statistical analysis
Statistical analysis was performed using the sas Version 8.2 (SAS Institute Inc.). To evaluate the effect of alogliptin in the short-term study, statistical differences were analysed using the one-tailed Williams' or one-tailed Shirley–Williams test. To evaluate if combination treatment with alogliptin and pioglitazone had significant additive or synergistic effects, a two-way anova was performed, which generates main effects and interaction effect of alogliptin and pioglitazone. The evaluation of interaction effect aims to detect statistically any synergistic or attenuation effects through the combination of alogliptin and pioglitazone. The results of two-way anovas were interpreted as follows: (i) When a significant interaction effect (alogliptin × pioglitazone, P ≤ 0.05) was observed, the effect of combination treatment with alogliptin and pioglitazone was assessed to be synergistic (when the effect of the combination therapy exceeds the sum of the effect of the monotherapy) or attenuated (when the effect of the combination therapy falls below the sum of the effect of the monotherapy), which was determined from observed values. (ii) When no significant interaction was observed, the effect of combination treatment with alogliptin and pioglitazone was assessed to be neither synergistic nor attenuated. On the basis of no interaction observed, when both main effects of alogliptin treatment and pioglitazone treatment were significant (P ≤ 0.05), the effect of combination treatment with alogliptin and pioglitazone was assessed to be additive (when the effect of the combination therapy equals the sum of the effect of the monotherapy). (iii) When only one significant (P ≤ 0.05) main effect was observed, the effect was assessed to be induced by only one drug, which was not affected by the other drug. Direct comparison among study groups was not statistically tested in the combination study. All data are presented as the mean ± SD.
Materials
Alogliptin benzoate (2-[[6-[(3R)-3-amino-1-piperidinyl]-3,4-dihydro-3-methyl-2,4-dioxo-1(2H)-pyrimidinyl]methyl]benzonitrile monobenzoate) was synthesized by Albany Molecular Research Institute (Albany, NY, USA). Pioglitazone hydrochloride was synthesized by Takeda Pharmaceutical Company, Ltd. (Osaka, Japan). The doses of alogliptin and pioglitazone are expressed as the free base form. All other reagents were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Sigma-Aldrich (Tokyo, Japan).
Results
Effects of short-term administration of alogliptin on plasma DPP-4 activity and active GLP-1 levels
A short-term study was conducted in 8 week old db/db mice to determine effects of alogliptin on plasma DPP-4 activity. After 2 days of treatment, alogliptin at doses of 0.01%, 0.03% or 0.1% in the diet significantly (P ≤ 0.025) and dose-dependently inhibited plasma DPP-4 activity by 69%, 79% and 84%, respectively, and significantly (P ≤ 0.025) increased plasma active GLP-1 levels by 4.9-, 4.4- and 4.9-fold, respectively, compared with vehicle alone (Figure 1). However, the increases in plasma active GLP-1 levels did not result in significant decreases in plasma glucose levels in the db/db mice under fed conditions (data not shown).
Figure 1.
