Abstract
Feline immunodeficiency virus (FIV) causes AIDS in the domestic cat (Felis catus) but has not been explicitly associated with AIDS pathology in any of the eight free-ranging species of Felidae that are endemic with circulating FIV strains. African lion (Panthera leo) populations are infected with lion-specific FIV strains (FIVple), yet there remains uncertainty about the degree to which FIV infection impacts their health. Reported CD4+ T-lymphocyte depletion in FIVple-infected lions and anecdotal reports of lion morbidity associated with FIV seroprevalence emphasize the concern as to whether FIVple is innocuous or pathogenic. Here we monitored clinical, biochemical, histological and serological parameters among FIVple-positive (N = 47) as compared to FIVple-negative (N = 17) lions anesthetized and sampled on multiple occasions between 1999 and 2006 in Botswana. Relative to uninfected lions, FIVple-infected lions displayed a significant elevation in the prevalence of AIDS-defining conditions: lymphadenopathy, gingivitis, tongue papillomas, dehydration, and poor coat condition, as well as displaying abnormal red blood cell parameters, depressed serum albumin, and elevated liver enzymes and gamma globulin. Spleen and lymph node biopsies from free-ranging FIVple-infected lions (N = 9) revealed evidence of lymphoid depletion, the hallmark pathology documented in immunodeficiency virus infections of humans (HIV-1), macaques, and domestic cats. We conclude that over time FIVple infections in free-ranging lions can lead to adverse clinical, immunological, and pathological outcomes in some individuals that parallel sequelae caused by lentivirus infection in humans (HIV), Asian macaques (SIV) and domestic cats (FIVfca).
Keywords: FIV, Lentivirus, Lions, Wild, Free-ranging, Pathology, Immune depletion
Introduction
Pathological conditions associated with lentivirus infection in human and animal models include immune depletion, oral lesions caused by opportunistic infections, wasting, renal disease, and frequently a chronic inflammatory response. These conditions have been described as shared disease sequelae in humans infected with HIV, in macaques with SIV or chimeric S/HIV, and in domestic cats with FIV (Table 1 ). AIDS-defining conditions in HIV and SIV include immunodeficiency indicators such as CD4+ depletion (Freeman et al., 2004, Pandrea et al., 2007, Varbanov et al., 2006), lymphadenopathy (McClure et al., 1989, Quijano et al., 1997, Wang et al., 2003, Yanai et al., 2006), and progressive changes in histopathology consisting of lymphoid hyperplasia, involution, and atrophy (McClure et al., 1989, Quijano et al., 1997, Wang et al., 2003). In humans, loss of condition, or cachexia, is also AIDS-defining (Eid and Orenstein, 2006, Faintuch et al., 2006, Freeman et al., 2004, Kotler et al., 1984). Oral lesions such as gingivitis and papillomavirus associated warts (papillomas) are common in HIV infection and can be useful diagnostic indicators of HIV status since they parallel decline in CD4+ counts and rising viral load (Greenspan and Greenspan, 1997, Greenspan and Greenspan, 2002, Hodgson et al., 2006, Pantanowitz et al., 2006, Reddy, 2007, Woodman et al., 2007, zur Hausen, 2002). The documentation of gingivitis and papillomas in HIV-positive patients has increased in recent years possibly due to the long-term survival of patients receiving anti-retroviral therapy (Reznik, 2005). Papillomavirus is also associated with aggressive cervical cancer in HIV coinfected humans (Hawes et al., 2003, Pantanowitz et al., 2006, Woodman et al., 2007, zur Hausen, 2002). Although the incidence of papilloma viruses in SIV-infected rhesus macaques (Prospero-Garcia et al., 1996) is unknown, gingivitis has also been observed in this animal model (L. Colenda, personal communication).
Table 1.
Comparative sequelae of HIV, SIV, and FIV.
| Medical condition present in: | HIV (refs) human | SIV (refs) macaque | FIVFCA (refs) domestic cat | Lions |
|||||
|---|---|---|---|---|---|---|---|---|---|
| FIVple-negative |
FIVple-positive |
Odds ratio | p-value | ||||||
| % Affectedb | (No. lions)c,d | % Affectedb | (No. lions)e | ||||||
| Immunodeficiency | |||||||||
| CD4 depletiona | |||||||||
| Absolute number of CD4+ T-cells/mm3 in peripheral whole blood ± s.e. | (47) | (15, 33) | (1, 2, 22, 25, 32, 45, 46, 49) | 0 | (5) | 100f | (8) | nag | 0.00015 |
| Oral manifestations | |||||||||
| Gingivitis | (7, 16, 17, 38, 39) | (36) | (3, 25, 35) | 40.00 | (15) | 88.40 | (43) | 11.40 | 0.00016 |
| Papillomavirus | (7, 16, 17, 18, 21, 34, 38, 39, 52, 55) | ndh | (11, 44) | 14.30 | (14) | 53.19 | (47) | 6.82 | 0.01009 |
| Chronic inflammatory response | |||||||||
| Lymphadenopathy | (37, 48) | (29, 54) | (3, 6, 35) | 41.67 | (12) | 76.60 | (47) | 4.58 | 0.01900 |
| Hyperglobulinemia (serum globulin ≥ 2 s.d. above mean)j | (7, 8) | (51) | (1, 22, 43) | 0 | (14) | 85.71 | (46) | na | < 2 × 10− 9 |
| Elevated erythrocyte sedimentation rate (ESR ≥ 2 s.d. above mean)j | (24, 27, 40) | nd | nd | 13.33 | (15) | 64.86 | (37) | 12.00 | 0.00076 |
| Dehydration (≥ 4%) | (41) | (4, 20) | (10) | 26.67 | (15) | 63.04 | (46) | 4.69 | 0.01408 |
| Non-specific indicators of Ill-health | |||||||||
| Hair and coat abnormalities | nd | nd | nd | 13.30 | (15) | 52.27 | (44) | 7.12 | 0.00840 |
| Hypoalbuminemia (marker for cachexia) (serum albumin ≥ 2 s.d. below mean)j | (5, 14, 42) | nd | nd | 0 | (14) | 46.94 | (46) | na | 0.00129 |
| Anemia (Hemoglobin and/or PCV ≥ 2 s.d. below mean)j | (30, 42) | (19) | (22, 25, 43) | 11.11 | (18) | 55.77 | (52) | 10.09 | 0.00101 |
| Cachexia/unexplained weight loss | (12, 13, 26) | (15) | (3, 23, 25, 28, 53) | nd | Observed in 3 FIV+ populationsk | na | na | ||
| Histopathologic evidence of lymphoid response | |||||||||
| Histopathologic evidence of: lymphoid activation | (37, 48) | (29) | (6, 9) | nd | Yes | na | na | ||
| Histopathologic evidence of: lymphoid atrophy and depletion | (37, 48) | (29) | (6, 9) | nd | Yes | na | na | ||
Detailed data for CD4 depletion in wild lions is presented in Roelke et al. (2006). Other FIV entries are in this report.
References used in table
1. Ackley et al. (1990); 2. Barlough et al. (1991); 3. Bendinelli et al. (1995); 4. Board et al. (2003); 5. Bonarek et al. (2001); 6. Brown et al. (1991); 7. Coogan et al. (2005); 8. De Milito et al. (2004); 9. Diehl et al. (1995); 10. Egberink et al. (1990); 11. Egberink et al. (1992); 12. Eid and Orenstein (2006); 13. Faintuch et al. (2006); 14. Feldman et al. (2000); 15. Freeman et al. (2004); 16. Greenspan and Greenspan (1997); 17. Greenspan and Greenspan (2002); 18. Hawes et al. (2003); 19. Hillyer et al. (1993); 20. Hodge et al. (1998); 21. Hodgson et al. (2006); 22. Hofmann-Lehmann et al. (1997); 23. Hutson et al. (1991); 24. Kim et al. (2006); 25. Kohmoto et al. (1998); 26. Kotler et al. (1984); 27. Lifson et al. (1995); 28. Matsumura et al. (1993); 29. McClure et al. (1989); 30. Mocroft et al. (1999); 31. Nagase et al. (2001); 32. Novotney et al. (1990); 33. Pandrea et al. (2007); 34. Pantanowitz et al. (2006); 35. Pedersen et al. (1987); 36. Prospero-Garcia et al. (1996); 37. Quijano et al. (1997); 38. Reddy (2007); 39. Reznik (2005); 40. Schwartlander et al. (1993); 41. Selwyn and Rivard (2003); 42. Shah et al. (2007); 43. Sparkes et al. (1993); 44. Sundberg et al. (2000); 45. Tompkins et al. (1991); 46. Torten et al. (1991); 47. Varbanov et al. (2006); 48. Wang et al. (2003); 49. Willett et al. (1993); 50. Willett et al. (1997); 51. Wong et al. (1999); 52. Woodman et al. (2007); 53. Yamamoto et al. (1989); 54. Yanai et al. (2006); 55. zur Hausen (2002).
Medical condition for each lion was scored as “affected” if the individual was ever found to be abnormal (on physical exam) or have a blood value 2 standard deviations or more away from the mean of the FIV-negative lions (toward a less fit value).
Number of individual lions examined (for each parameter only a single observation date was used per lion).
FIV-negative lions = 23 lions (27 observation dates evaluated).
FIV-positive lions = 54 lions (84 observation dates evaluated).
Two standard deviations below the mean for the FIV-negative lions is 810 CD4+ cells.
na — not applicable.
nd — not documented.
| Globulin (measured and/or calculated) | |
| Measured | 4.44 ± 0.90 g/dL |
| T. protein minus albumin | 3.99 ± 0.72 g/dL |
| ESR (EDTA whole blood) | 10.5 ± 28.0 mm/h |
| ESR (heparin whole blood) | 23.9 ± 37.3 mm/h |
| used both sample types to determine abnormal values | |
| Albumin | 3.7 ± 0.76 g/dL |
| Hemoglobin | 12.26 ± 0.90 g/dL |
| Packed cell volume (PCV) | 40.0 ± 7.0% |
Serengeti National Park (MER), Okavango Delta (P. Kat, personal communication), Krugar National Park (Ide 2002).
FIV is an important natural model for HIV/AIDS since FIV-infected cats develop AIDS-like illnesses (Pedersen et al., 1987, Willett et al., 1997). As with HIV and SIV infection, lymphadenopathy, immunodeficiency, progressive lymphoid depletion, associated oral manifestations, loss of condition (wasting/cachexia), and chronic inflammatory response are characteristic of FIV infection (Ackley et al., 1990, Barlough et al., 1991, Brown et al., 1991, Diehl et al., 1995, Egberink et al., 1992, Hutson et al., 1991, Kohmoto et al., 1998, Matsumura et al., 1993, Novotney et al., 1990, Sundberg et al., 2000, Tompkins et al., 1991, Torten et al., 1991, Willett et al., 1993, Yamamoto et al., 1989). Progressive stages can last months to years after initial infection, during which time the cat may be an asymptomatic carrier or may exhibit persistent generalized lymphadenopathy, AIDS-related complex, or feline AIDS (Bendinelli et al., 1995).
