Abstract
In animals, multisystemic eosinophilic disease is a rare condition characterized by eosinophilic and lymphoplasmacytic infiltrates in various organs. This disorder resembles the human disease known as hypereosinophilic syndrome, a condition defined by prolonged peripheral eosinophilia in the absence of recognizable etiology and associated with end-organ damage. In this report we describe a research-naïve, colony-born, juvenile female owl monkey (Aotus vociferans) who presented clinically with severe respiratory distress and histologically with multiple end-organ infiltration with phenotypically mature eosinophils, plasma cells, and lymphocytes. No tumors or infectious agents were noted either macroscopically or microscopically. Cultures from lung samples revealed no bacteria or fungi. Histologic examination of lung, heart, thymus, liver, spleen, kidney, adrenal, pancreas, stomach, small intestine, and colon revealed no migrating nematode larvae, other parasites, or foreign material that might trigger eosinophilia, nor was there any evidence of or history consistent with an allergic etiology. Given that we ruled out most exogenous and endogenous triggers of eosinophilia, the signs, symptoms, and pathologic findings support the diagnosis of multisystemic eosinophilic disease. To our knowledge, this report is the first description of presumptive hypereosinophilic syndrome in a nonhuman primate.
Abbreviation: HES, hypereosinophilic syndrome
Multisystemic eosinophilic disease is a rare condition characterized by eosinophilic and lymphoplasmacytic infiltrates in multiple organs.19 The clinical signs vary according to the organs affected.19 This disorder resembles the human disease known as hypereosinophilic syndrome (HES), a condition defined by prolonged peripheral eosinophilia (greater than 1.5 × 109/L for more than 6 mo) in the absence of recognizable etiology and associated with end-organ damage.11,15,18 Clinically this disease has been a diagnosis of exclusion, but because blood and tissue eosinophilia typically are detected in association with Th2 cytokines elicited in response to parasitic, allergic, and hypersensitivity disorders, the recent identification of the hematopoeitic stem cell microdeletion in a subset of HES patients has aided in both diagnosis and in selecting therapeutic options.5,14 This gene rearrangement has not yet been reported in animals in association with multisystemic eosinophilic disease–hypereosinophilic syndrome, which remains a diagnosis of exclusion.19
Here we describe an owl monkey (Aotus vociferans) with multisystemic tissue eosinophilia resembling hypereosinophilic syndrome. To our knowledge, this report is the first description of presumptive hypereosinophilic syndrome in a nonhuman primate.
Case Report
The Center for Reproduction and Conservation of Nonhuman Primates is located close to Iquitos in the tropical rain forest of Peru. Briefly, colony animals were housed as breeding pairs with their offspring in 2 m (height) × 1 m (depth) × 1 m (width) cages, fed an in-house prepared biscuit, and kept in a mosquito-proof building screened with wire mesh under natural temperature and photoperiod conditions. All animal procedures followed the institutional guidelines of Universidad Nacional Mayor de San Marcos on animal care and use. The Center for Reproduction and Conservation of Nonhuman Primates is a breeding colony, and no experimental manipulation is performed on the animals. Quarantine procedures for newly arrived monkeys included a 30-d quarantine, during which animals received a physical examination; were weighed, tattooed, tuberculin-tested, and dewormed with thiabendazole; and underwent parasitologic examinations by direct fecal exams and fecal flotation methods. Colony animals were examined quarterly, when they received a physical exam and fecal parasitologic tests. If diarrhea was noted, fecal samples were collected and cultured for pathogenic enterobacteria and the animal treated accordingly.
A research-naïve, colony-born, 19-mo-old juvenile female owl monkey (Aotus vociferans) presented with respiratory distress. Severe increased inspiratory effort on auscultation was accompanied by mucosal cyanosis. The clinical condition declined rapidly, so the decision was made to euthanize the monkey without pursuing further clinical tests and to perform a necropsy. At necropsy, the animal's weight was 868 g, which was appropriate for age and gender, and body condition was good. A foamy clear discharge was observed at the nares, and similar foamy clear discharge was noted when the trachea was exposed. Lungs were diffusely congested, and on cut section, a clear foamy discharge in the bronchi was noted. All other organs appeared normal on gross examination. A preliminary diagnosis was made of acute lobar pneumonia. Tissue samples from lungs and major organs were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin for light microscopy examination. In addition, samples taken from the lungs were cultured in blood agar, nutrient agar, McConkey agar, and Saboureaud agar under aerophilic conditions, incubated at 37 °C, and evaluated at 24, 48, 72, 96, and 120 h. Colonies were identified by using routine differential biochemical media.
