Abstract
This prospective longitudinal study evaluated the effect of prenatal cocaine exposure (PCE) on executive functioning in 5- and 7-year-old children. In total, 154 pregnant cocaine users, identified by urine toxicology and structured interviews, were matched to 154 nonusers. Children were assessed by certified masked evaluators, and caregivers were interviewed by experienced staff during home visits. In approximately 90% of the surviving sample tested at ages 5 and 7 years, structural equation modeling demonstrated that an increased head circumference at birth (adjusted for gestation) significantly predicted better performance on executive functioning, and that PCE was indirectly related to executive functioning through its significant negative effect on head circumference at birth. At age 5 years, quality of environment also predicted executive functioning, and the R2 for the total model was 0.24. At 7 years, caregiver functioning predicted quality of environment, which in turn was positively related to executive functioning, and girls had better executive functioning. The total model at age 7 years accounted for 30% of the variance in executive functioning.
Keywords: Cocaine exposure, prenatal; Children; Executive functioning
Background and Theoretical Framework
The cocaine epidemic that began in the mid-1980s [1] was due in part to the development of crack cocaine, a more affordable, accessible and highly addictive form of free-based cocaine, resulting in the spread of use to all strata of society. As the prevalence of cocaine use among pregnant women increased, both animal and human studies were undertaken to explore the potential effects of prenatal cocaine exposure (PCE) on the developing fetus. However, in the search for rapid answers about cocaine’s possibly harmful effects on human fetuses, initial clinical studies were limited by design problems, e.g. small samples and the inability to control for confounding variables [2]. With the advent of several prospective longitudinal studies, it became possible to evaluate not only teratogenic effects, but to examine the influence of PCE relative to other variables known to affect child development. As children with PCE in these cohorts enter school, questions remain about the extent of PCE effects on their cognitive functioning.
Pharmacological studies of fetal cocaine exposure in animal models using a variety of species have shown that cocaine easily crosses both the placenta and the blood-brain barrier, and thus is a potential teratogen that, directly or indirectly, may affect the developing fetus [3]. Studies suggest that the effects of PCE most likely result from interference with the neurotrophic roles of monoaminergic transmitters during brain development [4-7], which can significantly affect cortical neuronal development and may lead to abnormalities in several brain areas [7]. Insults to the nervous system during neurogenesis, before homeostatic regulatory mechanisms are fully developed, are more likely to result in permanent changes in brain structure and function. Preclinical studies have supported this potential for PCE-related abnormalities that result in early and lasting deficits [6, 8]. It also appears that certain brain regions are more sensitive to cocaine effects than others, in particular, frontal lobe areas involved in attention and executive functioning. Thus, functions such as arousal, attention and memory may be particularly vulnerable to PCE [4, 9-12]. Indeed, preclinical studies have demonstrated a relationship between PCE and alterations in several aspects of neuronbehavioral functioning, e.g. reaction to stress, attention, memory, learning and inhibitory regulation [7, 8, 12-19]. Thus, PCE may significantly impede adaptive responses to environmental challenges that over the course of development require increasingly sophisticated reasoning and problem-solving abilities [19].
Findings from the first decade of research on the neurobehavioral effects of PCE on human offspring from birth to 3 years old were summarized in a review by Eyler and Behnke [2]. Reports included PCE effects on newborn irritability and lability of state, decreased autonomic and behavioral regulation, and poor alertness and orientation. Beyond the neonatal period, several studies reported specific PCE-related problems in visual expectancy, recognition memory, information processing, and arousal and responsiveness in learning tasks [2]. More recent studies of infants from birth to 4 months old demonstrated similar negative effects of PCE on neurobehavior [20-28], and, in infants 4–36 months old, a few but not all reported PCE-related poorer autonomic regulation [29], psychomotor development and cognition [30-32]. Among preschoolers, studies found alterations in various aspects of intellectual functioning [33], reactivity and regulation related to PCE [34] and selective attention related to PCE/tobacco [35]. With few exceptions [33], studies of children from infancy to school age have not found negative effects of PCE on general tests of overall development [2, 36], IQ [37-44], school readiness [41] or standardized tests of achievement [37, 44, 45].
However, as children reach school age, increasing demands are placed upon their reasoning and problem solving abilities. Given the problems described above in infants and preschoolers, there remains concern about the possible negative effects of PCE on executive functioning abilities in elementary school-aged children. Executive functioning has been defined as a diverse set of skills needed to engage in independent purposeful goal-directed behavior [46]. Such skills are thought to guide and manage cognitive, behavioral, and emotional functions, especially during active novel problem-solving [47]. The underlying neural substrate for such functioning appears to be the prefrontal cortex, and its subcortical connections that, as noted above, have been shown in preclinical studies to be particularly vulnerable to the effects of gestational cocaine exposure [4-19].
Several of the prospective, longitudinal studies of PCE examining executive functioning in 5- to 10-year-old children have reported negative effects of PCE on attention and inhibitory control, as measured by computerized continuous performance tasks [45, 48-51]. A study of response inhibition, using the contrary tapping task, found that 5-year-olds with PCE performed significantly worse than controls [52]. Another demonstrated negative effects of PCE on tests of general visual-motor ability and fine motor coordination [40]. However, in subsamples of several longitudinal cohorts, there have been mixed results when assessing visuo-motor planning, sequencing and set-shifting using the Trail Making Test (TMT) [53]. One group found no differences on trail making between PCE and non-PCE children [50], but as reported previously, we demonstrated PCE-related differences which were associated with frontal white matter integrity [54]. We also found that response inhibition as measured by the Stroop color-word test [55] was related to frontal white matter development [54]; another subsample of children with PCE demonstrated a relationship between a variation of the Stroop test and event-related responses [56].
Our longitudinal study [57] was designed not only to investigate any teratogenic effects [58] of PCE, but to examine a broader array of variables known to affect child development including the child’s caregiving environment as it changes over time [59]. This report of executive functioning in school-aged children with PCE is of particular significance as our sample was enrolled from an understudied population of rural/small-town women. Unlike urban cohorts, neither cocaine users nor matched controls used other illicit drugs, except marijuana. Although marijuana, alcohol and/or tobacco use was higher among cocaine users, their limited exposure to illicit drugs and the statistical control of the effects of these other substances increases the certainty with which negative outcomes can be attributed to PCE. However, such conclusions can only be drawn within the context of examination of the effects of environmental factors on a child’s development. In particular, the overall sample of pregnant women, both cocaine users and nonusers, was largely comprised of African-American, impoverished and underserved families. It is likely that this situation affected physical and psychological health as well as access to prenatal and general health care. Limited education among subjects, similar in both groups, was also of concern for its possible effects on the primary caregivers’ understanding of child development and ability to provide nurturing and enriched environments. Especially worrisome were the potential effects of the increased number of caregiver changes and foster care among PCE children. Thus, in examining the factors affecting executive functioning in school-aged children in this study, it was considered especially important to examine the effects of primary caregiver functioning, and the quality of the environment, as well as prenatal drug exposures and related risk variables, all of which we hypothesized would directly or indirectly affect executive functioning.
Specifically, the caregiver functioning variables chosen for this study included measures of depression, locus of control and a variant of self-esteem (the caregiver’s sense of competence in parenting). The fact that maternal depression is a risk factor for problems in cognitive development of young children has been well established [60]. For example, severe and chronic maternal depressive symptoms have been shown to negatively affect cognitive functioning by school entry [61, 62]. Others have shown that when depressive symptoms are high, maternal sensitivity is decreased, and this resulted in poorer cognitive skills [63].
Theoretically, it was expected that a caregiver’s psychological well-being would enable her to provide support and stimulation that would enhance the quality of the child’s caregiving environment. For example, a study of working mothers of 3 to 6-year-olds demonstrated that personal resources brought to caregiving, including self-esteem and internal locus of control, had a significant positive effect on the child’s home environment [64]. Furthermore, a meta-analysis found measures of functioning similar to those used in the current study; self-esteem, self-efficacy, locus of control, and neuroticism were highly related and explained by 1 factor [65].
There also has been a large body of research on the effect of a child’s home environment on development [66, 67]. A study of children, from birth to age 13 years, from poor and nonpoor ethnic groups, including African-Americans (the racial/ethnic group predominant in our study), found that learning stimulation in the home significantly affected child development, particularly among younger children [68].
All of the previously mentioned reports, as well as findings in our own studies, informed our development of the latent factors of caregiver functioning and quality of the home environment. Figure 1 outlines the theoretical model developed to evaluate executive functioning at ages 5 and 7 years.
Fig. 1.