Immunodeficiency viruses closely related to FIV have been documented in free-ranging populations of eight non-domestic felid species (Olmsted et al., 1992, Troyer et al., 2005, Osofsky et al., 1996). Of these, lions have the highest seroprevalence, approaching 100% of adult animals in many populations (Brown et al., 1994, Troyer et al., 2005). Multiple highly divergent strains of lion FIV (FIVple) are found across much of Africa (O'Brien et al., 2006, Troyer et al., 2005). Because of the high levels of genetic diversity both within and between the six known FIVple strains, as well as the phylogeographic structure of viral sequences that mimics patterns of lion population structure and migration (Antunes et al., 2008, Pecon-Slattery et al., 2008b), FIVple is thought to be a relatively old virus, perhaps infecting lions for thousands of years.
The presumed age of FIVple coupled with ecological studies suggesting that there are no population-level correlates to infection (i.e. reduced lifespan, or reduced fecundity) has led to the current paradigm of FIVple as a host-adapted virus that is no longer pathogenic to lions (Biek et al., 2006, Carpenter and O'Brien, 1995, Hofmann-Lehmann et al., 1996, Packer et al., 1999). This assumption has been reinforced by laboratory experiments demonstrating that domestic cats infected with FIVple seem to recover completely after transient lymphadenopathy and plasma viremia, despite persistent cell-associated viremia throughout the study period (VandeWoude et al., 2002, VandeWoude et al., 1997). Similarly, naturally SIV-infected hosts such as sooty mangabeys and African green monkeys are rarely symptomatic, in contrast to AIDS-like pathology in non-native SIV-infected hosts such as Asian macaques, and AIDS in humans (Pandrea et al., 2006, VandeWoude and Apetrei, 2006).
Recent studies demonstrating significantly reduced CD4+ counts in both captive and free-ranging lions infected with FIVple challenge the assumption of a completely innocuous infection (Bull et al., 2002, Bull et al., 2003, Roelke et al., 2006). Neurological effects and cachexia have also been associated with FIVple infection in captive lions (Brennan et al., 2006, Bull et al., 2003). In this study, three free-ranging Botswana lion populations, as well as lions from the Serengeti Tanzania population, were examined for thirteen physiological and pathological correlates to FIVple infection (Fig. 1 ; Table 1). We examined clinical, hematological and biochemical profiles of FIVple-infected and FIVple-seronegative free-ranging lions and performed histopathological analysis on a suite of tissues from a subset of FIVple-infected lions. Relative increases in the occurrence of specific and non-specific clinical symptoms including lymphadenopathy, gingivitis, papillomas, dehydration, and loss of coat condition were found in FIVple-infected lions, as were biochemical profiles indicative of hyperglobulinemia, anemia, and hypoalbuminemia. Similarly, histopathological changes in FIV-infected lion lymphoid tissue were consistent with an FIVple-associated pathogenesis.
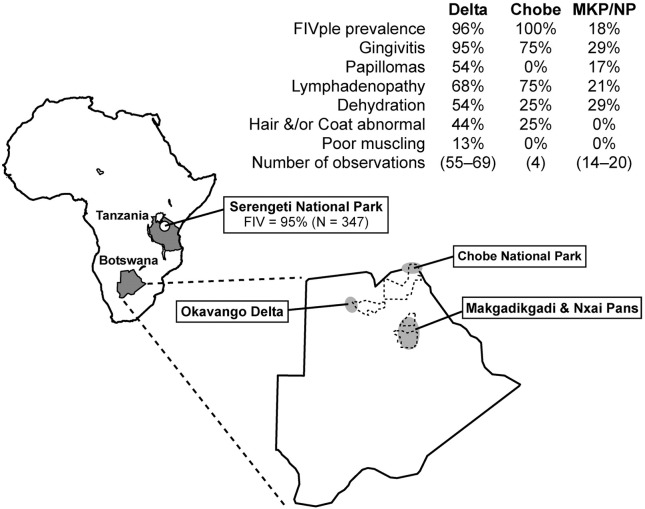
Fig. 1.
Study sites in Botswana and Tanzania. Dotted outlines designate national parkland, while shaded ovals encompass the general areas where lions were sampled. N indicates the number of lions tested for FIV. Not all parameters were determined for every animal handled at every time point, therefore N was not the same for all clinical observations from a region and is given as a range.
Results
FIV seroprevalence
A total of 64 lions were anaesthetized, examined, and sampled from 1999 to 2006 in three distinct Botswana ecosystems: Makgadikgadi National Park and Nxai Pans (MKP/NP; 20 individual lions handled on 24 occasions), Okavango Delta (Delta; 40 lions, 70 observations), and Chobe National Park (Chobe; 4 lions, 4 observations; Fig. 1). FIV status was compared between populations. A limited number of lions from Serengeti National Park in Tanzania (SNP; 8 lions, 8 observations, sampled from 1994 to 1996; (Roelke et al., 2006) were also included. FIVple prevalence in our sampling was significantly reduced in study samples from the MKP relative to other lion study populations; 18% in MKP/NP compared to 96%, 100% and 100% in the Delta, Chobe, and SNP, respectively (p < 0.0001 in all cases). These FIVple sample prevalences reflect those of the larger populations (Brown et al., 1994, Carpenter and O'Brien, 1995, Olmsted et al., 1992, Troyer et al., 2005). Mean age of FIVple-positive and FIVple-negative lions was 7.3 (range = 0.8–14 years) and 6.3 (range = 1.8–8 years) respectively (no significant difference between groups, p = 0.32) and likewise the proportion of females was similar in the two FIV groups (0.706 for FIVple-positive and 0.846 for FIVple-negative; p = 0.50; for age and sex of each lion see Fig. S1). Immunodeficiency, as indicated by low CD4 counts, has been previously documented in the SNP lions included in this study (Roelke et al., 2006). To further document specific immune effects, eight FIVple-positive lions were collared and sampled sequentially by a full medical exam including laparoscopic surgery to obtain biopsy samples from lymph nodes, spleen, liver, and kidney for histopathological analysis.
A summary of the prevalence of various AIDS-defining conditions in FIVple-negative versus FIVple-positive lions discussed below are summarized in Table 1. We also list in Table 1 citations for AIDS-defining conditions in human AIDS, in SIV-infected macaques, and FIVfca infected domestic cats. Detailed clinical parameters for individual FIVple-positive and FIVple-negative lion are presented in Fig. S1.
Oral manifestations; gingivitis and papillomas
On gross clinical examination of FIVple-infected lions, the most striking observation was their poor oral health. Gingivitis and sublingual papillomas were more frequently observed during physical examinations of seropositive compared to seronegative animals (p = 0.00016 and p = 0.01009, respectively; Table 1; Fig. 2 ). Virtually all FIVple-positive animals had some manifestation of gingivitis. In some younger lions (≤ 3 years old) the gingivitis presented as a bright red line along the gingival edge (very similar in appearance to the linear gingival erythema seen in HIV-associated gingivitis) (Reznik, 2005). This inflammation was associated with a narrow zone of depigmentation (especially noticeable along the molars; Fig. 2). All older FIVple-positive lions had well-developed chronic granulomatous gingival lesions with varying degrees of depigmentation that, in some cases, involved the entire alveolar mucosa (Fig. 2) although no lions in the study presented with the extreme necrotizing gingival lesions seen in human AIDS patients. In contrast, the mild gingivitis observed in six (out of fifteen) FIVple-negative lions tended to present as minor gingival irritation with limited depigmentation; more severe gingival lesions were not observed in these lions.
Fig. 2.
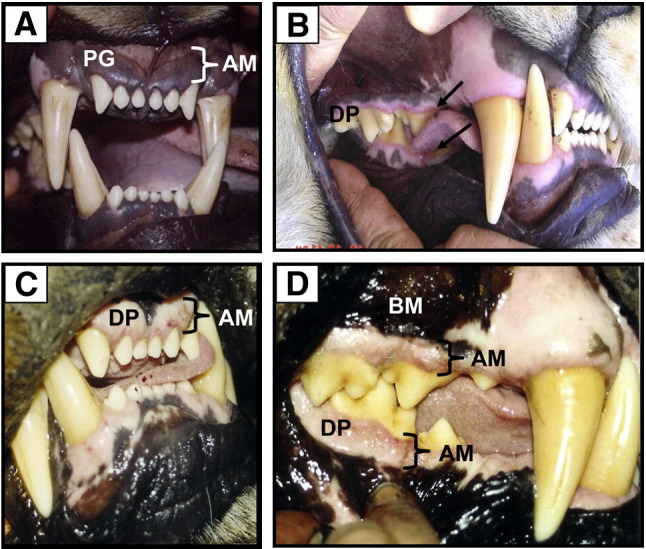
Gingival tissue of normal (A) and FIV-positive lions with varying stages of gingivitis (B–D); frontal incisors (A and C) and lateral molar (B and D) views. (A) Normal, healthy gingiva in an FIVple-negative lion. Note darkly pigmented (PG) alveolar mucosa (AM) (adhered to bone). (B) Early stage FIVple gingivitis in a young adult female lion showing linear gingival erythema (arrow), an inflammatory process that starts to erode the normal darkly pigmented alveolar mucosa resulting in a narrow zone of depigmentation (DP) along free edge of gingiva adjacent to molars and lower outside incisors. (C and D) Granulomatous gingivitis typically seen in all mature, chronically FIV-infected lions. Note extensive depigmented zone affecting virtually all alveolar gingival mucosa (AM) but sparing the bucal mucosa (BM). The depigmented area above the upper canine teeth is normal.
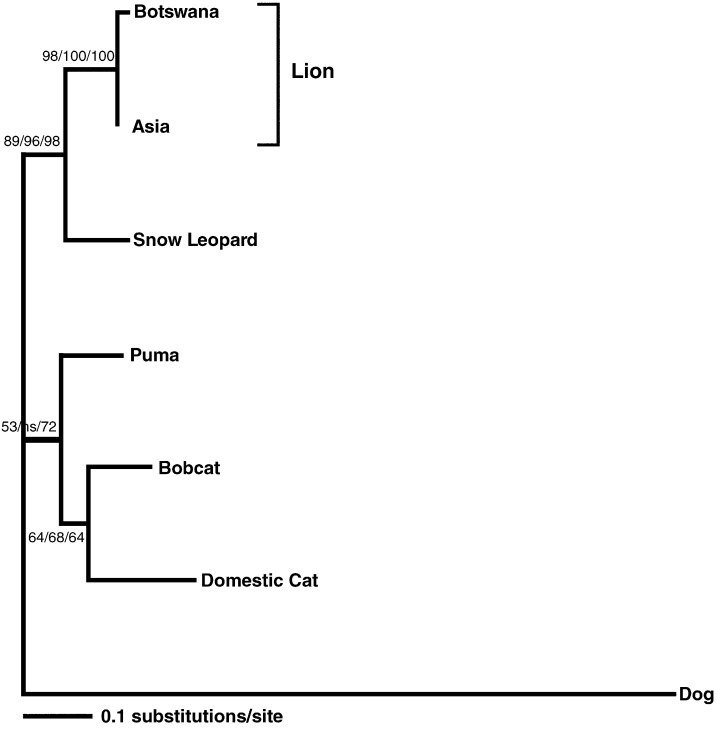
The majority of FIVple-infected lions (53.19% compared to only 14.3% of uninfected lions) presented with wart-like papillomatous lesions on the underside of the tongue (1–6 lesions of 2 mm–2 cm in diameter). Histopathologic examination of biopsied lesions from 12 lions was consistent with papilloma viral infection (diagnostic eosinophilic intracytoplasmic inclusions were observed in all cases). Plaques were removed and DNA was extracted and amplified with feline papilloma virus-specific primers. Resultant PCR products from two feline papilloma virus genes (early and late: ∼ 900 bp) were sequenced and shown to be feline papilloma viruses almost identical to an isolate from a Gir Forest India Asiatic lion (Rector et al., 2007) (Fig. 3 ).