Histologically, the lungs were diffusely congested, and small areas with increased numbers of alveolar macrophages containing cellular debris were present. Small aggregates of mature eosinophils, plasma cells, and lymphocytes in association with bronchi were noted (Figure 1). In addition, perivascular and peribronchial edema was found (Figure 2). Moderate to large numbers of mature eosinophils, plasma cells, and lymphocytes were diffusely scattered in the submucosa, lamina propria, and mucosa of the stomach and small and large intestines (Figure 3). The region of the pancreas adjacent to the duodenum showed extensive coagulative necrosis (Figure 4). The kidneys showed trace focal mononuclear cell infiltration and small numbers of mature eosinophils lining the papilla and, in some areas, infiltrating the cortex and glomeruli (Figures 5 and 6). Acute, mild, multifocal, renal tubular epithelial necrosis characterized by loss of cellular detail, cytoplasmic vacuolation, and pycnotic nuclei was present also. Several tissues contained eosinophils undergoing degranulation (Figure 7). The spleen had slight myeloid hyperplasia composed of increased numbers of cells diffusely distributed in the red pulp. Occasionally mature eosinophils were present in the lumen of large blood vessels. No evidence of parasitic, bacterial, fungal, viral, or inorganic material was noted grossly at necropsy or microscopically during histologic examination of lungs, gastrointestinal tract, and kidneys. No bacterial or fungal organisms were isolated from the lung samples submitted for microbiologic examination.
Figure 1.
Aotus vociferans. Lung. Bronchus. Small aggregates of mature eosinophils, plasma cells, and lymphocytes are present between the cartilage and muscularis. Hematoxylin and eosin stain; magnification, ×1000.
Figure 2.
Aotus vociferans. Lung. Marked perivascular and peribronchial edema (asterisk) and peribronchial cellular infiltrate (arrows). Hematoxylin and eosin stain; magnification, ×40.
Figure 3.
Aotus vociferans. Small intestine. Large numbers of mature eosinophils, plasma cells, and lymphocytes diffusely scattered in the submucosa, lamina propria, and mucosa. Hematoxylin and eosin stain; magnification, ×400.
Figure 4.
Aotus vociferans. Pancreas. Region of the pancreas adjacent to the duodenum showing extensive coagulative necrosis. Hematoxylin and eosin stain; magnification, ×40.
Figure 5.
Aotus vociferans. Kidney. Mature eosinophils infiltrating the corticomedullary junction. Note the renal tubular epithelial necrosis and protein deposits in the Bowman capsule space. Hematoxylin and eosin stain; magnification, ×400.
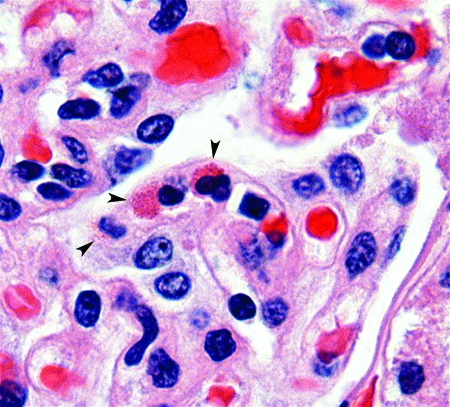
Figure 6.
Aotus vociferans. Kidney. Mature eosinophils in glomerulus (arrowheads). Hematoxylin and eosin stain; magnification, ×1000.
Figure 7.
Aotus vociferans. Small intestine. Lamina propria. Eosinophils undergoing degranulation (arrow) next to a crypt (arrowhead). Hematoxylin and eosin stain; magnification, ×1500.
Discussion
Multisystemic eosinophilic disease has been identified in humans,17 dogs,2,20 cats,8,12,13,21 ferrets,3 and horses.19 In animals, the diagnosis can be complicated because multiple conditions can be accompanied by eosinophilia, and clinical histories are not always easy to obtain. In humans, traditionally the diagnosis of HES has required that 3 conditions be met. First, the subject must have at least 6 mo of sustained blood eosinophilia. Second, all other known etiologies for eosinophilia must be ruled out, and third, signs and symptoms of end-organ involvement must be present.17 Our improved understanding of the molecular basis of human HES has permitted these criteria to be appreciated within a larger context. We now understand HES as a heterogeneous group of disorders, some of which are better understood than others. The most clearly defined are those that arise from rearrangements in the gene for platelet-derived growth factor receptor α, in which a microdeletion on chromosome 4q12 in a hematopoietic stem cell results in fusion of this receptor with the sequence of FIP1L1. Persons with this form of HES respond readily to the tyrosine-kinase inhibitor imatinib, in contrast to patients with the more poorly defined clonal T cell disorders, whose eosinophilia is secondary to overproduction of the Th2 cytokine IL5—this patient subset responds more readily to antiIL5 therapy.11,15,18 These subsets of HES have not yet been defined or explored in nonhuman animal species.
