Abstract
A novel neurochemical method was applied for studying the activity of sympathetic nerves in the human cerebral vascular system. The aim was to investigate whether noradrenaline plasma kinetic measurements made with internal jugular venous sampling reflect cerebrovascular sympathetic activity. A database was assembled of fifty-six healthy subjects in whom total body noradrenaline spillover (indicative of whole body sympathetic nervous activity), brain noradrenaline spillover and brain lipophlic noradrenaline metabolite (3,4-dihydroxyphenolglycol (DHPG) and 3-methoxy-4-hydroxyphenylglycol (MHPG)) overflow rates were measured. These measurements were also made following ganglion blockade (trimethaphan, n= 6), central sympathetic inhibition (clonidine, n= 4) and neuronal noradrenaline uptake blockade (desipramine, n= 13) and in a group of patients (n= 9) with pure autonomic failure (PAF). The mean brain noradrenline spillover and brain noradrenaline metabolite overflow in healthy subjects were 12.5 ± 1.8, and 186.4 ± 25 ng min−1, respectively, with unilateral jugular venous sampling for both. Total body noradrenaline spillover was 605.8 ng min−1± 34.4 ng min−1. As expected, trimethaphan infusion lowered brain noradrenaline spillover (P= 0.03), but perhaps surprisingly increased jugular overflow of brain metabolites (P= 0.01). Suppression of sympathetic nervous outflow with clonidine lowered brain noradrenaline spillover (P= 0.004), without changing brain metabolite overflow (P= 0.3). Neuronal noradrenaline uptake block with desipramine lowered the transcranial plasma extraction of tritiated noradrenaline (P= 0.001). The PAF patients had 77% lower brain noradrenaline spillover than healthy recruits (P= 0.06), indicating that in them sympathetic nerve degeneration extended to the cerebral circulation, but metabolites overflow was similar to healthy subjects (P= 0.3). The invariable discordance between noradrenline spillover and noradrenaline metabolite overflow from the brain under these different circumstances indicates that the two measures arise from different sources, i.e. noradrenaline spillover originates from the cerebral vasculature outside the blood–brain barrier, and the noradrenaline metabolites originate primarily from brain noradrenergic neurons. We suggest that measurements of transcranial plasma noradrenaline spillover have utility as a method for assessing the sympathetic nerve activity of the cerebral vasculature.
The blood vessels of the brain are substantially innervated with sympathetic nerve fibres (Nielsen & Owman, 1967; Falck et al. 1968; Busija & Heistad, 1984; Edvinsson et al. 1993; Gulbenkian et al. 2001). Despite knowledge of their existence for centuries (Hamel, 2006), there is controversy surrounding the regulatory role that these sympathetic nerves play in the cerebral circulation (Zhang et al. 2002). On one hand it is argued that the sympathetic nerves of the cerebral vascular system have little or no biological significance (Strandgaard & Sigurdsson, 2008). On the other, it is maintained that they play a role in physiological homeostasis through dynamic autoregulation of the brain's circulation in response to changes in blood pressure and cerebral blood flow (Zhang et al. 2002; Ogoh et al. 2008; van Lieshout & Secher, 2008).
Given that noradrenaline is the principle neurotransmitter of sympathetic nerves, the rate of its release from nerve fibres is a well established means of assessing sympathetic nerve activity. Measuring noradrenaline kinetics (Esler et al. 1979, 1988, 1990; Grassi, 1998) has an advantage over measures of plasma noradrenaline, which do not factor in the rates of removal from plasma, the so called ‘clearance process’ (Grassi & Esler, 1999). Noradrenaline kinetics provides a measure of the apparent rate that noradrenaline enters into plasma, more aptly termed the ‘spillover rate’ (Esler et al. 1979). Techniques used to measure noradrenaline kinetics can be further divided into measurements to estimate whole body neural sympathetic activity (Esler et al. 1979; Grassi & Esler, 1999) and those to assess regional organ-specific neural sympathetic activity (Esler et al. 1988). These techniques are often simply referred to as ‘total’ and ‘organ-specific’ measures of noradrenaline spillover (Esler et al. 1984), respectively. The latter has been successfully applied to the heart, kidneys, and forearms (Esler et al. 1984, 1988; Sudhir et al. 1989). Similar techniques have been used to measure the rate at which the lipophilic metabolites of noradrenaline, specifically 3,4-dihydroxyphenolglycol (DHPG) and 3-methoxy-4-hydroxyphenylglycol (MHPG), enter the bloodstream (Lambert et al. 1995a,b; Aggarwal et al. 2003).
