Abstract
Scientists have long hypothesized the existence of tissue-specific (somatic) stem cells and have searched for their location in different organs. The theory that adrenocortical organ homeostasis is maintained by undifferentiated stem or progenitor cells can be traced back nearly a century. Similar to other organ systems, it is widely believed that these rare cells of the adrenal cortex remain relatively undifferentiated and quiescent until needed to replenish the organ, at which time they undergo proliferation and terminal differentiation. Historical studies examining cell cycle activation by label retention assays and regenerative potential by organ transplantation experiments suggested that the adrenocortical progenitors reside in the outer periphery of the adrenal gland. Over the past decade, the Hammer laboratory, building on this hypothesis and these observations, has endeavored to understand the mechanisms of adrenocortical development and organ maintenance. In this review, we summarize the current knowledge of adrenal organogenesis. We present evidence for the existence and location of adrenocortical stem/progenitor cells and their potential contribution to adrenocortical carcinomas. Data described herein come primarily from studies conducted in the Hammer laboratory with incorporation of important related studies from other investigators. Together, the work provides a framework for the emerging somatic stem cell field as it relates to the adrenal gland.
I. Introduction
II. Adrenal Anatomy
III. Adrenal Gland Development and Establishment of the Definitive (Adult) Cortex
IV. Anatomic Evidence in Support of Adrenocortical Stem/Progenitor Cells
V. Regenerative Capacity of Adrenal Cortex
VI. Clonal Relationship of Undifferentiated Subcapsular Cells to Differentiated Cortical Cells
VII. Dual Role of Sf1-Mediated Transcription in Adrenocortical Development and Steroidogenesis
- VIII. Regulation of Sf1-Dependent Gene Expression in the Subcapsular Cortex by Endocrine and Paracrine Signaling
- A. Sf1 and Dax1 interactions
- B. Endocrine signaling
- C. Paracrine signaling
- IX. Adrenocortical Carcinomas in the Context of Cancer Stem Cells
- A. Beckwith-Wiedemann syndrome and IGF-II
- B. Familial adenomatous polyposis
- C. Li-Fraumeni syndrome
- D. Telomeres, telomerase, stem cells, and cancer
- X. Future Directions
- A. Tissue-specific silencing of Sf1 gene expression
- B. Pod1 null mice
- C. Hedgehog signaling
- D. MicroRNAs
- E. Notch signaling pathway
XI. Summary
I. Introduction
Thomas Addison made a seminal contribution to the field of clinical endocrinology by defining the syndrome of primary autoimmune adrenal failure in the late 1830s. Over 40 years later, Gottschau described the processes of adrenal gland replenishment from the cells of an outer germinal layer and adrenal cellular breakdown in the “zona consumptive” at the interface of the adrenal cortex and medulla. In 1909, Bongomolez confirmed these findings and observed that proliferation in the adrenal cortex was restricted to the subcapsular gland and that cells from this region “migrated” centripetally to populate the inner cortex. Nearly 40 more years passed when Edward Kendall began purifying the major adrenocortical hormones and basic researchers were beginning to uncover the regenerative potential of the adrenal capsule/subcapsular unit through a series of innovative enucleation and lineage-tracing studies. Although these studies provided seminal observations in support of stem and/or progenitor-like cells in the adrenal cortex, work in this area was soon eclipsed by the availability of powerful cellular and molecular biology techniques that were applied to the growing field of steroidogenesis. Only recently, as gene-targeting technology has emerged to apply molecular approaches to whole organ studies, have scientists begun to readdress the questions raised by our scientific predecessors of the early 1900s. What are the mechanisms of adrenocortical cellular replenishment and maintenance? What are the mechanical and chemical stimuli that induce subcapsular proliferation? What is the relationship of the adrenal capsule to the proliferating subcapsular cells? In addition to their contribution to the development and the maintenance of the adrenal cortex, do these cells play a role in pathogenic states of the organ, namely hypoplasias and cancer? Such questions are at the heart of the burgeoning field of tissue stem/progenitor cells. This review will address the concept of adrenocortical somatic stem cells through an examination of the potential roles of such cells in development and homeostatic maintenance of the organ as well as their contributions to developmental pathogenesis and tumorigenesis. We will detail available data stemming from studies primarily conducted in the Hammer laboratory, with incorporation of related works from other groups contributing to this emerging field.
II. Adrenal Anatomy
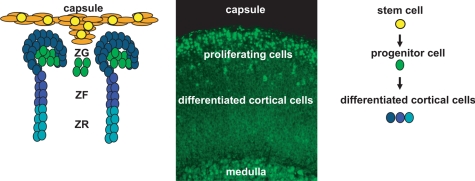
The adrenal gland, a component of the hypothalamic-pituitary-adrenal axis, is a major hormone-secreting organ. The gland is composed of two functionally distinct organs. The medulla, derived from neural crest cells of neuroectoderm lineage, synthesizes catecholamines that facilitate the acute mammalian stress or “fight-or-flight” response. The cortex, derived from the cells of the intermediate mesoderm, synthesizes steroid hormones that mediate body homeostasis and chronic stress responses. The cortex is organized into three concentric zones, zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR), each responsible for the production of different steroid hormones. In 1866, Arnold (2) first described the zonal organization of the cortex with nomenclature that is still in use today (2,3). The cells of the zG are organized in rounded clusters around capillary coils or glomeruli and synthesize mineralocorticoids. The cells of the zF synthesize glucocorticoids and are arranged in radial rows separated by trabeculae and blood vessels. The cells of the zR are arranged in a uniform reticular net of connective tissue and blood vessels and synthesize a subset of sex steroid precursors, including the neurohormone dehydroepiandrosterone sulfate. Although histological and functional differences exist between the adrenal cortices of various mammalian species (mainly the absence of zR and the presence of the fetal/X-zone in some rodents), common developmental principles appear to mediate the formation and homeostatic maintenance of the gland (2,3,4).
III. Adrenal Gland Development and Establishment of the Definitive (Adult) Cortex
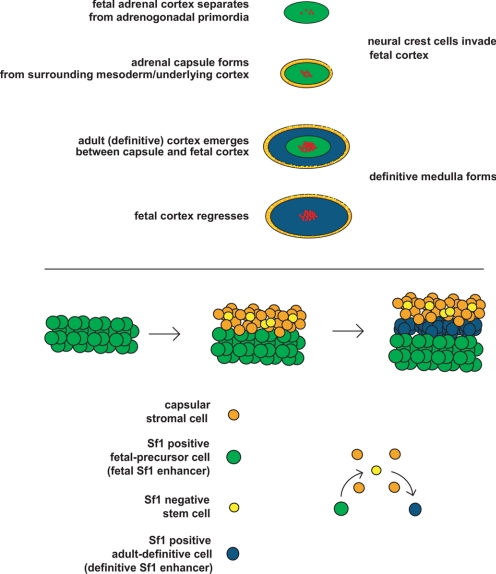
Adrenal gland development in the mammal is defined by discrete histological events (4,5,6). The first milestone is marked by the proliferation of the mesoderm-derived coelomic epithelia and underlying mesonephric mesenchymal cells, forming the embryonic adrenogonadal primordia (AGP) that reside between the primitive urogenital ridge and the dorsal mesentery (7,8) [reviewed by Else and Hammer (5) and Kim and Hammer (9)]. Essential for the formation of the adrenogonadal primordium is expression of the orphan nuclear receptor, Sf1 (Nr5a1, nuclear receptor subfamily 5, group A, member 1, or Ad4BP). Although Sf1 expression is critical, a variety of loss-of-function studies indicate that additional transcription factors including pre-B cell leukemia homeobox 1 (Pbx1), odd-skipped related 1, and polycomb group protein M33 also participate in specification and/or expansion of the AGP (10,11,12). The bilateral AGP then divide into the adrenal primordia (adrenal blastema, often referred to as the fetal adrenal or fetal zone) and the gonadal primordia. The molecular specification of the adrenal primordia is initiated through the up-regulation of Sf1 expression by Wilm’s tumor 1 and Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain 2 (13). Upon separation of the adrenal primordia from the AGP, a different transcription complex containing the homeobox protein PKNOX1, homeobox gene 9b, and Pbx1 is recruited to maintain fetal zone expression of Sf1 through activation of a fetal zone-specific Sf1 enhancer (FAdE). Subsequently, Sf1 itself provides feed-forward activation of its own expression in this compartment (14). Coincident with this transcriptional cascade is the coalescence of the mesenchymal capsule around the fetal cortex (5,9). Once encapsulation is complete, the development of the definitive cortex (definitive zone or adult cortex) becomes evident between the capsule and fetal cortex (Fig. 1).
Figure 1.
Top, Stages of adrenal gland development. Bottom, Model of capsular and definitive zone cell lineage.
Because the cells of the definitive cortex are continually renewed throughout life, a unified model for the formation and maintenance of this structure is paramount. In a previous review, we provided two distinct possibilities for the developmental establishment and postnatal maintenance of the definitive cortex (9). First, the definitive cortex arises from the precursors within the mesenchymal capsule (i.e., the capsule contains the adrenal cortical stem cells) (9). Second, the fetal cortex contains precursors, which give rise to the adult cortex [i.e., capsule serves the role of stem cell “niche” (9)]. Recent and emerging data allow for a reconciliation of these two models in the form of an adrenocortical stem/progenitor niche hypothesis.
In the burgeoning field of stem cell biology, the concept of stem cell residence is used to define the complex microenvironment composed of the stem cells and the supporting niche cells. The stem cell niche provides structural and trophic support, topographical information, and physiological cues to resident stem cells that govern stem cell fate (i.e., self-renewal and symmetric or asymmetric division) (15,16,17). Data from organ systems such as the blood, gonad, and hair follicle reveal conserved niche components that include stromal support cells, extracellular matrix, and vasculature (15,16). Multiple studies have characterized a complex network of capillary beds together with an enrichment of extracellular matrix components within the adrenal capsule (18,19,20,21,22,23), and we suggest that these components provide the needed structural and physiological support to the adrenocortical stem cells. The capsular niche would serve both to recruit stem cells during development and to regulate stem cell fate throughout the life of the organism (16,24). We favor a model whereby the adrenocortical stem cells reside within the capsule itself (i.e., the capsule serves as the physical site of the adrenocortical stem cell as well as the niche) and give rise to the underlying definitive cortex in response to a variety of capsular/subcapsular morphogenic signals (Wnt, Shh, Notch). Indeed, circumstantial evidence comes from chimeric mouse studies that detail concordant clonality of patches of capsular cells and underlying cortical radial stripes (i.e., chimeric patches in capsule lined up with chimeric radial stripes in the cortex), suggesting a shared origin (25,26,27,28).
Although these data are provocative, there remains no conclusive molecular or genetic evidence confirming whether the capsule serves as a stem/progenitor cell pool and/or as a niche for the subcapsular cells. Therefore, determining the lineage relationship between the fetal cortex (precursor population), capsule, and definitive cortex is of paramount importance. Recent data from the Morohashi lab (14,29) demonstrate that the definitive cortex is derived from the fetal adrenal cortex. The data suggest that the FAdE is first shut off (in fetal cells destined to become definitive cells), followed by the activation of a definitive Sf1 enhancer in the emerging definitive cells (29). However, data emerging from our laboratory suggest that Sf1 expression is actively repressed in a subset of capsular cells by the capsule-specific helix-loop-helix (HLH) factor Pod-1 and that genetic loss of Pod-1 results in activation of Sf1 in a subset of capsular cells and an increased number of Sf1-expressing cells in the underlying cortex. Because the definitive cortex only becomes evident between the developing capsule (Sf1 negative) and fetal adrenal cells (Sf1 positive), we propose a model whereby the fetal precursor cells give rise to Sf1-negative cells that participate in the formation of the capsule (stem cell residence). In such a model, mitogenic/morphogenic signals (endocrine and capsular paracrine/autocrine) activate the Sf1-negative capsular stem cells that exit from the capsular niche into the subcapsular environment, where they commence Sf1 expression. Current data suggest that this process requires the loss of capsular Pod-1 expression in the Sf1-negative cell, subsequent activation of the yet to be determined definitive Sf1 enhancer, and ultimate expression of Sf1 in subcapsular rapidly amplifying progenitor cells. Data in support of these models are discussed throughout this review.
IV. Anatomic Evidence in Support of Adrenocortical Stem/Progenitor Cells
What is the evidence for the existence of adrenocortical stem/progenitor cells? While the glomerulosa has been classically defined as a homogenous aldosterone-producing zone directly under the capsule, three observations suggest that the glomerulosa is composed of multiple cell types that participate in the homeostatic maintenance of the adrenal cortex. Early microscopic studies of the adrenal glands of different vertebrates suggested the presence of peripheral undifferentiated cells. For example, in the arctic seal (Phoca vitulina vitulina), clusters of rounded large cells, referred to as adrenocortical blastema, reside within both the capsule and trabeculae that arise from the capsule as perpendicular cords piercing into the outer cortex (30). These cells contain conspicuously large euchromatin-rich nuclei and have high ratios of both rough to smooth endoplasmic reticulum and cristae-type to tubulus-type mitochondria, all characteristic features of less-differentiated cells (30). Because these cells have the histological appearance of progressive centripetal transition to the juxtaposed glomerulosa cells (30), it has been hypothesized that the blastema define a population of undifferentiated cells that undergo both proliferation and differentiation in response to homeostatic stimuli. Recent observational studies in the grass snake (Natrix natrix L.) suggest a similar peripheral pool of undifferentiated transitional cells that have the potential to differentiate into steroidogenic adrenocortical cells (31). The glomerulosa of other species exhibit variable thicknesses and tortuosity with a wide range of embedded, undifferentiated clusters that at times coalesce into circumferential zones of undifferentiated cells. The restricted circumferential expression of preadipocyte factor 1 or Pref-1 (a factor that serves to inhibit differentiation in a variety of tissues) in the glomerulosa of the rat supports such a hypothesis that the glomerulosa layer is composed of cells of varying degrees of differentiation (i.e., progenitor cells). Specifically, an outer layer expresses both Pref-1 and aldosterone synthase, a middle inner layer expresses only Pref-1, and an inner layer above the fasciculata zone expresses no Pref-1, aldosterone synthase, or 11β-hydroxylase (32). In the rat, the two inner aldosterone synthase-negative zones of the glomerulosa are referred to as the zona undifferentiated or zona intermedia (33). In the mouse adrenal, where a defined undifferentiated zone is not observable, such cells might be scattered between differentiated glomerulosa cells. The paucity of expression of certain steroidogenic enzyme genes (Cyp11b1 and Cyp11b2) in these cells has fueled the hypothesis that these cells undergo a morphological transition into differentiated steroidogenic cells of the zG (34,35,36).
Indeed, early histological studies place new adrenocortical cells in the most peripheral position of cellular columns/projections arising from the capsule (37). It has been proposed that the slowly proliferating cells in the capsule generate daughter cells that eventually are centripetally displaced to populate the adrenal cortex (38,39). The robust mitosis and proliferation in the subcapsular region (40,41) is more consistent with transiently amplifying progenitor population. Results of experiments employing radioactive thymidine, bromodeoxyuridine pulse chase, and proliferating cell nuclear antigen staining are consistent with this notion and indicate that subcapsular cells of the rat adrenal replenish the neighboring steroidogenic zones, suggesting that this population provides a pool of progenitors that serve to maintain the functional capacity of the cortex (33,34,36,41,42,43).
