Abstract
Somatostatin (SS) released by hypothalamic neurons inhibits GH exocytosis noncompetitively. Therefore, we postulated that attenuation of GH feedback-induced SS outflow would help to unmask covariates of endogenous secretagogue drive. To this end, 42 healthy pre- and postmenopausal women were randomly assigned to receive leuprolide plus estradiol (E2) or leuprolide plus placebo. A putatively low-SS milieu was imposed by l-arginine infusion. Deconvolution and regularity analyses were applied to 6-h GH concentration-time profiles. By two-way ANOVA, age negatively (P < 0.001) and E2 positively (P = 0.001) determined pulsatile GH secretion in the presumptively SS-deficient milieu (P < 0.001). Comparable effects were exerted on the mass of GH secreted per burst per unit distribution volume (age P = 0.001, E2 P < 0.001, overall P < 0.001). E2 alone predicted basal (nonpulsatile) GH secretion (P = 0.004). Stepwise forward-selection multivariate regression demonstrated that age (P = 0.0017) and E2 (P = 0.0002) together explained 46% of intersubject variability in pulsatile GH secretion (P < 0.001) and fully replaced the negative univariate effect of abdominal visceral fat (r2 = 0.32, P < 0.001). Moreover, age and E2 (but not AVF) interacted to supervise GH regularity (P = 0.007). We conclude that age and E2 availability individually and together constitute primary predictors of basal, pulsatile, and patterned GH secretion in an inferentially feedback-silenced context in healthy women. Therefore, both factors must be considered in framing hypotheses of endogenous GH drive.
Keywords: growth hormone, somatotropin, insulin-like growth factor I, female, pituitary
intravenous injection of a pulse of growth hormone (GH) exerts reversible negative feedback by evoking hypothalamic somatostatin (SS) outflow (1, 7, 37). SS acts by noncompetitively blocking exocytosis of GH secretory vesicles from somatotrope cells (29, 40). Hypothalamic SS release varies markedly on a short time scale in the rat, sheep, and pig (27, 35, 38, 39, 43). Fluctuations in SS outflow seem necessary for the generation of large GH pulses (21, 24, 33) but at the same time may confound acute GH responses to exogenous secretagogues such as GH-releasing hormone (GHRH) and ghrelin (a GH-releasing peptide) (8, 32, 34).
One direct SS receptor antagonist was tested in the rat (4), but none is available for use in humans. However, infusion of l-arginine abolishes or significantly attenuates GH-induced as well as IGF-I-induced feedback inhibition of GHRH stimulation in humans (3, 15, 16). The capability of l-arginine to relieve negative feedback is prima facie evidence of a concomitant reduction in endogenous SS release or action (17, 48). Indeed, GH autofeedback is also muted 1) in SS receptor subtype 2 knockout animals (52), 2) after immunoneutralization of SS (22, 26), 3) when electrolytic lesions are placed to isolate the mediobasal hypothalamus from periventricular SS-ergic inflow (3, 15, 16, 48), and 4) when the GH receptor is mutated or genetically downregulated in the brain (48). Although the exact biochemical mediator of the silencing action of l-arginine on GH feedback remains unknown, infusion of a maximally effective amount of this amino acid evokes GH secretion and potentiates stimulation by GHRH and ghrelin (42, 45). Inasmuch as GHRH and ghrelin constitute two major stimulatory regulators of GH secretion (17, 48), exposure to l-arginine should provide an indirect means to assess factors that determine endogenous GHRH and ghrelin drive (secretion and action).