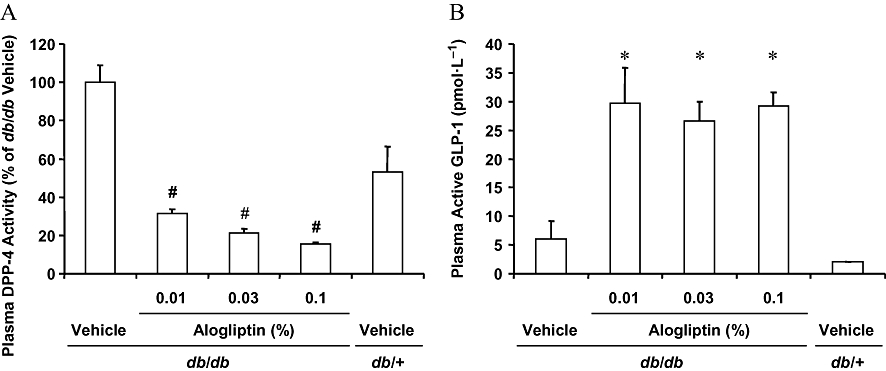
Effects of short-term treatment with alogliptin on plasma DPP-4 activity (A) and active GLP-1 levels (B). Eight week old mice were treated with vehicle or alogliptin at the indicated dosages for 2 days. After treatment, blood samples were collected and plasma DPP-4 activity and plasma active GLP-1 levels were determined. DPP-4 activity is presented as a relative percentage compared with vehicle-treated db/db mice. Plasma DPP-4 activity was significantly (#P ≤ 0.025) reduced at all dosages when compared with vehicle-treated db/db mice by one-tailed Shirley–Williams test. Plasma active GLP-1 was significantly (*P ≤ 0.025) increased at all dosages when compared with vehicle-treated db/db mice by one-tailed Williams' test. Data are presented as means ± SD (n = 8 for db/db mice, n = 5 for db/+ mice). DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
Effects of chronic administration of alogliptin plus pioglitazone on body weight and food consumption
Alogliptin (0.03%; equivalent to 76.4 mg·kg−1·day−1), pioglitazone (0.0075%;15.4 mg·kg−1·day−1) or alogliptin plus pioglitazone (0.03% and 0.0075%, 56.5 and 14.1 mg·kg−1·day−1 respectively) was given in the diet to 6 week old db/db mice for 3–4 weeks. Pioglitazone alone and combination treatment increased body weight by 15% and 23%, respectively, and decreased average food consumption by 7% and 12%, respectively, after nearly 4 weeks of treatment (Figure 2A,B: alogliptin, NS; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS). Alogliptin alone had no effects on these variables during the treatment period (Figure 2A,B).
Figure 2.
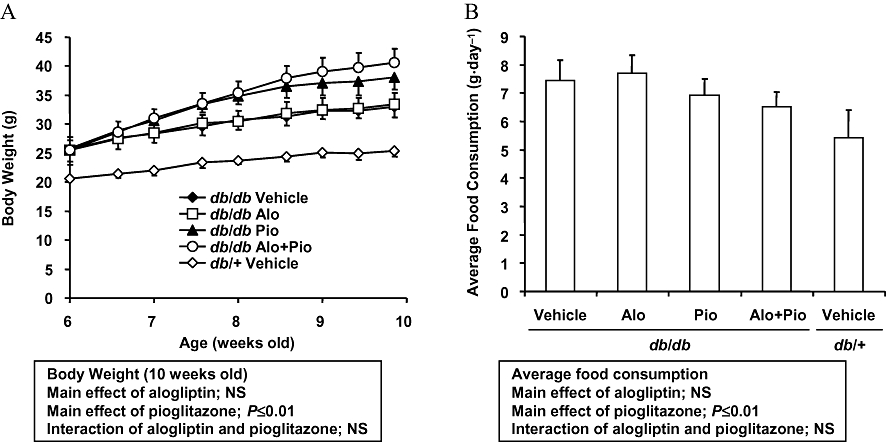
Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on body weight (A) and food consumption (B). Animals were fed a diet containing alogliptin (Alo; 0.03%), pioglitazone (Pio; 0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) during the study period. Body weight and food consumption were measured at designated intervals throughout the treatment period. Data are presented as means ± SD (n = 8 for db/db mice, n = 4 for db/+ mice). The results from a two-way anova are presented below the figure. NS, not significant.