V. Regenerative Capacity of Adrenal Cortex
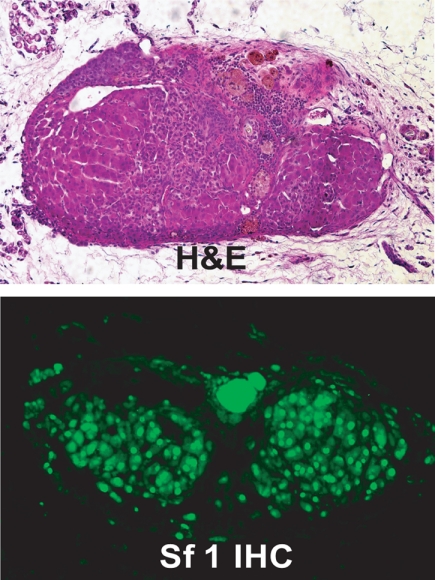
Does the adrenal cortex have regenerative capacity? Where do the adrenocortical stem/progenitors reside? The observations of undifferentiated adrenocortical cells proliferating and repopulating the organ are further supported by experiments assessing the regenerative potential of the adrenal gland. Although primary adrenocortical cells transplanted sc in immunodeficient mice form functional adrenocortical tissue within the grafted host and can respond to hormonal stimuli, the tissue lacks zonal organization and fails to undergo continued homeostatic cellular renewal (i.e., lacks viability after serial transplantation), presumably due to the absence of an intact capsular/subcapsular unit (44,45,46,47). Indeed, when the majority of the adrenal tissue in the living rat is removed, leaving only the capsule and the underlying subcapsular tissue (adrenal enucleation), functional adrenal tissue still regenerates. Moreover, ongoing studies in our group suggest that even when the capsule/subcapsular unit of a donor mouse is removed and sc implanted into syngeneic mice, regenerative potential is maintained, as evidenced by regrowth of Sf1-positive tissue (Fig. 2). Together these data support an essential role of the capsular/subcapsular unit in adrenocortical growth maintenance.
Figure 2.
Histological analysis of a regenerating mouse adrenal gland. Top, Hemotoxylin and eosin (H&E) staining of an enucleated mouse adrenal gland transplanted into the kidney capsule. Bottom, Immunohistochemistry with an anti-Sf1 antibody.
We believe that the proliferating cells arising in the remaining capsule/subcapsular unit initiate the reformation of the functional adrenal cortex. The majority of the regeneration takes place within first 15 d of the enucleation, and repopulation of the entire cortex is accomplished in approximately 3 months (48,49,50,51). Intuitively, the process of regeneration requires both the proliferation and differentiation of these cells. Although the endocrine signals (angiotensin II and ACTH) that define the differentiation or steroidogenic fate of the subcapsular cells have been well studied for decades (48), the autocrine, paracrine, and endocrine proliferative signals have just recently begun to emerge as major regulators of stem/progenitor cell populations. The requirement for ACTH in differentiation is highlighted by the adrenal insufficiency and adrenal hypoplasia in Pomc knockout mice and patients with proopiomelanocortin, Mc2R or Mc2R accessory protein mutations (5,52,53,54,55,56). Studies detailed below have begun to dissect how various signaling pathways regulate the capsular/subcapsular unit to maintain the dynamic balance of proliferation and differentiation that is essential to the function of the adrenal cortex as a central mediator of the mammalian stress response.
VI. Clonal Relationship of Undifferentiated Subcapsular Cells to Differentiated Cortical Cells
Although enucleation studies indicate that the capsular/subcapsular unit has the capacity to proliferate and differentiate, experiments have not been designed to establish the lineage relationships between inner and outer cells of the intact cortex. Immunohistochemical analyses of the adrenal glands of chimeric and transgenic mice (utilizing β-galactosidase reporters under the control of either the cytomegalovirus or steroidogenic gene promoter) reveal variegated expression of chimeric or reporter genes in cord-like radial stripes extending from the periphery to the corticomedullary boundary, consistent with a clonal origin of cells within each radial stripe. The data support the hypothesis that the adrenal cortex is maintained through proliferation and clonal replenishment of peripheral cells that undergo centripetal displacement and differentiation in response to endocrine stimulation (26,57,58,59,60).
VII. Dual Role of Sf1-Mediated Transcription in Adrenocortical Development and Steroidogenesis
Studies in which ACTH mediates steroidogenesis/differentiation coupled with the appropriate molecular tools have provided our laboratory and many others with the means to examine the processes of differentiation and proliferation in organ homeostasis. The independent discoveries by the Parker and Morohashi groups (7,61,62) of Sf1/Ad4BP as a critical transcriptional mediator of both steroidogenesis and organ development have provided seminal findings in the field of adrenocortical stem/progenitor biology. The orphan nuclear receptor Sf1 was discovered in both laboratories by its ability to interact with and transactivate promoters of ACTH-activated steroidogenic enzyme genes (61,63). Soon after it was shown to be essential for adrenocortical development, as evidenced by the adrenal aplasia of Sf1 null mice (7,63,64,65).
The subcapsular cells of the adrenal cortex are predicted to have the capacity to both proliferate and differentiate. Studies in other systems have detailed the processes by which quiescent undifferentiated cells undergo rapid and dynamic nuclear architectural changes as they become engaged in proliferative or differentiation programs. Preceding the necessary formation of the euchromatin and initiation of active transcription, many covalent modifications occur on both DNA and transcriptional factors that enable cell cycle-specific gene activation (66). In the mid 1990s, with no definitive Sf1 ligand, the mechanism by which peptide hormone-initiated signaling cascades result in activation of Sf1-dependent transcription was not entirely clear. We began exploring whether posttranslational modifications of Sf1 differentially activated proliferative and/or differentiation programs.
We first built on earlier observations in Holly Ingraham’s laboratory by Hammer et al. (67) that phosphorylation of Sf1 serine 203 by Erk1/2 plays a critical role in cofactor recruitment and Sf1-dependent transcriptional activation. Although we posited that phosphorylation of Sf1 in response to ACTH may be critical for gene activation, other work by Sewer and Waterman (68) soon demonstrated clearly that dephosphorylation of Sf1 also served a critical role in the activation of certain ACTH-dependent targets. We wondered whether these observations were mutually exclusive or whether both phosphorylation and dephosphorylation are essential for different phases of transcriptional activation. Chromatin immunoprecipitation (ChIP) experiments had recently revealed that transcriptional activation by ligand-dependent nuclear receptors is a dynamic process, occurring in an ordered, stepwise, and cyclical fashion (69,70). Therefore, our lab sought to explore whether ACTH might coordinate Sf1-mediated transcription through a series of phosphorylation and dephosphorylation events that could regulate cofactor assembly/disassembly and subsequent transactivation of target gene promoters. Using ChIP in mouse adrenocortical Y1 cells, our lab demonstrated that Sf1 is actively recruited to the promoters of a variety of steroidogenic enzyme genes after ACTH stimulation (71). Moreover, Sf1 exhibited a cyclical occupancy on the Mc2r gene promoter that was coincident with phosphorylation of Sf1 and recruitment of coactivator proteins (71), supporting a dynamic model of ACTH-mediated phosphorylation of Sf1 that participates in the regulation of steroidogenesis (differentiation).
It has recently become apparent that Sf1 can be repressed and activated through a variety of mechanisms that might serve cell and/or gene-specific functions. For example, several lines of evidence have identified Sf1 as a SUMO target protein (72,73,74). Sf1 is SUMOylated at Lysine 194 (K194), which lies within a synergy control motif in the hinge region of Sf1. Mutation of the synergy control motif leads to an enhancement of synergistic transcription from a promoter containing multiple Sf1 sites (73). This result is further supported by evidence that mutations of SUMOylation sites enhance the transcriptional activity of Sf1 but do not affect the DNA binding affinity of Sf1. Therefore, the SUMOylation site of Sf1 functions as an intrinsic repression domain (72). Moreover, overexpression of SUMO-1 directs wild-type Sf1, but not SUMOylation-deficient Sf1 mutant, to nuclear speckles (72). These results demonstrate that SUMOylation is an important posttranslational modification that regulates the transcriptional activity of Sf1. Two E3 SUMO ligases, PIAS1 and PIAS3 (73), and the helicase ARIP4 (K. Morohashi, personal communication) have been found to interact with Sf1 and promote Sf1 SUMOylation. More recently, the DEAD-box RNA helicase DP103 (Dxd20/Gemin-3) has been found to promote PIAS-dependent SUMOylation and intranuclear relocalization of Sf1 (74). These studies provide evidence that helicases are directly coupled to Sf1 transcriptional repression by protein SUMOylation.
Although the context in which these modifications participate in the regulation of Sf1 remains unclear, a few recent observations provide the groundwork for future studies along these lines. The Bakke laboratory (75) has recently revealed that in the highly proliferative Y1 adrenocortical cell, CDK7 as opposed to Erk1 is the primary kinase responsible for phosphorylation of Sf1 serine 203. Whether such phosphorylation serves an in vivo function to uniquely engage Sf1 in proliferative (as opposed to differentiation) programs is unknown. Moreover, recent findings from others and from our lab in collaboration with the laboratory of Jorge Iniguez indicate that SUMOylation of Sf1 K194 serves to both inhibit phosphorylation of Sf1 by CDK7 and prevent occupancy of Sf1 on a variety of steroidogenic promoters (76,77). The data support a general model in which SUMOylation participates in transcriptional repression in part by preventing additional activating posttranslational modifications of nuclear receptors. Similarly, the Sewer laboratory (78,79) has reported that, after ACTH stimulation, endogenous adrenocortical sphingolipids in the Sf1 binding pocket are repressive and displaced by activating phospholipids [as characterized by the West, Xu, and Ingraham labs (80,81,82)]. Together these results indicate that posttranslational modifications and ligand availability may play coordinating roles in Sf1 activation. Because CDK7 is well-known to be activated only in the context of cell cycle activation and proliferation is restricted to the subcapsular cortex, a critical issue to resolve is the physiological context in which these modifications activate Sf1 in proliferating vs. differentiated cells of the intact adrenal cortex. The first clue to this riddle came in the form of a paradox.
VIII. Regulation of Sf1-Dependent Gene Expression in the Subcapsular Cortex by Endocrine and Paracrine Signaling
A. Sf1 and Dax1 interactions
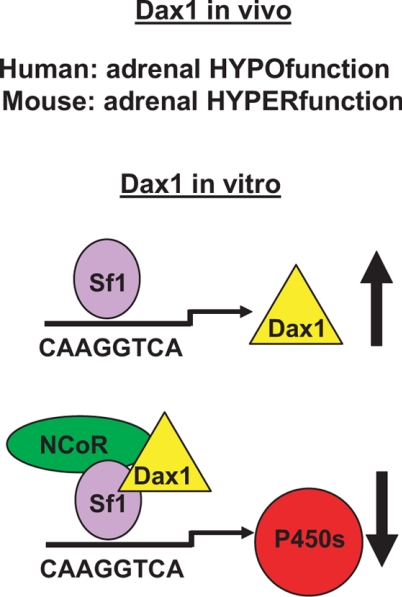
DAX1 (NR0B1; dosage-sensitive sex reversal, adrenal hypoplasia congenita critical region, on chromosome X, gene 1) was initially cloned as the gene responsible for X-linked congenital adrenal hypoplasia (83,84). Homozygous mutations in SF1 were later found in patients with a similar congenital adrenal aplasia/hypoplasia (85,86,87), suggesting a genetic or molecular interaction between these two atypical nuclear receptors. Consistent with this prediction, the Dax1 gene was determined to be a bona fide Sf1 target gene by multiple laboratories. However, the gene product of Dax1 was surprisingly found to serve as a repressor of Sf1-dependent transactivation (88). How such a repressive function could be reconciled with the phenotype of patients with mutations in DAX1 that partially phenocopied patients with SF1 mutations was unclear (Fig. 3). We hoped to gain insight into these potentially mutually exclusive observations by comparative studies of Sf1 haploinsufficient mice and combined Sf1 haploinsufficient/Dax1 null mice.
Figure 3.
Role of Dax1 in adrenal gland physiology. Model of Sf1- and Dax1-mediated transcription.
Although homozygous loss of the Sf1 gene in mice results in perinatal death of mice due to complications arising from adrenal aplasia, Sf1 heterozygous mice are viable with small adrenal glands (89,90). Using the paradigm of compensatory adrenal growth after unilateral adrenalectomy, Felix Beuschlein et al. (38) in our lab determined that Sf1+/− mice are not able to mount a compensatory proliferative response in the subcapsular cortex. Consistent with this result, mice engineered to express multiple copies of Sf1 in the adrenal cortex have recently been shown to exhibit pronounced subcapsular proliferation (91). Together, these studies provide evidence that Sf1 dosage is critical to the proliferative capacity of subcapsular cells and ultimately provide insight into the interactions of Sf1 and other transcription factors in these cells.
The Sf1 target genes Pbx1 and Dax1 provide examples of how Sf1 serves to regulate both a transcriptional activator and a repressor within the same cellular compartment. Studies in the Beuschlein group (92) demonstrated that Pbx1 is a direct target of Sf1, that Pbx1 expression was reduced 50% in the Sf1 haploinsufficient mice, and that Pbx1+/− mice phenocopied the subcapsular defect in Sf1 haploinsufficient mice after unilateral adrenalectomy, suggesting that Pbx1 is a downstream mediator of Sf1-dependent proliferation of subcapsular cells. Dax1, however, created a unique challenge. If Dax1 serves a primary role to inhibit Sf1-mediated transcription, loss of Dax1 would be expected to compensate for Sf1+/− haploinsufficiency. Alternatively, if Dax1 serves as a mediator of Sf1-dependent adrenal growth, we would expect either no change or a more severe adrenal hypoplasia. We examined these alternate hypotheses using Dax1 null mice generated in the laboratory of Larry Jameson. Surprisingly, Dax1 deficiency in mice did not appear to phenocopy the X-linked congenital adrenal hypoplasia seen in patients with a DAX1 mutation, but instead the combined Sf1+/−/Dax1−/Y mice almost completely rescued the hypoplasia and reduced steroidogenesis observed in Sf1+/− mice (93).