When GH autofeedback is intact, age, estrogen availability, and relative adiposity [defined by body mass index (BMI) or abdominal visceral fat (AVF)] determine pulsatile GH secretion in a complex manner (17, 48). For example, estradiol (E2) amplifies the submaximally stimulatory effects (potencies) of GHRH and ghrelin and diminishes the inhibitory potency of exogenous SS (5, 42, 45). Conversely, increased age and BMI/AVF reduce GH responses to most secretagogues. The extent to which the effects of age, estrogen, and adiposity are mediated via altered outflow of endogenous secretagogues compared with SS remains unclear. To address this point, the present study utilizes a triple paradigm comprising 1) infusion of l-arginine to presumptively restrict GH- and IGF-I feedback-induced SS outflow, 2) evaluation of young [premenopausal (PRE)] and older [postmenopausal (POST)] women to appraise age-related effects, and 3) administration of a gonadotropin-releasing hormone receptor agonist to downregulate the pituitary-ovarian axis before back placebo or a late follicular-phase amount of E2 is added transdermally to clamp the estrogen milieu. The postulate is that this protocol will unmask the relative impact of age, estrogen, and AVF in a low-SS-mediated feedback milieu.
METHODS
Subjects.
Women provided voluntary written informed consent approved by the Mayo Human Subjects Institutional Review Board. Exclusion criteria comprised sex steroid exposure within 1 mo of study; symptoms or signs of ischemic or inflammatory arteriovenous disease; acute or chronic hepatic, renal, immunologic, pulmonary, malignant, or infectious illness; cholelithiasis; known or suspected breast neoplasm; hemoglobin <11.8 g/dl; concomitant psychiatric treatment; drug or alcohol abuse; exposure to neuroactive drugs such as antidepressants, antihypertensives, or anticonvulsants within six half-lives; >3 kg weight change in 6 wk; regular nightshift work; transmeridian travel (>3 time zones traversed within the preceding 5 days); and inability to provide informed consent. Inclusion criteria were community-living, mentally competent, healthy women aged 18–30 or 50–80 yr. PRE status was defined by cyclic menses with a history of normal puberty. Pregnancy was excluded by serum human chorionic gonadotropin measurement. POST status was defined by amenorrhea for ≥2 yr, FSH >45 IU/l, LH >20 IU/l, and E2 ≤35 pg/ml (multiply by 3.68 for pmol/l).
Computerized axial tomography at L4–L5 was used to estimate abdominal visceral fat, as described earlier (9).
Clinical protocol.
The study was a prospectively randomized, parallel-cohort comparison of the effects of age and E2 on GH secretion during putatively reduced GH feedback-induced SS secretion. To achieve estrogen depletion, all 42 subjects received depot leuprolide acetate (3.75 mg im) twice 3 wk apart (10). The first injection was given in younger volunteers within 8 days of menses onset and 48 h of a negative blood pregnancy test and ≥3 wk following discontinuation of sex hormone-containing contraception. Older women received their first injection ≥3 wk after withdrawal of any sex hormone supplements. Placebo patch (n = 10 PRE, n = 12 POST) vs. graded transdermal E2 repletion (n = 10 PRE, n = 10 POST) was accomplished on an outpatient basis starting on the day of the second leuprolide injection (day 0). In subjects randomized to receive estrogen, the initial transdermal E2 dose was 0.05 mg/day for 4 days. The dose was increased to 0.10, 0.15, and 0.20 mg/day every 4 days (Estraderm; Novartis, Basel, Switzerland). The highest E2 dose (0.2 mg/day) was administered for 10 days (days 14–23 inclusive). l-arginine infusion and repetitive blood sampling were performed on days 17–23, when serum E2 concentrations were expected to be in the late-follicular phase range (9, 10). At the end of the study, micronized progesterone (100 mg orally) was administered for 12 days to women with an intact uterus according to standards of good medical practice.
Infusion and sampling schedule.
At 1800 the night before the study, volunteers received a standardized outpatient meal of 8 kcal/kg distributed as 20% protein, 50% carbohydrate, and 30% fat. Subjects then remained fasting overnight and until the end of sampling. At 0700 the next morning, two intravenous (iv) catheters were placed in (contralateral) forearm veins to allow simultaneous l-arginine infusion and blood sampling (1 ml) every 10 min for 6 h from 0800 to 1400. The infusion comprised iv saline (20 ml/h) from 0800 to 1000 followed by l-arginine (30 g) delivered from 1000 to 1030 at a constant rate. This dose is maximally stimulatory (25).