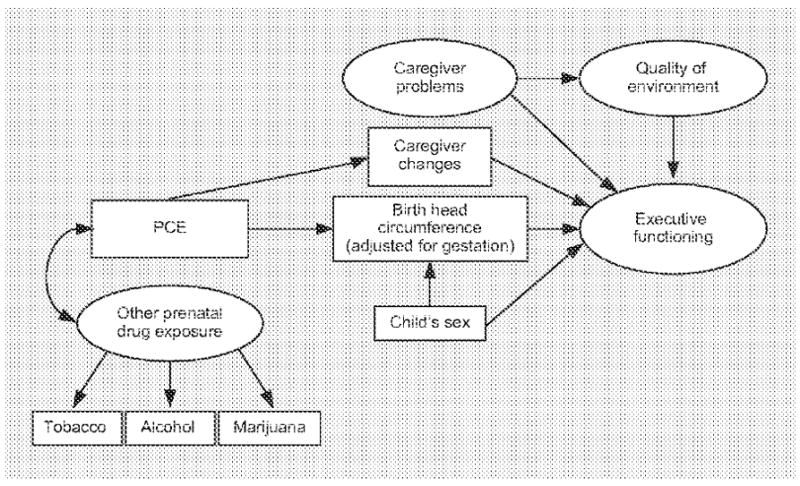
Theoretical model developed to predict executive functioning at 5 and 7 years old. Arrows represent the direction of hypothesized relationships; double-ended arrows depict variables allowed to covary.
Methods
Subject Selection, Identification and Enrollment
To enroll a representative sample of cocaine-using pregnant women in our catchment area, research staff, over a 2-year period, approached all women from the 2 closest public health prenatal clinics whose patients were designated to deliver in our tertiary care hospital, as well as those women referred to the hospital’s high risk clinic. Women who had no prenatal care or those who were unable to be enrolled/interviewed in the prenatal clinics were recruited when they arrived to deliver at the hospital. To reduce maternal factors that occurred uncommonly, but might confound effects of cocaine on pregnancy outcome or child development, a priori exclusion criteria included: illicit drug use beyond cocaine and marijuana, chronic use or abuse of prescription or other medications, and any major illness present prior to pregnancy. Chronic use/abuse of medications and illnesses likely to affect outcomes were determined by the physician investigator (M.B.) who was blinded to the cocaine-use history of the questionable subject. Due to the potential difficulty in obtaining informed consent and valid interviews, we also excluded non-English speaking women (<1% in our delivery population) and those under 18 years old (<10% in our delivery population and less likely to use cocaine than pregnant adults in our hospital) [69].
User and comparison groups were matched on more commonly occurring variables found in pilot studies [69] to be potential confounders of cocaine effects: self-reported race (Black vs. non-Black), parity (first vs. subsequent births), level of adapted Hollingshead Index of Socioeconomic Status (scored 1–5, with 5 being the most disadvantaged) [Hollingshead AB, unpublished work] and clinic-designated high or low pregnancy risk.
Study design included 2 methods to identify drug use of potential subjects: the collection of biologic specimens to increase identification of women reluctant to admit drug use, and extensive interviewing of the women to obtain details of use, as well as to attempt to identify users with negative toxicology screens. Urine specimens were collected at 2 unanticipated times: the day of enrollment (maternal) and at delivery (child). The 13 women who were not able or willing to provide the initial urine specimen were not enrolled. Toxicologists, masked to drug history, used fluorescence polarization immunoassay to screen specimens for cocaine, marijuana and, for purposes of study exclusion, 7 other categories of commonly abused drugs. Samples positive for cocaine or its major metabolites were confirmed by gas chromatography/mass spectrometry techniques [70].
To facilitate recall of details of drug use and any problems experienced in early pregnancy, subjects were recruited prenatally and interviewed at the end of each trimester in which they were receiving care. Interviews at the end of trimesters one and two were administered in the clinics; third trimester interviews were carried out in hospital following delivery. In private settings, experienced female staff used structured interviews [71] to obtain maternal histories that included timing and amount (or cost) of typical drug usage and changes in patterns over pregnancy and the 12 weeks prior to pregnancy. It was expected that the amount a woman admitted using during the 12 weeks prior to pregnancy might better reflect her actual (compared to her reported) use in the first trimester. Interviewers developed subject-calendars and asked questions about timing of use relative to dates and events memorable for each woman (e.g. birth dates). To be less threatening, interviewers asked questions at the end of each trimester about previous rather than current use, a technique which has been shown to facilitate disclosure [71]. Given the patterns of use of prenatal care, 77% of subjects were interviewed in the prenatal clinic once or twice before delivery. About 42% of women began prenatal care by the end of their first trimester, and thus were available to be interviewed at the end of each trimester. Women who began prenatal care during the second trimester (35%) were interviewed about all prior use at the end of the second trimester. Women receiving prenatal care only in the third trimester or arriving to deliver without care (23%) were first interviewed in the hospital following birth about all prior use. Because prenatal cocaine users entered the prenatal care system later on average, a significantly higher percentage of them had their first interview following delivery (40%) compared to the percentage of nonusers (6%).
At enrollment, Institutional Review Board-approved consent forms were read and explained to each potential subject. In addition to the usual efforts taken to insure confidentiality of data, special precautions to protect subjects were warranted in the health care settings, e.g. explaining the distinction between researchers and care providers and publicly presenting the research as a child health and development (not specifically a drug) study. Additionally, a certificate of confidentiality was obtained from the National Institute on Drug Abuse to protect against subpoena of research data. Although enrollment (1991–1993) predated the requirements of the Health Insurance Portability and Accountability Act, researchers did not have access to patient files prior to consent. Staff in the prenatal clinics and hospitals directed interested subjects to research staff who explained the study and obtained informed consent.
Of the 2,526 potential subjects approached, 85% consented to participate. Almost 8% were identified as using cocaine sometime during their pregnancy. As each cocaine user was identified, a woman with no evidence of use by interview or toxicology results, who was closest in gestation to the user, was selected from consenting subjects in the appropriate match pool category. After the initial interview and toxicology screening, 16 subjects were eliminated for use of illicit drugs and 3 for use of potentially confounding prescription medications. Rematches were made as needed and the final user/nonuser groups each included 154 subjects.
Description of Overall Sample Enrolled
The sample was predominantly Black, multiparous and in the most disadvantaged education/employment category of socioeconomic status. Compared to nonusers, cocaine users began prenatal care later in gestation and consumed higher average amounts of tobacco, alcohol and marijuana. There was a wide range in the amount of cocaine use reported, with crack cocaine predominantly identified as the drug of choice. Among cocaine-using women reporting details of use, 29% used only during their first trimester; 15% reported they used during first and second, but not third trimesters; and 32% admitted to use throughout pregnancy. The remaining women reported various other combinations of use.
There was no significant difference between groups in perinatal deaths, but PCE neonates were born on average at an earlier gestational age, had lower birth weights and smaller head circumferences (HC), even when HC was adjusted for gestational age. Controlling for tobacco, alcohol and marijuana, there were no longer cocaine-related differences in gestational age or birth weight; however, the mean daily amount of reported cocaine use was significantly negatively related to birth HC and to regulation of behavioral state, a finding that becomes relevant for this report. These and other details of birth outcome measures have been previously published [57, 72, 73].
Demographically, many families lived in poverty and moved frequently; at times some were homeless and a few were incarcerated. Compared to controls, the biological mothers of children with PCE reported more hopelessness, depression and low self-esteem, and subscribed to a more simplistic understanding of child development [74]. By the end of the first year, 5 nonexposed children (but nearly half of the PCE children) had been in the care of someone other than their biological mother [75]. At 7 years old, 74 PCE and 11 non-PCE children were in some type of foster care. Only 51 PCE children had always been with their biological mother.
Description of Current Study Sample
By 5 years old, 297 of the original 308 children were still living, and 266 (89%) of their families were located and willing to participate. Caregivers provided interviews and all but 5 children, including 2 who were too disabled to be tested, completed the age 5 years outcome measures (n = 261; PCE = 128). At 7 years old, 270 families (91% of 296 surviving children) participated. Of those, 260 had all caregiver interviews completed and child tests administered. However, there were twelve 7-year-olds who could not grasp the concept or did not know the few numbers and letters needed to successfully complete, within the time limit, one of the executive functioning outcome measures: the TMT part A. Thus, data from 248 children (PCE = 122) were available for inclusion in the structural equation model. Subjects participating in the current study did not differ from the original sample on the initial match variables, prenatal drug exposures and child growth measures at birth. As in the original sample, the PCE groups were exposed to significantly more tobacco, alcohol and marijuana, and were younger and smaller at birth (table 1).
Table 1.