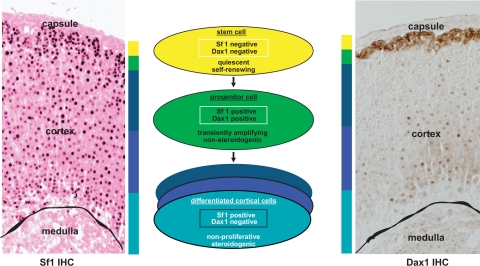
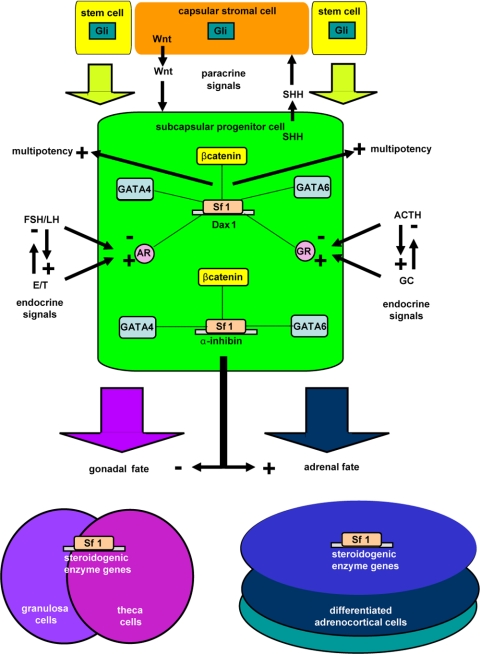
Several recent findings have provided insights to help explain these apparent discrepant observations. First, although Dax1 is expressed in the fetal zone of the adrenal, as the definitive zone emerges, expression becomes restricted to the subcapsular cortex. Sf1 would only be predicted to regulate Dax1 gene expression in this cell population. Secondly, we have recently observed that whereas the early enhanced differentiation in the adrenal glands of Dax1−/Y mice is coincident with early enhanced growth, the aging organ does not sustain homeostatic proliferation and ultimately develops biochemical adrenal failure with histological adrenal cytomegaly (our unpublished observation). The above data, together with the burgeoning role of Dax1 as a mediator of mouse embryonic stem cell pluripotency (94), have led us to begin examining the paracrine and endocrine signals that regulate homeostatic proliferation and differentiation of adrenocortical subcapsular cells with a particular interest in Sf1 and the restricted expression of Dax1. The unique expression pattern of Sf1 and Dax1 in capsular (Sf1 negative, Dax1 negative), subcapsular (Sf1 positive, Dax1 positive), and mature steroidogenic (Sf1 positive, Dax1 negative) cortical cells predicts that binary decisions participate in paracrine and endocrine regulation of lineage relationships in these cellular populations (Fig. 4).
Figure 4.
Model of hierarchical organization of adrenocortical cells. Left, Immunohistochemistry (IHC) with anti-Sf1 reveals cortical staining. Middle, Model of Sf1 and Dax1 expression in stem/progenitor/differentiated adrenal cortex cells. Right, Immunohistochemistry with anti-Dax1 reveals membranous subcapsular expression and nuclear intracortical expression.
B. Endocrine signaling
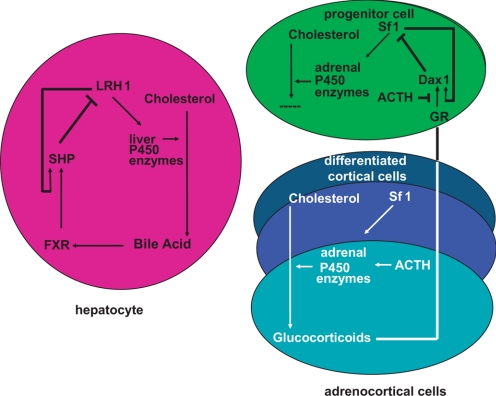
The structural and biochemical regulation of a nuclear receptor can often provide insight into the mechanism of action of a related gene family member. The phylogenetic relationship of the NR0B factors, DAX1 and small heterodimer partner (SHP), and of the NR5A factors, SF1 and liver receptor homolog (LRH), has provided a number of clues to the regulatory circuits controlling SF1 and DAX1 action in the adrenal cortex. The metabolism of cholesterol in the adrenal cortex parallels the metabolism of cholesterol in the liver. Both require the P450 enzymes for the conversion of cholesterol into its end products as well as transcription factors that regulate expression of these enzymes (31). Pioneering work of the Mangelsdorf lab (95) defined an intracellular biochemical feedback loop in the hepatocyte that regulates the synthesis of bile acid through the coordinated expression of LRH and SHP. LRH-1 was shown to regulate expression of CYP7A1, the rate-limiting P450 enzyme for bile acid production (96,97). LRH-1 also induces expression of a transcriptional repressor, SHP (98,99,100), which is analogous to the SF1-dependent regulation of DAX1 expression. The observations that 1) bile acid production in the liver was found to be accompanied by an increase in SHP expression, and 2) bile acids are activating ligands for the farnesoid X receptor, led to the discovery that farnesoid X receptor binds to the promoter for the SHP gene and synergizes with LRH-1 to activate SHP transcription. The increase in SHP levels results in SHP-mediated repression of LRH-1-dependent transcription of CYP7A1 and provides a biochemical basis of feedback control of bile acid synthesis (Fig. 5) (95).
Figure 5.
Comparative paradigm of nuclear receptor-mediated transcriptional feedback loop. The intracellular feedback regulation of adrenocortical steroid production parallels the regulation of bile acid synthesis in the liver.
These parallels suggested to us that similar mechanisms might participate in cholesterol metabolism in the adrenal cortex. However, if glucocorticoids inhibited adrenocortical steroidogenesis through a synergistic glucocorticoid receptor (GR)/Sf1-dependent activation of Dax1 transcription in adrenocortical cells, it seemed likely that such an intraadrenal system was a vestigial regulatory network in the presence of the robust hypothalamic-pituitary-adrenal axis that is defined by endocrine-regulated long feed-forward and long feedback control. We ultimately defined an intraadrenal regulatory loop whereby glucocorticoid-bound GR synergistically activated Sf1-dependent Dax1 transcription (101). Moreover, quantitative ChIP analysis in synchronized cells allowed us to observe that: 1) Sf1 was bound constitutively to the Dax1 promoter at baseline; and 2) ACTH induced the clearance of both GR and Sf1 from the Dax1 promoter, resulting in transcriptional silencing of the Dax1 gene (101). This finding was in direct contrast to the near universal robust Sf1 recruitment and gene activation of other Sf1 target genes after ACTH treatment (101). Moreover, it held a critical clue to the Dax1 riddle. Because Dax1 is primarily expressed in the subcapsular region of the adrenal cortex coincident with the location of proliferating undifferentiated progenitor cells, we proposed a model whereby the differentiated cortical cell generates glucocorticoids that provide an endocrine signal (via centrifugal adrenal blood flow) to the undifferentiated progenitor cells to activate Dax1, which in turn inhibits Sf1-mediated differentiation (steroidogenesis). ACTH stimulation inactivates Dax1 and initiates steroidogenesis (Fig. 5). Because the presentation of adrenal failure in patients with DAX1 mutations is stochastic (even within the same kindred) (84,94,102), we would predict that such adrenal failure results from intrinsic differences in progenitor cell reserve. Loss of subcapsular cells would be reflected in premature differentiation at the expense of depletion of the progenitor pool. Indeed, a number of reports document persistent postnatal presence of 11-deoxycoritsol and testosterone in patients with X-linked adrenal hypoplasia congenita before the development of failure (103,104), consistent with this hypothesis.
However, the broad subcapsular expression of Dax1 in the mouse is not consistent with a solitary role of Dax1 in such an undifferentiated progenitor cell. Instead, two possibilities seem likely. Dax1, akin to Pref-1, is expressed in multiple types of granulosa cells (aldosterone synthase positive and aldosterone synthase negative), implying that Dax1 has roles in aldosterone synthase-positive glomerulosa cells that extend beyond its proposed role in the inhibition of differentiation of progenitor cells (aldosterone synthase-negative cells). Alternatively, the aldosterone synthase positive glomerulosa cell is the progenitor cell in the mouse, and Dax1 serves to inhibit the differentiation of a glomerulosa cell into ACTH-dependent fasciculata cells.
In addition to Pbx1 and Dax1 (105,106), Sf1 has been shown to interact with other transcription factors [i.e., cAMP-responsive element binding protein (107), androgen receptor (108), Sp1 (109), and β-catenin (101)] on a variety of gene promoters. Although these interactions (except Dax1) result in gene activation, the above data lend credence to the possibility that Sf1 might repress and Dax1 might activate transcription of certain genes in subcapsular cells. Indeed, in recent microarray analysis of Sf1-overexpressing cells, a number of transcripts proposed to be direct Sf1 targets were down-regulated (91). In collaboration with Bernard Schimmer (University of Toronto), we have shown that an Sf1/Sp1 interaction represses transcription of the type 4 adenylate cyclase, Adcy4 (110). Moreover, the human DAX1 gene generates an alternate transcript that lacks the repressive domain and therefore acts as a transcriptional activator (111). Our recent collaborative work with Ronald Koenig’s laboratory confirms that mouse Dax1 can function as a bona fide coactivator of Nr5a-dependent transcription at high doses in both adrenocortical cells and mouse embryonic stem cells (220). Although a better understanding of the cellular context of these interactions is required, these data may provide an additional layer of complexity to the regulation of Sf1-dependent transcription pertinent to organ homeostasis.
C. Paracrine signaling
A network of highly conserved developmental signaling pathways often orchestrates complex developmental events during embryogenesis as well as homeostatic events of adult organ system maintenance. Defects in TGFβ, hedgehog (Hh), Notch, and wingless-type MMTV integration site (Wnt) signaling pathways have been found to participate in the etiology of both developmental abnormalities and cancer (112). To explore the importance of such pathways in the adrenal cortex, we focused our efforts on Wnt and TGFβ signaling pathways in adrenocortical homeostasis.
1. Wnt signaling pathway.
Wnt signaling is categorized into two distinct intracellular pathways with different molecular mediators and cellular consequences: canonical Wnt signaling and noncanonical/planar-cell polarity pathway. Transcriptional activation after canonical pathway stimulation is mediated through the stabilization of the effector protein β-catenin, after the binding of a Wnt ligand to its respective Frizzled (Fzd) receptor. In the absence of Wnt ligands, the pool of β-catenin is sequestered to the cellular membrane/cell adherence junctions, and a low cytoplasmic concentration is maintained by ubiquitin-mediated proteolysis through a degradation complex consisting of Axin/adenomatous polyposis coli (APC)/glycogen synthase kinase 3 β. Upon Wnt ligand binding, disruption of the degradation complex permits cytoplasmic and nuclear accumulation of β-catenin. Inside the nucleus, β-catenin interacts with members of the lymphoid enhancer-binding factor/T cell factor (Tcf) family of transcription factors to activate expression of target genes. Through transcription of target genes, the canonical Wnt pathway regulates processes such as proliferation, specification of cell fate, stem cell maintenance, and differentiation. Recent studies have suggested that a number of Wnt ligands and Fzd receptors play roles in adrenocortical development and/or homeostasis. For example, loss of Wnt 4 (primarily a noncanonical Wnt) results in the aberrant migration of adrenocortical cells into the developing gonad (113). Due to the combinatorial complexity of the Wnt signaling pathway (19 ligands and 10 receptors in mouse), we focused our efforts on the role of the canonical Wnt signaling pathway (i.e., β-catenin-dependent transcription) in adrenocortical development and maintenance.
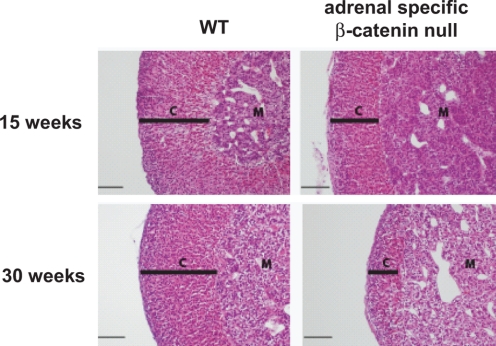
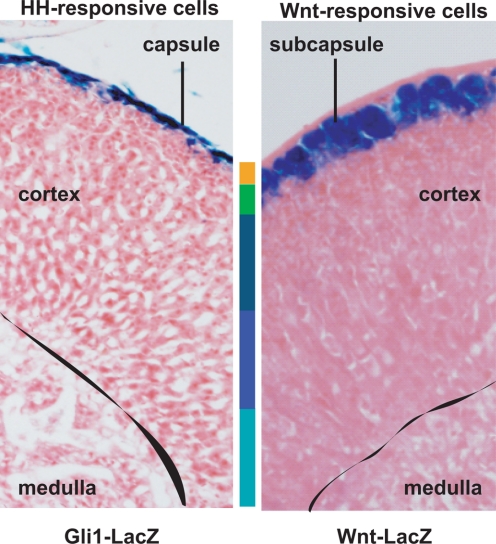
Initially, we examined the temporal and spatial expression of the β-catenin protein as well as the localization of the active β-catenin signaling using the Wnt-Gal transgenic reporter mouse line that expresses β-galactosidase only in cells with active β-catenin signaling. At embryonic day (E) 12.5, although β-catenin protein was expressed (as determined by immunohistochemistry) in nearly all cells of the newly formed fetal adrenal cortex, active signaling (as determined by the presence of lacZ) was absent (114). However, as the capsule of the adrenal formed, β-catenin protein and active β-catenin signaling became strictly localized to cells under the newly emerging adrenal capsule, the proposed location of the adrenocortical somatic progenitor cells. To further examine the role of β-catenin in the adrenal cortex, in collaboration with the laboratory of Keith Parker, we used the Cre-loxP conditional knockout strategy to inactivate β-catenin alleles in the adrenal cortex. In the study, we mated Ctnnb1tm2kem transgenic mice carrying floxed β-catenin alleles to two lines of transgenic mice, one carrying a low-expressing Sf1-Cre transgene (Sf1-Crelow) and one carrying a high-expressing Sf1-Cre transgene (Sf1-Crehigh) (114). With near complete excision of β-catenin alleles in the adrenocortical cells using the Sf1-Crehigh mice, we observed the absence of the adrenal gland at postnatal day zero. When examining the developing adrenal glands of these mice, we observed a marked failure of cellular proliferation under the developing capsule between E12.5 and E14.5 (114). With excision of β-catenin in only 50% of adrenocortical cells in the Sf1-Crelow mice, we observed normal development of the gland at birth followed by progressive cortical thinning (Fig. 6) and decreased steroidogenic capacity (as determined by steroidogenic enzyme mRNA levels) over a timeframe of 30 wk, presumably due to loss of proliferating subcapsular cells (114). Building upon this observation that canonical Wnt signaling is essential for the homeostatic maintenance of the adult gland, current efforts are focused on the isolation of Wnt-responsive subcapsular cells via flow cytometry and identification by cDNA expression arrays of downstream target genes that may contribute to subcapsular cell fate. Identifying the roles of specific Wnt ligands and their respective Fzd receptors responsible for canonical signaling in the adrenal cortex together with an exploration of the roles of noncanonical Wnt signaling pathway in the adrenal cortex are logical areas of future investigation.
Figure 6.
Role of canonical Wnt signaling in adrenocortical homeostasis. Hematoxylin and eosin staining of conditional β-catenin knockout adrenals. Progressive depletion in the adrenal cortex is evident at 30 wk of age. [Reproduced with permission of Development (114)].
2. TGFβ signaling and inhibin.
The shared origin of the adrenal and gonad, the existence of pluripotent stem/progenitor cells for steroidogenic lineage in both tissues, and the presence of 1) ACTH-driven adrenal rests in the gonads of patients with long-standing untreated congenital adrenal hyperplasia, and 2) gonadotropin-driven thecal metaplasia in the adrenal cortex of estrogen-naive postmenopausal women predict a mechanism that guides the stem/progenitor cells of the each tissue (gonad vs. adrenal) to differentiate selectively in response only to the appropriate peptide hormone and not the other (FSH/LH vs. ACTH). The adrenocortical defects in inhibin-α null mice provided a unique opportunity to evaluate this hypothesis.
The TGFβ superfamily encompasses approximately 30 growth and differentiation ligands that include TGFβs, activins, inhibins, and bone morphogenetic proteins, many of which have been shown to play essential roles in stem cell fate commitment (115). This highly conserved signaling pathway commences with the binding of a ligand to a TGFβ type II receptor. The type II receptor is a serine/threonine receptor kinase, which in turn phosphorylates the type I receptor. The type I receptor is then able to phosphorylate and activate the receptor-dependent Smad proteins upon which these mediators translocate to the nucleus, recruit other transcription factors to activate, and/or antagonize the expression of target genes (Fig. 7) (116).