Effects of chronic administration of alogliptin plus pioglitazone on plasma levels of DPP-4 activity, insulin, glucagon and adiponectin
As often observed in patients with type 2 diabetes, db/db mice exhibit decreased insulin secretion, high plasma glucagon levels and low plasma adiponectin levels, compared with age-matched non-diabetic mice. After 3 weeks of treatment, plasma DPP-4 activity was potently and equally inhibited by 79% and 78% in alogliptin- and the combination-treated db/db mice, compared with vehicle-treated db/db mice. In addition, plasma DPP-4 activity was 7% lower in pioglitazone-treated db/db mice, compared with the vehicle-treated db/db mice (Figure 3A: alogliptin, P ≤ 0.01; pioglitazone, NS; alogliptin × pioglitazone, NS). Plasma insulin levels were increased by 1.2-, 2.4- and 3.8-fold in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (Figure 3B: alogliptin, NS; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS). Compared with the vehicle-treated db/db mice, plasma glucagon levels were decreased by 16% and 41% (Figure 3C: alogliptin, NS; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS), and plasma adiponectin levels were increased by 2.2- and 2.4-fold (Figure 3D: alogliptin, NS; pioglitazone, P ≤ 0.01, alogliptin × pioglitazone, NS) in pioglitazone- and the combination-treated db/db mice respectively. Alogliptin alone did not affect plasma glucagon and adiponectin levels (Figure 3C,D).
Figure 3.

Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on the plasma levels of DPP-4 activity (A), insulin (B), glucagon (C) and adiponectin (D). Animals were fed a diet containing alogliptin (Alo; 0.03%), pioglitazone (Pio; 0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) during the study period. Plasma DPP-4 activity was determined after 21 days, plasma insulin levels were determined after 14 and 21 days, and plasma glucagon and adiponectin levels were determined after 23 days. Alogliptin treatment decreased plasma DPP-4 activity and pioglitazone treatment increased plasma adiponectin levels. Although the combination of alogliptin with pioglitazone was more effective at increasing plasma insulin levels and decreasing glucagon levels, these effects were not statistically additive or synergistic by two-way anova. Data are presented as means ± SD (n = 8 for db/db mice, n = 4 for db/+ mice). The results from a two-way anova are presented below the figure. DPP-4, dipeptidyl peptidase-4; NS, not significant.
Effects of chronic administration of alogliptin plus pioglitazone on glycaemic parameters
After 3 weeks of treatment, glycosylated haemoglobin levels were decreased by 0.4%, 1.2% and additively decreased by 2.2% in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (Figure 4A: alogliptin, P ≤ 0.01; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS). There was no difference in non-fasting plasma glucose levels for the alogliptin-treated db/db mice compared with the vehicle-treated db/db mice (Figure 4B). In contrast, non-fasting plasma glucose levels were decreased by 23% in pioglitazone-treated db/db mice and synergistically decreased by 52% in the combination-treated db/db mice compared with the vehicle-treated db/db mice (Figure 4B: alogliptin, P ≤ 0.01; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, P ≤ 0.01). Fasting plasma glucose levels prior to an oral glucose load (time = 0) were decreased by 1%, 28% and 50% in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (Figure 4C: alogliptin, NS; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS). Although this effect was not determined to be a significant additive effect, combination treatment showed a more potent effect than each drug alone on fasting plasma glucose levels. In addition, evaluation of glycaemic control using an OGTT showed that glucose area under the curve levels after an oral glucose load were decreased by 6%, 14% and additively decreased by 40% in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (Figure 4D: alogliptin, P ≤ 0.05; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS).
Figure 4.

Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on glycosylated haemoglobin (A), non-fasting plasma glucose (B), fasting plasma glucose and glucose excursion during an OGTT (C) and glucose AUC during an OGTT (D). Animals were fed a diet containing alogliptin (Alo; 0.03%), pioglitazone (Pio; 0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) during the study period. Glycosylated haemoglobin was determined after 21 days, non-fasting plasma glucose levels were determined after 14 and 21 days, and fasting plasma glucose, glucose excursion and glucose AUC during an OGTT were determined after 25 days followed by 17 h fasting. Combination treatment with alogliptin and pioglitazone resulted in additive improvements in glycosylated haemoglobin and glucose AUC during an OGTT, and a synergistic improvement in non-fasting plasma glucose. Although the combination of alogliptin with pioglitazone was more effective at lowering fasting plasma glucose levels, the effect was not statistically additive or synergistic. Data are presented as means ± SD (n = 8 for db/db mice, n = 4 for db/+ mice). The results from a two-way anova are presented below the figure. AUC, area under the curve; NS, not significant; OGTT, oral glucose tolerance test.