Hormone assays.
GH concentrations were measured in duplicate by automated double-monoclonal immunoenzymatic chemiluminescence assay using 22-kDa recombinant human GH as assay standard (Sanofi Diagnostics Pasteur Access, Chaska, MN) (10). Samples (n = 25) from any given subject were analyzed together. Sensitivity was 0.010 μg/l (defined as 3 standard deviations above the zero-dose tube). Interassay coefficients of variation (CVs) were 7.9 and 6.3% at GH concentrations of 3.4 and 12 μg/l, respectively. Intra-assay CVs were 4.9% at 1.1 μg/l and 4.5% at 20 μg/l. No values fell below 0.020 μg/l. Cross-reactivity with 20-kDa GH is <5%.
Screening serum E2, LH, and FSH concentrations were quantified by automated chemiluminescence assay (ACS 180; Bayer, Norwood, MA) using as standards E2 and the First and Second International Reference Preparations, respectively (10). Procedural sensitivities for E2, LH, and FSH are 35 pg/ml (189 pmol/l), 0.2 IU/l, and 0.4 IU/l, respectively. Intra-assay CVs for LH were 4.7, 3.5, and 3.8% and interassay CVs 8, 3.7, and 4.7% at 4.4, 18, and 39 IU/l, respectively. For FSH measurements, intra-assay CVs were 5.6, 4.3, and 3.5% and interassay CVs 6, 4, and 2.8% at 4.6, 25, and 62 IU/l, respectively.
Study-day values of E2 were quantified by liquid chromatography-tandem mass spectrometry as described (47). Total IGF-I, IGF-binding protein (IGFBP)-1, and IGFBP-3 concentrations were assayed by immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX) as presented (9, 10, 41).
Model-free analysis.
Unstimulated GH concentrations were averaged over the 2-h saline-infusion interval (0800–1000) in each subject.
Deconvolution analysis.
GH concentration time series (all 6 h) were analyzed using a recently developed automated deconvolution method, which was mathematically verified by direct statistical proof and empirically validated using hypothalamo-pituitary sampling and simulated pulsatile time series (6, 20). The MatLab-based algorithm first detrends the data and normalizes concentrations to the unit interval (0, 1) (19). Second, the program creates multiple successive potential pulse-time sets each containing one fewer via a smoothing process (a nonlinear adaptation of the heat-diffusion equation). Third, a maximum-likelihood expectation estimation method computes all secretion and elimination parameters simultaneously conditionally on each of the candidate pulse-time sets. Deconvolution parameters comprised basal secretion (β0), secretory burst mass (η0, η1), random effects on burst mass (σA), procedural/measurement error (σɛ), and a three-parameter flexible γ-secretory burst waveform (β1, β2, β3). The fast GH half-life was represented as 3.5 min constituting 37% of the decay amplitude and the slow half-life as 20.8 min (12). Statistical model selection was performed to distinguish among fits of the multiple candidate pulse-time sets using the Akaike information criterion (2). Outcomes evaluated were basal and pulsatile GH secretion (concentration units/session), mass secreted per burst (concentration units), and waveform shape (mode or time delay to maximal secretion after objectively estimated burst onset, min).
Statistical methods.
The design was a prospectively randomized, placebo-controlled, masked parallel-cohort assessment of the effects of age and E2 on pulsatile GH secretion unleashed by l-arginine infusion. Subjects, investigators, and infusion administrators were masked until closure of the study. Prestudy power analyses predicted >90% statistical power to detect a 30% effect of age or E2 by unpaired two-tailed Student's t-test if 40 subjects completed the study.