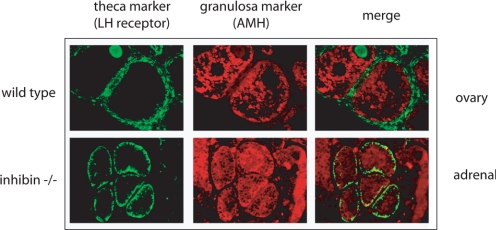
Figure 7.
The role of TGFβ signaling in adrenal vs. gonadal fate determination. Histological analysis of adrenal cortex revealing follicular-like structures in the adrenal cortex of inhibin null mice (red, granulosa cell staining for anti-mullerian hormone; green, theca cell staining for LH receptor). [Reproduced with permission. Copyright 2006, The Endocrine Society (119)].
Our interest in this signaling pathway began with studies describing gonadal tumors and subsequent adrenocortical blastomas after gonadectomy in both inhibinα null mice (Inha−/−) (117) and in mice with overexpression of the simian virus 40 T-antigen gene under control of the inhibinα promoter (221). In the collaboration with the laboratory of John Nilson, we demonstrated that gonadectomy of Inha−/− mice (kindly provided by Marty Matzuk) resulted in the formation of large tumors in the adrenal glands that we ultimately showed were dependent upon chronically high levels of LH (118). Further analysis revealed that the tumors were actually newly differentiated ovarian tissue that was formed from LH-stimulated undifferentiated adrenal subcapsular progenitor cells. Two events were found to be critical for this ultimate manifestation of ovarian fate in the adrenal cortex of these mice. First, it appears that the adrenocortical subcapsular cells of wild-type adrenal cortex have the innate capacity to respond to gonadal-specific differentiation signals (LH and FSH) consistent with the singular origin of the adrenal cortex and gonad—the adrenogonadal primordium. Specifically, LH induces expression of gonadal-restricted Gata4 in the subcapsular cells of the adrenal with concomitant loss of adrenal-restricted Gata6. LH and Gata4 are both necessary (but not sufficient) to drive adrenocortical progenitor cells to ovarian fate. Indeed, these adrenal tumors display a global reprogramming of their cellular identity, manifesting in expression of genes normally restricted to theca and granulosa cells of the ovary (119). Second, we were able to show genetically that it is the specific loss of inhibin-α and consequent unopposed Smad3 activation that leads to expansion of these Gata4-positive cells and ultimate generation of ovarian tissue in the adrenal cortex (119). Consistent with this paradigm, the LH-driven gonadal-programmed subcapsular cells in wild-type mice are prevented from expanding and forming true ovarian tissue through the actions of inhibin (119). When Smad3 is genetically ablated in the context of compound inhibin-α-null/Smad3-null mice, while Gata4-positive cells still reside in the subcapsular progenitor cell region (in response to LH), no expansion occurs, indicating the importance of inhibin-α as a gatekeeper of adrenogonadal stem/progenitor cell fate (120).
It remains equally plausible that cells of the developing gonadal primordia are mislocalized in the adrenal cortex and hence respond normally to gonadotropins, albeit in an ectopic location. Indeed, in a recent paper by the Swain group (113), such a model has been proposed for the expression of adrenal-specific genes in the gonadal primordia of Wnt4 knockout mice. We would posit that the activation of gonadal genes in the subcapsular cells of nongenetically modified mice in multiple strains of mice argues against mislocalization and rather argues in favor of bona fide multipotency.
The dependence of adrenal-specific ovarian differentiation upon Smad3 indicated that inhibin-α loss allowed unopposed receptor activation by a stimulatory ligand of the TGFβ superfamily, namely a TGFβ or an activin, both of which signal primarily through Smad2 and-3. Inhibin is an atypical member of the TGFβ family of signaling ligands and is classically understood to function via competitive antagonism of activin ligand binding. However, because activin is not expressed in the adrenal cortex before tumor formation and is not induced by elevated LH levels, we are currently examining the hypothesis that adrenocortical tumorigenesis in Inha−/− mice is explained by a novel interaction between inhibin-α and the TGFβ2 ligand. Taken together, these observations provide rationale for the fetal adrenal production of massive amounts of inhibin-α. We predict that this is to ensure the specification of adrenal fate by preventing human chorionic gonadotropin-mediated ovarian differentiation of the gland.
IX. Adrenocortical Carcinomas in the Context of Cancer Stem Cells
Benign adrenal tumors are relatively common, with occurrences of 3–7% of the population. Malignant adrenal tumors or adrenocortical carcinomas (ACC) are relatively rare, with the incidence rate of approximately two cases per million people per year and representing only 0.2% of cancer deaths in the United States. Although rare, this form of cancer is highly malignant and presents with extremely poor prognosis as a consequence of metastasis or local invasion at the time of diagnosis (121,122,123). The recent findings that most ACC are monoclonal in origin (123) suggest that an initiating mutation within a single dividing cell might give rise to adrenal cancer (123). These suggestions, together with our observations that adrenocortical subcapsular somatic progenitor cells alone have a robust proliferation potential, support a stem and/or progenitor origin of adrenal cancer.
The cancer stem cell theory hypothesizes that a distinct subpopulation of cancer cells maintains the stem cell potential (124). These cells, termed “cancer stem cells,” are uniquely endowed with the ability to self-renew and differentiate into multiple different cell types within a cancer, analogous to normal tissue stem cells. Cancer stem cells have been identified in blood, breast, brain, colon, and pancreatic cancers, and the list is expanding (124). Furthermore, evidence is mounting that aberrant epigenetic reprogramming of stem/progenitor cells is another level of dysregulation contributing to the pathogenesis in human cancers (125). Although cancer stem cells are predicted to be the only cells within a tumor that can give rise to new tumor cells, they are also predicted to be the only cells that are not responsive to standard chemotherapeutic protocols. This theory challenges current dogma of cancer therapeutics that all tumor cells possess equal malignant potential. Thus, drug discovery is challenged to shift treatment paradigms toward targeting this tumorigenic subpopulation of cancer stem cells for potential cure.
Armed with emerging data on adrenocortical stem/progenitor cells and our data describing the important molecular mechanisms regulating adrenocortical organ maintenance, we began to examine the biology of genes known to be mutated in hereditary ACC syndromes such as Beckwith-Wiedemann syndrome (BWS), familial adenomatous polyposis, and Li-Fraumeni syndrome. Because these genes are known to play various roles in the regulation of somatic stem cell fate in a variety of systems (122,123), we hoped to gain insights into both the normal and pathological roles of these factors in adrenocortical stem and/or progenitor cells.
A. Beckwith-Wiedemann syndrome and IGF-II
BWS is a rare embryonic overgrowth disorder that manifests with macrosomia, macroglossia, abdominal wall defects, renal abnormalities, cleft palate, and an increase in a variety of childhood cancers, including ACC. It has a frequency in the United States of about 1 in 14,000, and about 10–15% of cases are familial (126). Approximately 85% of BWS patients have causative defects in the genomic imprinting of five genes located in two chromosomal clusters within the 11p15.5 region (127). The two genes comprising the distal cluster are the IGF-II and H19 (an untranslated mRNA) genes. The proximal cluster includes the CDKN1C (cyclin-dependent kinase inhibitor p57kip2), KCNQ1 (potassium voltage-gated channel) and KCNQ1OT1 (KCNQ1 overlapping transcript) genes. Several studies have demonstrated the association of IGF-II expression with malignant vs. benign adrenal tumors (128,129), and DNA microarray expression analyses in the laboratory of our collaborator Tom Giordano implicate IGF-II as the single highest expressed gene in the majority of sporadic ACC (130). Moreover, in these samples, H19 and p57 are characteristically down-regulated, consistent with a somatic epigenetic defect. Mouse models have also confirmed that overexpression of the IGF-II gene results in a BWS-like syndrome (131) that includes adrenal hyperplasias and cytomegaly (132).
IGF-II is often seen as a key growth factor employed during early fetal development, whereas postnatal IGF-I expression is used for maintaining normal growth as a downstream effector of growth hormone stimulation. Both IGF-II and IGF-I bind to the receptor tyrosine kinase, IGF-1R (IGF-I receptor) (and the insulin receptor to a lesser degree) to exert their physiological effects. Extracellular ligand binding causes IGF-1R autophosphorylation of its intracellular domain. Subsequently, the MAPK and phosphatidylinositol-3-kinase/Akt signaling pathways are activated to induce expression of numerous genes that regulate progrowth/survival pathways in the cell (133). Because in the adrenal gland IGF-II is uniquely expressed in the capsule (http://www.GenePaint.org; ID MH523) (134) and has recently been shown to be a critical mediator of the embryonic and tissue stem/progenitor cell niche (135), the prediction that abnormal imprinting of the IGF-II locus in the capsule/subcapsular unit serves as an initiator of the disease becomes a testable hypothesis and the IGF-II pathway a possible target for therapeutic intervention in ACC. Supporting this hypothesis is the finding that the transcription factor Zac1, which regulates the IGF-II network of imprinted genes in tissue stem cells (223), is the most down-regulated gene in pediatric adrenal cancer (222). Extending similar findings of others (136), we have shown that antagonizing this pathway with two different pharmacological agents that target IGF-IR leads to a potent inhibition of growth of ACC cells in culture and in human ACC xenografts. We also demonstrated that the addition of mitotane (currently the only U.S. Food and Drug Administration-approved therapy for ACC), increased the cytostatic effects of either compound on human ACC xenografts in mice (137). The above data support the testable hypothesis that aberrant epigenetic reprogramming of 11p15.5 and potentially other loci in stem/progenitor cells participates in the etiology of a subset of cancers including ACC. These studies have resulted in both a phase I trial of IGF-IR inhibition in refractory ACC patients and a current multi-institutional phase II trial using this targeted approach as front-line therapy in stage 4 ACC.
B. Familial adenomatous polyposis
Familial adenomatous polyposis is a hereditary disorder in which patients typically present with innumerable colonic polyps and ultimate colonic cancer. This disorder is characterized by mutations in the APC gene on chromosome 5q21-q22 that result in a truncated and/or nonfunctional APC protein (138,139). These patients also have increased risk of other cancers in the extracolonic organs, including the pancreas, thyroid, and adrenal glands (140,141,142,143).
The molecular mechanism of tumorigenesis resulting from APC gene mutations has been widely characterized (138,139,143,144). The product of the APC gene functions as a tumor suppressor mainly through regulation of β-catenin protein stability and hence β-catenin-mediated transcription. Inactivating mutations of APC result in up-regulation of the β-catenin target genes, subsequent unabated growth, and ultimate tumor formation. Although recent evidence suggests additional functions of APC in cell adhesion, cell polarity and migration, chromosome segregation, and mitochondria-mediated apoptosis, this review addresses the role of APC only as a member of the β-catenin degradation complex (144). Predicated on the known role of APC in the regulation of the canonical Wnt signaling pathway in tissue development and homeostasis, our lab and others began analyzing the status of β-catenin in sporadic adrenocortical tumors. In earlier work, the Bertherat laboratory (145) had observed an increase in the nuclear/cytoplasmic ratio of β-catenin in sporadic ACC. In collaboration with the Giordano laboratory, we began screening a panel of ACC samples for stabilization of β-catenin and observed a subset of carcinoma samples with strong nuclear accumulation of β-catenin indicative of active signaling. Utilizing expression array profiling, we compared carcinomas with strong nuclear β-catenin staining to carcinomas with only membranous β-catenin staining. In the analysis, we observed marked up-regulation of classical β-catenin-mediated transcription targets, but only in ACC samples did we observe increased nuclear β-catenin. Such results support a potential role of active β-catenin signaling in a subset of patients with ACC.
To further test a hypothesis that active β-catenin signaling may initiate adrenocortical carcinogenesis through aberrant activation in adrenocortical subcapsular cells, we are using a conditional knockout approach to delete the Apc gene in the mouse adrenal cortex. We hypothesize that canonical Wnt signaling regulates the proliferation and the undifferentiated state of adrenocortical stem/progenitor cells. With constitutive activation of the canonical Wnt pathway, expansion of this population is predicted to result in ultimate tumor formation.
The additional mutations and signaling pathways contributing to these phenotypes are areas of active investigation. IGF and Wnt ligands are two such signals generated in the adrenal capsule that have been shown to be essential in the regulation of stem cell niches and/or the communication with neighboring stem/progenitor cells in multiple organ systems. Specifically, IGF is a critical mediator of the embryonic and tissue stem/progenitor cell niche, whereas Wnt signaling is essential for stem/progenitor cell self-renewal in multiple systems (135,146). Together with our above studies utilizing IGF-1R inhibition in ACC treatment, recent preclinical studies by the laboratory of Enzo Lalli using β-catenin antagonists (224) hold promise for new therapies for ACC that target defective signaling pathways in adrenocortical stem/progenitor cells that are predicted to participate in the initiation of adrenal cancer.
C. Li-Fraumeni syndrome
This familial syndrome arises from mutations in the p53 tumor suppressor gene and is associated with soft tissue sarcomas, osteosarcomas, as well as breast, brain, and adrenal cancer (147). p53 is often regarded as the “guardian of the genome” because it is a critical transcriptional mediator of cell cycle arrest, DNA repair, and programmed cell death in response to DNA damage (148). Loss of p53 function confers impaired apoptosis, genomic instability, and loss of cell cycle control. Indeed, loss of p53 function is the most common mutation in human cancer, accounting for nearly half of all sporadic cancers (148). Recent work suggests that p53 is an important regulator of differentiation of both embryonic stem cells and adult tissue stem cells, where it functions as a rheostat that balances the induction of differentiation vs. apoptosis (149,150). Our recent work examining p53 in adrenocortical cancer is discussed below in the context of the roles of telomerase in adrenocortical homeostasis and cancer.
D. Telomeres, telomerase, stem cells, and cancer
The phenotype of adrenocortical dysplasia (Acd) mice consists of large cells with nuclear pleomorphy, nucleomegaly, and nuclear inclusion bodies, reminiscent of the adrenocortical histology of the cytomegalic form of congenital adrenal hypoplasia in patients with DAX1 mutations or IMAGe syndrome (151). As detailed below, the Acd phenotype in mice is caused by telomere dysfunction and subsequent senescence (Fig. 8).
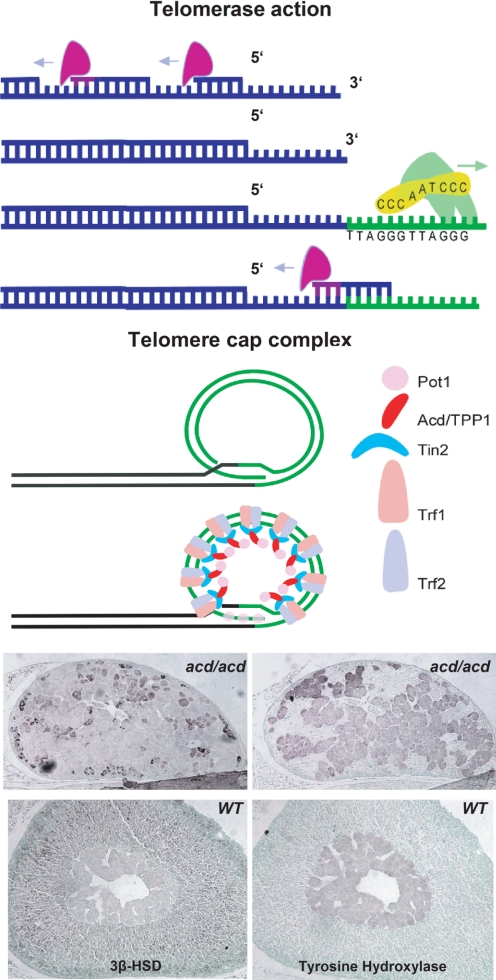
Figure 8.