Effects of chronic administration of alogliptin plus pioglitazone on lipid profiles
As observed in patients with type 2 diabetes, the db/db mice exhibited elevated triglyceride levels. After 3–4 weeks of treatment, non-fasting and fasting plasma triglyceride levels were decreased by 30% and 13%, respectively, in the alogliptin-treated db/db mice, by 65% and 28%, respectively, in the pioglitazone-treated db/db mice, and additively decreased by 77% (Figure 5A: alogliptin, P ≤ 0.01; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS) and 67% (Figure 5B: alogliptin, P ≤ 0.05; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS), respectively, in the combination-treated db/db mice compared with the vehicle-treated db/db mice. The non-fasting NEFA levels were decreased by 12%, 30% and additively decreased by 48% in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (Figure 5C: alogliptin, P ≤ 0.05; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, NS).
Figure 5.

Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on plasma triglyceride (A), fasting plasma triglyceride (B) and non-fasting plasma NEFA (C). Animals were fed a diet containing alogliptin (Alo; 0.03%), pioglitazone (Pio; 0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) during the study period. Non-fasting plasma triglyceride levels were determined after 14 and 21 days, fasting plasma triglyceride levels were determined after 25 days followed by 17 h fasting, and non-fasting plasma NEFA levels were determined after 21 days. Combination treatment with alogliptin and pioglitazone resulted in additive improvements on these parameters. Data are presented as means ± SD (n = 8 for db/db mice, n = 4 for db/+ mice). The results from a two-way anova are presented below the figure. NEFA, non-esterified fatty acids; NS, not significant.
Effects of chronic administration of alogliptin plus pioglitazone on pancreatic hormone content
At approximately 10 weeks of age, after the 26 day study period, combination treatment increased pancreatic insulin content when compared with either drug alone (Figure 6). Pancreatic insulin content was increased by 1.1-, 1.8- and synergistically increased by 4.5-fold, in alogliptin-, pioglitazone- and the combination-treated db/db mice, respectively, compared with the vehicle-treated db/db mice (alogliptin, P ≤ 0.05; pioglitazone, P ≤ 0.01; alogliptin × pioglitazone, P ≤ 0.05). Pancreatic insulin content in the combination-treated db/db mice was equivalent to that of the vehicle-treated db/+ mice. In contrast, pancreatic glucagon content was not significantly changed by any of the treatments (data not shown).
Figure 6.

Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on pancreatic insulin content. Animals were fed a diet containing alogliptin (Alo; 0.03%), pioglitazone (Pio; 0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) during the study period. After 25 days of treatment, the animals were fasted for 17 h and then given an OGTT. Upon the completion of the OGTT, the pancreas was isolated for processing and analysis of insulin content. Combination treatment with alogliptin and pioglitazone resulted in a synergistic increase in pancreatic insulin content. Data are presented as means ± SD (n = 8 for db/db mice, n = 4 for db/+ mice). The results from a two-way anova are presented below the figure. OGTT, oral glucose tolerance test.
Effects of chronic administration of alogliptin plus pioglitazone on insulin-staining, β-cell/α-cell architecture and PDX1 expression in pancreatic islets
Type 2 diabetes is associated with characteristic and progressive changes in the structure of pancreatic islets. Such changes include reduced insulin synthesis in β-cells, increased α-cell mass and a widespread disruption of islet-specific gene expression (Del Prato and Marchetti, 2004; Kjorholt et al., 2005). Pancreata isolated from the db/db mice were analysed by immunohistochemistry using anti-insulin, anti-glucagon and anti-PDX1 antibodies before treatment (at 6 weeks of age) and after the 26 day study period (at approximately 10 weeks of age). At 6 weeks of age, islets of db/db mice showed degranulation of β-cells (Figure 7A), whereas glucagon-producing α-cells were localized at their normal peripheral position in the islet (Figure 7G). The transcription factor PDX1, which appears to play a pivotal role in β-cell differentiation and function as well as in pancreatic regeneration (Melloul, 2004), was expressed in β-cells (Figure 7M).
Figure 7.