Descriptive statistics for age 5 and age 7 samples
| Characteristic | Age 5 years
|
Age 7 years
|
||||
|---|---|---|---|---|---|---|
| cocaine-exposed | nonexposed | p value | cocaine-exposed | nonexposed | p value | |
| Mother | ||||||
| African-American, % | 80 | 83 | n.s. | 79 | 82 | n.s. |
| Multiparous, % | 85 | 89 | n.s. | 85 | 88 | n.s. |
| Socioeconomic status score | 4.7 ± 0.6 | 4.7 ± 0.5 | n.s. | 4.7 ± 0.6 | 4.7 ± 0.5 | n.s. |
| Prenatal drug use | ||||||
| Cigarettes, n/day | 8.8 ± 9.1 | 2.1 ± 5.9 | <0.0001 | 8.6 ± 9.1 | 2.1 ± 5.5 | <0.0001 |
| Absolute alcohol, oz/day | 0.2 ± 0.4 | 0.0 ± 0.1 | <0.0001 | 0.2 ± 0.4 | 0.0 ± 0.1 | <0.0001 |
| Marijuana, joints/day | 0.1 ± 0.4 | 0.0 ± 0.1 | <0.0001 | 0.1 ± 0.4 | 0.0 ± 0.1 | <0.0001 |
| Cocaine, % weeks use1 | 0.5 ± 0.3 | – | 0.5 ± 0.3 | – | ||
|
| ||||||
| Child | ||||||
| Gestational age at birth, weeks | 38.6 ± 2.1 | 39.2 ± 1.7 | 0.021 | 38.7 ± 2.0 | 39.2 ± 1.8 | 0.024 |
| Birth weight, g | 3,050 ± 605 | 3,242 ± 584 | 0.011 | 3,083 ± 591 | 3,271 ± 565 | 0.013 |
| BHC, cm | 33.6 ± 1.9 | 34.2 ± 1.7 | 0.014 | 33.7 ± 1.9 | 34.3 ± 1.7 | 0.019 |
| BHC (adjusted for gestational age), cm | 33.9 ± 1.4 | 34.3 ± 1.5 | 0.017 | 34.0 ± 1.4 | 34.4 ± 1.5 | 0.027 |
Statistical tests between the 2 study groups were conducted separately for ages 5 and 7 years.
Number of weeks of reported use during each individual pregnancy + 12 weeks prior/total weeks in same time period.
Assessment of Child Outcome
At ages 5 and 7 years, the children underwent comprehensive standardized neuropsychological assessments by examiners masked to drug histories. A senior-level school psychologist administered or supervised all developmental and psychological testing; a licensed clinical neuropsychologist provided training and oversight for all neuropsychological evaluations. With consent from families and school systems, the tests were administered in a mobile evaluation unit parked outside each child’s school during class hours or outside the child’s home when assessments were performed during school holidays. At the end of each clinic, children were offered their choice of a toy or game and received certificates noting their contributions to science.
Executive Functioning Variables
At age 5 years, the children were administered the Wechsler Preschool and Primary Scale of Intelligence, Revised Edition (WPPS1-R) [76]. Two WPPSI-R subscales assessing aspects of emerging executive functioning were included in analyses at age 5 years: block design, a timed task involving spatial relations, and mazes, a measure of motor planning and perceptual organization. The 5-year-olds were also given the Hooper Visual Organization Test [77], a measure of visual perceptual ability that does not involve motor skills or speed.
The Wechsler Intelligence Scale for Children, Third Edition (WISC-III) [78], was administered at age 7 years. Included in this report is the WISC-III block design subtest, also used at age 5 years, and the coding subtest, which assesses several aspects of executive functioning (including attention, visual-motor coordination, tracking and short-term memory). The TMT [79], is a timed pencil-and-paper test that consists of 2 parts. Part A, which was used in the age 7 model, involves sequencing of over-learned material (drawing pencil lines to connect the numbers 1–15 scattered randomly on a page) and measures attention and speed in visual search and motor functions. The scores are numbers of seconds to successful completion of the task within the 5-min time limit.
Predictor Variables
Prenatal Drug Exposure
The quantities of drugs used during pregnancy were expected to negatively affect executive functioning. A woman’s average daily use of tobacco cigarettes, ounces of absolute alcohol, and marijuana joints were calculated from the usual daily amounts she reported during her pregnancy and the 12 weeks prior to pregnancy, prorated by the frequency of occasions when she reported more or less than usual. To control for the possible effects of these 3 substances, the calculated amounts of exposure to each were included in analyses as components of a latent factor that was allowed to covary with PCE.
To quantify prenatal cocaine use, a ratio of ‘weeks of PCE’ was calculated: the number of weeks during which a woman reported use over her individual length of gestation plus the 12 weeks prior was divided by the total number of weeks during that same time period. For example, if a woman reported use for each of the 12 weeks prior to, and during, each week of her 38-week pregnancy, her ratio of weeks use would be 50/50 = 1.0 or 100%. If a woman reported she used weekly prior to pregnancy (12 weeks) but stopped using after her 10th week of a full-term pregnancy (40 weeks), her ratio would be 22/52 = 0.42, or 42%. Previous work from this research team suggested a predictive advantage to using the ‘weeks of PCE’ ratio over using the average amount of reported use [80]. Of the 154 cocaine users, only 22 did not admit or give details of use; they were instead identified by confirmed positive urine specimen(s). To avoid any bias that might result from eliminating quantifiable data on these subjects, the amount and frequency of their cocaine use was imputed using as estimates the median amount of use of women who had the same pattern of toxicology results, e.g. positive at study entry but not at birth.
Adjusted Birth HC
HC at birth was measured as part of the research protocol by pediatric nurse practitioners masked to drug exposure of the newborns. The HC variable was included as a potential predictor in modeling executive functioning because of its significant negative relationship to PCE in this study. Because including both HC and gestational age measures in the same model was likely to result in the statistical problem of multicollinearity due to their shared variance, an adjustment was made that preserved the distributional form of the HC variable, while controlling for the effect of gestational age on infant size. Infants born at 40 weeks or later were assigned the HC measured just after birth. If born before term, infants’ HC at full term were projected from their positions on the normative newborn size/growth charts of the Centers for Disease Control, e.g. if born at 34 weeks with HC at the 45th percentile, the infant was assigned the full-term HC corresponding to the 45th percentile.
Child’s Sex
Each child’s sex was included as a predictor variable in the models to account for sex differences in HC at birth (male newborns are generally larger than females), as well as possible sex differences in the executive functioning outcome measures.
Caregiver Functioning
Because the prenatal cocaine users in this cohort reported significantly more psychosocial problems that might affect the quality of their caregiving [74, 81], consideration was given to the possible effects of caregiver functioning on executive functioning in school-aged children. The psychosocial surveys included in this report were obtained from primary caregivers concurrent with the child assessments at ages 5 and 7 years. The primary caregiver was defined as the person who had cared for the child for the longest amount of time since the previous interview. Familiar female staff arranged a home visit to conduct interviews and observe the caregiving environment. To standardize protocols and increase validity of data collected in a population where illiteracy is common, interviewers read all questions, and, as appropriate, used cue cards listing response choices for each question. Out of respect and appreciation for the time commitment and effort made by the caregivers, each was given monetary compensation for their participation. Assistance with referrals (for caregivers or their children) was offered when problems were discovered or disclosed.
The Center for Epidemiologic Studies Depression Scale (CES-D) [82], a sound and widely used self-report measure that screens for symptoms of depression [83, 84], was administered and included in the analyses at ages 5 and 7 years.
At age 7 years, additional measures of caregiver functioning were available. The Levenson Locus of Control (LOC) Scale [85], measured and included in the age 7 model, assesses the belief that life events are dependent upon one’s own behavior (internal LOC) versus luck, fate or powers beyond control (external LOC) [86], Internal LOC has been related to physical and mental health [87, 88], and acceptance of responsibility [89, 90]. External LOC has been related to maternal likelihood for child abuse [91].
The Parenting Sense of Competence Scale [92] was administered and included in the analyses at age 7 years; this assesses a person’s appraisal of their efficacy and satisfaction with parenting, which has been related to other aspects of self-esteem and feelings of social support [ 93, 94; Halpern F, unpublished work].
Quality of Caregiving Environment
The Home Observation for Measurement of the Environment (HOME) Inventory [95], a tool that uses observation and a semistructured interview of the primary caregiver during an extended home visit, was designed to measure the aspects of nurturance and stimulation in the environment that have been shown to be important for cognitive development [96-98]. At the 5-year visit, interviewers administered the early childhood version of the HOME [99]. For the model developed at age 5 years, the learning, language and academic stimulation subscales of the HOME comprised the quality of environment latent factor. At age 7 years, the middle-childhood version of the HOME [100] was administered, and the subscales growth-fostering materials and experiences, provision for active stimulation, and family participation in developmentally stimulating experiences were the variables included in the quality of environment latent factor.