Importance of telomere stability in adrenal gland development. Top, Model of telomerase-mediated telomere elongation. Middle, Model of telomere cap complex. Bottom, Histological analysis of Acd-deficient mouse adrenals. Immunohistochemistry using anti-3β-HSD and antityrosine hydroxylase reveals aberrant adrenal development.HSD, Hydroxysteroid dehydrogenase. [The bottom panel is reproduced with permission from Oxford University Press (151)].
Telomerase is a ribonucleoprotein that adds telomeric repeats to the 3′ ends of chromosomes to prevent the loss of genetic material over consecutive cell divisions due to the inability of the DNA replication machinery to copy the ends of chromosomes fully (152). There is no active telomerase activity in resting somatic human cells. It can only be detected in rapidly dividing and renewing tissues such as germ cells, basal layers of the skin, and the hematopoietic system. Critically short or dysfunctional telomeres induce a p53-sensitive pathway to senescence or apoptosis, which removes these cells from the proliferating cell pool to prevent the accumulation of genomic aberrations (153). Dysfunctional telomeres can be detected and eventually joined by the DNA repair machinery, leading to dicentric chromosomes that can lead to amplification and loss of genetic material through breakage-fusion-bridge cycles. Telomerase activity, although usually confined to stem cell compartments, can be reactivated in neoplastic cells to prevent the progressive shortening of chromosomes over time and inhibit the subsequent induction of apoptosis or senescence (153). In the human adrenal cortex, the RNA component of telomerase is spatially exclusively expressed in the proliferative subcapsular cortex. The requirement of telomerase activity in stem cell compartments has been shown in human embryonic stem cells and can also be deduced from the progressive failure of bone marrow stem cells in patients with dyskeratosis congenita who harbor germline mutations in the telomerase gene (154).
Recent research has focused on the role of a six-protein complex termed shelterin that protects the telomere from being recognized as damaged DNA and prevents the potentially deleterious processing by the DNA repair machinery. Cloned by our laboratory, the gene responsible for adrenocortical dysplasia is the murine homolog of TPP1, a component of the shelterin complex (151). One can speculate that human syndromes accompanied by cytomegalic adrenocortical hypoplasia may represent the same endpoint of different forms of adrenocortical stem cell failure.
Multiple chromosomal fusions are observed in analyses of metaphases from acd mouse embryonic fibroblasts (225). These telomere dysfunction-derived fusions can potentially serve as starting points for breakage-fusion- bridge cycles and lead to a further shuffling of the genome generating oncogenic genomic losses and gains. To further examine the role of telomere decapping in the observed adrenal failure in acd mice and to analyze the impact of telomere dysfunction-derived chromosomal abnormalities in carcinogenesis, acd mice are being crossed to a p53-deficient background. This approach prevents the induction of telomere dysfunction-induced senescence through p53-sensitive pathways and, hence, leads to the accumulation of potential oncogenic mutations and finally a procancer genome. Such studies will help determine whether telomere dysfunction together with other deleterious events in the stem/progenitor cells (i.e., perturbations of the p53 pathway, defective IGF imprinting, gain-of-function β-catenin) serve as the pathogenetic basis of human adrenocortical carcinogenesis.
A two-step model for the role of telomere dysfunction has been proposed in human carcinogenesis: 1) an early event that leads to shuffling of the genome and the acquisition of oncogenic mutations ultimately leading to a procancer genome; and 2) a late event in which telomeres are maintained either by telomerase activity or alternative mechanisms of telomere maintenance. The latter is a rather ill-defined mechanism of telomere maintenance that is independent of telomerase activity. Most likely this mechanism includes homologous recombinations between chromosomes. An increase in telomere length and the colocalization of telomere sequences with promyelocytic leukemia bodies have been described as surrogate parameters of alternative telomere maintenance. We recently studied a large cohort of benign and malignant ACC and showed that the vast majority of ACC employ active telomere maintenance mechanisms, predominantly telomerase, but to a lesser extent also alternative, telomerase- independent mechanisms (155). Together these findings highlight the importance of the telomere-telomerase function in adrenocortical physiology to ensure the genomic integrity necessary for organ replenishment from the stem/progenitor cell compartment. In the case of carcinogenesis, the work underscores 1) the role of telomere dysfunction in generating oncogenic genomic alterations, and 2) the role of telomerase in maintenance of a malignant phenotype.
X. Future Directions
A. Tissue-specific silencing of Sf1 gene expression
1. Methylation.
Early data supported a role of the Sf1 E-box in the proximal promoter in the “basal” expression of Sf1 in Sf1-expressing tissue (156,157). In adrenocortical cell lines, it was shown that upstream stimulatory factor (USF) 1 and USF2 could bind this site and facilitate transcriptional activation (158). The context in which such activation or repression of activation is important has been unclear until recently. Recent data by the Bakke group (159) reveal that methylation of CpG sites in the E-box-containing proximal promoter is responsible for promoter silencing and the resultant restricted expression pattern of Sf1. Sf1 is only expressed in tissues where the region is hypomethylated. Chromatin immunoprecipitation analyses revealed that USF2 and RNA polymerase II were only recruited to this DNA region when hypomethylated (159). Moreover, recent studies indicate that the ectopic expression of Sf1 in human endometriotic cells, but not normal endometrial cells, is due to abnormal hypomethylation of the proximal promoter (160). Lastly, forced overexpression of Sf1 in transgenic mice induces adrenocortical hyperplasia of the subcapsular region of the mouse adrenal cortex (91) and amplification of 9q34, and an increased copy number of the embedded Sf1 gene has been observed in a high percentage of pediatric ACC and adrenocortical cancer cell lines (161,162). Whether epigenetic methylation of the Sf1 gene serves to regulate Sf1 levels as a normal mechanism in organ maintenance of the capsular/subcapsular unit remains unclear.
2. POD1.
Although the steroidogenic adrenocortical cell is identified by expression of Sf1, the mesenchymal capsular cell is devoid of Sf1. In the proposed model (Fig. 9) whereby the capsule serves as the residence and niche, a small number of quiescent cortical stem cells (derived from the Sf1-expressing fetal zone cells) would be predicted to actively repress the expression of the Sf1 gene until the cells differentiate into Sf1-positive proliferating progenitor cells. Indeed, histological evidence indicates differentiation of Sf1-positive steroidogenic cells within areas of Sf1-negative spindle cell hyperplasia of the capsule that extends into the outer parenchyma of zG (163). A number of basic HLH transcriptional regulatory proteins have been identified that govern similar processes of cellular differentiation and fate determination in various tissues. Pod1/capsulin/Tcf21 belongs to a subfamily of basic HLH proteins that control mesodermal development (164,165). In the embryonic gonads, Pod1 is expressed in regions from which progenitor cells migrate into the gonads to generate several somatic lineages of the testes. Loss of Pod1 in the testis leads to enhanced expression of Sf1, subsequently resulting in premature commitment of progenitor cells to a steroidogenic lineage. In Pod1-deficient testes, the Leydig cell population expands coincident with an increase in Sf1 and Cyp11a1 (a downstream target of Sf1) expression. This is presumably associated with the concomitant loss of peritubular myoid cells and pericytes. Moreover, Sf1 expression is observed in the LacZ-expressing Pod1 null cells (LacZ knock-in), indicating that the loss of Pod1 induces the expression of Sf1 (166,167). Therefore, it is proposed that Pod1 represses Sf1 expression in a multipotent cell precursor, allowing the differentiation of several interstitial lineages, such as Leydig cells, peritubular myoid cells, and pericytes. Indeed, Pod1 has been shown to specifically inhibit the expression of Sf1 by antagonizing the activity of USF1 on the proximal E-box of the Sf1 promoter (167). Within the adrenal gland, Pod1 is uniquely expressed in the developing capsule of the E11.5 mouse cortex, consistent with its known expression in mesenchymal cells within the developing kidney and gonad (164,165,166,167).
Figure 9.
Cellular organization of the adrenal cortex. Left, Model of adrenocortical homeostatic growth maintenance (right, color key for model). Middle, Immunohistochemistry using anti-PCNA reveals subcapsular localization of proliferating adrenocortical cells.
It is important to relate these Pod1/Sf1 interactions in the maintenance of the adult gland to the development of the adrenal capsule. At E11.5–12.5, loose condensations of flattened cells appear over the surface of the mouse fetal adrenal cortex. As these cells coalesce to form the capsule (coincident with Pod1 expression), the adult cortex slowly emerges between the fetal cortex and capsule (9). The temporal appearance of these three structures (and ultimate disappearance of the fetal cortex at birth) leaves open three possibilities regarding the developmental origin and fate of capsular cells: 1) capsule is unrelated to cortical lineages and the fetal cortex gives rise to the adult cortex; 2) capsule gives rise to the adult cortex, and the fetal zone is an unrelated lineage; and 3) the fetal zone cells give rise to stem cells that assume residence within the capsule, and these cells give rise to the subsequent definitive cortex. The ultimate residence of adult cortical progenitor cells within or underneath the capsule is consistent with each model. Sf1 expression defines both fetal and adult cortical cells, but the transcriptional activation of Sf1 utilizes unique enhancers in each tissue (14). If the cortical stem cells within the capsule arise from the underlying fetal cortex, Pod1 (which acts at the proximal E-box of the common basal promoter) would serve to actively repress the fetal and/or definitive enhancer-mediated Sf1 expression in the capsule. Reactivation of an adult Sf1 enhancer would serve to form the underlying adult cortex. Regardless of the developmental origins of capsular lineages (stromal and adrenocortical stem cells), two possible nonmutually exclusive mechanisms seem likely for how adrenal capsular Pod1 regulates the maintenance of the adult subcapsular adrenal cortex: 1) Pod1 represses Sf1 expression in capsular stem cells (i.e., differentiation of Sf1-negative capsular cells is mediated by a down-regulation of Pod1); and/or 2) Pod1 maintains the integrity of the capsular niche cell (164,165).
An additional mechanism for how Sf1 expression is extinguished in the fetal zone (utilizing the fetal Sf1 enhancer) before being actively repressed in capsular stem cells rests on the ability of Dax1 to antagonize Sf1-mediated gene expression. Because Sf1 maintains its own expression in the fetal adrenal, Dax1 (a target gene of Sf1) might be predicted to initially down-regulate the expression of the Sf1 gene itself in a fetal zone cell as it establishes residence in the coalescing capsule. However, once the Sf1-negative cell is in capsular residence, it is difficult to envision how Dax1 would participate in the maintenance of this Sf1-negative state because Dax1 is not expressed in this cellular compartment.
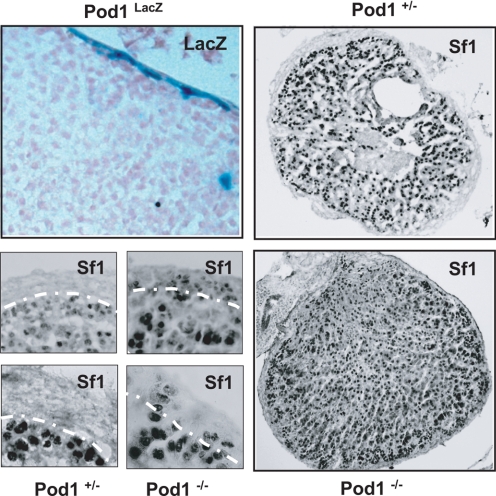
B. Pod1 null mice
The Pod1 locus was targeted by homologous recombination in embryonic stem cells to generate a Pod1 null allele that expresses β-galactosidase or rtTA (tetracycline transactivator) under the regulation of the endogenous Pod1 promoter (168). We first examined LacZ expression in the adrenal glands of 4-month-old heterozygous Pod1/LacZ [LacZ knock-in (KI)] male mice and found robust LacZ expression restricted to the capsule as revealed by X-gal histochemistry. We then compared rtTA and Sf1 expression in E18.5 heterozygous Pod1 (rtTA KI) vs. Pod1 null (rtTA KI) mice. Immunohistochemistry confirmed mutually exclusive expression of rtTA (proxy for Pod1) in the capsule (VP16 epitope of rtTA) and Sf1 in the cortex in heterozygous Pod1 mice. However, in Pod1 null mice we now observe both rtTA expression in cortical cells and Sf1 expression in capsular cells. Together with an increased Sf1 mRNA in Pod1 null adrenals (1.64 ± 0.13-fold above Pod1+/− adrenals; P > 0.01), the data are consistent with the hypothesis that under normal circumstances Pod1 serves to inhibit Sf1 expression in a small subset of cells of the capsule (our unpublished observation, in collaboration with Sue Quaggin, University of Toronto) (Fig. 10). In our recent published expression data set of ACC tissue samples, we see down-regulation of 84 genes including POD1 at 6q23. POD1 is markedly down-regulated in ACC vs. ACA (Adrenocortical Adenoma) and normal adrenal tissue (219). Although these data do not prove that capsular Pod1-positive/Sf1-negative cells become the subcapsular Pod1-negative/Sf1 positive cortical cells or that capsular Pod1 has additional niche effects, the data provide a valuable tool that will allow us to examine the roles of the capsule as niche and residence of adrenocortical stem cells in more detail.
Figure 10.
Role of Pod1 in adrenocortical development. Top left, LacZ activity staining in Pod1-LacZ adrenals reveals preferential capsular staining. Bottom left, Comparative immunohistochemical analysis of heterozygous and homozygous Pod1 knockout adrenals. Staining reveals presence of Sf1-positive cells in the capsule of homozygous Pod1 knockout adrenals. Right, Low-power magnification of anti-Sf1 staining in Pod1 knockout adrenals reveals expansion of Sf1 positive cells in homozygous Pod1 knockout adrenals.
C. Hedgehog signaling
The Hh developmental signaling pathways are well-conserved and important in vertebrate development, such as foregut, neural tube, limbs, lungs, and skin (112,169). The Hh pathway has been implicated in the regulation of both embryonic and adult stem cell fate (112,170,171,172,173,174). Activating and inhibiting mutations of the Hh pathway members demonstrate their importance in cancers, including as pancreatic, basal cell, and medulloblastoma (174,175,176,177,178). The activation of the pathway occurs through binding of Hh morphogens [Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh)] to the Patched-1 (Ptch1) receptor (112,169). In the absence of Hh ligands, Ptch inhibits downstream activation of the Smoothened receptor. The binding of Hh molecules to Ptch allows for activation of Smoothened and ultimate activation of the Gli transcription factors.
Recent studies indicate that Hh signaling is also a critical mediator of adrenogonadal development and growth maintenance. In the testis, the cell-restricted expression of Dhh in Sertoli cells and Ptch1 in fetal Leydig cells is consistent with the observations that Dhh is required for fetal Leydig cell formation and sex cord formation in mice (179,180,181,182). The decreased expression of Sf1 in the precursor Leydig cell population of Dhh null mice despite no change in the size of this population (182) suggests a defect in the lineage progression of the Sf1-positive precursors to bona fide Leydig cells. In the ovary, however, the dual (and potentially redundant) expression of both Dhh and Ihh in granulosa cells (with Ptch1 and Gli expressed in theca) (183) might explain the normal fertility of Dhh null mice (179). Nonetheless, loss-of function mutations in DHH resulting in human gonadal dysgenesis (184,185,186) indicate the clinical significance of these modeling studies.