The difficulty in defining a functional importance for neural cerebral vascular regulation exist partly because of the limitations of available technology for studying sympathetic nerve effects on the brain blood vessels of humans. We aimed to evaluate the regional noradrenaline spillover methodology. Organ-specific ‘regional noradrenaline spillover’ and ‘noradrenaline metabolite overflow’ can be applied to the brain through unilateral jugular venous sampling by estimating the rate that noradrenaline spillover enters the cerebral circulation and by measuring the overflow of noradrenaline metabolites. Previous studies have tended to pool the noradrenaline spillover and its metabolites (Ferrier et al. 1993). In the measurements of ‘brain noradrenaline turnover’ we postulated here, however, that given the lipophobic nature of noradrenline and therefore its very limited ability to cross the blood–brain barrier, brain spillover measured from jugular sampling is derived primarily from the sympathetic nerves of the cerebral vasculature. In contrast the noradrenaline metabolites are lipophilic, and their entry into the internal jugular venous plasma comes from both the sympathetic nerves of the cerebral vasculature and from noradrenergic brain neurons.
In this article we evaluate brain noradrenaline spillover as a measure of sympathetic activity of the cerebral circulation, in an assembled data base drawn from 79 healthy subjects, in whom we made measurements at rest, and with various drug interventions known to modify sympathetic nerve firing (trimethaphan, clonidine) and neuronal noradrenaline uptake (desipramine) (Ferrier et al. 1992, 1993; Lambert et al. 1995b, 1998). We also analysed the results from a small group of subjects with pure autonomic failure (PAF), for the purpose of studying a comparative group with known generalised sympathetic nerve degeneration. By simultaneously quantifying the internal jugular venous overflow of noradrenaline metabolites in the same subjects we aimed to identify whether noradrenaline metabolites have an origin different from that of noradrenaline spillover.
Methods
Ethics approval
The experimental protocol was explained to all participants in detail, and written consent obtained for the investigation. The research was approved by the Ethics Review Committee of the Alfred Hospital in compliance with the Declaration of Helsinki.
Participants
Seventy-nine healthy adult subjects (seven females), were recruited into the studies from which this data base was assembled (Ferrier et al. 1992, 1993; Lambert et al. 1995a, 1998) after providing written informal consent. All subjects had a detailed clinical evaluation which included a medical history, clinical examination and routine hematology and biochemistry testing. None were receiving prescribed or over the counter medications. Fifty-six (six females) of the healthy recruits were not assigned to an additional intervention. Six (all males) received an infusion of trimethaphan, four (all males) had clonidine infused, and 13 (one female), received an intravenous infusion of desipramine.
Additionally, results from nine subjects with a diagnosis of PAF were analysed. In them, the diagnosis of PAF was confirmed on clinical grounds and with plasma noradrenline kinetics measurements (Esler et al. 1979; Meredith et al. 1991). PAF subjects were not assigned an additional drug intervention (Fig. 1).
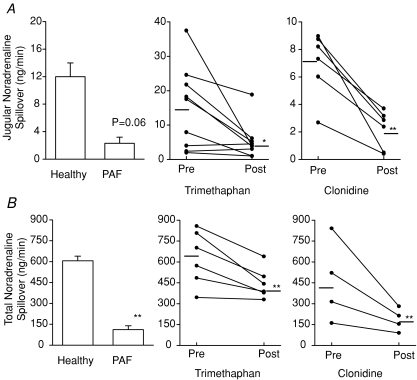
Figure 1. Jugular venous noradrenaline spillover and whole body noradrenaline spillover.
A, jugular venous noradrenaline spillover; *Trimethaphan intervention (P= 0.03); **clonidine intervention (P= 0.004). B, whole body noradrenaline spillover; **Healthy subjects vs. PAF (P < 0.001); **Trimethaphan (P= 0.01); **Clonidine (P= 0.008). When noradrenaline spillover rates from the brain (A) and for the whole body (B) was compared, both responded concordantly to the same interventions and study groups. Trimethaphan and clonidine infusions both lowered regional brain noradrenaline spillover and whole body noradrenaline spillover. Healthy subjects at rest had higher noradrenaline spillover from the brain and whole body noradrenaline spillover than PAF patients. All values are expressed as means ±s.e.m. PAF, pure autonomic failure.
General procedure
The experiments were performed in the morning, as an out-patient procedure, in an experimental room maintained at an ambient temperature of between 21°C and 23°C, following a 12 h abstinence from alcohol, nicotine and caffeinated beverages. On the morning of the study, all subjects had a standardised light meal (345 kcals), insufficient to cause postprandial sympathetic nervous activation (Cox et al. 1995).