Number of Caregiver Changes
To account for the effects on child outcomes of the caregiver, whether biological or foster, we have included, as described previously, the primary caregiver’s reported functioning and HOME assessment. However, evaluating the potential positive and negative effects of foster care is complex. For example, having a nurturing caregiver and enriching home environment may not account for the earlier and often multiple caregiver changes experienced by some of the children which could possibly have affected their sense of attachment as well as being a chaotic factor in their lives. Thus, we have included in the model a variable consisting of the total number of caregiver changes that have been reported by the family. For the 5-year model the changes were totaled from birth to 5 years; for the 7-year model, any additional changes between 5 and 7 years of age were added.
Statistical Analyses
Initially, raw data were inspected for violations of normality and a square root transformation used when indicated, e.g. for the distribution of prenatal drug use and caregiver changes. SAS 8.2 (SAS Institute, Gary, N.C., USA) was used for statistical analyses. The significance criterion was set at α = 0.05, two-tailed. The Wilcoxon rank-sum test was used for comparisons of continuous variables. Categorical data were compared using the χ2 statistic.
Structural equation modeling (SEM), a path-analytic procedure that uses multiple regression analyses of factors, was employed to determine if our theoretical model of the effects of multiple variables on executive functioning at ages 5 and 7 years could successfully account for the observed relationships in our sample data. In addition to the inclusion of individual predictor variables, multivariable theoretical constructs were developed for the models. These constructs were operationalized by the formation of unobserved or latent factors derived from the reliable shared variance of observable indicator variables [101]. As considered optimal, 3 indicator variables were used in the development of each latent factor [102]. The structure of the models at each age and the variables included as single predictors were based on the theoretical framework, extant literature and our previous research, as outlined previously. We also examined correlations among a number of variables chosen to represent constructs of interest, and used these analyses to guide the selection of variables for each latent factor.
At each age, a measurement model that allowed for all possible correlations between the observed variables was developed first, and this served as a baseline model. Then the proposed structural equation model illustrated in figure 1 specified the hypothesized relationships among the observed and latent variables; thus, reducing the number of parameters to be estimated. Model fit for both the measurement models and the structural equation models was evaluated using multiple fit indices [103-107]. After acceptable fit was obtained for the measurement models, SEM was used to test the structural models. The χ2 difference test was used to determine whether the structural models adequately accounted for the relationships between the observed variables as well as the measurement models. Given the sample size included in the SEM analyses, power was determined to be >0.80 [108].
Results
Group comparisons were made between the PCE and non-PCE subjects for the caregiver functioning, quality of the environment, number of caregiver changes experienced by the children, and executive functioning variables used in the SEM at ages 5 and 7 years, as well as the child’s sex (table 2). There were only 4 significant differences between cocaine exposure groups on the predictor and outcome measures used in the SEM. At age 5, the PCE children scored significantly lower on the WPPSI-R block design subtest than the non-PCE children. At 7 years old, the caregiving environment of the children with PCE was rated significantly higher on growth-fostering materials and experiences. At ages 5 and 7 years, PCE children had experienced a significantly higher number of changes in caregivers. Otherwise, at both ages, the groups were very similar and did not differ on male/female ratio.
Table 2.
Caregiver problems, home environment, executive functioning and child’s sex by cocaine-exposure status
| Cocaine-exposed | Nonexposed | p value | |
|---|---|---|---|
| Age 5 years | |||
| Caregiver functioning | |||
| CES-D score | 19.7 ± 10.2 | 20.0 ± 9.9 | n.s. |
| Quality of environment, HOME score | |||
| Learning stimulation | 7.3 ± 2.3 | 6.7 ± 2.5 | n.s. |
| Language stimulation | 6.5 ± 0.9 | 6.3 ± 1.1 | n.s. |
| Academic stimulation | 4.5 ± 0.8 | 4.4 ± 0.9 | n.s. |
| Executive functioning | |||
| WPPSI-R block design | 7.3 ± 2.5 | 8.0 ± 2.8 | 0.039 |
| WPPSI-R mazes | 7.2 ± 2.7 | 7.2 ± 3.0 | n.s. |
| Hooper Visual Organization Test | 13.4 ± 3.6 | 13.6 ± 3.7 | n.s. |
| Female sex, % | 50 | 42 | n.s. |
| Number of caregiver changes (up to age 5 years) | 1.2 ± 1.5 | 0.2 ± 0.7 | <0.0001 |
|
| |||
| Age 7 years | |||
| Caregiver functioning | |||
| CES-D score | 18.7 ± 10.5 | 19.8 ± 11.4 | n.s. |
| Internal LOC score (Levenson Scale) | 33.2 ± 4.3 | 33.6 ± 4.6 | n.s. |
| Parenting Sense of Competence score (satisfaction) | 33.9 ± 6.0 | 35.2 ± 6.4 | n.s. |
| Quality of environment, HOME scores | |||
| Growth-fostering materials and experiences | 5.1 ± 1.6 | 4.6 ± 1.5 | 0.004 |
| Provision for active stimulation | 3.9 ± 1.9 | 3.7 ± 1.8 | n.s. |
| Family participation in stimulating experiences | 6.4 ± 2.0 | 6.6 ± 2.1 | n.s. |
| Executive functioning | |||
| Trail Making Test time (part A) | 41.2 ± 23.9 | 40.3 ± 27.0 | n.s. |
| WISC-III coding score | 10.2 ± 3.5 | 10.5 ± 3.5 | n.s. |
| WISC-III block design score | 7.9 ± 2.6 | 8.1 ± 3.2 | n.s. |
| Female sex, % | 51 | 43 | n.s. |
| Number of caregiver changes (up to age 7 years) | 1.4 ± 1.7 | 0.2 ± 0.9 | <0.0001 |
Data presented as means ± SD or percentages.
In the age 5 years SEM, the number of caregiver changes variable was not predictive of outcome and precluded a successful fit of the SEM to the measurement model. Eliminating the caregiver changes variable resulted in the desirable high fit indices (>0.95) and low residual and error indices (<0.05) indicating goodness of fit for the measurement and structural models. The χ2 test comparing the measurement and structural models was not significant, indicating that the 2 models did not differ in adequately accounting for the underlying structure of the observed data (table 3).
Table 3.
Fit indices for measurement and theoretical models of the effect of prenatal cocaine exposure on executive functioning
| Model
|
Comparison to measurement model
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | d.f. | p | CFI | MCI | RMR | RMSEA | χ2diff | d.f. | p | |
| Age 5 model | ||||||||||
| Measurement model | 65.72 | 48 | 0.05 | 0.97 | 0.97 | 0.02 | 0.04 | – | – | – |
| Structural model | 77.63 | 57 | 0.04 | 0.96 | 0.96 | 0.03 | 0.04 | 11.91 | 9 | n.s. |
| Age 7 model | ||||||||||
| Measurement model | 114.62 | 80 | 0.01 | 0.93 | 0.93 | 0.53 | 0.04 | – | – | – |
| Structural model | 141.26 | 97 | 0.00 | 0.91 | 0.91 | 0.51 | 0.04 | 26.64 | 17 | n.s. |
CFI = Comparative fit index; MCI = McDonald’s centrality index; RMR = root-mean-square residual; RMSEA = root-mean-square error of approximation; χ2diff = χ2 difference lest between measurement and structural models.
Figure 2 displays the age 5 SEM used to predict executive functioning. Single predictor variables (in rectangles) included the calculated ratio of weeks of PCE, Adjusted Birth Head Circumference (ABHC), Child Sex (males were set to 0, females to 1), and Caregiver Depression represented by depressive symptoms reported on the CES-D. Latent constructs (in ovals) were developed for Other Prenatal Drug Exposure, Quality of Environment and Executive Functioning. Included in figure 2 are the loadings of the indicators on their respective latent factors. The latent factor for Other Prenatal Drug Exposure included reported amounts of tobacco, alcohol and marijuana used prenatally which loaded positively. As other drug exposure was confounded with PCE, the Other Prenatal Drug Exposure latent factor was allowed to covary with PCE. Variables that loaded positively onto the latent construct representing Quality of Environment were the Learning, Language, and Academic Stimulation subscales of the HOME. The variables representing Executive Functioning included the WPPSI-R Block Design and Mazes subtests and the Hooper Visual Organization Test, each of which loaded positively on the latent outcome construct.
Fig. 2.