Two clinical syndromes have provided insight into the importance of Shh in proper adrenocortical development. Adrenal hypoplasia/aplasia has been reported often in Pallister-Hall syndrome due to loss-of-function GLI3 mutations (187) and in Smith-Lemli-Opitz syndrome due to cholesterol synthetic defects that result in altered Hh signaling (188,189). The resultant adrenal aplasia after the introduction of the Gli3 mutation in mice (190) supports such a role of Shh in adrenal development. The spatially restricted expression of Shh in the subcapsular adrenocortical cells that is temporally coincident with formation of the adrenal capsule (191) and the emergence of adrenocortical defects in Shh null mice precisely at E12.5 when the capsule is forming lends credence to a role of Shh in adrenocortical lineage progression dictated by capsule/subcapsule relationships (192). Indeed, the enriched expression of Gli1 in the adrenal capsule suggests a potential reciprocal relationship between these two compartments (our unpublished observation, in collaboration with Dr. A. Dlugosz, University of Michigan) (Fig. 11).
Figure 11.
Comparative histology of transgenic Hedgehog and Wnt reporter mouse adrenals. Left, LacZ activity staining in Gli1-LacZ mouse adrenals (218) reveals preferential capsular staining. Right, LacZ activity staining reveals preferential subcapsular staining in Wnt-Gal reporter adrenals.
D. MicroRNAs
MicroRNAs (miRNAs) are endogenous, short (19–25 nucleotides) RNAs that have been implicated in numerous cellular processes such as proliferation, differentiation, and transformation. miRNAs derive from endogenous genomic loci and reside in both independent and clustered transcription units. Additionally, nearly half of the known mammalian miRNAs reside within introns of protein-coding genes, and are expressed coordinately with their host genes (193,194). RNA polymerase II is responsible for the generation of primary transcripts that are recognized for cleavage by the RNAse III, Drosha. The resulting pre-miRNA is subsequently exported from the nucleus into the cytoplasm and undergoes further modification by another RNAse III, Dicer, to generate an RNA duplex of approximately 22 nucleotides in length. From here, one strand is selectively loaded into the RISC (RNA-induced silencing complex), which then down-regulates target mRNA expression by recognizing an imperfectly matched target sequence in the 3′ untranslated region of mRNAs (193,194).
Although several hundred miRNAs have been reported in humans, the function of these small noncoding RNAs is not yet fully understood. Further contributing to the complexity of miRNA-mediated gene regulation is the fact that owing to the presence of base pair mismatches between miRNAs and target 3′ untranslated region sequences, a single miRNA can be predicted to interact with literally hundreds of gene transcripts. Despite this complexity, recent advances into understanding the function of miRNAs in development, normal physiology, as well as pathophysiology have been made. Mice lacking the Dicer RNAse III die in utero at E7.5, just before axis formation, consistent with the notion that miRNAs are important in embryonic patterning and morphogenesis (195). Tissue-specific Dicer knockout mice have subsequently demonstrated the importance of miRNAs in the development of several tissue types including the heart and skin, and in murine limb morphogenesis (196,197,198,199,200). Additionally, miRNAs are important in the normal physiology of mammalian cells because knock-down of specific miRNAs in the heart and immune system results in pathological phenotypes (200,201,202). Further evidence supporting the notion that miRNA perturbation can lead to pathophysiology comes from the observations that miRNA expression is often altered and dysregulated in transformation and carcinogenesis. For example, the let-7 miRNA was shown to repress the oncogenes RAS and HMGA2 and has been reported to be down-regulated in several tumor types, including lung, melanoma, and Burkitt’s lymphoma (203,204,205,206,207,208). However, let-7 is not the only known miRNA whose dysregulation is thought to be involved in carcinogenesis, and as research into these small RNA mediators continues, more miRNA species will be implicated in either the suppression or promotion of oncogenic activity. Finally, recent miRNA expression profiles of human and mouse demonstrate that miRNA can be preferentially or exclusively expressed in certain tissues, suggesting that the presence (or absence) of individual miRNAs may be critical in the physiological maintenance of specific cells and tissues. Of particular interest to us, these miRNA profiles also included adrenal tissue (209,210).
Based on the current research and understanding of miRNA, our laboratory has begun studies to address the role of miRNAs in the normal physiological development and maintenance of the adrenal gland, as well as tumorigenesis associated with ACC. We have generated adrenal-specific Dicer knockout mice to study the phenotypic effects of perturbing global miRNA processing in vivo. Preliminary data suggest that Dicer-dependent miRNA processing is critical for the development and/or maintenance of the adrenal cortex. Adrenal-specific Dicer-deficient mice die shortly after birth and exhibit a phenotype consistent with a failure of adrenocortical development or organ maintenance. Currently, we are in the process of further characterizing these mice by performing timed pregnancy analyses to determine the exact time of cortical failure. Additionally, studies into the miRNA profile of the adrenal gland will hopefully shed further light on specific miRNAs that may be crucial in adrenal development, maintenance, and cancer.
E. Notch signaling pathway
Notch is a complex signaling pathway that has been implicated in diverse processes from cancer to development to implications in stem cell biology. Notch involves the interaction between a ligand, expressed in a particular cell type, and a receptor, expressed in an adjacent cell, which results in proteolytic cleavage of the Notch receptor, release of the active signaling molecule, the Notch intracellular domain (NICD), and induction of Notch-responsive genes in the receptor-presenting cell (211). It is currently unclear whether there is any molecular impact of Notch signaling on the ligand-presenting cell or whether ligands/receptors have functions independent of classical signaling. Four Notch receptors (Notch1–4) and five Notch ligands (Dll1/3/4 and Jagged1/2) exist in mammals, and the ultimate impact of Notch signaling appears to depend on the complement of receptors and ligands expressed in addition to a constant degradation and regeneration of the NICD. Notch signaling is highly context dependent because it has been shown to be both tumor suppressive and oncogenic, in addition to promoting differentiation and quiescence of stem cells in different systems (212). Little has been published about a role of Notch signaling in the adrenal gland. Recently, using our microarray of adrenocortical tumors, we identified a significant up-regulation of Jagged1 in all ACC when compared with normal and ACA (219). Interestingly, Jagged1 up-regulation has been seen in other cancers, such as breast, where its expression is correlated to prognosis (213,214). Jagged1 is expressed in the subcapsular zone of the adrenal cortex, placing it in a prime location to interact with and influence putative stem cells or niche cells in the adrenal capsule. Furthermore, active Notch signaling is present in the mouse adrenal, as indicated by the presence of the NICD, and a subsequent elevation in NICD proteins levels was observed in various ACC cell lines (our unpublished data). We are currently assessing the functional significance of Notch signaling in the normal biology of the adrenal gland and the up-regulation of Jagged1 in ACC.
XI. Summary
This comprehensive review has detailed historical data and subsequent experiments that provide support for the existence of undifferentiated pluripotent adrenocortical cells that underlie the regenerative potential of the adrenal cortex. Although the exact origin of the regenerating cells still remains uncertain, we believe that most data support the assertion that steroidogenic/differentiated cells within the concentric zones of the cortex arise from capsular and subcapsular cells in the outer periphery of the adrenal cortex. Whether fibroblast-like mesenchymal capsular cells themselves or rare undifferentiated adrenocortical cells (possibly residing within capsule) give rise to differentiated adrenocortical cells remains unknown. Additionally, we cannot exclude the possibility that the capsule does not provide undifferentiated cells but rather acts as a supporting milieu/niche for the undifferentiated cells within the subcapsule that are the bona fide tissue-specific stem cells of the adrenal cortex. Moreover, further insights remain to be clearly defined through investigations of other pathways involved in normal adrenal maintenance and implicated in adrenal pathologies such as aplasias and carcinomas.
Based on the evidence presented above, we propose the following model (Fig. 12). Adrenocortical cell repopulation in the context of homeostatic tissue maintenance is regulated by the reciprocal relationship between the adrenal capsule and subcapsular cells. The adrenocortical fate of a subset of capsular cells is transcriptionally repressed through Pod1/capsulin/Tcf21-directed inhibition of Sf1. Following mitogenic stimuli and/or through other extrinsic cues, the capsule undergoes asymmetric division, resulting in production of another capsular cell and a subcapsular progenitor cell. These subcapsular cells subsequently gain Sf1 expression but, due to the Wnt and glucocorticoid activated Sf1-dependent expression of Dax1, these cells are limited in their steroidogenic activity. These cells continually proliferate, as evidenced by proliferating cell nuclear antigen and bromodeoxyuridine studies, to maintain adrenocortical homeostasis. Dax1 is repressed by ACTH stimulation, resulting in a centripetal migration of subcapsular cells toward the corticomedullary boundary coincident with commencement of steroidogenic activity as differentiated adrenocortical cells. Additionally, inhibin serves as a gatekeeper of maintaining the fidelity of adrenal-specific programming by inhibiting the expansion of LH-primed Gata4-positive progenitors destined for an ovarian fate.
Figure 12.
Summary figure.
Although the studies described in this review have provided data in support of the presence of adrenal adult stem and progenitor cells, defining undifferentiated adrenocortical cells as true tissue-specific adult stem cells is premature without further in vitro and in vivo characterization utilizing existing tools from tissues with molecular- and cellular-defined stem cells and their respective niches, such as those in the intestinal, mammary, hematopoietic, dermal, and neuronal systems (215). For example, investigation of conserved signaling pathways such as Wnt, TGFβ, IGF, Shh, and Notch that are shown to regulate somatic stem cells in other systems should be conducted (216). The cre/loxP-conditional knockout technology can be used to elucidate the roles of these pathways in adrenocortical lineage determination. Lastly, recent investigations of epigenetic regulation revealing a role of epigenetic regulators, such as Bmi1, in maintenance of somatic stem cells suggest that such factors would be important additional targets of inquiry (217).
Acknowledgments
This review constitutes the efforts of many people over the last 10 yr who have contributed to work in our laboratory and those of our collaborators. We have listed below all members of the laboratory who have participated in published research projects. Due to the nature of this review series, although numerous members of our collaborator’s laboratories were essential to many of the projects described, we could only list the names of each laboratory’s principal investigator. Similarly, we could not provide an exhaustive review that included all relevant studies and investigators in the adrenal field but instead have attempted to incorporate the work of many colleagues in the field whose laboratories have laid the groundwork for (and continue to advance) the burgeoning field of adrenocortical stem and progenitor cell biology.
Hammer Laboratory Members and Collaborators, 1999–2009: Research Staff, Joanne Heaton, M.S. Faculty: Claudimara Lotfi, Ph.D. Graduate Students: Ferdous Barlaskar, B.S.; Isabella Finco, B.S.; Victoria Kelly, B.A.; Alex Kim, B.S.; Kenneth Krill, B.S.; Joshua Scheys, B.S.; Derek Simon, B.S.; and Alessia Trovato, M.D. Prior Research Staff: David Bavers, B.S. Prior Postdoctoral Fellows: Poda Suresh Babu, Ph.D.; Felix Beuschlein, M.D.; Tobias Else, M.D.; Catherine Keegan, M.D., Ph.D.; and Wei-Hsiung Yang, Ph.D. Prior Clinical Fellows: Daniel Elsholz, M.D.; and Yanping Kong, M.D. Prior Graduate Students: Brian Gummow, Ph.D.; Brendan Looyenga, Ph.D.; and Jonathon Winnay, Ph.D. Prior Undergraduate Students: Janna Hutz, Ph.D.; Chris Mutch, M.D., Ph.D.; Kerri Serecky, B.S.; and Sonalee Shah, B.S., D.O. Collaborators: Edgar Ben-Josef, M.D.; Sally Camper, Ph.D.; Anj Dlugosz, M.D., Ph.D.; David Ferguson, M.D., Ph.D.; Tom Giordano, M.D., Ph.D.; Jorge Iniguez, Ph.D.; Ron Koenig, M.D., Ph.D.; Rork Kuick, M.A.; Peter Lucas, M.D., Ph.D.; Ormond MacDougald, Ph.D.; and Aaron Spalding, M.D., Ph.D. (University of Michigan); John Achermann, M.D. (University College London); Wes Beamer, M.D. (Jackson Laboratories); Adrian Clark, Ph.D. (Royal London School of Medicine and Dentistry); Titia de Lange, Ph.D. (Rockefeller University); William Engeland, Ph.D. (University of Minnesota); Angela Huebner, Ph.D. (Children’s Hospital, Technical University Dresden); Larry Jameson, M.D., Ph.D. (Northwestern University); Edward McCabe, M.D., Ph.D. (University of California, Los Angeles); John Nilson, Ph.D. (Washington State University); Bert O’Malley, M.D. (Baylor University); Keith Parker, M.D., Ph.D. (University of Texas Southwestern); Susan Quaggin, Ph.D., Bernard Schimmer, Ph.D. (University of Toronto); and Constantine Stratakis, M.D., D.Sc. (Section on Endocrinology and Genetics, Developmental Endocrinology Branch, National Institute of Child Health and Human Development, and National Institutes of Health).
Footnotes
Support for the work in our laboratory has been provided by: the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health; the American Cancer Society; and by the University of Michigan’s Millie Schembechler Adrenal Cancer Research Fund, Garry Betty Adrenal Cancer Scholar Program, Urology O’Brien Center, Michigan Diabetes Research and Training Center, Office of the Vice President for Research, Comprehensive Cancer Center Pilot Grant Program, Undergraduate Research Opportunity Program, BioMedical Research Council Grant Program, Internal Medicine Innovative Grant Program, and Center for Organogenesis.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 29, 2009
Abbreviations: ACC, Adrenocortical carcinoma(s); Acd, adrenocortical dysplasia; AGP, adrenogonadal primordia; APC, adenomatous polyposis coli; BWS, Beckwith-Wiedemann syndrome; ChIP, chromatin immunoprecipitation; Dhh, Desert hedgehog; E, embryonic day; Fzd, Frizzled; GR, glucocorticoid receptor; Hh, hedgehog; HLH, helix-loop-helix; Ihh, Indian hedgehog; KI, knock-in; LRH, liver receptor homolog; miRNA, microRNA; NICD, Notch intracellular domain; Pbx1, pre-B cell leukemia homeobox 1; Pref-1, preadipocyte factor 1; Ptch1, Patched-1; rtTA, tetracycline transactivator; Shh, Sonic hedgehog; SHP, small heterodimer partner; Tcf, T cell factor; USF, upstream stimulatory factor; Wnt, wingless-type MMTV integration site; zF, zona fasciculata; zG, zona glomerulosa; zR, zona reticularis.