In each case simultaneous blood samples were obtained from a central venous and arterial catheter inserted percutaneously under local anaesthesia (Ferrier et al. 1992, 1993; Lambert et al. 1995a, 1998). Central venous catheter placement was performed via an 8.5F percutaneous introducing sheath (Arrow International Inc., Reading, PA, USA) inserted into the ante-cubital vein of the forearm, through which a 7F type CCS-7U coronary sinus thermodilution catheter was inserted (Webster Laboratories, Baldwin Park, CA, USA). The catheter was guided fluoroscopically (Model DC 12MB-1, Toshiba Industries, Osaka, Japan) into an internal jugular vein and the position of the catheter tip confirmed with the injection of 2 ml radio-opaque contrast medium (Omnipaque, Winthrop Pharmaceuticals, NY, USA). The catheter tip was beyond the angle of the jaw, to exclude sampling from the tissues of the face (Ferrier et al. 1993). Venous blood samples were obtained from the right or left internal jugular vein, or both. Five to ten minutes was allowed for stabilisation after each catheter placement (Cox et al. 1995). We have previously provided evidence that catheter repositioning does not constitute a material stressor, in experienced hands (Cox et al. 1995). Jugular blood flow was obtained by thermodilution (Ferrier et al. 1993). Arterial blood samples were obtained from the 21G arterial cannula in the radial or brachial artery.
Throughout the course of the experiment a tracer infusion of 3H-labelled l-noradrenaline (specific activity 11–25 Ci mmol−1, New England Nuclear, Boston, MA, USA) was administered intravenously at 0.8 μCi min−1 via a dorsal vein in the hand (Ferrier et al. 1992, 1993; Lambert et al. 1995a, 1998).
Interventions
In each interventional study blood samples were obtained in the same manner already outlined in the general procedure.
Trimethaphan
The effects of an intravenous infusion of the ganglion blocker, trimethaphan was available for six healthy subjects. Trimethaphan was infused through a peripheral vein at a rate producing a reduction of approximately 20 mmHg in supine systolic blood pressure (0.4–1.2 mg min−1). Blood samples were taken before the infusion and on reaching the effect of the blockade intervention, when blood pressure reduction was maximal. This occurred at between 30 and 60 min of infusion. Ganglion blockade was confirmed by abolition, or material reduction, of the diastolic blood pressure overshoot in phase IV of the Valsalva manoeuvre.
Clonidine
In four healthy subjects, clonidine (Boehringer-Ingelheim), an imidazoline with α2-adrenergic agonist activity which inhibits sympathetic outflow from the CNS, was infused via a peripheral vein over 10 min to a total dose of 200–300 μg. Blood samples were taken at rest and at 20 min post-infusion. This protocol was derived from the observation that the hemodynamic effects of a single dose of clonidine peak at 15–20 min and return to baseline by 45 min (Giles et al. 1981).
Desipramine
After blood samples had been taken at rest in 13 healthy subjects, the noradrenaline neuronal uptake blocker desipramine was administered through a peripheral vein over 20 min (total dose of free base 0.5 mg kg−1). Blood samples were taken immediately after the infusion.
Neurochemical analysis
Neurochemical assays were performed according to previously described methods for measurements of plasma noradrenaline, tritiated noradrenaline and the noradrenaline metabolites, DHPG and MHPG (Eisenhofer et al. 1988; Esler et al. 1988).
Calculations
Total body plasma noradrenaline clearance and spillover were calculated using the formulae: (i) plasma noradrenaline clearance =3H-labelled norepinephrine infusion rate (d.p.m. min−1)/plasma 3H-labelled noradrenaline concentration (d.p.m. ml−1), and (ii) noradrenaline spillover =3H-labelled noradrenaline infusion rate (d.p.m. min−1)/plasma noradrenaline specific activity (d.p.m. pg−1) (Esler et al. 1979).
Brain noradrenaline spillover was calculated using the methodology established for other organs (Esler et al. 1984) with the following equation:
 |
where NAa and NAv are the arterial and internal jugular venous concentrations of noradrenaline and NAex is the fractional extraction of plasma tritiated noradrenaline across the brain. The derived value represented the unilateral measurement.
Regional DHPG overflow for brain was calculated using the equation (Lambert et al. 1995b):
where DHPGa and DHPGv are the arterial and internal jugular venous concentrations of DHPG.
Regional MHPG overflow for brain was calculated using the equation (Lambert et al. 1995b):  Where MHPGa and MHPGv are the arterial and internal jugular venous concentrations of MHPG.
Where MHPGa and MHPGv are the arterial and internal jugular venous concentrations of MHPG.