The effects of age stratum and estrogen status and their interaction were evaluated using a two-way (2 × 2 factor) least-squares general-linear ANOVA model (51). Analysis of covariance was not used, since the putative covariate (mean 2-h prestimulus baseline GH concentration in each subject) was not significant. Departure of the variance-covariance matrix from compound symmetry was adjusted for the use of the Huynh-Feldt statistic. Wilk's lambda was applied to evaluate the significance of possible interactions between age and status. The null hypothesis was that neither age stratum nor E2 condition determines GH secretion. Post hoc contrasts were made using Tukey's honestly significantly different (HSD) test (13). Significance was construed for experiment-wise P < 0.05. Data are presented as means ± SE (n).
Regression analysis.
Linear regression analysis was employed to explore correlations between GH secretion and age, E2 levels, and AVF or BMI. Stepwise, forward-selection, multivariate, linear regression analysis was applied to identify the principal determinant(s) of pulsatile GH secretion from among age, E2 concentration, and AVF in the combined cohorts (n = 42 subjects). Computations were made using Systat Version 11 (Systat, Point Richmond, CA).
Approximate entropy.
Approximate entropy (ApEn) is a scale- and model-independent univariate regularity statistic used to quantify the orderliness (subpattern consistency) of serial measurements. Mathematical models and feedback experiments establish that pattern orderliness monitors feedback and/or feedforward interactions within an interlinked axis with high sensitivity and specificity (both >90%) (31, 49).
RESULTS
PRE women had (means ± SE) ages and BMIs of 23 ± 0.62 and 25 ± 1.1 yr and 26 ± 0.75 and 23 ± 1.0 kg/m2, respectively, in the placebo and E2 limbs. Corresponding values in POST women were 63 ± 2.2 and 64 ± 1.4 yr and 25 ± 1.1 and 26 ± 1.2 kg/m2, respectively.
Table 1 shows the comparability of E2 concentrations in PRE and POST women subjected to a given low- or high-E2 clamp. IGF-I concentrations were lower in POST than in PRE women whether compared in the setting of leuprolide plus E2 or leuprolide plus placebo. IGFBP-1 was higher in POST than in PRE volunteers with comparable E2 status. Additionally, POST subjects supplemented with E2 had higher IGFBP-1 concentrations than PRE volunteers given placebo. IGFBP-3 was maximal in PRE − E2 individuals. Exploratory univariate regression analysis indicated that fasting (2-h mean unstimulated) GH concentrations before l-arginine infusion correlated positively with E2 levels (r2 = 0.14, P = 0.015), and negatively with AVF (r2 = 0.10, P = 0.041), but not with age (P = 0.21). By stepwise forward-selection regression, E2 concentrations alone explained the effects of E2 and AVF on fasting unstimulated GH concentrations (r2 = 0.14, P = 0.015).
Table 1.
Hormone measurements during leuprolide/E2 and leuprolide/Pl clamps
| Subject Groups | E2, pg/ml* | IGF-I, μg/l | IGFBP-1, μg/l | IGFBP-3, mg/l |
|---|---|---|---|---|
| Pre − E2 (n = 10) | 11±1.3a | 352±29a | 14±2.3a | 5.1±0.3a |
| Post − E2 (n = 12) | 8.8±1.1a | 187±24b | 22±2.5a,b | 4.1±0.2b,c |
| PRE + E2 (n = 10) | 147±7.3b | 418±46a | 28±2.4a,b | 4.8±0.3a,c |
| POST + E2 (n = 10) | 116±10b | 176±24b | 38±6.6b | 3.4±0.2c |
| P values† | <0.001 | <0.001 | 0.015 | <0.001 |
Data are means ± SE. E2, estradiol; Pl, placebo; IGFBP-1 and -3, IGF-binding protein-1 and -3, respectively; PRE, premenopausal; POST, postmenopausal.
Multiply by 3.68 for pmol/l.