Chronic effects of alogliptin-, pioglitazone- and alogliptin plus pioglitazone combination treatment on insulin-staining, β-cell/α-cell architecture and PDX1 expression in pancreatic islets. Animals were fed a diet containing alogliptin (0.03%), pioglitazone (0.0075%) or alogliptin (0.03%) plus pioglitazone (0.0075%) for 25 days and were fasted for 17 h. After completing an OGTT, the pancreas was isolated and stained with anti-insulin antibody (A–F), anti-glucagon antibody (G–L) and anti-PDX1 antibody (M–R). Representative images for each group of mice are shown. The combination-treated db/db mice exhibited increased expression of insulin and PDX1, and normal β-cell/α-cell distribution, which were comparable to those observed in the vehicle-treated non-diabetic db/+ mice. Scale bars = 200 µm for insulin and glucagon, and 100 µm for PDX1. PDX1, pancreatic and duodenal homeobox 1.
At approximately 10 weeks of age, severe degranulation of β-cells was observed in the vehicle-treated db/db mice (Figure 7B) compared with the vehicle-treated non-diabetic db/+ mice (Figure 7F). Treatment with alogliptin or pioglitazone alone failed to produce a clear inhibition of β-cell degranulation (Figure 7C,D). However, consistent with higher pancreatic insulin content levels (Figure 6), extensive insulin staining of β-cells was observed in the combination-treated db/db mice (Figure 7E) and insulin staining in this group of mice was comparable to that observed in the vehicle-treated non-diabetic db/+ mice.
Glucagon-producing α-cells were located at their normal peripheral position in the islets of the vehicle-treated non-diabetic db/+ mice at nearly 10 weeks of age (Figure 7L). In contrast, α-cell replication and widespread distribution throughout the islets were observed in the vehicle-treated db/db mice and in the mice treated with alogliptin or pioglitazone alone (Figure 7H–J). However, the α-cells were located at their normal peripheral position in the islets of the combination-treated db/db mice (Figure 7K).
At nearly 10 weeks of age, PDX1 expression was reduced throughout the islets in the vehicle-treated db/db mice (Figure 7N). Treatment with alogliptin or pioglitazone alone did not produce detectable changes in the expression of PDX1 in the db/db mice (Figure 7O,P). However, in the combination-treated db/db mice, PDX1 protein was highly expressed throughout the islets (Figure 7Q) and its expression was comparable to that observed in the vehicle-treated non-diabetic db/+ mice (Figure 7R).
Discussion and conclusions
In the present study, db/db mice, a model of type 2 diabetes, were used to evaluate the effects of combination treatment with alogliptin and pioglitazone on glycaemic control, lipid and hormone profiles, pancreatic β-cell function and islet structure. In this model, diabetic phenotypes are accelerated, at least in part, due to insufficient β-cell compensation for age-dependent increases in obesity and insulin resistance. The hyperglycaemic, hyperinsulinemic, hyperglucagonemic and hyperlipidemic phenotypes observed in db/db mice resemble those commonly observed in patients with type 2 diabetes (Dunning and Gerich, 2007; Tomkin, 2008).
In this study, alogliptin treatment showed marked inhibition of plasma DPP-4 activity and an increase in plasma active GLP-1 levels in db/db mice. However, alogliptin alone showed only marginal improvement in basal plasma insulin levels and glycaemic control, compared with pioglitazone alone. Consistent with these observations, the DPP-4 inhibitors sitagliptin and vildagliptin also failed to improve these parameters in similarly designed db/db mice studies (Y. Moritoh, unpubl. obs.). In addition, alogliptin alone did not induce a clear effect on insulin and PDX1 expression in the islets, and pancreatic insulin content in the db/db mice. Taken together, this study suggests that the increased circulating active GLP-1 levels, which were induced by DPP-4 inhibition, may not provide significant trophic or protective effects against glucose toxicity in β-cells of this model. In contrast to its effects in db/db mice, DPP-4 inhibitors improved both glycaemic control and β-cell function in ob/ob mice (Moritoh et al., 2008) and high-fat-diet-fed streptozotocin mice (Mu et al., 2006). These findings suggest that undefined variables influence the effectiveness of DPP-4 inhibitors in the different animal models.