Total noradrenaline metabolite overflow for the brain was calculated using the equation:
where DHPGΔ is DHPG regional overflow for brain and MHPGΔ is MHPG regional overflow for brain. Again, this represents a unilateral measurement.
Statistical analysis
All results where appropriate are expressed as means ±s.e.m. Comparative data before and after an intervention were analysed using Student's paired t-test. Inter-group comparisons were analysed using Student's unpaired t-test. In the case of some smaller sample sizes, normality testing for distribution failed. In this case data are analysed non-parametrically using the Mann–Whitney test for inter-group comparisons and are expressed as the median value. The null hypothesis was rejected at P < 0.05. The data was analysed using SigmaStat (Systat Software Inc., San Jose, CA, USA).
Results
Healthy subjects values at rest
Of the 56 healthy subjects who participated without pharmacological interventions 17 underwent bilateral jugular venous sampling resulting in a total of 73 jugular venous samples. The age of participants was 38.0 ± 2.2 years. There were 50 male and 6 female participants. The mean total body noradrenaline spillover for healthy subjects was 605.8 ± 34.4 ng min−1 (n= 49) with the unilateral regional noradrenaline spillover from brain being 12.1 ± 2.3 ng min−1 for right jugular venous sampling (n= 47) and 13.2 ± 2.9 ng min−1 for the left (n= 26). Regional brain spillover of noradrenaline, summing the bilateral values, represented 4.0% of total body spillover. The mean noradrenaline metabolite overflow for unilateral jugular venous sampling, derived from right and left jugular sampling, was 186.4 ± 25 ng min−1 (n= 54). Total brain noradrenaline metabolite overflow doubles this value and was approximately 373 ng min−1 (Tables 1 and 2).
Table 1.
Jugular venous noradrenaline spillover in healthy subjects at rest
| Plasma NA |
Transcranial trititiated NA plasma extraction | Plasma Flow (ml min−1) | NA spillover rate (ng min−1) | ||
|---|---|---|---|---|---|
| Art NA (pg ml−1) | JV NA (pg ml−1) | ||||
| RJV (47) | 230.5 ± 14.3 | 251.7 ± 16.8 | 0.15 ± 0.03 | 233.2 ± 14.6 | 12.1 ± 2.3†* |
| LJV (26) | 246.0 ± 14.9 | 265.3 ± 15.1 | 0.15 ± 0.02 | 220.5 ± 15.5 | 13.2 ± 2.9†* |
The mean noradrenaline (NA) spillover for brain utilising both left and right internal jugular venous (JV) samples was 12.5 ± 1.8 ng min−1.
Noradrenaline spillover for brain using combined left and right JV samples is approximately double the mean unilateral spillover measures, i.e. approximately 25 ng min−1.
Table 2.
Noradrenaline metabolite overflow in healthy subjects (DHPG and MHPG)
| Plasma concentration |
Blood flow (ml min−1) | MHPG and DHPG overflows for L and R jugular (ng min−1) | Total metabolite overflow for pooled R jugular (ng min−1) | ||
|---|---|---|---|---|---|
| ART (pg ml−1) | JV (pg ml−1) | ||||
| MHPG | 2783.1 ± 86.03 | 3119.4 ± 103.5 | 421.2 ± 26.1 | 114.9 ± 28.6 | 180 ± 29.8*† |
| RJV | (n= 37)‡ | (n= 37)‡ | (n= 47)‡ | (n= 37)‡ | |
| DHPG | 947.2 ± 27.4 | 1065.6 ± 37.9 | 421.2 ± 26.1 | 47.3 ± 10.4 | (Right side, n= 36)‡ |
| RJV | (n= 46)‡ | (n= 46)‡ | (n= 47)‡ | (n= 45)‡ | |
| MHPG | 2935.4 ± 186.3 | 3362.8 ± 252.2 | 384.8 ± 27.2 | 166.6 ± 43.9 | 199.2 ± 46.9*† |
| LJV | (n= 18)‡ | (n= 18)‡ | (n= 25)‡ | (n= 18)‡ | |
| DHPG | 959.2 ± 29.7 | 1080.35 ± 41.0 | 384.8 ± 27.2 | 38.3 ± 10.2 | (Left side, n= 18)‡ |
| LJV | (n= 26)‡ | (n= 26)‡ | (n= 25)‡ | (n= 25)‡ | |
The mean noradrenaline metabolite overflow for both left and right JV samples was 186.4 ± 25 ng min−1.
The total metabolite overflow for the brain is therefore approximately double the mean unilateral measurement, i.e. approximately 373 ng min−1.
We were not able to obtain DHPG or MHPG assays and blood flows on all healthy subjects. Hence the number obtained per unilateral sample is expressed as (n=x).