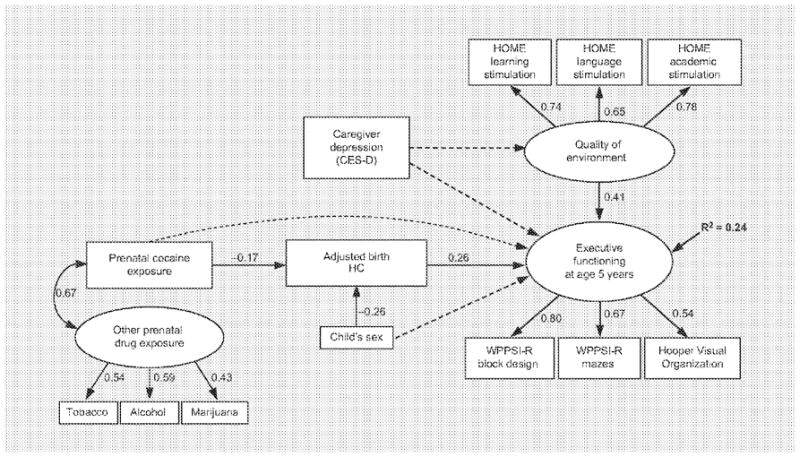
SEM predicting executive functioning at age 5 years. Solid lines represent significant paths, while dashed lines indicate hypothesized but nonsignificant paths. Paths from latent constructs to indicators are factor loadings.
SEM pathways tested and statistically significant are noted by solid-line arrows with adjacent standardized path coefficients representing the direction and strength of the relationships (fig. 2; table 4). The model at age 5 demonstrates that increased PCE was significantly related to smaller ABHC. Child sex also was significantly related to ABHC with larger BHC generally found among males (set to zero, thus the negative relationship). Unexpectedly, CES-D was not significantly related to the Quality of Environment or to Executive Functioning. However, higher Quality of Environment (more Learning, Language, and Academic Stimulation as measured by the HOME) did significantly predict better performance on the Executive Functioning latent factor. Although there was no direct relationship of PCE to Executive Functioning, PCE had an indirect effect through its significant negative relationship with ABHC. In turn, ABHC significantly predicted Executive Functioning tasks such that smaller birth head size was related to worse scores. The model accounted for 24% of the variance in Executive Functioning at age 5.
Table 4.
Path coefficients, standard errors and t values for SEM
| Unstandardized path coefficient | Standard error | t value | Standardized path coefficient | |
|---|---|---|---|---|
| Age 5 model | ||||
| PCE | ||||
| Other prenatal drug exposure | 0.21 | 0.02 | 8.88 | 0.67 |
| ABHC | ||||
| PCE | −0.83 | 0.29 | −2.88 | −0.17 |
| Child’s sex | −0.78 | 0.18 | −4.42 | −0.26 |
| Quality of home environment | ||||
| Caregiver depression | −0.08 | 0.06 | −1.52 | −0.11 |
| Executive functioning | ||||
| PCE | 0.01 | 0.25 | 0.05 | 0.00 |
| ABHC | 0.20 | 0.05 | 3.58 | 0.26 |
| Child’s sex | −0.06 | 0.16 | −0.40 | −0.03 |
| Caregiver depression | 0.05 | 0.06 | 0.89 | 0.06 |
| Quality of home environment | 0.46 | 0.10 | 4.80 | 0.41 |
|
| ||||
| Age 7 model | ||||
| PCE | ||||
| Other prenatal drug exposure | 0.20 | 0.02 | 8.29 | 0.65 |
| ABHC | ||||
| PCE | −0.72 | 0.30 | −2.43 | −0.15 |
| Child’s sex | −0.68 | 0.18 | −3.72 | −0.23 |
| Quality of home environment | ||||
| Caregiver functioning | 0.40 | 0.10 | 3.77 | 0.37 |
| Executive functioning | ||||
| PCE | −0.56 | 0.36 | −1.59 | −0.14 |
| ABHC | 0.24 | 0.08 | 3.00 | 0.30 |
| Child’s sex | 0.71 | 0.23 | 3.02 | 0.30 |
| Caregiver functioning | −0.07 | 0.14 | −0.50 | −0.06 |
| Quality of home environment | 0.42 | 0.15 | 2.82 | 0.38 |
At age 7, because the first SEM and measurement models significantly differed, the Stepwise Multivariate Wald Test was used to eliminate nonsignificant paths one at a time. The first deletion, the nonsignificant path from Caregiver Functioning to Executive Functioning, resulted in the desired nonsignificant χ2 difference test. This indicated that the SEM did not differ from the measurement model in adequately accounting for the underlying structure of the observed data. The indices of goodness of fit of the measurement and structural models were appropriately high and the error indices were low, except for the root-mean-square residual (RMR), which was the only index not meeting conventional criteria for good fit. (table 3)
Figure 3 is the SEM developed to predict executive functioning at age 7 years. As in the 5-year model, the individual predictor variables include the calculated ratio of weeks of PCE, ABHC, child’s sex (males = 0; females = 1) and, in addition, the total number of caregiver changes from birth to age 7 years. The latent construct Other Prenatal Drug Exposure (on which reports of amount of tobacco, alcohol and marijuana use loaded positively) was allowed to covary with PCE. In addition to the CES-D caregiver-reported depressive symptoms used in the model at age 5 years, other psychosocial reports were available at age 7 years, and thus a caregiver functioning latent construct was developed, with higher scores denoting better emotional functioning. The additional indicators included the caregivers’ positive belief in an internal LOG and satisfaction with their parenting competence (measured by the Parenting Sense of Competence Scale), both of which loaded positively with caregiver functioning, while depressive symptoms loaded negatively. Variables that loaded positively onto the latent construct representing quality of environment were the growth-fostering materials and experiences, provision for active stimulation and family participation in developmentally stimulating experiences subscales of the HOME. At age 7 years, the variables representing executive functioning were the WISC-III coding and block design subscales (scaled scores), and the TMT part A (time scores). High performance on coding and block design loaded positively on the latent outcome construct, and the amount of time taken to complete TMT part A loaded negatively. Figure 3 includes the factor loadings of each of the indicators on their respective latent constructs.
Fig. 3.
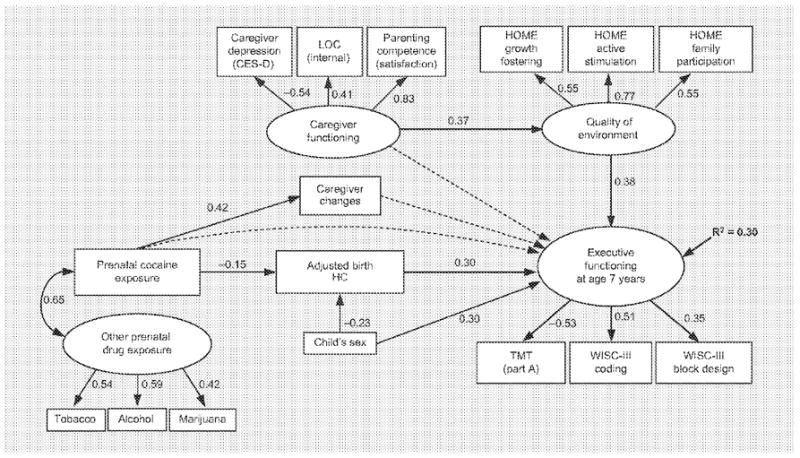
SEM predicting executive functioning at age 7 years. Solid lines represent significant paths, while dashed lines indicate hypothesized but nonsignificant paths. Paths from latent constructs to indicators are factor loadings.
As above, SEM pathways tested and statistically significant are noted by solid-line arrows with adjacent standardized path coefficients representing the direction and strength of the relationships (fig. 3; table 4). The model of executive functioning at age 7 years demonstrated the same relationships of higher levels of PCE with smaller ABHC and the child’s sex (males) with larger ABHC. PCE was also positively related to number of caregiver changes, which was not related to executive functioning. Caregiver functioning was not directly related to executive functioning, but had an indirect effect through a significant negative relationship to quality of environment. In turn, higher quality of environment significantly predicted better performance on executive functioning tasks. Smaller ABHC predicted worse executive functioning, while the amount of PCE had an indirect effect through its negative influence on ABHC. Furthermore, the child’s sex not only had an indirect influence on outcome through its relationship with ABHC, but was directly and significantly predictive of executive functioning. The positive relationship indicates that females generally performed better on tasks of executive functioning at age 7 years. The model accounted for 30% of the variance in executive functioning at age 7 years.