Chronic treatment with pioglitazone partially improved glycaemic control and potently reduced lipid profiles in db/db mice in the present study. Pioglitazone treatment also specifically increased plasma adiponectin levels in this model. As expected, pioglitazone alone had no dominant inhibitory effect on DPP-4 activity but partially preserved basal circulating insulin and increased pancreatic insulin content in the db/db mice, which may have been due to improved glucose control, as discussed below.
Combination treatment with alogliptin and pioglitazone resulted in either additive or synergistic effects. After 3–4 weeks of treatment, the alogliptin plus pioglitazone combination increased plasma insulin levels and decreased plasma glucagon levels, more so than monotherapy with either agent alone, while the increase in circulating adiponectin was probably due to a pioglitazone effect. The combination treatment improved glycosylated haemoglobin, plasma glucose levels, glucose excursion during OGTT and lipid levels. Again, the improvements observed in these parameters were greater in the combination-treated db/db mice than the db/db mice treated with alogliptin or pioglitazone alone. In addition, combination treatment elevated insulin and PDX1 expression in the islets and increased pancreatic insulin content, more so than with each drug alone. Taken together with the increased insulin circulation, combination treatment seems to have improved β-cell function in the db/db mice. In addition to improving β-cell function, combination treatment maintained normal β-cell/α-cell distribution in the islets, indicating this strategy is also effective for preserving islet structure in the db/db mice.
Because robust glycaemic control by itself is sufficient to improve β-cell characteristics in db/db mice (Kjorholt et al., 2005), the improved glycaemic control from the combination treatment in this study may have contributed to the improvement in β-cell function. Recently, Xu et al. (2007) reported that expression of the GLP-1 and GIP receptors are significantly decreased in islets of pancreatectomized hyperglycaemic rats, which are recovered when glucose levels are normalized. These authors also reported that db/db mice show reduced gene expression of the Glp1r and Gipr in islets (Xu et al., 2007). It is thus conceivable that improved glycaemic control induced by alogliptin plus pioglitazone may have contributed to the improved expression of GLP-1 and GIP receptors, thus potentiating the effects of alogliptin-sustained incretin activity on combination with pioglitazone. In fact, alogliptin in combination with pioglitazone may have enhanced efficacy on improving glycaemic control and β-cell function, and may be more potent than the expected efficacy with alogliptin alone when administered to db/db mice. However, this hypothesis remains to be tested.
In the normal healthy individual, glucagon secretion is regulated by changes in systemic glucose and insulin concentrations. Hyperglucagonemia and/or an elevated plasma glucagon-to-insulin ratio have been reported in diabetic patients (Sloop et al., 2005) and animals (Sloop et al., 2004), and contribute to hyperglycaemia by increasing hepatic glucose production. GLP-1-induced inhibition of glucagon secretion has been reported to be mediated directly via GLP-1 receptors expressed on α-cells (Gromada and Rorsman, 2004) and indirectly via stimulation of insulin and somatostatin secretion (Heller and Aponte, 1995; Strowski et al., 2000; Cejvan et al., 2003; Xu et al., 2006). In the present study, combination treatment preserved functional β-cells and decreased plasma glucagon levels, whereas alogliptin alone increased active GLP-1 levels but failed to suppress circulating glucagon as well as to preserve functional β-cells, highlighting the role of functional β-cells for suppression of glucagon concentrations in this model. These observations are consistent with the previous report demonstrating that mice with β-cell-specific inactivation of the Pdx1 gene exhibit impaired β-cell function and defective suppression of glucagon secretion following treatment with a GLP-1 analogue (Li et al., 2005).