The influence of trimethaphan, clonidine and PAF on noradrenaline spillover from the brain
Of the six subjects who had the trimethaphan intervention, three had bilateral sampling yielding a total of nine samples. Trimethaphan infusion lowered the unilateral internal jugular spillover from brain from a mean of 14.9 ± 4.1 ng min−1 to 5.2 ± 1.8 ng min−1 (P= 0.03) (Fig. 1).
Of the four subjects who had clonidine intervention, two had bilateral sampling yielding a total of six samples. Clonidine infusion lowered the unilateral internal jugular noradrenaline spillover from brain, from 7.0 ± 1 ng min−1 to 2.1 ± 1.5 ng min−1 (P= 0.004) (Fig. 2).
Figure 2. Tritiated noradrenaline extraction across the cerebrovascular circulation in healthy subjects, PAF patients and with desipramine administration.
Healthy vs. PAF (P= 0.4); **Desipramine intervention (P= 0.001). Tritiated noradrenaline extraction across the cerebral circulation was lowered significantly by desipramine infusion. In contrast, [3H]noradrenaline fractional extraction was only marginally lower in PAF patients, and not significantly so. Values are expressed as means ±s.e.m. All units are decimal fractions. PAF, pure autonomic failure.
Of the nine subjects with PAF, one had bilateral sampling resulting in a total of 10 samples. The mean age was 60.2 ± 4.5 years (n= 9). There were five male and four female participants. The median noradrenaline spillover for brain was 1.8 ± 0.9 ng min−1vs. 12.0 ± 1.9 ng min−1 in healthy subjects (P= 0.06) (Fig. 2).
The influence of trimethaphan, clonidine and PAF on the total body noradrenaline spillover rate
Trimethaphan infusion lowered the total body noradrenaline spillover (29% lower) from 628.2 ± 80.4 ng min−1 to 445.7 ± 45.2 ng min−1 (P= 0.01). With clonidine infusion, total body noradrenline spillover was 59% lower, falling from 459.3 ± 147.1 ng min−1 to 184.8 ± 40.9 ng min−1 (P= 0.08). The total body spillover for PAF was 111.9 ± 28.4 ng min−1vs. 605.8 ± 34.4 ng min−1 in healthy subjects (P < 0.001) (Fig. 1).
The influence of desipramine and PAF on the transcranial extraction of plasma tritiated noradrenaline
Of the 13 subjects undergoing DMI infusion, three had bilateral sampling making a total of 16 samples. DMI lowered plasma tritiated noradrenaline fractional extraction across the brain from 0.18 ± 0.03 to 0.08 ± 0.03 (P= 0.001).
The extraction fraction in PAF compared with that in healthy subjects was somewhat lower but not statistically so. Failing tests for normality, a Mann–Whitney rank sum test showed the median in PAF was 0.07 vs. 0.14 (P= 0.4) (Fig. 2).
The influence of trimethaphan, clonidine and PAF on brain noradrenaline metabolite overflow
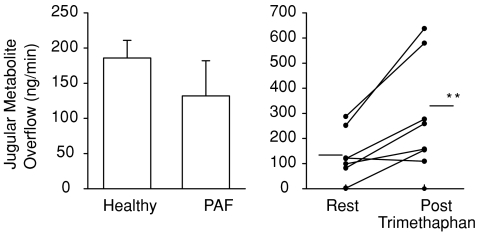
Trimethaphan infusion increased noradrenaline metabolite turnover from 137.5 ± 37.7 ng min−1 to 310 ± 80.4 ng min−1 (P= 0.01). The noradrenaline metabolite overflow was unchanged with clonidine. Mean metabolite overflow before and after clonidine infusion was 105.4 ± 27.4 ng min−1 compared with 58.7 ± 19.6 ng min−1 (P= 0.3). There was no statistically significant difference in noradrenaline metabolite overflow between PAF and healthy subjects. The median noradrenaline metabolite overflow was 88.6 ng min−1 in PAF, compared with 142.8 ng min−1 (P= 0.3) (Fig. 3).
Figure 3. Internal jugular venous metabolite overflow (MHPG and DHPG combined) from the brain in healthy and PAF subjects, and with trimethaphan administration.
*Healthy vs. PAF (P= 0.3); **Trimethaphan (P= 0.01). Jugular venous noradrenaline metabolite overflow is discordant with the previously illustrated brain noradrenaline spillover rates and noradrenaline whole body spillover rates (Fig. 1), specifically trimethaphan infusion significantly elevated noradrenaline metabolite brain overflow, and noradrenaline metabolite overflow in PAF patients was not significantly different from that of healthy patients. Clonidine, not shown, did not alter noradrenaline metabolite overflow from the brain. All data are given in ng min−1. PAF, pure autonomic failure.