References
- Gottschau M 1883 Structur und embryonale Entwickelung der Nebennieren bei Saugethieren. Archiv fur Anat und Phys S:412–458 [Google Scholar]
- Arnold J 1866 Ein Beitrag zur feineren Struktur und dem Chemismus der Nebennieren. Virchow Arch Patholog Anatomie Physiol Klin Med 35: 64–107 [Google Scholar]
- Evelyn H-M 1927 A transitory zone in the adrenal cortex which shows age and sex relationships. Am J Anat 40:251–293 [Google Scholar]
- Keegan CE, Hammer GD 2002 Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab 13:200–208 [DOI] [PubMed] [Google Scholar]
- Else T, Hammer GD 2005 Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16:458–468 [DOI] [PubMed] [Google Scholar]
- Uotila UU 1940 The early embryological development of the fetal and permanent adrenal cortex in man. The Anatomical Record 76:183–203 [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K 1996 Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1:663–671 [DOI] [PubMed] [Google Scholar]
- Kim AC, Hammer GD 2007 Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol 265–266:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM 2006 Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133:2995–3004 [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, Toshimori K, Morohashi K 2005 Mouse polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 106:1612–1620 [DOI] [PubMed] [Google Scholar]
- Schnabel CA, Selleri L, Cleary ML 2003 Pbx1 is essential for adrenal development and urogenital differentiation. Genesis 37:123–130 [DOI] [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A 2007 Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134:2349–2358 [DOI] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K 2006 Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ 2008 No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 9:11–21 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G 2004 Socializing with the neighbors: stem cells and their niche. Cell 116:769–778 [DOI] [PubMed] [Google Scholar]
- Sneddon JB, Werb Z 2007 Location, location, location: the cancer stem cell niche. Cell Stem Cell 1:607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JR, West SH 1997 Vascularization of the adrenal cortex: its possible involvement in the regulation of steroid hormone release. Microsc Res Tech 36:546–557 [DOI] [PubMed] [Google Scholar]
- Chamoux E, Bolduc L, Lehoux JG, Gallo-Payet N 2001 Identification of extracellular matrix components and their integrin receptors in the human fetal adrenal gland. J Clin Endocrinol Metab 86:2090–2098 [DOI] [PubMed] [Google Scholar]
- Magennis DP, McNicol AM 1998 Vascular patterns in the normal and pathological human adrenal cortex. Virchows Arch 433:69–73 [DOI] [PubMed] [Google Scholar]
- Otis M, Campbell S, Payet MD, Gallo-Payet N 2007 Expression of extracellular matrix proteins and integrins in rat adrenal gland: importance for ACTH-associated functions. J Endocrinol 193:331–347 [DOI] [PubMed] [Google Scholar]
- Tokunaga H 1996 Postnatal development of the blood vasculature in the rat adrenal gland: a scanning electron microscope study of microcorrosion casts. Arch Histol Cytol 59:305–315 [DOI] [PubMed] [Google Scholar]
- Virtanen I, Korhonen M, Petajaniemi N, Karhunen T, Thornell LE, Sorokin LM, Konttinen YT 2003 Laminin isoforms in fetal and adult human adrenal cortex. J Clin Endocrinol Metab 88:4960–4966 [DOI] [PubMed] [Google Scholar]
- Whetton AD, Graham GJ 1999 Homing and mobilization in the stem cell niche. Trends Cell Biol 9:233–238 [DOI] [PubMed] [Google Scholar]
- Weinberg WC, Howard JC, Iannaccone PM 1985 Histological demonstration of mosaicism in a series of chimeric rats produced between congenic strains. Science 227:524–527 [DOI] [PubMed] [Google Scholar]
- Iannaccone P, Morley S, Skimina T, Mullins J, Landini G 2003 Cord-like mosaic patches in the adrenal cortex are fractal: implications for growth and development. FASEB J 17:41–43 [DOI] [PubMed] [Google Scholar]
- Landini G, Iannaccone PM 2000 Modeling of mosaic patterns in chimeric liver and adrenal cortex: algorithmic organogenesis? FASEB J 14:823–827 [DOI] [PubMed] [Google Scholar]
- Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, Heikinheimo M 2002 Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology 143:3136–3143 [DOI] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K 2008 Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragulla H, Hirschberg RM, Schlotfeldt U, Stede M, Budras KD 2004 On the structure of the adrenal gland of the common seal (Phoca vitulina vitulina). Anat Histol Embryol 33:263–272 [DOI] [PubMed] [Google Scholar]
- Rupik W 2002 Early development of the adrenal glands in the grass snake Natrix natrix L. (Lepidosauria, Serpentes). Adv Anat Embryol Cell Biol 164:I-XI, 1–102 [DOI] [PubMed] [Google Scholar]
- Halder SK, Takemori H, Hatano O, Nonaka Y, Wada A, Okamoto M 1998 Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology 139:3316–3328 [DOI] [PubMed] [Google Scholar]
- Vinson GP, Whitehouse B, Hinson J 1992 The adrenal cortex. Upper Saddle River, NJ: Prentice Hall Inc. [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y 1999 Development of functional zonation in the rat adrenal cortex. Endocrinology 140:3342–3353 [DOI] [PubMed] [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y 2003 The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta 1619:317–324 [DOI] [PubMed] [Google Scholar]
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y 1994 A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology 135:431–438 [DOI] [PubMed] [Google Scholar]
- Baker BL 1952 A comparison of the histological changes induced by experimental hyperadrenocorticalism and inanition. Recent Prog Horm Res 7:331 [Google Scholar]
- Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD 2002 Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology 143:3122–3135 [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Ferreira J, Vendeira P, Magalhaes MC, Vinson GP 2002 Proliferation of capsular stem cells induced by ACTH in the rat adrenal cortex. Endocr Res 28:683–691 [DOI] [PubMed] [Google Scholar]
- Race GJ, Green RF 1955 Studies on zonation and regeneration of the adrenal cortex of the rat; effects of sodium restriction, potassium intoxication, corticotropin, and orchiectomy when studied with colchicine. AMA Arch Pathol 59:578–586 [PubMed] [Google Scholar]
- Zajicek G, Ariel I, Arber N 1986 The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol 111:477–482 [DOI] [PubMed] [Google Scholar]
- Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y 1992 Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 β in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology 130:2971–2977 [DOI] [PubMed] [Google Scholar]
- Wright NA, Voncina D, Morley AR 1973 An attempt to demonstrate cell migration from the zona glomerulosa in the prepubertal male rat adrenal cortex. J Endocrinol 59:451–459 [DOI] [PubMed] [Google Scholar]
- Thomas M, Northrup SR, Hornsby PJ 1997 Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals’ adrenal glands. Nat Med 3:978–983 [DOI] [PubMed] [Google Scholar]
- Thomas M, Hornsby PJ 1999 Transplantation of primary bovine adrenocortical cells into scid mice. Mol Cell Endocrinol 153:125–136 [DOI] [PubMed] [Google Scholar]
- Thomas M, Yang L, Hornsby PJ 2000 Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat Biotechnol 18:39–42 [DOI] [PubMed] [Google Scholar]
- Thomas M, Hawks CL, Hornsby PJ 2003 Adrenocortical cell transplantation in scid mice: the role of the host animals’ adrenal glands. J Steroid Biochem Mol Biol 85:285–290 [DOI] [PubMed] [Google Scholar]
- Nickerson PA, Brownie AC, Skelton FR 1969 An electron microscopic study of the regenerating adrenal gland during the development of adrenal regeneration hypertension. Am J Pathol 57:335–364 [PMC free article] [PubMed] [Google Scholar]
- Pellegrino C, Ricci PD, Tongiani R 1963 A quantitative cytochemical and physiological study of the rat adrenal cortex in hypertrophy after unilateral adrenalectomy. Exp Cell Res 31:167–182 [DOI] [PubMed] [Google Scholar]
- Perrone RD, Bengele HH, Alexander EA 1986 Sodium retention after adrenal enucleation. Am J Physiol 250:E1–E12 [DOI] [PubMed] [Google Scholar]
- Skelton FR 1959 Adrenal regeneration and adrenal-regeneration hypertension. Physiol Rev 39:162–182 [DOI] [PubMed] [Google Scholar]
- Coll AP, Challis BG, Yeo GS, Snell K, Piper SJ, Halsall D, Thresher RR, O'Rahilly S 2004 The effects of proopiomelanocortin deficiency on murine adrenal development and responsiveness to adrenocorticotropin. Endocrinology 145:4721–4727 [DOI] [PubMed] [Google Scholar]
- Karpac J, Ostwald D, Bui S, Hunnewell P, Shankar M, Hochgeschwender U 2005 Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 146:2555–2562 [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A 1998 Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Krude H, Gruters A 2000 Implications of proopiomelanocortin (POMC) mutations in humans: the POMC deficiency syndrome. Trends Endocrinol Metab 11:15–22 [DOI] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, Cheetham ME, Clark AJ 2005 Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC 1999 Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology 140:5609–5618 [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, Weinberg WC 1987 The histogenesis of the rat adrenal cortex: a study based on histologic analysis of mosaic pattern in chimeras. J Exp Zool 243:217–223 [DOI] [PubMed] [Google Scholar]
- Iannaccone PM 1987 The study of mammalian organogenesis by mosaic pattern analysis. Cell Differ 21:79–91 [DOI] [PubMed] [Google Scholar]
- Morley SD, Viard I, Chung BC, Ikeda Y, Parker KL, Mullins JJ 1996 Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol Endocrinol 10:585–598 [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T 1992 A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- Morohashi KI, Omura T 1996 Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J 10:1569–1577 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL 1993 Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7:852–860 [DOI] [PubMed] [Google Scholar]
- Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez A 2003 SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KL, Hormaeche I, Licht JD 2007 Epigenetic regulation of normal and malignant hematopoiesis. Oncogene 26:6697–6714 [DOI] [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA 1999 Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Waterman MR 2002 Adrenocorticotropin/cyclic adenosine 3′,5′-monophosphate-mediated transcription of the human CYP17 gene in the adrenal cortex is dependent on phosphatase activity. Endocrinology 143:1769–1777 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Winnay JN, Hammer GD 2006 Adrenocorticotropic hormone-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor 1-dependent transcriptional activation. Mol Endocrinol 20:147–166 [DOI] [PubMed] [Google Scholar]
- Chen WY, Lee WC, Hsu NC, Huang F, Chung BC 2004 SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K 2004 Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol 18:2451–2462 [DOI] [PubMed] [Google Scholar]
- Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA 2005 The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol 25:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AE, Rusten M, Hoivik EA, Vikse EL, Hansson ML, Wallberg AE, Bakke M 2008 Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol Endocrinol 22:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LA, Faivre EJ, Show MD, Ingraham JG, Flinders J, Gross JD, Ingraham HA 2008 Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1). Mol Cell Biol 28:7476–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Heaton JH, Brevig H, Mukherjee S, Iniguez-Lluhi JA, Hammer GD 2009 SUMOylation inhibits SF-1 activity by reducing CDK7 mediated serine 203 phosphorylation. Mol Cell Biol 29:613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Sewer MB 2006 Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology 147:5249–5258 [DOI] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Kelly S, Wang E, Merrill Jr AH, Sewer MB 2007 Steroidogenic factor-1 is a sphingolipid binding protein. Mol Cell Endocrinol 265–266:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL 2005 The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA 102:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE 2005 Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA 2005 Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G 1994 An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641 [DOI] [PubMed] [Google Scholar]
- Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwarz HP, Kaplan JC, Camerino G, Meitinger T, Monaco AP 1994 Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372:672–676 [DOI] [PubMed] [Google Scholar]
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL 1999 A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Schoenle EJ 2000 Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JK, McCabe ER 2001 Mutations in NR0B1 (DAX1) and NR5A1 (SF1) responsible for adrenal hypoplasia congenita. Hum Mutat 18:472–487 [DOI] [PubMed] [Google Scholar]
- Lalli E, Sassone-Corsi P 2003 DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol Endocrinol 17:1445–1453 [DOI] [PubMed] [Google Scholar]
- Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA 2000 Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, Fowkes RC, Ingraham HA 2004 Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18:941–952 [DOI] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E 2007 Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- Lichtenauer UD, Duchniewicz M, Kolanczyk M, Hoeflich A, Hahner S, Else T, Bicknell AB, Zemojtel T, Stallings NR, Schulte DM, Kamps MP, Hammer GD, Scheele JS, Beuschlein F 2007 Pre-B-cell transcription factor 1 and steroidogenic factor 1 synergistically regulate adrenocortical growth and steroidogenesis. Endocrinology 148:693–704 [DOI] [PubMed] [Google Scholar]
- Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, Hammer GD 2002 Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143:665–673 [DOI] [PubMed] [Google Scholar]
- Niakan KK, Davis EC, Clipsham RC, Jiang M, Dehart DB, Sulik KK, McCabe ER 2006 Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol Genet Metab 88:261–271 [DOI] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ 2000 Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515 [DOI] [PubMed] [Google Scholar]
- del Castillo-Olivares A, Gil G 2000 α1-Fetoprotein transcription factor is required for the expression of sterol 12α-hydroxylase, the specific enzyme for cholic acid synthesis. Potential role in the bile acid-mediated regulation of gene transcription. J Biol Chem 275:17793–17799 [DOI] [PubMed] [Google Scholar]
- Nitta M, Ku S, Brown C, Okamoto AY, Shan B 1999 CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7α-hydroxylase gene. Proc Natl Acad Sci USA 96:6660–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Thomsen JS, Damdimopoulos AE, Spyrou G, Gustafsson JA, Treuter E 1999 The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERα and ERβ. J Biol Chem 274:345–353 [DOI] [PubMed] [Google Scholar]
- Lee YK, Parker KL, Choi HS, Moore DD 1999 Activation of the promoter of the orphan receptor SHP by orphan receptors that bind DNA as monomers. J Biol Chem 274:20869–20873 [DOI] [PubMed] [Google Scholar]
- Seol W, Choi HS, Moore DD 1996 An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336–1339 [DOI] [PubMed] [Google Scholar]
- Gummow BM, Scheys JO, Cancelli VR, Hammer GD 2006 Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol 20:2711–2723 [DOI] [PubMed] [Google Scholar]
- Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC 2006 Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab 91:3048–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achermann JC, Silverman BL, Habiby RL, Jameson JL 2000 Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. J Pediatr 137:878–881 [DOI] [PubMed] [Google Scholar]
- Peter M, Viemann M, Partsch CJ, Sippell WG 1998 Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J Clin Endocrinol Metab 83:2666–2674 [DOI] [PubMed] [Google Scholar]
- Ito M, Yu R, Jameson JL 1997 DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Dorn C, Sadovsky Y, Milbrandt J 1998 Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 18:2949–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Park Y, Weck J, Mayo KE, Jameson JL 2000 Synergistic activation of the inhibin α-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol 14:66–81 [DOI] [PubMed] [Google Scholar]
- Jorgensen JS, Nilson JH 2001 AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol Endocrinol 15:1505–1516 [DOI] [PubMed] [Google Scholar]
- Liu Z, Simpson ER 1997 Steroidogenic factor 1 (SF-1) and SP1 are required for regulation of bovine CYP11A gene expression in bovine luteal cells and adrenal Y1 cells. Mol Endocrinol 11:127–137 [DOI] [PubMed] [Google Scholar]
- Rui X, Tsao J, Scheys JO, Hammer GD, Schimmer BP 2008 Contributions of Sp1 and steroidogenic factor 1 to Adcy4 expression in Y1 mouse adrenal cells. Endocrinology 149:3668–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Li C, Saunders GF 2004 Generation of two distinct functional isoforms of dosage-sensitive sex reversal-adrenal hypoplasia congenita-critical region on the X chromosome gene 1 (DAX-1) by alternative splicing. Mol Endocrinol 18:1428–1437 [DOI] [PubMed] [Google Scholar]
- Blank U, Karlsson G, Karlsson S 2008 Signaling pathways governing stem-cell fate. Blood 111:492–503 [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A 2003 Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130:3663–3670 [DOI] [PubMed] [Google Scholar]
- Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD 2008 Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135:2593–2602 [DOI] [PubMed] [Google Scholar]
- Dreesen O, Brivanlou AH 2007 Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 3:7–17 [DOI] [PubMed] [Google Scholar]