Discussion and Conclusions
Although there was only 1 significant difference in a simple comparison of PCE and non-PCE children on the executive functioning tasks included in this study, SEM enabled us to demonstrate that our theoretical model of the effects of multiple variables on the executive functioning of 5- and 7-year-old children successfully accounted for the actual relationships observed in our data. At both 5 and 7 years, SEM demonstrated that the weeks of prenatal exposure to cocaine had an indirect effect on performance of executive functioning tasks through cocaine’s negative effect on ABHC. Smaller HC at birth significantly predicted poorer outcomes at both ages. The relationships between PCE, adjusted HC and developmental outcomes were initially observed at birth in our longitudinal study [57]. Although measurements taken since birth have shown no differences in head size between the PCE and non-PCE groups, smaller ABHC, significantly related to increased levels of PCE, has continued to be significantly related to poorer development in our sample as measured at 6 months [109], at 3 years [110], and now at 5 and 7 years of age. Other have demonstrated that even with good postnatal head growth, an impairment in acquisition of cognitive and academic skills was found among 7- to 9-year-old children who experienced compromised head growth in utero [111]. Another study found a significant relationship between relatively smaller HC at birth and lower developmental scores at 7 years old, which was lessened by inclusion of family factors, social class and sex [112], Although there is little evidence in the literature specifically of PCE-related HC predicting executive functioning, Azuma and Chasnoff [113] reported a significant negative relationship of PCE to IQ at age 4 years that was mediated by BHC.
In SEM at ages 5 and 7 years, the quality of the environment also was found to be a significant predictor of executive functioning. In fact at age 5 years, the path coefficient between quality of environment and executive functioning latent factors is larger than that between ABHC and executive functioning. By 7 years old, caregiver functioning began to demonstrate an indirect effect on outcome through its negative effect on the quality of the environment. These findings are consistent with our earlier report that poor caregiver functioning predicted the quality of the environment which in turn was significantly related to developmental outcome at age 3 years [110]. Others investigating the relationship of PCE to development have also reported significant effects on outcomes of various aspects of the caregiving environment [114].
At 5 and 7 years old, the child’s sex had an indirect effect on outcome through a significant relationship with HC (boys being larger at birth). More interestingly, by age 7 years, the child’s sex independently predicted executive functioning, with females generally performing better on tasks. While there have been reports of differences between boys and girls in some ability and achievement measures in school-aged children, we had not predicted a specific effect of sex on executive functioning at these ages.
Although subtle, findings of this study are consistent with the putative effects of PCE on the developing fore-brain. According to animal models, PCE appears to interfere with the neurotrophic roles of monoaminergic transmitters, alter cortical neuronal development, and result in permanent structural and functional abnormalities [4-8]. Brain regions most sensitive to the effects of PCE are areas that are involved in control of executive functioning abilities [4, 7-19]. Thus, clinical researchers and practitioners have been particularly concerned about the neurobehavioral effects of PCE on exposed children as they reach school age and must respond to increasing environmental challenges. Clinical studies have begun to demonstrate PCE-associated problems in executive functioning, including several aspects of attention, visual-motor abilities and memory [40, 45, 48-52].
The current study contributes to this literature in that our impoverished but nonurban subjects who used a range of cocaine, some marijuana but no other illicit drugs represent an understudied population of pregnant cocaine users. Subjects were prospectively recruited early in pregnancy from all attendees at prenatal care clinics whose patients delivered at our tertiary care hospital and from those women arriving to deliver at our hospital without prenatal care; 85% gave consent to participate; and thus the sample represents the population in our catchment area well. Additional strengths of the study include: a priori exclusion of rare but potential confounding factors; a comparison group matched on common risk variables; identification of prenatal drug exposure using both urine specimens, confirmed for cocaine, and structured interviews by experienced staff; standard testing conditions and evaluators masked to drug histories; use of sophisticated statistical modeling, SEM, that included prenatal drug exposures and birth outcomes, as well as caregiver functioning and the quality of the home environment, as predictors of executive functioning; and a longitudinal design with 90% of surviving children and their primary caregivers remaining in the study and no evidence of any bias in attrition.
Limitations of the study include those common in research on the effects of PCE. Cocaine users reported significantly more marijuana, alcohol and tobacco use during pregnancy than women in the comparison group, requiring us to statistically control for these substances in modeling outcomes. From the beginning of the study, the biological mothers of PCE children reported more psychosocial problems, which were likely to affect their parenting. Subsequently, children with PCE were significantly more likely to be placed in relative or formal foster care, a situation also likely to affect development. We attempted to address these potentially confounding parenting factors by using, as possible predictors in the model, the primary caregiver’s report of psychological functioning, the observed quality of the home environment and the total number of caregiver changes experienced by the children from birth.
While this predominantly rural, poor, African-American sample represents a relatively unique population that has been understudied (with the particular strength of including cocaine users with limited other illicit drug use), it is possible that the results may not generalize to cocaine users in urban areas, those less impoverished or of a different racial/ethnic mix.
Furthermore, data in this study do not support a true mediation effect, as PCE does not directly predict executive functioning with or without the inclusion of ABHC in SEM. Thus, we have used the less specific term, indirect effect, in order to note that PCE significantly predicts ABHC and, in multiple analyses across several ages, ABHC has predicted various developmental outcomes. It is possible that a smaller head size at birth, shown to be related to the amount of prenatal cocaine exposure, may be a marker of the deleterious effects of cocaine on fetal development that subsequently result in poorer executive functioning. More research will be needed to fully understand the meaning of this particular relationship.
As children move to middle and high school, where even more demands are made upon the frontal association areas, early effects of PCE on cognitive functioning may be exacerbated. Given the poorer performance of boys on executive functioning tasks, further research will be needed to consider a possible interaction effect, i.e. to determine if male infants may be more vulnerable to the effects of prenatal drug exposure. Deficits in executive functioning not only affect cognitive performance, but also can alter perception of, judgment about and behavioral responses to interpersonal and other environmental situations. Furthermore, there is empirical support [115-121] that PCE as well as other environmental factors may have significant adverse effects on behavior as well as cognition. Thus, youths with PCE may be less likely to perceive that a behavior would be inappropriate or risky, judge the possible negative consequences, or inhibit an impulsive reaction. This raises a specific concern about the potential effects of PCE on adolescent risk-taking behaviors, including the possibility of early drug use. Therefore, it will be important to continue to investigate the effects of PCE and other risk variables on both cognitive and psychosocial development in older children.
Although subtle, the findings of this study are consistent with the hypothesized effects of PCE on the developing brain. They also demonstrate the importance of caregiver well-being and a stimulating environment for child functioning. Continued evaluation of relationships with family, friends and mentors may identify protective factors that can facilitate optimal development for these and other children at risk.
Acknowledgments
Supported by National Institute on Drug Abuse, National Institutes of Health (DA05854) and General Clinical Research Centers (RR00082).