Discussion
Whilst the sympathetic innervation of cerebral blood vessels is a well known anatomical feature, the role these nerves play in vascular regulation in humans remains disputed. There is a collective body of research which has attempted to establish the functional role of sympathetic nerves within cerebral blood vessels, but also a great deal of heterogeneity within the research methods used, and the results obtained and their interpretation. Measurements of cerebral blood flow, commonly studied using transcranial Doppler applied to the middle cerebral artery, have been used as an indicator of cerebrovascular neural response. Changes in mean blood pressure in relationship to middle cerebral artery blood flow velocity have been measured during ganglion blockade with trimethaphan (Zhang et al. 2002). Similarly middle cerebral artery blood flow velocity has been studied using transcranial Doppler whilst hypotension was induced non-pharmacologically by releasing thigh cuffs, following which prazosin was given. This was to study the effects of α1-adrenoceptor blockade on cerebral blood flow during a hypotensive state (Ogoh et al. 2008). Doppler ultrasound has been used to measure middle cerebral artery blood flow velocity under the effects of cycling exercise and β1-adrenoceptor blockade (Ide et al. 2000). One difficulty throughout is that cerebral blood flow is the outcome measure, and a relationship to vascular control by the sympathetic nervous system is only inferred. Earlier experimental studies had surgically removed the sympathetic innervation of the brain in animal models and recorded the consequence on cerebral blood flow (Busija & Heistad, 1984; Kissen & Weiss, 1989; Wei et al. 1993), but issues of a potential lack of interspecies similarity remain.
Analysing an assembled data base of measurements, we tested whether this neurochemical technique could be validly used to study the cerebrovascular sympathetic nerves. It was possible to demonstrate a clear concordance between noradrenaline spillover values from in the brain and for thewhole body. In healthy resting people regional internal jugular venous noradrenaline spillover from the brain represented approximately 4% of whole body spillover. When pharmacological interventions were applied, the measurements responded in parallel. Clonidine and trimethaphan, which both cause a reduction in postganglionic sympathetic nerve traffic, lowered both jugular venous and whole body noradrenaline spillover. All results were statistically significant with the exception of the affect on whole body spillover with clonidine intervention, which lowered total body noradrenaline spillover from 459.3 ± 147.1 ng min−1 to 184.8 ± 40.9 ng min−1 (P= 0.08). The whole body spillover under the influence of clonidine was 59% lower than the baseline and given the small sample (n= 4), this reduction is likely to reflect a real trend.
The sympathetic innervations of the cerebral vessels can be delineated into an extrinsic and an intrinsic system. Extracerebral blood vessels receive postganglionic sympathetic fibres arising from the superior cervical ganglion (‘extrinsic innervation’). Within the brain parenchyma, the cerebral arteries lose their extrinsic innervation and are supplied by noradrenergic neurons within the brain (‘intrinsic innervation’) (Paulson et al. 1999; Hamel, 2006). Our assumption is that brain noradrenaline spillover derives from the extrinsic cerebrovascular sympathetic nerves.
The similarity in brain and whole body noradrenaline spillover is further clarified by the comparative analysis of healthy and PAF subjects. PAF subjects demonstrate widespread sympathetic denervation. We expected both the brain and whole body noradrenaline spillover would be less than that seen in a healthy population. The mean total body spillover for PAF was 111.9 ± 28.4 ng min−1 compared with 605.8 ± 34.4 ng min−1 in healthy subjects (P < 0.001), and the median regional spillover for brain in PAF was 1.8 ng min−1 compared with 7.9 ng min−1 (P= 0.06), demonstrating approximate parity between the brain and whole body noradrenaline spillover results.
In another arm of the study, DMI was used to test for the presence of neuronal uptake of noradrenaline in cerebral blood vessels. This would provide an indicator of the existence of functional sympathetic nerves in the walls of the cerebral blood vessels. The postulated lowering of plasma tritiated noradrenaline extraction during transcranial passage of blood was found with DMI infusion. DMI significantly lowered plasma tritiated noradrenaline extraction in transit through the brain from 18 ± 3% to 8 ± 3% (P= 0.001, n= 9). Pharmacologically induced changes in both brain noradrenaline spillover and transcerebral noradrenaline removal thus are in each case as would be expected in the presence of functional sympathetic nerves.