ANOVA within each column. Different (unshared) superscripted letters denote significantly different means by Tukey's honestly significantly different post hoc test for multiple comparisons.
Pulsatile GH secretion was quantified by deconvolution analysis. Based upon two-way ANOVA, both age (P < 0.001) and E2 status (P = 0.001) determined pulsatile GH responses to l-arginine infusion (overall P < 0.001; Fig. 1). Post hoc comparisons via Tukey's HSD test revealed that maximal pulsatile GH secretion occurred in PRE + E2 (P = 0.016 vs. POST + E2, P = 0.027 vs. PRE − E2, and P < 0.001 vs. POST − E2). The interaction between age and E2 trended toward significance (P = 0.067).
Fig. 1.
Age and estradiol (E2) determine pulsatile growth hormone (GH) secretion in a putatively low-somatostatin milieu. Two-way analysis of variance in 42 women given leuprolide with placebo or E2 addback transdermally and then intravenous infusion of l-arginine. Volunteers were sampled every 10 min for 6 h, which included a 2-h baseline. Tukey's honestly significantly different (HSD) test was applied to compare means post hoc. P values are as indicated. The number of subjects in each of the 4 groups is stated within each bar. Data are means ± SE. PRE, premenopausal; POST, postmenopausal.
The opposing effects of age and E2 on pulsatile GH secretion during putatively muted GH negative feedback were explicable by modulation of the mass of GH secreted per burst. According to two-way ANOVA, age had a significantly negative (P = 0.001) and E2 a significantly positive (P < 0.001) effect (overall P < 0.001). Post hoc multiple-comparison contrasts revealed that GH secretory burst mass in PRE + E2 significantly exceeded that in all three other study cohorts (0.001 ≤ P ≤ 0.036; Fig. 2).
Fig. 2.
E2 augments the mass of GH secreted per burst in PRE and POST women, with ∼3-fold greater responses in PRE than in POST individuals. Data are otherwise presented as described in Fig. 1.
Basal (nonpulsatile) GH secretion was determined statistically by E2 (P = 0.004) but not by age (P = 0.80) (overall ANOVA, P = 0.037; Fig. 3). Basal secretion in PRE + E2 women was similar to that in POST + E2 individuals, and both values exceeded those in the two E2-deficient cohorts. Basal secretion represented 1.4, 4.3, 3.8, and 12% of pulsatile GH secretion in the PRE − E2, PRE + E2, POST − E2, and POST + E2 categories, respectively.
Fig. 3.
Impact of E2 and PRE vs. POST status on basal (unstimulated nonpulsatile) GH secretion in 42 women studied under a leuprolide clamp. The format is that of Fig. 1.
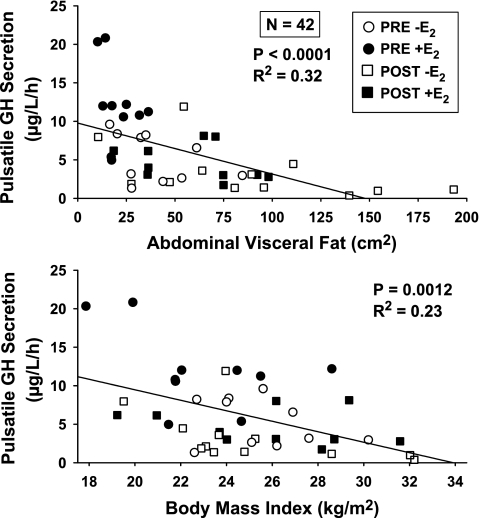
Univariate linear regression analysis disclosed that l-arginine-induced pulsatile GH secretion correlated negatively with computerized tomography-estimated AVF (P < 0.0001) and BMI (P = 0.0012), which explained 32 and 23%, respectively, of interindividual response variability (Fig. 4). Age was a strongly positive univariate predictor of AVF (P < 0.0001, r2 = 0.32). Stepwise forward-selection multivariate regression analysis (n = 42 subjects) was used to assess the concomitant contribution(s) of age and E2 (if any) to the effect of AVF. Figure 5 is three-dimensional plot of the concerted influences of age (negatively; P = 0.0017) and E2 concentrations (positively; P = 0.0002) on stimulated pulsatile GH secretion (P < 0.0001 overall). In the multivariate regression model, AVF vanished as a contributor, leaving age and E2 together to explain 46% of interindividual response variability. When GH secretory burst mass was used as the dependent variable, the effects of age (P = 0.029) and E2 (P = 0.0004) were also significant (overall P = 0.0001), together accounting for 37% of intersubject variance. The mode (time delay in minutes from GH secretory burst onset to maximal secretion) was not significantly related to age, E2, AVF, or BMI.