Fig. 3.
Phylogeny of concatenated early and late genes (900 bp) of a Botswana lion papilloma virus compared to papilloma viruses isolated from several felid species. Canine papilloma virus was used as an outgroup. Shown here is the maximum likelihood (ML) tree. Minimum evolution (ME) and maximum parsimony (MP) trees gave similar topologies. Bootstrap values are given at all nodes (ML/ME/MP; ns = not significant).
Chronic inflammatory response
Lymphadenopathy was observed more frequently during physical examinations of seropositive animals. As in HIV-infected people and FIV-infected domestic cats, lymphadenopathy was intermittent (i.e. varied by time point in lions examined more than once), and present in 77% of FIVple-positive lions versus 42% of FIVple-negative animals (p = 0.019; Table 1).
Total protein, as well as globulin and gammaglobulin levels, were significantly higher in FIVple-positive samples than in FIVple-negative ones (p = 0.048, p < 0.001, and p < 0.001 respectively; Fig. 4 ) but albumin was notably lower (p = 0.001; Fig. 4). Serum protein electrophoretic profiles showed a polyclonalgammopathy (data not shown). This protein profile resulted in a large and significant (p = 0.00006) difference in albumin/globulin ratios between FIVple-negative and positive lions (Table S1). The skewed ratio, hypergammaglobulinemia, and hypoalbuminemia are classic signatures of chronic inflammatory disease. Elevated erythrocyte sedimentation rate (ESR) can also indicate a persistent inflammatory state and was observed significantly more often in the seropositive lions (abnormally fast ESR seen in 64.86% of FIVple-positive lions and 13.33% of FIVple-negative lion; p = 0.00076; Table 1).
Fig. 4.
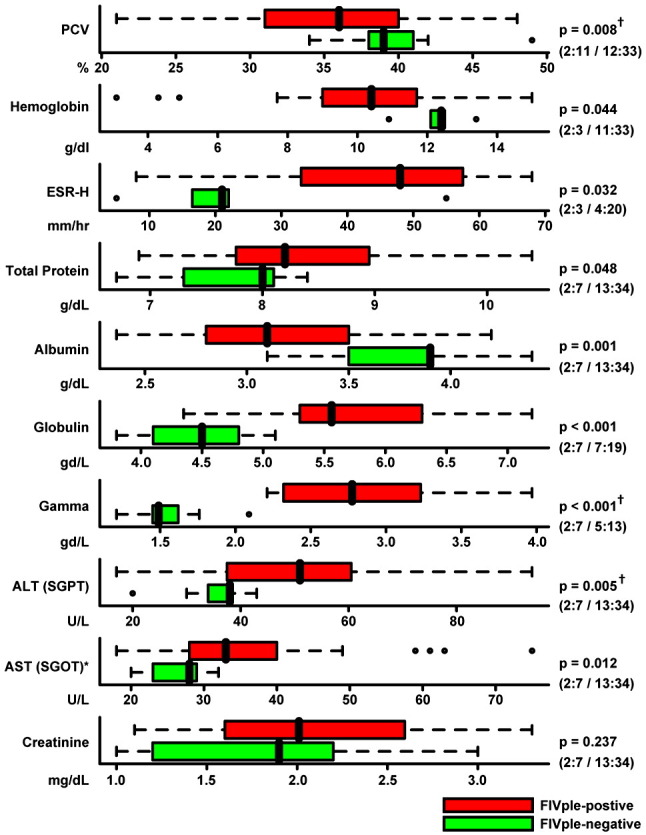
Comparison of blood parameters, protein levels, and chemistry values from FIV-positive (red) and negative (green) lions. The black vertical line represents the median, colored bars represent the interquartile range (IQR), and dotted lines represent the range up to 1.5 times the IQR. Outliers are represented by open circles, and biomarkers with an asterisk (ALT and AST) had extreme outliers that are not shown here in the interest of scale. Due to the non-normal distribution of most biomarkers, non-parametric Wilcoxon rank-sum statistics were used to determine p values. Number of FIVple-negative and positive male and female lions is indicated in parentheses (negative males:negative females/positive males:positive females). Among FIVple-positive lions, all of the parameters in this figure were examined for age-dependant effects. The three traits marked with a “†” had a significant difference in values between adult (4–8 years) and older (9+) age classes, with older lions trending further away from the mean of the FIVple-negative lions. However, when older lions were removed from the analysis of these three parameters, significant differences still remained between FIVple-positive and -negative lions (p = 0.029, p = 0.0001, and p = 0.032 for PCV, globulin, and ALT, respectively). Abbreviations: PCV — packed cell volume; ESR — erythrocyte sedimentation rate; ALT — alanine aminotransferase (aka SGPT); AST — aspartate aminotransferase (aka SGOT).
Dehydration was seen more frequently in FIVple-positive lions (63.04% of the FIVple-positive lions in contrast to 26.67% of FIV-negative lions; a significant difference; p = 0.014; Table 1). Of the 30 lions where the percentage of clinical dehydration was specifically recorded, 33% were minimally dehydrated (3%), 53% were moderately dehydrated (4–5%), 13% were severely dehydrated (6–10%), and none were gravely dehydrated (> 10%). Remarkably, dehydrated FIVple-positive lions in this study live primarily in swampland with ample drinking water, while most FIV-negative lions reside in scrub and arid areas. Dehydration does not appear to be the result of impaired renal function since urine from 27 of 29 FIVple-positive lions was sufficiently concentrated (specific gravity ≥ 1.035) with only 5 animals presenting with proteinuria (urine protein:creatine ratio> 0.4; Fig. S1).
Ten liver biopsies from seven lions and four kidney biopsies from three lions were obtained from Okavango Delta FIVple-positive animals for histopathology. Mild kidney abnormalities were observed, with evidence of multifocal segmental to diffuse membranoproliferative glomerulonephropathy (MPGN) with no evidence of tubular or interstitial lesions in all three lions (Table S2). This histopathology was not considered to be of sufficient severity to account for the high incidence of dehydration in FIVple-positive lions (see discussion). Seven liver biopsies from Delta lions had portal inflammation, often with mineralization and a high proportion of eosinophils (Table S3). Remnants of parasite eggs were seen in two biopsies. Liver enzymes ALT and AST were also significantly elevated in FIVple-infected samples (p = 0.005 and p = 0.012; Fig. 4). Among FIVple-positive samples, ALT and AST also varied by habitat, with the lowest values seen in samples from lions residing in arid/savannah environments with intermittent water supplies (MKP/NP and SNP), followed by samples from lions living with a constant riverine water source (Chobe), and the highest levels seen in samples from lions in swampland (the Delta).
Clinical and biochemical non-specific indicators of poor health
Cachexia (poor condition or wasting) is a common finding in humans and domestic cats infected with pathogenic immunodeficiency viruses (Bendinelli et al., 1995, Eid and Orenstein, 2006, Faintuch et al., 2006, Kohmoto et al., 1998, Kotler et al., 1984, Matsumura et al., 1993, Yamamoto et al., 1989). Therefore the relative condition of FIVple-positive and -negative lions was assessed. Hair and coat condition was abnormal in 52.27% of positive lions and only 13.3% of negative animals (p = 0.008; Table 1). Poor muscle condition was only seen in a single FIVple-negative animal (Ple-1035, a 10 month-old Delta lion whose litter mates had both seroconverted; Fig. S1) and was observed in 9 positive animals, but this difference was not significant (p = 0.5; data not shown). Severe cachexia (wasting) has been observed in FIVple-endemic locations (Kruger National Park and SNP; Fig. 5 ), but also has been reported in other lion populations due to prey limitations or other outbreaks such as tuberculosis.
Fig. 5.

Varying degrees of body condition in FIVple-infected animals. While most uninfected and infected lions appear to be in good to excellent physical condition (A; 94-184 Ple July 1994, Serengeti National Park; photo credit Christopher Ratier). However, occasionally lions with unexplained weight loss (cachexia) are observed in populations with FIVple-positive lions (B; 94-060 Ple Mar 1994, Serengeti National Park; similar “poor doers” have been seen in Kruger National Park and the Okavango Delta; photo credit Melody Roelke), often in the context of secondary disease outbreaks. Both these lions were photographed in the spring of 1994 during a CDV epidemic. CDV alone is not commonly associated with wasting.
The criteria for cachexia in humans include > 5% unintentional weight loss, body mass index (BMI) < 20, low fat free mass, evidence of cytokine excess, and low blood albumin levels (< 3.5 mg/dL is considered poor prognosis for long-term survival in humans with a variety of conditions including HIV and AIDS) (Feldman et al., 2000, Morley et al., 2006, Sabin et al., 2002, Sullivan et al., 2005). Although study lions were weighed at capture, most were evaluated at only one time point. Some lions were weighed at multiple time points, however age of animals and seasonal differences in prey abundance complicate determination of weight loss. Thus we could not accurately document changes in weight. Similarly, the lack of standard measurements and benchmarks for BMI and low fat free mass and standardized assays for cytokines in lions makes measures of these parameters unfeasible in these populations. An index of cachexia that could be measured in study lions was albumin; therefore we used low albumin levels as a proxy for low BMI. None of the FIVple-negative animals presented with abnormally low albumin levels, while 46.94% of FIVple-positive animals had hypoalbuminemia (defined as ≥ 2 s.d. lower than the mean of the controls; p = 0.00129; Table 1; Fig. 4); 70% of infected animals had levels 1 s.d. or more below the mean of the controls (< 3.3 mg/dL; Fig. 4; Fig. S1). Within FIVple-positive animals, there was no significant difference in albumin levels between study sites despite varying prey densities across sites.
Anemia was observed more often in FIVple-positive lions (p = 0.001; Table 1), which presented with significantly lower hemoglobin values (p = 0.044) and packed cell volume (PCV: p = 0.008) than FIVple-negative individuals (Fig. 4; Table S1). These values were used to calculate red cell indices (MCV, MCH, and MCHC; Table S1), which were highly variable with 56–74% of infected animals presenting with abnormal values (≥ 2 s.d. above or below the uninfected mean; Fig. S1). The erythrocyte sedimentation rate trended higher in FIVple-positive animals in EDTA whole blood, but was only significantly higher in heparinized whole blood (p = 0.032; Fig. 4, Fig. S1, Table S1). There was no significant difference in total white cell count (Fig. S1, Table S1).