In contrast to the parallels that we observed in brain and whole body noradrenaline spillover under the influence of sympathetic inhibition and in PAF subjects, measurements of noradrenaline metabolite overflow from the brain were discordant. Clonidine did not significantly change noradrenaline metabolite overflow. PAF subjects did not have significantly lower levels of noradrenaline metabolite overflow than healthy subjects. The most intriguing result is that trimephathan significantly elevated the noradrenaline metabolite overflow post-infusion from 137 ± 37 ng min−1 to 310 ± 80 ng min−1 (P= 0.01, n= 9). We have previously interpreted this as representing activation of brain noradrenergic neurons activated by the blood pressure fall (Lambert et al. 1998).
What we appear to be demonstrating is that whilst noradrenaline spillover is responding in the manner we would predict if it were derived from cerebrovascular extrinsic sympathetic nerves, noradrenaline metabolite overflow is not. This discordance shows that the brain noradrenaline spillover is derived from extrinsic sympathetic nerves within cerebral blood vessels, whilst the lipophilic noradrenaline metabolites sampled in the internal jugular vein are in fact derived from both sides of the blood–brain barrier. The results indicate that the proportional input of noradrenaline metabolites from extrinsic sympathetic nerves must be small; the bulk appears to be derived from brain noradrenergic neurons. The contribution from the intrinsic sympathetic innervation of the intracerebral vessels is unknown but is presumably also small. We suggest that in the brain, noradrenaline spillover measurements provide the clinical investigator with a valid tool to study the extrinsic sympathetic nerves.
In previously published studies when we measured brain noradrenaline turnover, aiming to investigate noradrenaline release from brain noradrenergic neurons, the measurement was derived from summing brain noradrenaline spillover with the overflow of noradrenaline metabolites. We now believe that this is incorrect, but any introduced error would be small, as noradrenaline spillover values are only 5–10% of the noradrenaline metabolite overflow value. The true brain turnover figure should be derived from the noradrenaline metabolite overflow values. The contribution to this from intrinsic sympathetic neurons is unknown, but as suggested, presumably small.
Ultimately if sympathetic nerves serve a regulatory function in cerebral blood vessels, there may potentially be clinical ramifications of cerebral sympathetic dysregulation, but this remains to be tested. If sympathetic nerves play a role in physiological homeostasis of cerebral vessels, then pathological conditions that alter sympathetic activity would be likely to alter this control. Sympathetic dysregulation might then perhaps be a precursor of other pathology in the cerebral vascular system. Abnormal cerebral vascular regulation could perhaps be a possible precursor to stroke, migraine and vascular dementia. This speculation remains untested. We believe that brain noradrenaline spillover measurements now provide a tool for assessing the sympathetic innervation of cerebral vessels in a range of clinical settings.
Acknowledgments
M.E. and G.L. are recipients of an NHMCR Fellowship Grant. We also acknowledge the support of the NHMRC Baker Institute Program Grant in helping to fund this study.
Glossary
Abbreviations
- DHPG
3,4-dihydroxyphenolglycol
- DMI
Desipramine
- MHPG
3-methoxy-4-hydroxyphenylglycol
- PAF
pure autonomic failure
Author contributions
D.M., collation and analysis of data, writing of first draft and study design. G.L. conducted studies on subjects, chemical analysis, writing and editing of paper. N.S., P.R. and J.V.L., study design, writing and editing of the paper. M.E., recruitment of subjects, conducted studies on subjects, editing and writing of the paper.