References
- 1.Farrar HC, Kearns GL. Cocaine: clinical pharmacology and toxicology. J Pediatr. 1989;115:665–675. doi: 10.1016/s0022-3476(89)80640-7. [DOI] [PubMed] [Google Scholar]
- 2.Eyler FD, Behnke M. Early development of infants exposed to drugs prenatally. Clin Perinatol. 1999;26:107–150. [PubMed] [Google Scholar]
- 3.Mayes LC. Neurobiology of prenatal cocaine exposure effect on developing monoamine systems. Infant Ment Health J. 1994;15:121–133. [Google Scholar]
- 4.Dow-Edwards DL. Developmental toxicity of cocaine: mechanisms of action. In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale: Lawrence Erlbaum Associates; 1995. pp. 5–17. [Google Scholar]
- 5.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- 6.Whitaker-Azmitia PM. Role of the neurotrophic properties of serotonin in the delay of brain maturation induced by cocaine. Ann NY Acad Sci. 1998;846:158–164. doi: 10.1111/j.1749-6632.1998.tb09734.x. [DOI] [PubMed] [Google Scholar]
- 7.Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Dev Brain Res. 2003;147:23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Lauder JM. Discussion: neuroteratology of cocaine-relationship to developing monoamine systems. In: Kilbey MM, Ashgar J, editors. Methodological Issues in Controlled Studies on Effects of Prenatal Exposure to Drug Abuse (NIDA Research Monograph No 114) Bethesda: National Institute on Drug Abuse; 1991. pp. 233–247. [PubMed] [Google Scholar]
- 10.Woods JR., Jr Adverse consequences of prenatal illicit drug exposure. Curr Opin Obstet Gynecol. 1996;8:403–411. [PubMed] [Google Scholar]
- 11.Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 12.Malanga CJ, Kosofsky BE. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin Perinatol. 1999;26:17–37. [PubMed] [Google Scholar]
- 13.Spear LP. Alterations in cognitive functioning following prenatal cocaine exposure: studies in an animal model. In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale: Erlbaum; 1995. pp. 207–227. [Google Scholar]
- 14.Vorhees CV. A review of developmental exposure models for CNS stimulants: cocaine. In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale: Lawrence Erlbaum Associates; 1995. pp. 71–94. [Google Scholar]
- 15.Morris P, Gillam MP, Allen RR, Paule MG. The effect of chronic cocaine exposure during pregnancy on the acquisition of operant behaviors by rhesus monkey offspring. Neurotoxicol Teratol. 1996;18:155–166. doi: 10.1016/0892-0362(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel M, Taylor C. Prenatal exposure to cocaine impairs neuronal coding of attention and discriminative learning. Ann NY Acad Sci. 1998;846:194–212. [PubMed] [Google Scholar]
- 17.Wilkins AS, Genova LM, Posten W, Kosofsky BE. Transplacental cocaine exposure. 1. A rodent model. Neurotoxicol Teratol. 1998;20:215–226. doi: 10.1016/s0892-0362(97)00125-6. [DOI] [PubMed] [Google Scholar]
- 18.Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci. 2000;114:725–738. [PubMed] [Google Scholar]
- 19.Stanwood GD, Levitt P. Drug exposure early in life: functional repercussions of changing neuropharmacology during sensitive periods of brain development. Curr Opin Pharmacol. 2004;4:65–71. doi: 10.1016/j.coph.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Chiriboga C, Brust J, Bateman D, Hauser A. Dose response effect of fetal cocaine exposure on newborn neurologic function. Pediatrics. 1999;103:79–80. doi: 10.1542/peds.103.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Coles CD, Bard KA, Platzman KA, Lynch ME. Attentional response at eight weeks in prenatally drug-exposed and preterm infants. Neurotoxicol Teratol. 1999;21:527–537. doi: 10.1016/s0892-0362(99)00023-9. [DOI] [PubMed] [Google Scholar]
- 22.Swanson MW, Streissguth AP, Sampson PD, Olson HC. Prenatal cocaine and neuromotor outcome at four months: effect of duration of exposure. J Dev Behav Pediatr. 1999;20:325–334. doi: 10.1097/00004703-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Dev Psychobiol. 2000;36:194–212. [PubMed] [Google Scholar]
- 24.Singer LT, Arendt R, Minnes S, Farkas K, Salvator A. Neurobehavioral outcomes of cocaine-exposed infants. Neurotoxicol Teratol. 2000;22:653–666. doi: 10.1016/s0892-0362(00)00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes ML. Influence of prenatal cocaine exposure on full-term infant neurobehavioral functioning. Neurotoxicol Teratol. 2001;23:533–544. doi: 10.1016/s0892-0362(01)00173-8. [DOI] [PubMed] [Google Scholar]
- 26.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110:1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 27.Lester BM, Lagasse L, Seifer R, Tronick EZ, Bauer CR, Shankaran S, Bada HS, Wright LL, Smeriglio VL, Liu J, Finnegan LP, Maza PL. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2003;142:279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- 28.Schuetze P, Eiden RD. The association between maternal cocaine use during pregnancy and physiological regulation in 4- to 8-week-old infants: an examination of possible mediators and moderators. J Pediatr Psychol. 2006;31:15–26. doi: 10.1093/jpepsy/jsj022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: effects on infant autonomic regulation at 7 months of age. Dev Psychobiol. 2007;49:276–289. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyssen Van Beveren T, Little BB, Spence MJ. Effects of prenatal cocaine exposure and postnatal environment on child development. Am J Human Biol. 2000;12:417–428. doi: 10.1002/(SICI)1520-6300(200005/06)12:3<417::AID-AJHB12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, Kliegman R. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiriboga CA, Kuhn L, Wasserman GA. Prenatal cocaine exposures and dose-related cocaine effects on infant tone and behavior. Neurotoxicol Teratol. 2007;29:323–330. doi: 10.1016/j.ntt.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Hurt H, Malmud E, Betancourt LM, Brodsky NL, Giannetta JM. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. J Dev Behav Pediatr. 2001;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol Teratol. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- 38.Delaney-Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, Clark N, Surendran A, Martier S, Sokol RJ. Expressive language development of children exposed to cocaine prenatally: literature review and report of a prospective cohort study. J Commun Disord. 2000;33:463–480. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 39.Hurt H, Giannetta J, Brodsky NL, Malmud E, Pelham T. Are there neurologic correlates of in utero cocaine exposure at age 6 years? J Pediatr. 2001;138:911–913. doi: 10.1067/mpd.2001.113709. [DOI] [PubMed] [Google Scholar]
- 40.Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, Carlson L, Min MO, Klein N, Flannery D. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Behav Pediatr. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulsifer MB, Radonovich K, Belcher HM, Butz AM. Intelligence and school readiness in preschool children with prenatal drug exposure. Child Neuropsychol. 2004;10:89–101. doi: 10.1080/09297040490911104. [DOI] [PubMed] [Google Scholar]
- 42.Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min MO, Kirchner HL. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA. 2004;291:2448–2456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank DA, Rose-Jacobs R, Beeghly M, Wilbur M, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48-month IQ: importance of preschool enrichment. Neurotoxicol Teratol. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Morrow CE, Culbertson JL, Accornero VH, Xue L, Anthony JC, Bandstra ES. Learning disabilities and intellectual functioning in school-aged children with prenatal cocaine exposure. Dev Neuropsychol. 2006;30:905–931. doi: 10.1207/s15326942dn3003_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicol Teratol. 2005;27:203–211. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- 47.Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychol. 2002;8:121–137. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- 48.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year olds. Neurotoxicol Teratol. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 49.Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 50.Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Behav Pediatr. 2005;26:42–47. [PubMed] [Google Scholar]
- 51.Accornero VH, Amado AJ, Morrow CE, Xue L, Anthony JC, Bandstra ES. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–351. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Interpretation. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 54.Warner TD, Behnke M, Eyler FD, Padgett K, Leonard C, Hou W, Garvan CW, Schmalfuss IM, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golden CJ, Freshwater SM, Golden Z. Stroop Color and Word Test Children’s Version for ages 5–14: A Manual for Clinical and Experimental Uses. Wood Dale: Stoelting Company; 2003. [Google Scholar]
- 56.Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: I. Interactive and dose effects on health and growth. Pediatrics. 1998;101:229–236. doi: 10.1542/peds.101.2.229. [DOI] [PubMed] [Google Scholar]
- 58.Vorhees CV. Concepts in teratology and developmental toxicology derived from animal research. Ann NY Acad Sci. 1989;562:31–41. doi: 10.1111/j.1749-6632.1989.tb21005.x. [DOI] [PubMed] [Google Scholar]
- 59.Sameroff AJ, Chandler MJ. Reproductive risk and the continuum of caretaking casualty. In: Horowitz FD, editor. Review of Child Development Research. Chicago: University of Chicago Press; 1975. pp. 187–244. [Google Scholar]
- 60.Downey G, Coyne JC. Children of depressed parents: an integrative review. Psychol Bull. 1990;108:50–76. doi: 10.1037/0033-2909.108.1.50. [DOI] [PubMed] [Google Scholar]
- 61.The course of maternal depressive symptoms, maternal sensitivity, and child outcomes. NICHD Early Child Care Research Network. Dev Psychol. 1999;35:1297–1310. doi: 10.1037//0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- 62.Brennan PA, Hammen C, Andersen MJ, Bor W, Najman JM, Williams GM. Chronicity, severity, and timing of maternal depressive symptoms: relationships with child outcomes at age 5. Dev Psychol. 2000;36:759–766. doi: 10.1037//0012-1649.36.6.759. [DOI] [PubMed] [Google Scholar]