Histological lymphoid involvement
Lymphoid and non-lymphoid tissues were examined for 9 FIVple-positive lions from the Delta (8 biopsied lions and 1 hunter-killed lion that was opportunistically necropsied within 3 h of death). Histopathological changes in lymphoid tissues (lymph nodes and spleen) were common in FIVple-infected lions (Table 2 ), similar to HIV and FIVfca infection. One lion (Ple-1025), with both lymph node and spleen sampled 3 times over a period of 3 years, demonstrated the typical progression pattern from a hypertrophic state to an involuted one with increasing lymphoid depletion, hyalinosis, and development of plasmacytosis over time that is seen in HIV-infected humans and FIVfca infected domestic cats (Fig. 6 ; Table 2) (Matsumura et al., 1993, Quijano et al., 1997). In two lions (Ple-1738 and Ple-1756) there was evidence of follicular (cortex and/or pericortical areas) hyperplasia in both tissues. All nine individuals presented with evidence of moderate to severe follicular and/or pericortical atrophy either in lymph node, spleen, or both and most showed lymphocyte depletion with or without follicular hyalinosis (Table 2). Overall, there was more evidence of involution and depletion than hypertrophy in our biopsy samples. The single individual presenting with no evidence of splenic lymphoid depletion was the youngest lion sampled (Ple-1738; 3 years old).
Table 2.
Lymph node and splenic histopathology seen in FIV-positive lions from the Okavongo Delta (N = 9).
| Code name | Date | Lymph node histopathology |
Spleen histopathology |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node location | Hypertrophy/Hyperplasia and reactivity |
Involution and/or depletion |
Hypertrophy |
Involution and/or depletion |
Degeneration | ||||||||||
| Cortical follicular hypertrophy | Paracortical hypertrophy | Medullary plasmacytosis | Sinus histiocytosis (macrophages) | Cortical follicular atrophy | Paracortical atrophy | Cysts/dilated sinuses | Follicular hyalinosis | Follicular hypertropy | Follicular atrophy | Lymphocyte depletion | Follicular hyalinosis | ||||
| PLE-0700 | 2004 | Ca | – | – | + | + | – | ++ | – | – | – | + | + | – | Ap, Nc, PMN deg |
| PLE-1024 | 2003 | – P | + | +++ | + | ||||||||||
| PLE-1025 | 2002 | Ab | – | ++ | – | – | ++ | – | – | – | + | + | + | – | Ap, Lcy, PMN deg |
| 2003 | + | + | ++ | + | |||||||||||
| 2004 | Ca | – | – | + | ++ | nta | nta | – | – | – | + | ++ | ++ | ||
| PLE-1026 | 2001 | Ax-Asp | – | – | – | + | ++ | + | – | – | |||||
| PLE-1726 | 2001 | Ax-Asp | – | – | – | + | +++ | +++ | – | – | |||||
| PLE-1738 | 2002 | Ma | – | – | – | – | ++ | +++ | – | + | + | – | – | – | Ap, PMN deg |
| 2002 | Ab | + | + | – | + | ++ | – | – | + | ||||||
| PLE-1744 | 2004 | – | + | + | + | Ap, PMN deg | |||||||||
| PLE-1750 | 2003 | – P | + | + | + | ||||||||||
| 2004 | – | + | ++ | + | |||||||||||
| PLE-1756 | 2006 | Pop | ++ | – | + | + | – | + | – | + | + | ++ | ++ | ++ | |
| 2006 | Ax | ++ | – | + | – | – | ++ | + | + | ||||||
| 2006 | Oth | – | – | – | – | +++ | +++ | +++ | + | ||||||
Scoring code (for tissue categories as in Ida 2002)
– not present
+ mild
++ moderate
+++ severe
nta no tissue available
P follicules are present
Lymph node location
Ca caudal abdomenal (mammary)
Asp asperate peripheral LN
Ab abdomenal (cecal) LN
Ma mandibular LN
Pop popliteal LN
Ax axillary LN
Oth other not specified
Splenic degeneration
Ap apoptosis
Nc necrosis
PMN deg PMN degeneration assoc with trabeculae
Lcy lymphocytolysis
Biopsies from FIV-negative aged match control lions were not available for histology.
Fig. 6.
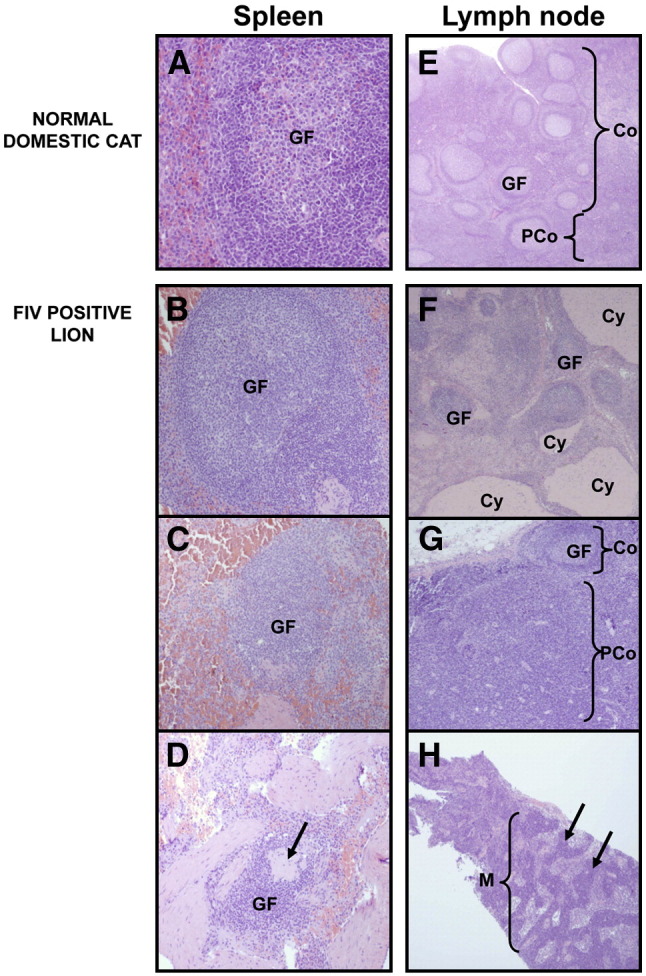
Spleen (A–D) and lymph node (E–H) tissue from normal, uninfected domestic cats (A and E) and FIVple-infected lions (B–D and F–H). GF = germinal follicle; Co = cortex; PCo = paracortex; Cy = cyst; M = medulla. (A) Healthy splenic tissue. (B) A hyperplastic follicle. (C) Follicular atrophy. (D) Severe follicular atrophy with pronounced lymphoid depletion. Arrow indicates an area of hyaline depletion. (E) A healthy lymph node with many follicles in the cortex and a smaller paracortical area. (F) Mixed hyperplastic follicles with cystic areas. (G) Cortical atrophy with paracortical hyperplasia. (H) Extensive medullary plasmacytosis. Arrows indicate cords of plasma cells.
Discussion
In this study we investigated the possibility that, despite inferences that FIVple-infected lions can live out normal life spans in the wild and reproduce successfully (Biek et al., 2006, Carpenter and O'Brien, 1995, Hofmann-Lehmann et al., 1996, Packer et al., 1999), there are significant pathological correlates to FIVple infection that have health consequences for individual lions. We extend previous reports of FIVple-associated CD4 depletion in peripheral blood (Bull et al., 2003, Roelke et al., 2006) by demonstrating lymphoid depletion via biopsies of lymphoid tissues from infected lions. We document evidence that lymphadenopathy, gingivitis, and papillomas are more common in FIVple-infected lions. In addition, we show that infected animals are more likely to be in poor condition, both with respect to their general physical health (hydration, coat condition, and albumin levels) as well having depressed hemoglobin and PCV. Finally, we demonstrate that infected lions have alterations in serum chemistry profiles and histopathologies that are consistent with immune deficiencies seen in humans, macaques, and domestic cats infected with HIV, SIV, and FIV, respectively.
Both gingivitis and papillomavirus have been implicated as AIDS-defining oral manifestations (Coogan et al., 2005, Greenspan and Greenspan, 1997). Papillomaviral-induced cervical cancer is also significantly increased in HIV-positive women (Hawes et al., 2003, zur Hausen, 2002) and papillomas have been described in domestic cats infected with pathological lentiviruses (Egberink et al., 1992, Sundberg et al., 2000). Fifty-three percent of FIVple-infected study lions presented with papillomas, while only 14.3% of uninfected lions had papillomas (Table 1). Gingivitis was documented in 88.4% of FIVple-positive lions and only 40% of FIVple-negative animals. In addition gingivitis persisted and progressed further in infected lions. Early stages of gingivitis in our study lions mimicked that seen in HIV-associated gingivitis with marked linear gingival erythema (LGE). Later stage gingivitis in lions was not similar to HIV-associated gingivitis as none of our study lions presented with the severe necrotizing gingivitis and periodontitis as seen in HIV-infected humans and FIV-infected domestic cats (Coogan et al., 2005, Kohmoto et al., 1998, Reddy, 2007). It is likely that these conditions would be mal-adaptive in a natural setting where healthy teeth and mouth are essential for survival.
Lymphoid depletion is a hallmark of immunodeficiency virus infections; CD4 count < 200 is the predominant AIDS-defining conditions that, in the absence of drug therapy, occurs in nearly all HIV-positive humans, SIV-infected macaques, and FIV-infected cats, although time to CD4 depletion can vary. Lions infected with FIVple undergo CD4 depletion (Bull et al., 2003, Roelke et al., 2006). Lymphadenopathy was also significantly increased in FIVple-infected animals. While it was not always observed in positive lions, not all lions are at the same stage of infection. Most lions in this study were over 6 years old and, given the 100% seroprevalence rate by 2 years of age, were probably infected for at least 4 years when first examined and therefore may not have been in an acute stage of lymphoid hyperplasia. Other signs of a chronic inflammatory response observed in FIVple-infected lions include ubiquitous hyperglobulinemia (primarily gammaglobulin), hypoalbuminemia, and anemia (low hemoglobin and/or PCV). These biochemical profiles are common in HIV-positive individuals (De Milito et al., 2004, Kim et al., 2006, Lifson et al., 1995, Nagase et al., 2001, Schwartlander et al., 1993) and have been observed in SIV and FIV infection (Ackley et al., 1990, Hillyer et al., 1993, Hofmann-Lehmann et al., 1997, Kohmoto et al., 1998, Sparkes et al., 1993, Wong et al., 1999). Further, low hemoglobin and albumin levels are significantly associated with progression to AIDS and death in human pre-AIDS patients (Bonarek et al., 2001, Mocroft et al., 1999, Shah et al., 2007).