Hypertriglyceridemia is a metabolic abnormality in many patients with type 2 diabetes (Taskinen, 2003) and contributes to an increased risk of cardiovascular disease (Pejic and Lee, 2006). This study indicates that alogliptin plus pioglitazone combination treatment may be useful in patients who show hypertriglyceridemia as the combination treatment additively decreased fasting and non-fasting plasma triglyceride levels. Combination treatment with alogliptin and pioglitazone also decreased plasma NEFA levels when compared with monotherapy with either agent. Considering the evidence that circulating NEFA levels are associated with an increase in insulin resistance and induce impaired β-cell function via lipotoxicity (Wilding, 2007), combination treatment may be helpful in treating abnormally high NEFA levels.
Alogliptin alone showed no effects on body weight in the db/db mice, which is consistent with human results with DPP-4 inhibitors (Drucker and Nauck, 2006). On the other hand, pioglitazone and alogliptin in combination with pioglitazone increased body weight, which may have been induced by high levels of plasma insulin.
In conclusion, this study demonstrates that combination treatment with alogliptin and pioglitazone at an early stage of diabetes resulted in improved insulin, glucagon and adiponectin secretion, enhanced glycaemic and lipid control, and improved β-cell function and islet structure in db/db mice when compared with either alogliptin or pioglitazone alone.
Acknowledgments
The authors would like to thank Toshikazu Ando for technical assistance, Masanori Watanabe, Masami Suzuki, Masatoshi Hazama for helpful discussions, and Michelle Kujawski and Elisabeth Wann for comments on the manuscript.
Glossary
Abbreviations:
- DPP-4
dipeptidyl peptidase-4
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- NEFA
non-esterified fatty acids
- OGTT
oral glucose tolerance test
- PDX1
pancreatic and duodenal homeobox 1
Conflicts of interest
Y.M., K.T., T.A., O.K. and H.O. are employees of Takeda Pharmaceutical Company Ltd. K.T. and O.K. own Takeda stock and/or stock options at the time of manuscript preparation.
References
- Cejvan K, Coy DH, Efendic S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes. 2003;52(5):1176–1181. doi: 10.2337/diabetes.52.5.1176. [DOI] [PubMed] [Google Scholar]
- Ceriello A. The possible role of postprandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia. 2003;46(Suppl.)(1):M9–M16. doi: 10.1007/s00125-002-0931-5. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Johns D, Widel M, Eckland DJ, Gilmore KJ, Tan MH. Comparison of effect of pioglitazone with metformin or sulfonylurea (monotherapy and combination therapy) on postload glycaemia and composite insulin sensitivity index during an oral glucose tolerance test in patients with type 2 diabetes. Diabetes Care. 2005;28(2):266–272. doi: 10.2337/diacare.28.2.266. [DOI] [PubMed] [Google Scholar]
- Charpentier G. Oral combination therapy for type 2 diabetes. Diabetes Metab Res Rev. 2002;18(Suppl.)(3):S70–S76. doi: 10.1002/dmrr.278. [DOI] [PubMed] [Google Scholar]
- Deacon CF. Therapeutic strategies based on glucagon-like peptide 1. Diabetes. 2004;53(9):2181–2189. doi: 10.2337/diabetes.53.9.2181. [DOI] [PubMed] [Google Scholar]
- Del Prato S, Marchetti P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm Metab Res. 2004;36(11–12):775–781. doi: 10.1055/s-2004-826163. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycaemia in type 2 diabetes and therapeutic implications. Endocr Rev. 2007;28(3):253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143(11):4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhang Z, Wallace MB, Stafford JA, Kaldor SW, Kassel DB, et al. Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem. 2007;50(10):2297–2300. doi: 10.1021/jm070104l. [DOI] [PubMed] [Google Scholar]
- Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9(2):166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Giorgino F, Laviola L, Leonardini A. Pathophysiology of type 2 diabetes: rationale for different oral antidiabetic treatment strategies. Diabetes Res Clin Pract. 2005;68(Suppl.)(1):S22–S29. doi: 10.1016/j.diabres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Gromada J, Rorsman P. New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res. 2004;36(11–12):822–829. doi: 10.1055/s-2004-826169. [DOI] [PubMed] [Google Scholar]