- 63.Campbell SB, Matestic P, von Stauffenberg C, Mohan R, Kirchner T. Trajectories of maternal depressive symptoms, maternal sensitivity, and children’s functioning at school entry. Dev Psychol. 2007;43:1202–1215. doi: 10.1037/0012-1649.43.5.1202. [DOI] [PubMed] [Google Scholar]
- 64.Menaghan EG, Parcel TL. Determining children’s home environments: the impact of maternal characteristics and current occupational and family conditions. J Marriage Fam. 1991;53:417–431. [Google Scholar]
- 65.Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? J Pers Soc Psychol. 2002;83:693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- 66.Bradley RH, Tedesco L. Environmental correlates of mental retardation. In: Lachenmeyer J, Gibbs M, editors. The Psychology of the Abnormal Child. New York: Gardner; 1982. pp. 155–189. [Google Scholar]
- 67.Parke R. Children’s home environments: social and cognitive effects. In: Altman I, Wohlhill J, editors. Children and the Environment. New York: Plenum; 1978. pp. 107–119. [Google Scholar]
- 68.Bradley RH, Corvyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States. II. Relations with behavioral development through age thirteen. Child Dev. 2001;72:1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- 69.Eyler FD, Behnke M, Conlon M, Woods NS, Frentzen B. Prenatal cocaine use: a comparison of neonates matched on maternal risk factors. Neurotoxicol Teratol. 1994;16:81–87. doi: 10.1016/0892-0362(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 70.Mule SJ, Casella GA. Confirmation of marijuana, cocaine, morphine, codeine, amphetamine, methamphetamine, phencyclidine by GC/MS in urine following immunoassay screening. J Anal Toxicol. 1988;12:102–107. doi: 10.1093/jat/12.2.102. [DOI] [PubMed] [Google Scholar]
- 71.Day NL, Wagener DK, Taylor PM. Measurement of substance use during pregnancy: methodologic issues. NIDA Res Monogr. 1985;59:36–47. [PubMed] [Google Scholar]
- 72.Behnke M, Eyler FD, Conlon M, Wobie K, Woods NS, Cumming W. Incidence and description of structural brain abnormalities in cocaine-exposed newborns. J Pediatr. 1998;132:291–294. doi: 10.1016/s0022-3476(98)70447-0. [DOI] [PubMed] [Google Scholar]
- 73.Eyler FD, Behnke M, Garvan C, Woods N, Wobie K, Conlon M. Newborn evaluations of toxicity and withdrawal related to prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:399–411. doi: 10.1016/s0892-0362(01)00166-0. [DOI] [PubMed] [Google Scholar]
- 74.Behnke M, Eyler FD, Woods NS, Wobie K, Conlon M. Rural pregnant cocaine users: an in-depth sociodemographic comparison. J Drug Issues. 1997;27:501–525. [Google Scholar]
- 75.Wobie K, Eyler FD, Garvan CW, Hou W, Behnke M. Prenatal cocaine exposure: an examination of out-of-home placement during the first year of life. J Drug Issues. 2004;34:77–94. [Google Scholar]
- 76.Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence – Revised. San Antonio: The Psychological Corporation; 1989. [Google Scholar]
- 77.Hooper EH. Hooper Visual Organization Test. Los Angeles: Western Psychology Services; 1965. [Google Scholar]
- 78.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 79.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- 80.Behnke M, Eyler FD, Garvan C, Wobie K, Marron J. The relationship of outcome to commonly used quantitative assessments of prenatal cocaine use. Pediatr Res. 1999;45:1394A. [Google Scholar]
- 81.Woods NS, Behnke M, Eyler FD, Conlon M, Wobie K. Cocaine use among pregnant women: socioeconomic, obstetrical, and psychological issues. In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Hillsdale: Lawrence Erlbaum Associates; 1995. pp. 305–332. [Google Scholar]
- 82.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Pscyhol Meas. 1977;1:385–401. [Google Scholar]
- 83.Comstock GW, Helsing KJ. Symptoms of depression in two communities. Psychol Med. 1976;6:551–563. doi: 10.1017/s0033291700018171. [DOI] [PubMed] [Google Scholar]
- 84.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 85.Levenson H. Activism and powerful others: distinctions within the concept of internal-external control. J Pers Assess. 1974;38:377–383. [Google Scholar]
- 86.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28. [PubMed] [Google Scholar]
- 87.Brown BR, Granick S. Cognitive and psychosocial differences between I and E locus of control aged persons. Exp Aging Res. 1983;9:107–110. doi: 10.1080/03610738308258435. [DOI] [PubMed] [Google Scholar]
- 88.Lefcourt HM, Martin RA, Fick CM, Saleh WE. Locus of control for affiliation and behavior in social interactions. J Pers Soc Psychol. 1985;48:755–759. [Google Scholar]
- 89.Johnson JH, Sarason IG. Life stress, depression and anxiety: internal-external control as a moderator variable. J Psychosom Res. 1978;22:205. doi: 10.1016/0022-3999(78)90025-9. [DOI] [PubMed] [Google Scholar]
- 90.Edwards JE, Waters LK. Relationships of locus of control to academic ability, academic performance, and performance-related attributions. Educ Psychol Meas. 1981;41:529–531. [Google Scholar]
- 91.Stringer S, La Greca A. Correlates of child abuse potential. J Abnorm Child Psychol. 1985;13:217–226. doi: 10.1007/BF00910643. [DOI] [PubMed] [Google Scholar]
- 92.Cutrona CE, Troutman BR. Social support, infant temperament, and parenting self-efficacy: a mediational model of postpartum depression. Child Dev. 1986;57:1507–1518. [PubMed] [Google Scholar]
- 93.Gibaud-Wallston J. Self-esteem and situational stress: factors related to sense of competence in new parents. Diss Abstr Int. 1977;39:379B. [Google Scholar]
- 94.Wandersman LP, Wandersman A, Kahn S. Social support in the transition to parenthood. J Community Psychol. 1980;8:332–342. [Google Scholar]
- 95.Caldwell BM, Bradley BH. Home Observation for Measurement of the Environment. Little Rock: University of Arkansas Press; 1984. [Google Scholar]
- 96.Bradley RH. The HOME Inventory: review and reflections. In: Reese H, editor. Advances in Child Development and Behavior. San Diego: Academic Press; 1994. pp. 241–288. [DOI] [PubMed] [Google Scholar]
- 97.Bradley RH, Corvyn RF, Whiteside-Mansell L. Life at home: same time, different places. An examination of the HOME Inventory in different cultures. Early Dev Parent. 1996;5:251–269. [Google Scholar]
- 98.Bradley RH, Corvyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States. II. Relations with behavioral development through age 13. Child Dev. 2001;72:1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- 99.Bradley RH, Caldwell BM. Home observation for measurement of the environment: a revision of the preschool scale. Am J Ment Defic. 1979;84:235–244. [PubMed] [Google Scholar]
- 100.Bradley RH, Caldwell BM, Rock SL, Hamrick HM, Harris P. Home Observation for Measurement of the Environment: development of a home inventory for use with families having children 6 to 10 years old. Contemp Educ Psychol. 1988;13:58–71. [Google Scholar]
- 101.Ullman JB. Structural equation modeling. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. 4. Boston: Allyn & Bacon; 2001. pp. 653–771. [Google Scholar]
- 102.Little TD, Lindenberger U, Nesselroade JR. On selecting indicators for multivariate measurement and modeling with latent variables: when ‘good’ indicators are bad and ‘bad’ indicators are good. Psychol Methods. 1999;4:192–211. [Google Scholar]
- 103.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 104.Hu LT, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–453. [Google Scholar]
- 105.Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. [Google Scholar]
- 106.McDonald RP. An index of goodness-of-fit based on noncentrality. J Classification. 1989;6:97–103. [Google Scholar]
- 107.MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annu Rev Psychol. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- 108.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1:130–149. [Google Scholar]
- 109.Behnke M, Eyler FD, Garvan CW, Wobie K, Hou W. Cocaine exposure and developmental outcome from birth to 6 months. Neurotoxicol Teratol. 2002;24:283–295. doi: 10.1016/s0892-0362(02)00191-5. [DOI] [PubMed] [Google Scholar]
- 110.Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frisk V, Amsel R, Whyte HE. The importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children. Dev Neuropsychol. 2002;22:565–593. doi: 10.1207/S15326942DN2203_2. [DOI] [PubMed] [Google Scholar]
- 112.Ounsted M, Moar VA, Scott A. Head circumference and developmental ability at the age of seven years. Acta Paediatr Scand. 1988;77:374–379. doi: 10.1111/j.1651-2227.1988.tb10663.x. [DOI] [PubMed] [Google Scholar]
- 113.Azuma SD, Chasnoff IJ. Outcome of children prenatally exposed to cocaine and other drugs: a path analysis of three-year data. Pediatrics. 1993;92:396–402. [PubMed] [Google Scholar]
- 114.Betancourt L, Fischer R, Giannetta J, Malmud E, Brodsky NL, Hurt H. Problem solving ability of inner-city children with and without in utero cocaine exposure. J Dev Behav Pediatr. 1999;20:418–424. doi: 10.1097/00004703-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Johnson AL, Morrow CE, Accornero VH, Xue L, Anthony JC, Bandstra ES. Maternal cocaine use: estimated effects on mother-child play interactions in the preschool period. J Dev Behav Pediatr. 2002;23:191–202. doi: 10.1097/00004703-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Accornero VH, Morrow CE, Bandstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–269. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Accornero VH, Anthony JC, Morrow CE, Xue L, Bandstra ES. Prenatal cocaine exposure: an examination of childhood externalizing and internalizing behavior problems at age 7 years. Epidemiol Psichiatr Soc. 2006;15:20–29. [PMC free article] [PubMed] [Google Scholar]
- 120.Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol RJ. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–791. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- 121.Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]