Fig. 4.
Univariate linear regression of pulsatile GH secretion unleashed by l-arginine infusion on abdominal visceral fat (top) or body mass index (bottom). Data were obtained from 42 women. P values reflect Pearson's correlation coefficient with corresponding r2.
Fig. 5.
Combined contributions of age and E2 concentrations to pulsatile GH secretion (top) and the mass of GH secreted per burst (bottom) in 42 women assigned to E2 or placebo addback after leuprolide suppression of the gonadal axis. Partial P values are indicated below the overall P value for the forward-selection stepwise multivariate analysis.
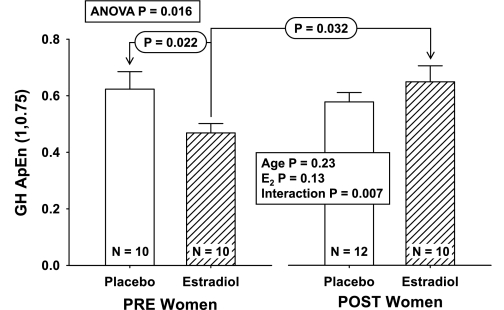
ApEn was employed to quantify GH secretory pattern regularity after time series were detrended by first differencing (36). ANOVA of GH ApEn values revealed a significant interaction between age and E2 (P = 0.007) but no independent effects of age (P = 0.23) or E2 (P = 0.13) (overall P = 0.016; Fig. 6). The interaction consisted of higher GH ApEn (greater irregularity = less orderliness) in POST + E2 than in PRE + E2 (P = 0.032) and lower GH ApEn (less irregularity) in PRE + E2 than in PRE − E2 (P = 0.022).
Fig. 6.
Interactive effects of PRE and POST status and E2 repletion/depletion on the regularity of GH secretion. Approximate entropy (ApEn) was used to quantify orderliness (regularity) of GH secretory patterns during l-arginine infusion. Increased ApEn denotes greater irregularity, which signifies less feedback control and/or less coordinated feedforward drive.
DISCUSSION
The present investigation evaluated the hypothesis that age, E2 availability, and body composition modulate endogenous feedforward drive of pulsatile GH secretion in 42 healthy women. Key outcomes in the presumptively low-SS (low GH feedback) milieu were that 1) age and E2 concentrations prominently (each P ≤ 0.001) control pulsatile GH output with a trend toward an interaction (P = 0.067); 2) the negative effect of age and the positive effect of E2 are mediated by corresponding changes in the size (mass) of GH secretory bursts (μg of GH released/unit distribution volume per burst); 3) a smaller positive effect of E2 operates on basal GH secretion (P = 0.004), which represented 1.4–12% of pulsatile GH secretion; 4) indexes of body composition explain significant (AVF 32%, BMI 23%) variability in pulsatile GH secretion; 5) by stepwise forward-selection multivariate analysis, the univariate effects of AVF and BMI are completely explained by age and E2, together accounting for 46% of intersubject variability in feedback-disinhibited, burst-like GH secretion (P < 0.0001); and 6) according to ApEn analysis, E2 enhances GH secretory pattern orderliness in PRE but not POST women under presumptively low-SS feedback. Measured E2 concentrations supported subject compliance with E2/placebo patches. Thus, the ensemble outcomes indicate that, to the extent that l-arginine mutes GH autofeedback-induced SS outflow, age and E2 represent major determinants of endogenous feedforward drive and, therefore, of physiological GHRH and ghrelin action.