Histological evidence suggests that some lions biopsied may have progressed to a chronic stage of infection or undergone atrophy of the lymph nodes, another consequence of pathologic immunodeficiency virus infections (Brown et al., 1991, Diehl et al., 1995, McClure et al., 1989, Quijano et al., 1997, Wang et al., 2003). In 2 lions, histopathology of lymphoid tissues (lymph nodes and spleen) was consistent with a progressive depletion of lymphocytes. While one could argue that lymphadenopathy may have other etiologies (fight wounds, injuries, etc.), wholesale lymphoid depletion is common during HIV, SIV, and FIV infection and is most compatible with an FIVple-associated immune deficiency.
FIVple-positive lions were often mildly to moderately dehydrated (Table 1). Major causes of dehydration include lack of water (an unlikely cause in the swampland of the Okavanga Delta) and gastrointestinal disease with vomiting and diarrhea, which have not been generally observed in these study lions by the field biologists. Also, significant renal disease can result in dehydration due to the kidney's inability to retain water and the high net protein loss by diseased glomerulus and tubules. However, very few infected lions (5 of 29 tested) presented with overt proteinuria or loss of functional concentrating ability (only 2 out of 29 tested had low specific gravity of urine). In addition, serum creatinine levels (which are indicative of glomerular dysfunction) were not significantly different between either FIVple-positive and negative lions or dehydrated vs. hydrated lions. Further, in the three lions for which we had renal biopsies, renal pathology was mild and restricted to the glomerulus. Taken together, these parameters of renal function do not support renal failure as the cause of widespread (63%) dehydration seen in FIVple-positive lions. The remaining typical reason that animals and humans become dehydrated are fever or depression/malaise (with associated inappetence and oligodipsia), both of which may be general signs of ill health and, along with dehydration itself, are well-documented in lentiviral infected humans and domestic cats (Bendinelli et al., 1995, Egberink et al., 1990, Faintuch et al., 2006, Pedersen et al., 1987, Selwyn and Rivard, 2003); dehydration is also documented in SIV-infected rhesus macaques (Board et al., 2003, Hodge et al., 1998).
Wasting, an extreme loss of condition, is common in humans, macaques, and domestic cats infected with pathogenic lentiviruses (Eid and Orenstein, 2006, Faintuch et al., 2006, Freeman et al., 2004, Hutson et al., 1991, Kohmoto et al., 1998, Kotler et al., 1984, Matsumura et al., 1993, Morley et al., 2006, Podell et al., 1998, Yamamoto et al., 1989). Many FIVple-infected lions in this study (Table 1, Appendix A) presented symptoms suggesting they were along a continuum between wellness and wasting as indicated by their general physical condition, anemic state and significantly depressed albumin levels. In addition, cachexic animals have been observed in FIV-endemic areas in Tanzania (SNP) and in South Africa (KNP). In both sites, the prevalence of wasting seemed to rise coincidental with the introduction of other immunosuppressive disease outbreaks; specifically tuberculosis (TB) in KNP (Ide, 2002, Renwick et al., 2007) and canine distemper virus (CDV) in SNP (Roelke-Parker et al., 1996). While TB causes a wasting syndrome on its own, CDV typically is not associated with wasting and it is possible there was a synergistic reaction or a release of FIVple from immune control in dually infected animals. Other indicators of general loss of condition have also been observed, including poor coat condition and hair quality (52.3% in FIVple-infected lions versus 13.3% in negative lions; p = 0.00840; Table 1) considered an indicator of wellness in veterinary practice. These markers are non-specific and can be influenced by many biological and environmental factors, but taken together show a general trend for FIVple-infected lions to be in poor health more often than FIVple-negative lions.
Opportunistic infections are the major cause of death in AIDS patients, and many of them are AIDS-defining conditions (Pantanowitz et al., 2006). While liver pathologies are often observed in HIV-infected humans due to viral infections, usually hepatitis C virus (HCV), they are rare in macaques or domestic cats, as these laboratory animals are sequestered from secondary infections in specific pathogen free environments. Elevated liver transaminase (ALT and AST) are indicative of hepatic insult and were observed more frequently in Okavango Delta FIVple-positive lions. The liver histopathology of a subset of those lions was consistent with parasite infection (see Table S3), as were the presence of parasite eggs in some samples. In this case we suspect the liver pathologies may have a primarily environmental component and may be due to a fluke or other parasite restricted to the marsh ecosystem (Okavango Delta). This does not rule out the possibility that, due to lymphocyte depletion, infected Botswana lions would be more susceptible to hepatic viruses or parasites such as schistomiasis, both of which are exacerbated by HIV infection in humans.
This study presents evidence that supports the conclusion that FIVple infection is associated with multiple pathologies seen in chronically infected patients at the pre-AIDS stages of HIV infection. We had previously speculated that FIVple is host-adapted (Brown et al., 1994, Carpenter and O'Brien, 1995, Troyer et al., 2004) and has little influence on lion survival, at least not in the Serengeti ecosystem (Hofmann-Lehmann et al., 1996). However, as with HIV-infected humans, there occurs variability in FIVple progression rates from acute to chronic disease states. Many lions may never progress to advanced symptoms, others may do so only after reproductive age or the natural lifespan of a lion in the wild, which is shorter than that of lions in captivity. Regardless of age, lions that are severely ill die or are killed very quickly in the wild; confounding attempts to document extreme pathologies, since bodies are seldom recovered for necropsies. Finally, the extensive genetic diversity observed among FIV strains, would raise the prospect of potential differences in pathogenicity between different FIVple strains, as is documented among both HIV and FIV strains (O'Brien et al., 2006, Pecon-Slattery et al., 2008a, Troyer et al., 2004, Troyer et al., 2005).
Given the high prevalence of FIVple in many lion populations, it is evident that in several different ecosystems many lions with FIVple have survived and thrived (Antunes et al., 2008, Brown et al., 1994, O'Brien et al., 2006, Olmsted et al., 1992, Troyer et al., 2005). However, in natural settings, small decreases in fitness can have large effects during times of stress. Thus, while FIVple-infected animals may do well under normal circumstances, they may potentially be more sensitive than uninfected animals to secondary assaults, such as new disease outbreaks. In fact, two documented disease outbreaks (TB and CDV) have occurred in lion populations where FIVple is endemic (Ide, 2002, Renwick et al., 2007, Roelke-Parker et al., 1996). We recommend that lions continue to be monitored for FIVple and that the possible correlates to FIVple infection presented here should be examined in other populations. We would also recommend that FIVple-infected animals should not be introduced into naive populations, and that care should be taken to prevent exposure of infected lion populations to domestic animals, which are a major documented source of harmful secondary infections in free-ranging lion populations.
Materials and methods
Sites and study animals
In Botswana, biologic samples were collected from free-ranging African lions radio-collared for monitoring studies from 1999 to 2006. Animals from MKP were captured on two collection trips (Nov. '99 and May '01). A total of 20 individual lions were darted, examined, and sampled. Four lions were captured on both trips allowing re-sampling. Seven collection trips were made to the Delta resulting in 70 separate examinations and samples from a total of 40 individual lions (1–5 samplings/animal). Four Chobe lions examined and sampled in May '01 and archived samples from 8 SNP lions (with one re-sample) collected from 1994 to 1996 were used as study site controls for the two major sites in this study. The majority of lions in all populations were mature adults between the ages of 6 to 9 years old. Cubs (up to 2 years old) were sampled from MKP and the Delta, and some sub-adults and young adults (2–6 years old) were sampled from each population. In MKP we sampled only 1 older adult (9 to 14 years), however in the Delta, we sampled over many years and 14 lions had reached old adult status by the time the study ended (see Fig. S1 for estimated age and age class of lions at each sample date). Samples from all populations were biased towards females to maximize recapture potential (Fig. S1). Within the Delta, 8 FIVple-positive animals were also selected for more extensive examination and surgical and percutaneous biopsies of lymphoid and other tissues. One individual, Ple-1025, was biopsied annually for 3 years for a progressive analysis of tissue pathology. Finally, one trophy-killed FIVple-positive male lion, Ple-1756, was necropsied for tissue collection within 3 h of death.
Anesthesia
Using a dart rifle, telazol and/or ketamine and/or medetomidine was injected into either the hind limb or shoulder muscle. After 10–20 min, the lion heart rate, respiratory rate, temperature, oxygen saturation, and venous blood pressure were monitored. If needed, additional administration of ketamine, or propofol, was administered. Lactated Ringers (1–4 L) was administered either IV or SQ. After approximately 1–2 h, atipamezol reversal was administered (if indicated).
For the surgical biopsies, the lionesses were moved to a mobile surgical unit using a wooden stretcher by all terrain vehicle or by helicopter. The lioness was then intubated and maintained under general gas anesthesia with isoflurane and positive pressure ventilation using a custom adapted large animal anesthetic machine (Mallard) for approximately 2–4 h. Lions were returned to point of capture and observed until appropriate (approximately 2–10 h) and located in the next days to assure they had returned to their pride.
Physical findings
Complete physical examinations of all major systems were conducted by a clinical veterinarian and recorded noting any abnormalities of the external, oral, lymphatic, heart and lung, abdomen, urogenital, musculoskeletal, and neurological systems. During exams, peripheral lymph nodes (LN) including popliteal, caudal abdominal (mammary) and axillary nodes were palpated and evaluated by the attending veterinarian. Nodes were considered abnormal (and animals are herein reported as having lymphadenopathy) if they were enlarged, excessively nodular and/or small, hard, and fibrotic.
Teeth, gums, and tongue were examined. Lions were scored as positive for gingivitis if they had any symptoms including the entire range from minimal linear gingival erythema to granular thickened chronic active inflammation of the alveolar mucosa. Lions were also scored for the presence or absence of papillomas (flat, white, plaque-like lesions), which were frequently observed under the tongue.
Hydration was assessed by observing the speed of skin retraction after a skin-tenting maneuver. Visibly slowed retraction was considered an indication of ≥ 3% dehydration. Hair and coat condition was considered normal if the coat was sleek, shiny and supple. Dry, bristly hair coat or evidence of serous atrophy of subcutaneous fat was scored as abnormal according to level of severity. Muscle condition was also evaluated; normal animals had no ribs showing, vertebrae not readily palpable, and musculature of the back level with or above spinous processes of the back vertebrae. Lack of muscle condition was scored by level of severity.
Sample collection and processing
Field samples (collected from all lions)
Whole blood (WB) was withdrawn from cephalic, lateral or medial (preferred) saphenous, or jugular veins via a 19 g butterfly infusion set with a luer and vacutainer adapters. Eighty to 120 ml of WB was drawn directly into vacutainer tubes containing ethylene-diaminetetraacetic acid (EDTA), sodium heparin, and/or clot activators (SST). After drawing, WB with EDTA and heparin were placed in an insulated, “cool” transport container, while the SST tubes were allowed to clot and retract before being placed in the container. Urine from both male and female lions was obtained by catheterization using sterile polypropylene catheters and aseptic technique. A human, female vaginal speculum and flashlight facilitated visualizing the external urethral meatus.
Laparoscopic surgery and biopsy collection
For eight of the lions from Delta, surgical biopsies of lymph node, liver, spleen, and/or kidney were collected via laparoscopy or percutaneously.