When recruited into tissues, eosinophils release the secretory mediators stored in specific granules and activate NADPH oxidase; the release of oxygen metabolites and cationic proteins from eosinophils may be partially or even wholly responsible for the tissue damage observed in hypereosinophilic syndrome.9 Interestingly, although the owl monkey eosinophil is morphologically indistinguishable from that of its human counterpart, 2 of the primary cationic secretory mediators of eosinophils, eosinophil cationic protein and eosinophil-derived neurotoxin, have undergone substantial Darwinian evolution (dN/dS > 1) within primate species; the owl monkey genome encodes only eosinophil-derived neurotoxin.16,23
In the present case, no tumors or infectious agents were noted either macroscopically or microscopically. The unexplained hyperproliferation of a single cell type always raises suspicion of neoplasia, but no promyelocytes or atypical eosinophils were observed during histologic examination of blood vessels, as would be expected if this animal had eosinophilic leukemia.7 However, a bone marrow sample is necessary to confirm the absence of neoplastic disease. Cultures from lung samples revealed no bacteria or fungi. In addition, histologic examination of lung and major organs did not reveal migrating nematode larvae or other parasites or foreign material that might trigger eosinophilia. The animal had not undergone grafts or transplants or been treated with drugs, permitting us to rule out graft-versus-host and drug-induced diseases resulting in eosinophilia. Because we observed no lesions consistent with systemic sclerosis and biliary cirrhosis, an autoimmune etiology is likewise unlikely.6,22 Allergic diseases such as rhinoconjunctivitis, atopic dermatitis, esophagitis, and colitis were not observed on gross specimen or microscopically and are inconsistent with systemic disease of such severity.9,10 Although no new food items were introduced, we were unable to rule out food allergy completely as the cause of the eosinophilia. However, this case would be an unusual picture of food allergy, because the diet provided to this animal has been used in this large nonhuman primate colony for more than 10 y with no signs of intolerance in owl monkeys. Similarly, although severe respiratory distress was noted in this animal and prompted its euthanasia, bronchial asthma was ruled out after microscopic examination because eosinophils were present in other organs in addition to the lungs, a disease picture that is likewise inconsistent with asthma alone.
Bolivian owl monkeys (Aotus azarae boliviensis) with peripheral blood eosinophilia (10-fold increased compared with other Aotus species) and increased bone marrow eosinophilopoiesis (increased 5 times that in other Aotus species) have been described, but, in contrast to the present report, no infiltration of tissues or evidence of end-organ damage has been reported.1 It would be intriguing to compare and contrast functional parameters, such as degranulation and serum cytokine levels, to determine why peripheral eosinophilia is readily tolerated in one Aotus species whereas organ damage clearly results in another. Recently, eosinophilic bronchitis in a colony-born macaque (Macaca mulatta), which died of severe respiratory distress unresponsive to therapy, was reported.4 However, in that case, eosinophils infiltrated the lungs only and were not present in other organs.
The owl monkey we describe presented and was evaluated in 1989, before the recent advances made toward understanding the genetic basis of human HES, and we do not have access to peripheral blood cells, serum, or bone marrow aspirates which might provide a definitive diagnosis. Regardless, as mentioned earlier, HES in animals remains a diagnosis of exclusion, and in our case, the evidence collected from this owl monkey with multiple end-organ infiltration with phenotypically mature peripheral eosinophils supports the diagnosis of multisystemic eosinophilic disease resembling HES. To our knowledge, this report is the first description of presumptive hypereosinophilic syndrome in a nonhuman primate.
Acknowledgments
This study was conducted as part of the activities of the Peruvian Primatological Project, supported by the Peruvian Government and the Pan American Health Organization (ICF/ZNS/010), and by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIAID), Comparative Medicine Branch, the Office of Research Support, and a NIAID contract to SoBran. We thank Dr Carmen Michaud for photographic assistance.