Under inferably reduced GH feedback imposed by l-arginine infusion, pulsatile GH secretion was 2.7-fold lower in POST than in PRE women despite statistically similar E2 concentrations. The mean E2 value (133 pg/ml = 489 pmol/l) is comparable with that observed in the late follicular phase of the normal menstrual cycle, when pulsatile GH secretion increases about 2.2-fold in healthy PRE women (11, 28, 50). An explanatory postulate would be that factors associated with POST status or aging diminish estrogen's capability to amplify endogenous GHRH and ghrelin's drive of burst-like GH release. In contrast, compared with placebo, E2 elevated basal (nonpulsatile) GH secretion equally in PRE and POST women. These data suggest that distinct E2- and age-related mechanisms regulate burst-like and basal GH secretion. GH deficiency may contribute to bone loss and muscle wasting in advancing age (48). Whether increased GH production secondary to E2 supplementation confers protection against catabolism has not been established. The question arises because E2 can also antagonize GH action on certain target tissues, such as liver (23), resulting in lower IGF-I and IGFBP-3 and higher IGFBP-1 concentration (44).
Univariate regression analysis identified a prominently negative correlation between AVF (or BMI) and low-feedback-associated pulsatile GH secretion. Notably, the independent covariates age and E2 together completely explained the statistical effect of AVF, possibly reflecting in part the positive correlation between age and AVF. However, whether age and AVF affect GH secretion via shared pathways has not yet been established.
ApEn is a regularity statistic that provides a barometer of the degree of network-like coordination driving orderly hormone output (14, 18). Earlier studies indicate that ApEn of GH release increases in midpuberty and after E2, testosterone, or GHRH administration (46). Increased ApEn signifies attenuation of feedback-coordinating signals on theoretical and empirical grounds (49). Under putatively low-GH feedback imposed by l-arginine infusion, ApEn of GH release was also high. Supplementation with E2 in PRE but not POST individuals reduced ApEn significantly, defining a more orderly GH release process (P = 0.007). The basis for the impaired feedback-modifying effect of E2 in POST individuals is not known. However, estrogen's enhancement of GH secretory regularity in PRE women is selective to the GH feedback-restricted milieu (30). This raises the possibility that E2 exposure evokes more regular GH secretion in PRE women by increasing feedforward by endogenous secretagogues.
Caveats include the somewhat small cohort size (n = 42), the use of a single E2 addback concentration, the relatively short duration of estrogen deprivation and repletion, the possibility that l-arginine might exert unknown effects, and the need to extend these findings to men and children and to corroborate outcomes prospectively so as to define a causal effect of aging.
In conclusion, analyses of basal, pulsatile, and entropic modes of GH secretion in PRE and POST women studied under a low- or high-E2 clamp in a putatively low-GH feedback milieu disclose 1) prominent joint effects of age (negative) and E2 (positive) on the summed mass of GH secreted in bursts, 2) significant univariate effects of AVF and BMI (both negative) on the same measurement, 3) a positive impact of E2 on basal (nonpulsatile) GH secretion, and 4) an age-by-E2 interaction in controlling the regularity of the GH secretory process. The collective outcomes motivate consideration of more complex models of hypothalamopituitary mechanisms regulating basal, pulsatile, and entropic GH secretion.
GRANTS
This work was supported in part via Clinical Translational Research Center Grant MO1-RR-00585 to the Mayo Clinic and Foundation from the National Center for Research Resources (Rockville, MD) and R01-NIA-AG-29362, R21-DK-072095, and DK-063609 from the National Institutes of Health (Bethesda, MD).