Anesthetized lions were placed in dorsal recumbency and the ventral abdomen was scrubbed with betadine and isopropyl alcohol, using standard asceptic, surgical techniques. The surgeon, surgical assistant, and lion were gowned, gloved, and draped respectively using sterile, disposable materials. Abdominal laparascopy and biopsies were performed using standard, acceptable medical procedures, with the following modifications. All equipment and laparoscopic instruments used were made by Stortz (Germany). Compressed medical grade CO2 was introduced into the abdomen via a 6″ Veress needle to a maximum pressure of 13–15 mmHg (controlled by a Stortz Endoflator). Once sufficient abdominal pressure was achieved, the lions were tipped head down at ∼ 30° to roll the viscera forward and a 11 mm trocar 10.5 cm long with a conical tip was used to penetrate the abdominal wall 3 to 5 cm caudal to the umbilicus and a 10 mm Hopkins 28 cm telescope was introduced. A fiberoptic cable carried light from a 300 W halogen light source to the telescope. A Storz camera system was attached to the eyepiece of the telescope and the image was visualized on a flat video screen (Samsung®). Two additional 5 mm ports were appropriately placed proximal and lateral to the camera port to accommodate instruments in order to biopsy the liver, spleen, right kidney, and cecal lymph nodes (also on the right). Blakesley and Manhes Biopsy forceps with 5 mm cups were used to collect tissue samples of liver, spleen and cecal lymph nodes (approximately 5 mm3 each). The preferred technique for the kidney biopsy was to tilt the animal to the left to expose the right kidney. Tru-cut biopsy needles were used to perform endoscopy guided sampling transcutaneously. When possible, multiple samples were taken from each organ and were placed in neutral buffered formalin and maintained at room temperature. Tissues from the necropsy of lion ple-1756 were treated in a similar way.
Percutaneous biopsies of peripheral lymph nodes (axillary, popliteal, and/or caudal mammary) was performed using either biopsy needles or 18 g needle and 10 cc syringe, depending on the size of the node.
Field blood processing
Samples were processed as soon after collection as was practical, ideally initiated within 8 h and no more than 24 h post draw. Supernatants from clot SST tubes (for serum) and 10 cm3 of urine were collected after centrifugation (2000 RPM for 10 min) and frozen in liquid nitrogen (in the field) for transportation to our laboratory then were stored at − 70 °C until analyzed.
Whole blood with EDTA was used for standard hematological procedures with modifications as indicated. White and red blood cell counts were determined using commercially available blood dilution reservoirs (Unopette, Becton Dickinson and Company, Franklin Lakes, New Jersey, USA), hemo-cytometers, and manual or electronic cell counters. Capillary tubes filled with whole blood were centrifuged for 5 min at 7000 RPM to determine the packed cell volume in % (PCV). The plasma portion of the PCV was used to determine the plasma proteins (gm/dL) utilizing a clinical refractometer (corrected for temperature). Dried blood smears were stained with Dif-Quick and differentials were read manually. Both WB EDTA and heparin were used to evaluate the erythrocyte sedimentation rates (ESR) using the Wintrobe method and 110 mm long glass tubes. The ESR value is derived from the total mm the red cells fell within 60 min.
Fresh urine samples were spun at 1000 RPM for 10 min. The specific gravity of the supernatant was determined with a refractometer, a multi-test urine dipstick was used to look for biochemical abnormalities, and the sediment was examined microscopically.
Laboratory blood analysis
Frozen, thawed serum, well homogenized whole blood (EDTA), and urine were submitted to one of two veterinary analytical laboratories (Antech Diagnostics in New York and the Laboratory Animal Science Program's Pathology/Histotechnology Laboratory, NCI-Frederick, MD) for the determination of serum biochemistries, hemoglobin, and urine creatinine and protein. The detection of antibodies to Toxoplasma gondii, herpesvirus, canine distemper virus, calicivirus, panleukopenia, and feline coronavirus was done by Washington Animal Disease Diagnostics Laboratory on frozen-thawed serum samples collected from 17 lions in 1999.
The presence of FIV antibodies was detected in the field by CITE ComboSNAP kit (IDEXX) and/or in the laboratory by immunoblot (Western blot) assays as described by Troyer et al. (2005). Serum (diluted 1:200), was tested against the viral proteins derived from isolates of domestic cat, puma (Puma concolor), and African lion FIV using a chemiluminescence Western blot. Test results (developed on X-ray film) were scored manually as positive, indeterminate, or negative based on the presence and intensity of antibody binding to the p24 gag capsid protein.
Papillomavirus detection and classification
From the frozen sublingual papilloma excised from Ple-1745, DNA was extracted using a commercially available kit (DNeasy Tissue Kit, Qiagen). PCR primers were designed from Felis domesticus papillomavirus type 1: genbank sequence NC_004765. (Early gene 358 bp: 5′GACACCCTGTATAAATCACGCG3′ and 5′CAGGACTAGCAATATACTTTCGTTTTA3′; Late gene 455 bp: 5′TCTCAAGGCCAAAACAATGG3′ and 5′ CCTCCACCCTGCAACACAT3′). PCRs were performed using approximately 50 ng of genomic DNA in a 50 μL reaction using 50 mM Kcl, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2, 0.25 mM concentrations of dATP, dCTP, dGTP, and dTTP, 2 mM concentrations of each primer, and 2.5 units of Platinum Taq polymerase (Applied Biosystems). PCR was run on a geneAmp PCR system 9700 thermocycler with the following touchdown conditions: 2 min at 95 °C followed by 3 cycles of 20 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C; annealing temperature was then dropped 2 °C every 5 cycles until it reached 50 °C, where it was kept for 22 cycles; followed by a final elongation at 72 °C for 2 min. PCR products were visualized on a 1% agarose gel, primers and unincorporated dNTPs were removed by Microcon YM (Millipore) technology and sequences were obtained using PCR primers in standard ABI BigDye terminator reactions. Nucleotide sequences were compiled and aligned for subsequent phylogenetic analysis by ClustalX (Thompson et al., 1997) and verified visually. Phylogenetic analyses in PAUP (Swofford, 1993) were performed as previously described (Troyer et al., 2005) for the following methods: minimum evolution, maximum parsimony, and maximum likelihood. Modeltest (Posada and Crandall, 1998) was used to estimate the optimal model of sequence evolution, and these settings were incorporated into subsequent analyses.
Histopathological examination of biopsied tissues
Formalin fixed (10% neutral buffered) tissue samples of spleen, lymph nodes, liver, and kidney were embedded in paraffin, cut in 5 μm sections, and routinely stained with hematoxylin-eosin and read by light microscopy. Lymphoid involution was evaluated according to histological criteria published for the domestic cat (Kipar et al., 2001). The severity of lymphoid depletion/animal (LD) was categorized as none, mild, moderate or severe, considering two variables: (a) number and type of lymphoid tissues affected and (b) grade of lymphoid depletion/tissue: normal, mild, moderate and severe (Table 2). Kidney tissues were also stained with PAS (periodic acid Schiff reagent), Masson's trichrome stain, and Congo red to evaluate renal basement membranes, collagen, and amyloid respectively. The pathologists on this study did not have access to any FIV-negative lion tissues to use as species controls, so used normal domestic cat tissue as baseline.
Statistics
Clinical outcomes were compared between FIVple+ and FIVple− lions using Chi-squared analysis. Animals were considered positive for any given outcome if they presented with that finding at any sampling in the study period. This was done since many of the parameters measured are transient, and not all parameters were measured at every sampling. Histological and biochemical parameters were also compared between FIVple+ and FIVple− lions. Because normal values for free-ranging lions have not been established, we determined values for uninfected lions and considered values for any sample to be abnormal if they were ≥ 2 standard deviations above or below the mean of the uninfected individuals.
Wilcoxon rank-sum tests were used to statistically test whether the various biomarkers explored tend to be in the same range in FIVple+ and FIVple− lions, and box plots were constructed to visualize the distributions of biomarkers in each group. Data from the last (oldest) sample date for each animal were included in the analysis with the exception of 7 animals, for which more complete data were available from an earlier sample date (see Fig. S1 for samples used in blood chemistry analysis). A total of 64 animals were included in the final analysis. Poisson regression was used to model the increase in number of secondary infections in FIVple+ lions, compared to FIVple− lions. These statistics were computed in R version 2.8.1 (R Development Core Team, 2008).
Acknowledgments
We would like to thank the following people for their logistic support, field sample collection, and processing: from the Laboratory of Genomic Diversity (NCI), Alison Pearks Wilkerson, Victor David, Ceth Parker, Cate Calson, Valerie Buckley-Beason, Brad Alger, Tyson Stull, and David Wells; in Botswana, Steve Ross, Ruth Kamnitzer, Albert Appelcryn, Nkgopolang Solomon, Pelotshweu P. Galebotswe, Mr. Alone and the staff of Moremi Air, La Roo La Tau, Rann Safari, and Air Botswana. We would also like to thank Dane Hawk, Piero Laricchiuta, Ingrid Steyns, Alan Klide, Joan Klenhaus, and Cindy Anderson for veterinary support in the field; Jan Martinson and Russ Hanson for expert assistance on import and CITES permits; and Richard Montali and Diana Haines for consultation on histopathology. Samples were collected in full compliance with specific federal permits (CITES; Endangered and Threatened Species) issued to the National Cancer Institute, principal investigator S.J. O'Brien, by the U.S. Fish and Wildlife Service of the Department of the Interior. Permission for Botswana sample collection was granted by the Department of Wildlife and National Parks and the Office of the President, Republic of Botswana research permit grant # OP 46/1 XCV (38), issued March 1, 2002. The Messerli Foundation of Switzerland provided funding and support for Serengeti collections. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.virol.2009.04.011.
Appendix A. Supplementary data
Comparison of blood parameters between FIV-negative and FIV-positive lions.
Renal biochemistry values, urine analysis, and kidney histopathology of FIV-infected lions.
Liver pathology and associated biochemical parameters in FIV-infected wild African lions.
Serologic evidence of exposure to select viral and protozoal disease agents in wild African lions with and out FIV infection.
Result of Clinical Physical examination findings, hematology, blood chemistry, and protein electrophoresis of study lions.