References
- 1.Albert DL, Brodie SJ, Sasseville VG, Ringler DJ. 1996. Peripheral blood eosinophilia in owl monkeys is associated with increased eosinophilopoeisis yet depressed recruitment kinetics to the Chemokine RANTES. Blood 88:1718–1724 [PubMed] [Google Scholar]
- 2.Aroch I, Perl S, Markovics A. 2001. Disseminated eosinophilic disease resembling idiopathic hypereosinophilic syndrome in a dog. Vet Rec 149:386–389 [DOI] [PubMed] [Google Scholar]
- 3.Blomme EA, Foy SH, Chappell KH, La Perle KM. 1999. Hypereosinophilic syndrome with Hodgkin's-like lymphoma in a ferret. J Comp Pathol 120:211–217 [DOI] [PubMed] [Google Scholar]
- 4.Christal JL, Hubbard GB, Dick EJ, Jr, Brasky KM, Jagirdar J. 2008. Eosinophilic bronchitis-like lesion as the cause of death in a Macaca mulatta: a first case report. J Med Primatol 37:63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, Cross NC, Tefferi A, Malone J, Alam R, Schrier SL, Schmid J, Rose M, Vandenberghe P, Verhoef G, Boogaerts M, Wlodarska I, Kantarjian H, Marynen P, Coutre SE, Stone R, Gilliland DG. 2003. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 348:1201–1214 [DOI] [PubMed] [Google Scholar]
- 6.Cox D, Earle L, Jimenez SA, Leiferman KM, Gleich GJ, Varga J. 1995. Elevated levels of eosinophil major basic protein in the sera of patients with systemic sclerosis. Arthritis Rheum 38:939–945 [DOI] [PubMed] [Google Scholar]
- 7.Gotlib J. 2008. Chronic eosinophilic leukemia–hypereosinophilic syndrome. Cancer Treat Res 142:69–106 [PubMed] [Google Scholar]
- 8.Hendrick M. 1981. A spectrum of hypereosinophilic syndromes exemplified by 6 cats with eosinophilic enteritis. Vet Pathol 18:188–200 [DOI] [PubMed] [Google Scholar]
- 9.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. 2008. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 38:709–750 [DOI] [PubMed] [Google Scholar]
- 10.Hogan SP, Rothenberg ME. 2006. Eosinophil Function in Eosinophil-associated Gastrointestinal Disorders. Curr Allergy Asthma Rep 6:65–71 [DOI] [PubMed] [Google Scholar]
- 11.Klion AD. 2007. Approach to the therapy of hypereosinophilic syndromes. Immunol Allergy Clin North Am 27:551–560 [DOI] [PubMed] [Google Scholar]
- 12.McEwen SA, Valli VE, Hulland TJ. 1985. Hypereosinophilic syndrome in cats: a report of 3 cases. Can J Comp Med 49:248–253 [PMC free article] [PubMed] [Google Scholar]
- 13.Muir P, Gruffydd-Jones TJ, Brown PJ. 1993. Hypereosinophilic syndrome in a cat. Vet Rec 132:358–359 [DOI] [PubMed] [Google Scholar]
- 14.Pardanani A, Ketterling RP, Li CY, Patnaik MM, Wolanskyj AP, Elliott MA, Camoriano JK, Butterfield JH, Dewald GW, Tefferi A. 2006. FIP1L1–PDGFRA in eosinophilic disorders: prevalence in routine clinical practice, long-term experience with imatinib therapy, and a critical review of the literature. Leuk Res 30:965–970 [DOI] [PubMed] [Google Scholar]
- 15.Peros-Golubicic T, Smojver-Jezek S. 2007. Hypereosinophilic syndrome: diagnosis and treatment. Curr Opin Pulm Med 13:422–427 [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg HF, Dyer KD, Tiffany HL, Gonzalez M. 1995. Rapid evolution of a unique family of primate ribonuclease genes. Nat Genet 10:219–223 [DOI] [PubMed] [Google Scholar]
- 17.Simon D, Simon HU. 2007. Eosinophilic disorders. J Allergy Clin Immunol 119:1291–1300 [DOI] [PubMed] [Google Scholar]
- 18.Simon HU, Cools J. 2007. Novel approaches to therapy of hypereosinophilic syndromes. Immunol Allergy Clin North Am 27:519–527 [DOI] [PubMed] [Google Scholar]
- 19.Singh K, Holbrook TC, Gilliam LL, Cruz RJ, Duffy J, Confer AW. 2006. Severe pulmonary disease due to multisystemic eosinophilic epitheliotropic disease in a horse. Vet Pathol 43:189–193 [DOI] [PubMed] [Google Scholar]
- 20.Sykes JE, Weiss DJ, Buoen LC, Blauvelt MM, Hayden DW. 2001. Idiopathic hypereosinophilic syndrome in 3 Rottweilers. J Vet Intern Med 15:162–166 [DOI] [PubMed] [Google Scholar]
- 21.Wilson SC, Thomson-Kerr K, Houston DM. 1996. Hypereosinophilic syndrome in a cat. Can Vet J 37:679–680 [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki K, Nakadate I, Suzuki K, Sato S, Masuda T. 1996. Eosinophilia in primary biliary cirrhosis. Am J Gastroenterol 91:516–522 [PubMed] [Google Scholar]
- 23.Zhang J, Rosenberg HF. 2002. Complementary advantageous substitutions in the evolution of an antiviral RNase of higher primates. Proc Natl Acad Sci USA 99:5486–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]