- Glasgow E, Mishra L 2008 Transforming growth factor-β signaling and ubiquitinators in cancer. Endocr Relat Cancer 15:59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A 1994 Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA 91:8817–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, Nilson JH, Hammer GD 2003 Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol Cell Biol 23:3951–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD 2006 Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol Endocrinol 20:2848–2863 [DOI] [PubMed] [Google Scholar]
- Looyenga BD, Hammer GD 2007 Genetic removal of Smad3 from inhibin-null mice attenuates tumor progression by uncoupling extracellular mitogenic signals from the cell cycle machinery. Mol Endocrinol 21:2440–2457 [DOI] [PubMed] [Google Scholar]
- Allolio B, Fassnacht M 2006 Clinical review: adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab 91:2027–2037 [DOI] [PubMed] [Google Scholar]
- Barlaskar FM, Hammer GD 2007 The molecular genetics of adrenocortical carcinoma. Rev Endocr Metab Disord 8:343–348 [DOI] [PubMed] [Google Scholar]
- Libe R, Fratticci A, Bertherat J 2007 Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer 14:13–28 [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF 2007 The biology of cancer stem cells. Annu Rev Cell Dev Biol 23:675–699 [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S 2006 The epigenetic progenitor origin of human cancer. Nat Rev Genet 7:21–33 [DOI] [PubMed] [Google Scholar]
- Weksberg R, Shuman C, Smith AC 2005 Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 137C:12–23 [DOI] [PubMed] [Google Scholar]
- Enklaar T, Zabel BU, Prawitt D 2006 Beckwith-Wiedemann syndrome: multiple molecular mechanisms. Expert Rev Mol Med 8:1–19 [DOI] [PubMed] [Google Scholar]
- Liu J, Kahri AI, Heikkila P, Ilvesmaki V, Voutilainen R 1995 H19 and insulin-like growth factor-II gene expression in adrenal tumors and cultured adrenal cells. J Clin Endocrinol Metab 80:492–496 [DOI] [PubMed] [Google Scholar]
- Gao ZH, Suppola S, Liu J, Heikkila P, Janne J, Voutilainen R 2002 Association of H19 promoter methylation with the expression of H19 and IGF-II genes in adrenocortical tumors. J Clin Endocrinol Metab 87:1170–1176 [DOI] [PubMed] [Google Scholar]
- Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM 2003 Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol 162:521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FL, Dean WL, Kelsey G, Allen ND, Reik W 1997 Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature 389:809–815 [DOI] [PubMed] [Google Scholar]
- Weber MM, Fottner C, Schmidt P, Brodowski KM, Gittner K, Lahm H, Engelhardt D, Wolf E 1999 Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology 140:1537–1543 [DOI] [PubMed] [Google Scholar]
- Nakae J, Kido Y, Accili D 2001 Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22:818–835 [DOI] [PubMed] [Google Scholar]
- Han VK, Lu F, Bassett N, Yang KP, Delhanty PJ, Challis JR 1992 Insulin-like growth factor-II (IGF-II) messenger ribonucleic acid is expressed in steroidogenic cells of the developing ovine adrenal gland: evidence of an autocrine/paracrine role for IGF-II. Endocrinology 131:3100–3109 [DOI] [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bosse M, Lajoie G, Bhatia M 2007 IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448:1015–1021 [DOI] [PubMed] [Google Scholar]
- Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC 2008 Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab 93:3524–3531 [DOI] [PubMed] [Google Scholar]
- Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD 2009 Preclinical targeting of the type 1 insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 94:204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finniear R, Markham A, Groffen J, Boguski MS, Altschul SF, Horii A, Ando H, Miyoshi Y, Miki Y, Nishisho I, Nakumura Y 1991 Identification of FAP locus genes from chromosome 5q21. Science 253:661–665 [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, Sargeant L, Krapcho K, Wolff E, Burt R, Hughes JP, Warrington J, Mc Pherson J, Wasmuth J, Paslier DL, Abderrahim H, Cohen OD, Leppert M, White R 1991 Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66:589–600 [DOI] [PubMed] [Google Scholar]
- Naylor EW, Gardner EJ 1981 Adrenal adenomas in a patient with Gardner’s syndrome. Clin Genet 20:67–73 [DOI] [PubMed] [Google Scholar]
- Naylor EW, Lebenthal E 1980 Gardner’s syndrome. Recent developments in research and management. Dig Dis Sci 25:945–959 [DOI] [PubMed] [Google Scholar]
- Painter TA, Jagelman DG 1985 Adrenal adenomas and adrenal carcinomas in association with hereditary adenomatosis of the colon and rectum. Cancer 55:2001–2004 [DOI] [PubMed] [Google Scholar]
- Fearnhead NS, Britton MP, Bodmer WF 2001 The ABC of APC. Hum Mol Genet 10:721–733 [DOI] [PubMed] [Google Scholar]
- Aoki K, Taketo MM 2007 Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci 120:3327–3335 [DOI] [PubMed] [Google Scholar]
- Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, Rene-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, Perret C, Bertherat J 2005 Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65:7622–7627 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W 2001 New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11:547–553 [DOI] [PubMed] [Google Scholar]
- Iwakuma T, Lozano G, Flores ER 2005 Li-Fraumeni syndrome: a p53 family affair. Cell Cycle 4:865–867 [DOI] [PubMed] [Google Scholar]
- Ljungman M 2000 Dial 9–1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia 2:208–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y 2005 A new role for p53 in maintaining genetic stability in embryonic stem cells. Cell Cycle 4:363–364 [DOI] [PubMed] [Google Scholar]
- Prochazkova J, Lichnovsky V, Kylarova D, Erdosova B, Vranka P 2004 Involvement of p53 and Bcl-2 family proteins in regulating programmed cell death and proliferation in human embryogenesis. Gen Physiol Biophys 23:209–229 [PubMed] [Google Scholar]
- Keegan CE, Hutz JE, Else T, Adamska M, Shah SP, Kent AE, Howes JM, Beamer WG, Hammer GD 2005 Urogenital and caudal dysgenesis in adrenocortical dysplasia (acd) mice is caused by a splicing mutation in a novel telomeric regulator. Hum Mol Genet 14:113–123 [DOI] [PubMed] [Google Scholar]
- Bianchi A, Shore D 2008 How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell 31:153–165 [DOI] [PubMed] [Google Scholar]
- Deng Y, Chan SS, Chang S 2008 Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8:450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS 2008 Telomere maintenance and human bone marrow failure. Blood 111:4446–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else T, Giordano TJ, Hammer GD 2008 Evaluation of telomere length maintenance mechanisms in adrenocortical carcinoma. J Clin Endocrinol Metab 93:1442–1449 [DOI] [PubMed] [Google Scholar]
- Nomura M, Bartsch S, Nawata H, Omura T, Morohashi K 1995 An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem 270:7453–7461 [DOI] [PubMed] [Google Scholar]
- Woodson KG, Crawford PA, Sadovsky Y, Milbrandt J 1997 Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol Endocrinol 11:117–126 [DOI] [PubMed] [Google Scholar]
- Harris AN, Mellon PL 1998 The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 12:714–726 [DOI] [PubMed] [Google Scholar]
- Hoivik EA, Aumo L, Aesoy R, Lillefosse H, Lewis AE, Perrett RM, Stallings NR, Hanley NA, Bakke M 2008 DNA methylation controls cell type specific expression of steroidogenic factor 1. Endocrinology 149:5599–5609 [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Cheng YH, Lin Z, Reierstad S, Yin P, Attar E, Xue Q, Imir G, Thung S, Trukhacheva E, Suzuki T, Sasano H, Kim JJ, Yaegashi N, Bulun SE 2008 Upstream stimulatory factor-2 regulates steroidogenic factor-1 expression in endometriosis. Mol Endocrinol 22:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, Zambetti G, DeLacerda L, Rodrigues GA, Haddad BR 2005 Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab 90:615–619 [DOI] [PubMed] [Google Scholar]
- Pianovski MA, Cavalli LR, Figueiredo BC, Santos SC, Doghman M, Ribeiro RC, Oliveira AG, Michalkiewicz E, Rodrigues GA, Zambetti G, Haddad BR, Lalli E 2006 SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer 42:1040–1043 [DOI] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB 2003 Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology 144:4123–4133 [DOI] [PubMed] [Google Scholar]
- Lu J, Richardson JA, Olson EN 1998 Capsulin: a novel bHLH transcription factor expressed in epicardial progenitors and mesenchyme of visceral organs. Mech Dev 73:23–32 [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Vanden Heuvel GB, Igarashi P 1998 Pod-1, a mesoderm-specific basic-helix-loop-helix protein expressed in mesenchymal and glomerular epithelial cells in the developing kidney. Mech Dev 71:37–48 [DOI] [PubMed] [Google Scholar]
- Tamura M, Kanno Y, Chuma S, Saito T, Nakatsuji N 2001 Pod-1/capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev 102:135–144 [DOI] [PubMed] [Google Scholar]
- Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE 2004 Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development 131:4095–4105 [DOI] [PubMed] [Google Scholar]
- Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, Rossant J 1999 The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126:5771–5783 [DOI] [PubMed] [Google Scholar]
- Xie K, Abbruzzese JL 2003 Developmental biology informs cancer: the emerging role of the hedgehog signaling pathway in upper gastrointestinal cancers. Cancer Cell 4:245–247 [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A 2008 Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11:277–284 [DOI] [PubMed] [Google Scholar]
- Komada M, Saitsu H, Kinboshi M, Miura T, Shiota K, Ishibashi M 2008 Hedgehog signaling is involved in development of the neocortex. Development 135:2717–2727 [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW 2008 A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 105:9349–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A 2008 Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol 317:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ 2008 Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell 14:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Dosch J, Simeone DM 2008 Pancreatic cancer stem cells. J Clin Oncol 26:2806–2812 [DOI] [PubMed] [Google Scholar]
- Brellier F, Bergoglio V, Valin A, Barnay S, Chevallier-Lagente O, Vielh P, Spatz A, Gorry P, Avril MF, Magnaldo T 2008 Heterozygous mutations in the tumor suppressor gene PATCHED provoke basal cell carcinoma-like features in human organotypic skin cultures. Oncogene 27:6601–6606 [DOI] [PubMed] [Google Scholar]
- Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A 2007 Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest 117:2929–2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau- Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D 2007 Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104:14466–14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP 1996 Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 6:298–304 [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD 2000 Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod 63:1825–1838 [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD 2001 A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod 65:1392–1402 [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B 2002 Desert hedgehog/patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA 2005 Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology 146:3558–3566 [DOI] [PubMed] [Google Scholar]
- Canto P, Soderlund D, Reyes E, Mendez JP 2004 Mutations in the desert hedgehog (DHH) gene in patients with 46,XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab 89:4480–4483 [DOI] [PubMed] [Google Scholar]
- Canto P, Vilchis F, Soderlund D, Reyes E, Mendez JP 2005 A heterozygous mutation in the desert hedgehog gene in patients with mixed gonadal dysgenesis. Mol Hum Reprod 11:833–836 [DOI] [PubMed] [Google Scholar]
- Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M 2000 A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet 67:1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Graham Jr JM, Olney AH, Biesecker LG 1997 GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 15:266–268 [DOI] [PubMed] [Google Scholar]
- Andersson HC, Frentz J, Martinez JE, Tuck-Muller CM, Bellizaire J 1999 Adrenal insufficiency in Smith-Lemli-Opitz syndrome. Am J Med Genet 82:382–384 [PubMed] [Google Scholar]
- Chemaitilly W, Goldenberg A, Baujat G, Thibaud E, Cormier-Daire V, Abadie V 2003 Adrenal insufficiency and abnormal genitalia in a 46XX female with Smith-Lemli-Opitz syndrome. Horm Res 59:254–256 [DOI] [PubMed] [Google Scholar]
- Bose J, Grotewold L, Ruther U 2002 Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet 11:1129–1135 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP 1995 Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172:126–138 [DOI] [PubMed] [Google Scholar]
- King PJ, Guasti L, Laufer E 2008 Hedgehog signalling in endocrine development and disease. J Endocrinol 198:439–450 [DOI] [PubMed] [Google Scholar]
- Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK 2003 RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 67:657–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Zamore PD 2005 microPrimer: the biogenesis and function of microRNA. Development 132:4645–4652 [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ 2003 Dicer is essential for mouse development. Nat Genet 35:215–217 [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE 2006 The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol 16:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X 2006 Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA 103:2208–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD 2007 Essential role for Dicer during skeletal muscle development. Dev Biol 311:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E 2006 Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38:356–362 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D 2007 Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1–2. Cell 129:303–317 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A 2007 Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN 2007 Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316:575–579 [DOI] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ 2005 RAS is regulated by the let-7 microRNA family. Cell 120:635–647 [DOI] [PubMed] [Google Scholar]
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T 2008 Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci USA 105:3903–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Dutta A 2007 The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 21:1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP 2007 Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315:1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T 2004 Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64:3753–3756 [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E 2007 let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131:1109–1123 [DOI] [PubMed] [Google Scholar]
- Liang Y, Ridzon D, Wong L, Chen C 2007 Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 8:166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T 2007 A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129:1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ 2006 Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol 7:678–689 [DOI] [PubMed] [Google Scholar]
- Bolos V, Grego-Bessa J, de la Pompa JL 2007 Notch signaling in development and cancer. Endocr Rev 28:339–363 [DOI] [PubMed] [Google Scholar]
- Dickson BC, Mulligan AM, Zhang H, Lockwood G, O'Malley FP, Egan SE, Reedijk M 2007 High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol 20:685–693 [DOI] [PubMed] [Google Scholar]
- Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE 2005 High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65:8530–8537 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA 2000 Stem cells: a new lease on life. Cell 100:143–155 [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H 2005 Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF 2003 Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423:302–305 [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL 2002 Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129:4753–4761 [DOI] [PubMed] [Google Scholar]
- Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G 2009 Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 15:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Yang W-H, Gerin I, Hu C-D, Hammer GD, Koenig RJ 2009 DAX-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidgenic factor-1 in steroidogenesis. Mol Cell Bio 29:1719–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen K, Markkula M, Mikola M, Rainio EM, McNeilly A, Huhtaniemi I 1996 Gonadectomy permits adrenocortical tumorigenesis in mice transgenic for the mouse inhibin α-subunit promoter/simian virus 40 T-antigen fusion gene: evidence for negative autoregulation of the inhibin α-subunit gene. Mol Endocrinol 10:1667–1677 [DOI] [PubMed] [Google Scholar]
- West AN, Neale GA, Pounds S, Figueredo BC, Rodriguez Galindo C, Pianovski MD, Oliveria Filho AG, Malkin D, Lalli E, Ribeiro R, Zambetti GP 2007 Gene expression profiling of Childhood adrenocortical tumors. Cancer Res 67:600–608 [DOI] [PubMed] [Google Scholar]
- Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, Severac D, Chotard L, Kahli M, Le Digarcher A, Pavlidis P, Journot L 2006 Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell 11:711–722 [DOI] [PubMed] [Google Scholar]
- Doghman M, Cazareth J, Lalli E 2008 The T cell factor/β-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab 93:3222–3225 [DOI] [PubMed] [Google Scholar]
- Else T, Theisen BK, Wu Y, Hutz JE, Keegan CE, Hammer GD, Ferguson DO 2007 Tppl/Acd maintains genomic stability through a complex role in telomere protection. Chromosome Res 15:1001–1013 [DOI] [PubMed] [Google Scholar]