References
- R Development Core Team, 2008. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Ackley C.D., Yamamoto J.K., Levy N., Pedersen N.C., Cooper M.D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J. Virol. 1990;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes A., Troyer J.L., Roelke M.E., Pecon-Slattery J., Packer C., Winterbach C., Winterbach H., Hemson G., Frank L., Stander P., Siefert L., Driciru M., Funston P.J., Alexander K.A., Prager K.C., Mills G., Wildt D., Bush M., O'Brien S.J., Johnson W.E. The evolutionary dynamics of lion Panthera leo revealed by host and viral population genomics. PLOS Genetics. 2008;4(11):e1000251. doi: 10.1371/journal.pgen.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J.E., Ackley C.D., George J.W., Levy N., Acevedo R., Moore P.F., Rideout B.A., Cooper M.D., Pedersen N.C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J. Acquir. Immune Defic. Syndr. 1991;4(3):219–227. [PubMed] [Google Scholar]
- Bendinelli M., Pistello M., Lombardi S., Poli A., Garzelli C., Matteucci D., Ceccherini-Nelli L., Malvaldi G., Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clin. Microbiol. Rev. 1995;8(1):87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R., Ruth T.K., Murphy K.M., Anderson C.R., Poss M. Examining effects of persistent retroviral infection on fitness and pathogen susceptibility in a natural feline host. Can. J. Cool-Revue Canadienne De Zoologie. 2006;84(3):365–373. [Google Scholar]
- Board K.F., Patil S., Lebedeva I., Capuano S., 3rd, Trichel A.M., Murphey-Corb M., Rajakumar P.A., Flynn J.L., Haidaris C.G., Norris K.A. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J. Infect. Dis. 2003;187(4):576–588. doi: 10.1086/373997. [DOI] [PubMed] [Google Scholar]
- Bonarek M., Morlat P., Chene G., Rapin D., Hilbert G., Pillet O., Gabinski C. Prognostic score of short-term survival in HIV-infected patients admitted to medical intensive care units. Int. J. STD AIDS. 2001;12(4):239–244. doi: 10.1258/0956462011922995. [DOI] [PubMed] [Google Scholar]
- Brennan G., Podell M.D., Wack R., Kraft S., Troyer J.L., Bielefeldt-Ohmann H., VandeWoude S. Neurologic disease in captive lions (Panthera leo) with low-titer lion lentivirus infection. J. Clin. Microbiol. 2006;44(12):4345–4352. doi: 10.1128/JCM.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.W., Yuhki N., Packer C., O'Brien S.J. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J. Virol. 1994;68(9):5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P.J., Hopper C.D., Harbour D.A. Pathological features of lymphoid tissues in cats with natural feline immunodeficiency virus infection. J. Comp. Pathol. 1991;104(4):345–355. doi: 10.1016/S0021-9975(08)80145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull M.E., Gebhard D.G., Tompkins W.A.F., Kennedy-Stoskopf S. Polymorphic expression in the CD8 alpha chain surface receptor of African lions (Panthera leo) Vet. Immunol. Immunopathol. 2002;84(3–4):181–189. doi: 10.1016/s0165-2427(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Bull M.E., Kennedy-Stoskopf S., Levine J.F., Loomis M., Gebhard D.G., Tompkins W.A. Evaluation of T lymphocytes in captive African lions (Panthera leo) infected with feline immunodeficiency virus. Am. J. Vet. Res. 2003;64(10):1293–1300. doi: 10.2460/ajvr.2003.64.1293. [DOI] [PubMed] [Google Scholar]
- Carpenter M.A., O'Brien S.J. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr. Opin. Genet. Dev. 1995;5(6):739–745. doi: 10.1016/0959-437x(95)80006-q. [DOI] [PubMed] [Google Scholar]
- Coogan M.M., Greenspan J., Challacombe S.J. Oral lesions in infection with human immunodeficiency virus. Bull. World Health Organ. 2005;83(9):700–706. [PMC free article] [PubMed] [Google Scholar]
- De Milito A., Nilsson A., Titanji K., Thorstensson R., Reizenstein E., Narita M., Grutzmeier S., Sonnerborg A., Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103(6):2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- Diehl L.J., Mathiason-Dubard C.K., O'Neil L.L., Obert L.A., Hoover E.A. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 1995;69(10):6149–6157. doi: 10.1128/jvi.69.10.6149-6157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberink H., Borst M., Niphuis H., Balzarini J., Neu H., Schellekens H., De Clercq E., Horzinek M., Koolen M. Suppression of feline immunodeficiency virus infection in vivo by 9-(2-phosphonomethoxyethyl)adenine. Proc. Natl. Acad. Sci. U. S. A. 1990;87(8):3087–3091. doi: 10.1073/pnas.87.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberink H.F., Berrocal A., Bax H.A., van den Ingh T.S., Walter J.H., Horzinek M.C. Papillomavirus associated skin lesions in a cat seropositive for feline immunodeficiency virus. Vet. Microbiol. 1992;31(2–3):117–125. doi: 10.1016/0378-1135(92)90070-a. [DOI] [PubMed] [Google Scholar]
- Eid A.J., Orenstein R. Metabolic and morphologic complications of HIV infection. J. Med. Liban. 2006;54(2):97–105. [PubMed] [Google Scholar]
- Faintuch J., Soeters P.B., Osmo H.G. Nutritional and metabolic abnormalities in pre-AIDS HIV infection. Nutrition. 2006;22(6):683–690. doi: 10.1016/j.nut.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Feldman J.G., Burns D.N., Gange S.J., Bacchetti P., Cohen M., Anastos K., Nowicki M., Delapena R., Miotti P. Serum albumin as a predictor of survival in HIV-infected women in the women's interagency HIV study. AIDS. 2000;14(7):863–870. doi: 10.1097/00002030-200005050-00013. [DOI] [PubMed] [Google Scholar]
- Freeman L.M., Mansfield K.G., Goldin B., Woods M., Gualtieri L., Li W., Bussell S., Lackner A., Gorbach S.L. Body-composition changes in the simian immunodeficiency virus-infected juvenile rhesus macaque. J. Infect. Dis. 2004;189(11):2010–2015. doi: 10.1086/386290. [DOI] [PubMed] [Google Scholar]
- Greenspan D., Greenspan J.S. Oral manifestations of HIV infection. AIDS Clin. Care. 1997;9(4):29–33. [PubMed] [Google Scholar]
- Greenspan J.S., Greenspan D. The epidemiology of the oral lesions of HIV infection in the developed world. Oral Dis. 2002;8(Suppl. 2):34–39. doi: 10.1034/j.1601-0825.2002.00009.x. [DOI] [PubMed] [Google Scholar]
- Hawes S.E., Critchlow C.W., Faye Niang M.A., Diouf M.B., Diop A., Toure P., Aziz Kasse A., Dembele B., Salif Sow P., Coll-Seck A.M., Kuypers J.M., Kiviat N.B. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J. Infect. Dis. 2003;188(4):555–563. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- Hillyer C.D., Klumpp S.A., Hall J.M., Lackey D.A., 3rd, Ansari A.A., McClure H.M. Multifactorial etiology of anemia in SIV-infected rhesus macaques: decreased BFU-E formation, serologic evidence of autoimmune hemolysis, and an exuberant erythropoietin response. J. Med. Primatol. 1993;22(4):253–256. [PubMed] [Google Scholar]
- Hodge S., Novembre F.J., Dewhurst S. Endogenous tumor necrosis factor-alpha contributes to lymphoproliferation induced by simian immunodeficiency virus variant, SIVsmmPBj14. Immunol. Lett. 1998;63(1):49–51. doi: 10.1016/s0165-2478(98)00050-9. [DOI] [PubMed] [Google Scholar]
- Hodgson T.A., Greenspan D., Greenspan J.S. Oral lesions of HIV disease and HAART in industrialized countries. Adv. Dent. Res. 2006;19(1):57–62. doi: 10.1177/154407370601900112. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Fehr D., Grob M., Elgizoli M., Packer C., Martenson J.S., O'Brien S.J., Lutz H. Prevalence of antibodies to feline parvovirus, calicivirus, herpesvirus, coronavirus, and immunodeficiency virus and of feline leukemia virus antigen and the interrelationship of these viral infections in free-ranging lions in east Africa. Clin. Diagn. Lab. Immunol. 1996;3(5):554–562. doi: 10.1128/cdli.3.5.554-562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann-Lehmann R., Holznagel E., Ossent P., Lutz H. Parameters of disease progression in long-term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: hematology, clinical chemistry, and lymphocyte subsets. Clin. Diagn. Lab. Immunol. 1997;4(1):33–42. doi: 10.1128/cdli.4.1.33-42.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson C.A., Rideout B.A., Pedersen N.C. Neoplasia associated with feline immunodeficiency virus infection in cats of southern California. J. Am. Vet. Med. Assoc. 1991;199(10):1357–1362. [PubMed] [Google Scholar]
- Ide A. (2002). University of Pretoria, Pretoria, South Africa.