- Heller RS, Aponte GW. Intra-islet regulation of hormone secretion by glucagon-like peptide-1-(7–36) amide. Am J Physiol. 1995;269(6)(1):G852–G860. doi: 10.1152/ajpgi.1995.269.6.G852. Pt. [DOI] [PubMed] [Google Scholar]
- Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycaemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54(9):2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- Lee B, Shi L, Kassel DB, Asakawa T, Takeuchi K, Christopher RJ. Pharmacokinetic, pharmacodynamic, and efficacy profiles of alogliptin, a novel inhibitor of dipeptidyl peptidase-4, in rats, dogs, and monkeys. Eur J Pharmacol. 2008;589(1–3):306–314. doi: 10.1016/j.ejphar.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54(2):482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- Melloul D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann N Y Acad Sci. 2004;1014:28–37. doi: 10.1196/annals.1294.003. [DOI] [PubMed] [Google Scholar]
- Miller S, St Onge EL. Sitagliptin: a dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40(7–8):1336–1343. doi: 10.1345/aph.1G665. [DOI] [PubMed] [Google Scholar]
- Mizushige K, Tsuji T, Noma T. Pioglitazone: cardiovascular effects in prediabetic patients. Cardiovasc Drug Rev. 2002;20(4):329–340. doi: 10.1111/j.1527-3466.2002.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Takeuchi K, Asakawa T, Kataoka O, Odaka H. Chronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycaemic control and beta-cell function in obese diabetic ob/ob mice. Eur J Pharmacol. 2008;588(2–3):325–332. doi: 10.1016/j.ejphar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic {beta}-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55(6):1695–1704. doi: 10.2337/db05-1602. [DOI] [PubMed] [Google Scholar]
- Nagakura T, Yasuda N, Yamazaki K, Ikuta H, Tanaka I. Enteroinsular axis of db/db mice and efficacy of dipeptidyl peptidase IV inhibition. Metabolism. 2003;52(1):81–86. doi: 10.1053/meta.2003.50014. [DOI] [PubMed] [Google Scholar]
- Pejic RN, Lee DT. Hypertriglyceridemia. J Am Board Fam Med. 2006;19(3):310–316. doi: 10.3122/jabfm.19.3.310. [DOI] [PubMed] [Google Scholar]
- Perfetti R, Hui H. The role of GLP-1 in the life and death of pancreatic beta cells. Horm Metab Res. 2004;36(11–12):804–810. doi: 10.1055/s-2004-826167. [DOI] [PubMed] [Google Scholar]
- Rendell MS, Jovanovic L. Targeting postprandial hyperglycaemia. Metabolism. 2006;55(9):1263–1281. doi: 10.1016/j.metabol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ristic S, Bates PC. Vildagliptin: a novel DPP-4 inhibitor with pancreatic islet enhancement activity for treatment of patients with type 2 diabetes. Drugs Today (Barc) 2006;42(8):519–531. doi: 10.1358/dot.2006.42.8.996570. [DOI] [PubMed] [Google Scholar]
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycaemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283(4):E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Baron MA, Camisasca RP, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9(2):175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. Antidiabetic agents in subjects with mild dysglycaemia: prevention or early treatment of type 2 diabetes? Diabetes Metab. 2007;33(1):3–12. doi: 10.1016/j.diabet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, et al. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest. 2004;113(11):1571–1581. doi: 10.1172/JCI20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop KW, Michael MD, Moyers JS. Glucagon as a target for the treatment of type 2 diabetes. Expert Opin Ther Targets. 2005;9(3):593–600. doi: 10.1517/14728222.9.3.593. [DOI] [PubMed] [Google Scholar]
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141(1):111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46(6):733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- Tomkin GH. Targets for intervention in dyslipidemia in diabetes. Diabetes Care. 2008;31(Suppl.)(2):S241–S248. doi: 10.2337/dc08-s260. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006;66(1):85–109. doi: 10.2165/00003495-200666010-00005. [DOI] [PubMed] [Google Scholar]
- Wilding JP. The importance of free fatty acids in the development of type 2 diabetes. Diabet Med. 2007;24(9):934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3(1):47–58. doi: 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycaemia: possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56(6):1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]