References
- Aggarwal A, Esler MD, Morris MJ, Lambert G, Kaye DM. Regional sympathetic effects of low-dose clonidine in heart failure. Hypertension. 2003;41:553–557. doi: 10.1161/01.HYP.0000055779.93635.A2. [DOI] [PubMed] [Google Scholar]
- Busija DW, Heistad DD. Effects of activation of sympathetic nerves on cerebral blood flow during hypercapnia in cats and rabbits. J Physiol. 1984;347:35–45. doi: 10.1113/jphysiol.1984.sp015051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox HS, Kaye DM, Thompson JM. Regional sympathetic nervous activation after a large meal in humans. Clin Sci. 1995;89:145–154. doi: 10.1042/cs0890145. [DOI] [PubMed] [Google Scholar]
- Edvinnson L, MacKenzie ET, Mcculloch J. Cerebral Blood Flow and Metabolism. New York: Raven Press; 1993. [Google Scholar]
- Eisenhofer G, Goldstein DS, Ropchak TG, Nguyen HQ, Keiser HR, Kopin IJ. Source and physiological significance of plasma 3,4-dihydroxyphenolglycol and 3-methoxy-4- hydroxyphenylglycol. J Auton Nerv Syst. 1988;24:1–14. doi: 10.1016/0165-1838(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Blombery P, Sacharias N, Leonard P. Measurement of total and organ-specific norepinepherine kinetics in humans. Am J Physiol Endocrinol Metab. 1984;247:E21–E28. doi: 10.1152/ajpendo.1984.247.1.E21. [DOI] [PubMed] [Google Scholar]
- Esler M, Jackman G, Bobik A, Kelleher D, Jennings G, Leonard P, Skews H, Korner P. Determination of norepinephrine apparent release rate and clearance in humans. Life Sci. 1979;25:1461–1470. doi: 10.1016/0024-3205(79)90371-0. [DOI] [PubMed] [Google Scholar]
- Falck B, Nielsen KC, Owman C. Adrenergic innervation of the pial circulation. Scand J Clin Lab Invest (Suppl) 1968;102:VI. doi: 10.3109/00365516809168994. [DOI] [PubMed] [Google Scholar]
- Ferrier C, Jennings G, Eisenhofer G, Lambert G, Cox HS, Kalff V, Kelly M, Esler MD. Evidence for increased noradrenaline release from subcortical brain regions in essential hypertension. J Hypertens. 1993;11:1217–1227. [PubMed] [Google Scholar]
- Ferrier C, Esler M, Eisenhofer G, Wallins G, Horne M, Cox H, Lambert G, Jennings G. Increased norepherine spillover into the cerbrovascular circulation in essential hypertension: Evidence of high central nervous system norepherine turnover? Hypertension. 1992;19:62–69. [Google Scholar]
- Giles TD, Iteld BJ, Mautner RK, Rognoni PA, Dillenkoffer RL. Short term effects of intravenous clonidine in congestive heart failure. Clin Pharmacol Ther. 1981;30:724–728. doi: 10.1038/clpt.1981.229. [DOI] [PubMed] [Google Scholar]
- Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–734. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the cerebral circulation. Peptides. 2001;22:995–1007. doi: 10.1016/s0196-9781(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Kissen I, Weiss HR. Cervical sympathectomy and cerebral microvascular and blood flow responses to hypocapnia hypoxia. Am J Physiol Heart Circ Physiol. 1989;256:H460–467. doi: 10.1152/ajpheart.1989.256.2.H460. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, Secher NH. Middle Cerebral artery blood velocity during exercise with β1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiologica Scandinavia. 2000;170:33–38. doi: 10.1046/j.1365-201x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Lefkovits J, Jennings GL, Turner AG, Cox HS, Esler MD. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation. 1995a;92:1813–1818. doi: 10.1161/01.cir.92.7.1813. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Vaz M, Cox HS, Turner AG, Jennings GL, Esler MD. Regional origins of 3-methoxy-4- hydroxyphenylglycol in plasma: effects of chronic sympathetic nervous activation and denervation, and acute reflex sympathetic stimulation. J Auton Nerv Syst. 1995b;55:169–178. doi: 10.1016/0165-1838(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Lambert GW, Kaye DM, Thompson JM, Turner AG, Kalff V, Kelly MJ, Cox HS, Vaz M, Jennnings GL, Wallin BG, Esler MD. Central control of sympathetic nervous system activity in humans: the importance of monoaminergic neuronal group. Acta Physiol Scand. 1998;163:155–163. doi: 10.1046/j.1365-201X.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- Meredith IT, Esler MD, Cox HS, Lambert GW, Jennings GL, Eisenhofer G. Biochemical evidence of sympathetic denervation of the heart in pure autonomic failure. Clin Auton Res. 1991;1:187–194. doi: 10.1007/BF01824986. [DOI] [PubMed] [Google Scholar]
- Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res. 1967;6:773–776. doi: 10.1016/0006-8993(67)90134-5. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers M, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Brain Metab Rev. 1999;2:161–192. [PubMed] [Google Scholar]
- Strandgaard S, Sigurdsson ST. Counterpoint: Sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;290:1366–1367. doi: 10.1152/japplphysiol.90597.2008a. [DOI] [PubMed] [Google Scholar]
- Van Liesshout JJ, Secher NH. Point: Sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;290:1364–1365. doi: 10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Jennings G, Esler M, Korner P, Blombery P, Lambert G, Whitworth J, Scoggins B. Hydrocortisone induced hypertension in man. Pressor responsiveness and sympathetic function. Hypertension. 1989;13:416–421. doi: 10.1161/01.hyp.13.5.416. [DOI] [PubMed] [Google Scholar]
- Wei HM, Sinha HK, Weiss HR. Cervical sympathectomy reduces the heterogeneity of oxygen saturation in small cerebrocortical veins. Am J Physiol Heart Circ Physiol. 1993;74:H1911–1915. doi: 10.1152/jappl.1993.74.4.1911. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]