- Kim A., Dadgostar H., Holland G.N., Wenby R., Yu F., Terry B.G., Meiselman H.J. Hemorheologic abnormalities associated with HIV infection: altered erythrocyte aggregation and deformability. Invest. Ophthalmol. Vis. Sci. 2006;47(9):3927–3932. doi: 10.1167/iovs.06-0137. [DOI] [PubMed] [Google Scholar]
- Kipar A., Kohler K., Leukert W., Reinacher M. A comparison of lymphatic tissues from cats with spontaneous feline infectious peritonitis (FIP), cats with FIP virus infection but no FIP, and cats with no infection. J. Comp. Pathol. 2001;125(2–3):182–191. doi: 10.1053/jcpa.2001.0501. [DOI] [PubMed] [Google Scholar]
- Kohmoto M., Uetsuka K., Ikeda Y., Inoshima Y., Shimojima M., Sato E., Inada G., Toyosaki T., Miyazawa T., Doi K., Mikami T. Eight-year observation and comparative study of specific pathogen-free cats experimentally infected with feline immunodeficiency virus (FIV) subtypes A and B: terminal acquired immunodeficiency syndrome in a cat infected with FIV Petaluma strain. J. Vet. Med. Sci. 1998;60(3):315–321. doi: 10.1292/jvms.60.315. [DOI] [PubMed] [Google Scholar]
- Kotler D.P., Gaetz H.P., Lange M., Klein E.B., Holt P.R. Enteropathy associated with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1984;101(4):421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- Lifson A.R., Allen S., Wolf W., Serufilira A., Kantarama G., Lindan C.P., Hudes E.S., Nsengumuremyi F., Taelman H., Batungwanayo J. Classification of HIV infection and disease in women from Rwanda. Evaluation of the World Health Organization HIV staging system and recommended modifications. Ann. Intern. Med. 1995;122(4):262–270. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- Matsumura S., Ishida T., Washizu T., Tomoda I., Nagata S., Chiba J., Kurata T. Pathologic features of acquired immunodeficiency-like syndrome in cats experimentally infected with feline immunodeficiency virus. J. Vet. Med. Sci. 1993;55(3):387–394. doi: 10.1292/jvms.55.387. [DOI] [PubMed] [Google Scholar]
- McClure H.M., Anderson D.C., Fultz P.N., Ansari A.A., Lockwood E., Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet. Immunol. Immunopathol. 1989;21(1):13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- Mocroft A., Kirk O., Barton S.E., Dietrich M., Proenca R., Colebunders R., Pradier C., Darminio Monforte A., Ledergerber B., Lundgren J.D. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- Morley J.E., Thomas D.R., Wilson M.M. Cachexia: pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006;83(4):735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- Nagase H., Agematsu K., Kitano K., Takamoto M., Okubo Y., Komiyama A., Sugane K. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin. Immunol. 2001;100(2):250–259. doi: 10.1006/clim.2001.5054. [DOI] [PubMed] [Google Scholar]
- Novotney C., English R.V., Housman J., Davidson M.G., Nasisse M.P., Jeng C.R., Davis W.C., Tompkins M.B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990;4(12):1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- O'Brien S.J., Troyer J.L., Roelke M., Marker L., Pecon-Slattery J. Plagues and adaptation: lessons from the Felidae models for SARS and AIDS. Biol. Conserv. 2006;131(2):255–267. doi: 10.1016/j.biocon.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R.A., Langley R., Roelke M.E., Goeken R.M., Adger-Johnson D., Goff J.P., Albert J.P., Packer C., Laurenson M.K., Caro T.M. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J. Virol. 1992;66(10):6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osofsky S.A., HIrsch K.J., Zuckerman E.E., Hardy W.D. Feline lentivirus and feline oncovirus status of free-ranging lions (Panthera leo), leopards (Panthera pardus), and cheetahs (Acinonyx jubatus) in Botswana: A regional perspective. Journal of Zoo and Wildlife Medicine. 1996;27(4):453–467. [Google Scholar]
- Packer C., Altizer S., Appel M., Brown E., Martenson J., O'Brien S.J., Roelke-Parker M., Hofmann-Lehmann R., Lutz H. Viruses of the Serengeti: patterns of infection and mortality in African lions. J. Anim. Ecol. 1999;68(6):1161–1178. [Google Scholar]
- Pandrea I., Apetrei C., Dufour J., Dillon N., Barbercheck J., Metzger M., Jacquelin B., Bohm R., Marx P.A., Barre-Sinoussi F., Hirsch M., Muller-Trutwin M.C., Lackner A.A., Veazey R.S. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 2006;80(10):4858–4867. doi: 10.1128/JVI.80.10.4858-4867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I., Apetrei C., Gordon S., Barbercheck J., Dufour J., Bohm R., Sumpter B., Roques P., Marx P.A., Hirsch M., Kaur A., Lackner A.A., Veazey R.S., Silvestri G. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109(3):1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantanowitz L., Schlecht H.P., Dezube B.J. The growing problem of non-AIDS-defining malignancies in HIV. Curr. Opin. Oncol. 2006;18(5):469–478. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- Pecon-Slattery J., McCracken C.L., Troyer J.L., VandeWoude S., Roelke M., Sondgeroth K., Winterbach C., Winterbach H., O'Brien S.J. Genomic organization, sequence divergence, and recombination of feline immunodeficiency virus from lions in the wild. BMC Genomics. 2008;9:66. doi: 10.1186/1471-2164-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecon-Slattery J., Troyer J.L., Johnson W.E., O'Brien S.J. Evolution of feline immunodeficiency virus in Felidae: implications for human health and wildlife ecology. Vet. Immunol. Immunopathol. 2008;123(1–2):32–44. doi: 10.1016/j.vetimm.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N.C., Ho E.W., Brown M.L., Yamamoto J.K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Podell M., Chen E., Shelton G.D. Feline immunodeficiency virus associated myopathy in the adult cat. Muscle Nerve. 1998;21(12):1680–1685. doi: 10.1002/(sici)1097-4598(199812)21:12<1680::aid-mus9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Posada D., Crandall K.A. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Prospero-Garcia O., Gold L.H., Fox H.S., Polis I., Koob G.F., Bloom F.E., Henriksen S.J. Microglia-passaged simian immunodeficiency virus induces neurophysiological abnormalities in monkeys. Proc. Natl. Acad. Sci. U. S. A. 1996;93(24):14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano G., Siminovich M., Drut R. Histopathologic findings in the lymphoid and reticuloendothelial system in pediatric HIV infection: a postmortem study. Pediatr. Pathol. Lab. Med. 1997;17(6):845–856. [PubMed] [Google Scholar]
- Rector A., Lemey P., Tachezy R., Mostmans S., Ghim S.J., Van Doorslaer K., Roelke M., Bush M., Montali R.J., Joslin J., Burk R.D., Jenson A.B., Sundberg J.P., Shapiro B., Van Ranst M. Ancient papillomavirus-host co-speciation in Felidae. Genome Biol. 2007;8(4):R57. doi: 10.1186/gb-2007-8-4-r57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. Control of HIV/AIDS and AIDS-related conditions in Africa with special reference to periodontal diseases. J. Int. Acad. Periodontol. 2007;9(1):2–12. [PubMed] [Google Scholar]
- Renwick A.R., White P.C., Bengis R.G. Bovine tuberculosis in southern African wildlife: a multi-species host-pathogen system. Epidemiol. Infect. 2007;135(4):529–540. doi: 10.1017/S0950268806007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik D.A. Oral manifestations of HIV disease. Top. HIV Med. 2005;13(5):143–148. [PubMed] [Google Scholar]
- Roelke M.E., Pecon-Slattery J., Taylor S., Citino S., Brown E., Packer C., Vandewoude S., O'Brien S.J. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J. Wildl. Dis. 2006;42(2):234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O'Brien S.J., Pospischil A., Hofmann-Lehmann R., Lutz H. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379(6564):441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin C.A., Griffioen A., Yee T.T., Emery C., Herrero-Martinez E., Phillips A.N., Lee C.A. Markers of HIV-1 disease progression in individuals with haemophilia coinfected with hepatitis C virus: a longitudinal study. Lancet. 2002;360(9345):1546–1551. doi: 10.1016/S0140-6736(02)11519-4. [DOI] [PubMed] [Google Scholar]
- Schwartlander B., Bek B., Skarabis H., Koch J., Burkowitz J., Koch M.A. Improvement of the predictive value of CD4+ lymphocyte count by beta 2-microglobulin, immunoglobulin A and erythrocyte sedimentation rate. The Multicentre Cohort Study Group. AIDS. 1993;7(6):813–821. [PubMed] [Google Scholar]
- Selwyn P.A., Rivard M. Palliative care for AIDS: challenges and opportunities in the era of highly active anti-retroviral therapy. J. Palliat. Med. 2003;6(3):475–487. doi: 10.1089/109662103322144853. [DOI] [PubMed] [Google Scholar]
- Shah S., Smith C.J., Lampe F., Youle M., Johnson M.A., Phillips A.N., Sabin C.A. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Med. 2007;8(1):38–45. doi: 10.1111/j.1468-1293.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- Sparkes A.H., Hopper C.D., Millard W.G., Gruffydd-Jones T.J., Harbour D.A. Feline immunodeficiency virus infection. Clinicopathologic findings in 90 naturally occurring cases. J. Vet. Intern. Med. 1993;7(2):85–90. doi: 10.1111/j.1939-1676.1993.tb03174.x. [DOI] [PubMed] [Google Scholar]
- Sullivan D.H., Roberson P.K., Bopp M.M. Hypoalbuminemia 3 months after hospital discharge: significance for long-term survival. J. Am. Geriatr. Soc. 2005;53(7):1222–1226. doi: 10.1111/j.1532-5415.2005.53369.x. [DOI] [PubMed] [Google Scholar]
- Sundberg J.P., Van Ranst M., Montali R., Homer B.L., Miller W.H., Rowland P.H., Scott D.W., England J.J., Dunstan R.W., Mikaelian I., Jenson A.B. Feline papillomas and papillomaviruses. Vet. Pathol. 2000;37(1):1–10. doi: 10.1354/vp.37-1-1. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Paup — a computer-program for phylogenetic inference using maximum parsimony. J. Gen. Physiol. 1993;102(6):A9. [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins M.B., Nelson P.D., English R.V., Novotney C. Early events in the immunopathogenesis of feline retrovirus infections. J. Am. Vet. Med. Assoc. 1991;199(10):1311–1315. [PubMed] [Google Scholar]
- Torten M., Franchini M., Barlough J.E., George J.W., Mozes E., Lutz H., Pedersen N.C. Progressive immune dysfunction in cats experimentally infected with feline immunodeficiency virus. J. Virol. 1991;65(5):2225–2230. doi: 10.1128/jvi.65.5.2225-2230.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer J.L., Pecon-Slattery J., Roelke M.E., Black L., Packer C., O'Brien S.J. Patterns of feline immunodeficiency virus multiple infection and genome divergence in a free-ranging population of African lions. J. Virol. 2004;78(7):3777–3791. doi: 10.1128/JVI.78.7.3777-3791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer J.L., Pecon-Slattery J., Roelke M.E., Johnson W., VandeWoude S., Vazquez-Salat N., Brown M., Frank L., Woodroffe R., Winterbach C., Winterbach H., Hemson G., Bush M., Alexander K.A., Revilla E., O'Brien S.J. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J. Virol. 2005;79(13):8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S., Apetrei C. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 2006;19(4):728. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S., Hageman C.A., O'Brien S.J., Hoover E.A. Nonpathogenic lion and puma lentiviruses impart resistance to superinfection by virulent feline immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 2002;29(1):1–10. doi: 10.1097/00126334-200201010-00001. [DOI] [PubMed] [Google Scholar]
- VandeWoude S., O'Brien S.J., Langelier K., Hardy W.D., Slattery J.P., Zuckerman E.E., Hoover E.A. Growth of lion and puma lentiviruses in domestic cat cells and comparisons with FIV. Virology. 1997;233(1):185–192. doi: 10.1006/viro.1997.8587. [DOI] [PubMed] [Google Scholar]
- Varbanov M., Espert L., Biard-Piechaczyk M. Mechanisms of CD4 T-cell depletion triggered by HIV-1 viral proteins. AIDS Rev. 2006;8(4):221–236. [PubMed] [Google Scholar]
- Wang M.C., Tsai T.L., Liu C.Y., Shu C.H., Lin C.Z. Nasopharyngeal lymphoid hyperplasia of an HIV carrier, mimicking nasopharyngeal cancer. J. Chin. Med. Assoc. 2003;66(3):189–191. [PubMed] [Google Scholar]
- Willett B.J., Flynn J.N., Hosie M.J. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today. 1997;18(4):182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- Willett B.J., Hosie M.J., Callanan J.J., Neil J.C., Jarrett O. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Wong S.W., Bergquam E.P., Swanson R.M., Lee F.W., Shiigi S.M., Avery N.A., Fanton J.W., Axthelm M.K. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J. Exp. Med. 1999;190(6):827–840. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman C.B., Collins S.I., Young L.S. The natural history of cervical HPV infection: unresolved issues. Nat. Rev. Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- Yamamoto J.K., Hansen H., Ho E.W., Morishita T.Y., Okuda T., Sawa T.R., Nakamura R.M., Pedersen N.C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 1989;194(2):213–220. [PubMed] [Google Scholar]
- Yanai T., Lackner A.A., Sakai H., Masegi T., Simon M.A. Systemic arteriopathy in SIV-infected rhesus macaques (Macaca mulatta) J. Med. Primatol. 2006;35(2):106–112. doi: 10.1111/j.1600-0684.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of blood parameters between FIV-negative and FIV-positive lions.
Renal biochemistry values, urine analysis, and kidney histopathology of FIV-infected lions.
Liver pathology and associated biochemical parameters in FIV-infected wild African lions.
Serologic evidence of exposure to select viral and protozoal disease agents in wild African lions with and out FIV infection.
Result of Clinical Physical examination findings, hematology, blood chemistry, and protein electrophoresis of study lions.