Acknowledgments
We thank Donna Scott for capable support of manuscript preparation, Ashley Bryant for excellent data analysis and graphics, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
REFERENCES
- 1.Aguila MC, McCann SM. Growth hormone increases somatostatin release and messenger ribonucleic acid levels in the rat hypothalamus. Brain Res 623: 89–94, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Akaike H A new look at the statistical model identification. IEEE Trans Autom Control 19: 716–723, 1974. [Google Scholar]
- 3.Alba-Roth J, Muller OA, Schopohl J, Von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab 67: 1186–1189, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Baumbach WR, Carrick TA, Pausch MH, Bingham B, Carmignac D, Robinson IC, Houghten R, Eppler CM, Price LA, Zysk JR. A linear hexapeptide somatostatin antagonist blocks somatostatin activity in vitro and influences growth hormone release in rats. Mol Pharmacol 54: 864–873, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Bray MJ, Vick TM, Shah N, Anderson SM, Rice LW, Iranmanesh A, Evans WS, Veldhuis JD. Short-term estradiol replacement in postmenopausal women selectively mutes somatostatin's dose-dependent inhibition of fasting growth hormone secretion. J Clin Endocrinol Metab 86: 3143–3149, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay S, Veldhuis JD, Keenan DM. Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Quarterly Appl Math 66: 401–421, 2008. [Google Scholar]
- 7.Chihara K, Minamitani N, Kaji H, Arimura A, Fujita T. Intraventricularly injected growth hormone stimulates somatostatin release into rat hypophysial portal blood. Endocrinology 109: 2279–2281, 1981. [DOI] [PubMed] [Google Scholar]
- 8.Clark RG, Robinson IC. Growth hormone responses to multiple injections of a fragment of human growth hormone-releasing factor in conscious male and female rats. J Endocrinol 106: 281–289, 1985. [DOI] [PubMed] [Google Scholar]
- 9.Erickson D, Keenan DM, Farhy LS, Mielke K, Bowers CY, Veldhuis JD. Determinants of dual secretagogue drive of burst-like GH secretion in premenopausal women studied under a selective estradiol clamp. J Clin Endocrinol Metab 90: 1741–1751, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson D, Keenan DM, Mielke K, Bradford K, Bowers CY, Miles JM, Veldhuis JD. Dual secretagogue drive of burst-like growth hormone secretion in postmenopausal compared with premenopausal women studied under an experimental estradiol clamp. J Clin Endocrinol Metab 89: 4746–4754, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Faria AC, Bekenstein LW, Booth RA Jr, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf) 36: 591–596, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Faria AC, Veldhuis JD, Thorner MO, Vance ML. Half-time of endogenous growth hormone (GH) disappearance in normal man after stimulation of GH secretion by GH-releasing hormone and suppression with somatostatin. J Clin Endocrinol Metab 68: 535–541, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Fisher LD, van Belle G. Descriptive statistics. In: Biostatistics: A Methodology for the Health Sciences. New York: John Wiley & Sons, 1996, p. 58–74.
- 14.Gevers E, Pincus SM, Robinson IC, Veldhuis JD. Differential orderliness of the GH release process in castrate male and female rats. Am J Physiol Regul Integr Comp Physiol 274: R437–R444, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Ghigo E, Arvat E, Valente F, Nicolosi M, Boffano GM, Procopio M, Bellone J, Maccario M, Mazza E, Camanni F. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology 54: 291–294, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Gianotti L, Maccario M, Lanfranco F, Ramunni J, Di Vito L, Grottoli S, Mueller EE, Ghigo E, Arvat E. Arginine counteracts the inhibitory effect of recombinant human insulin-like growth factor I on the somatotroph responsiveness to growth hormone-releasing hormone in humans. J Clin Endocrinol Metab 85: 3604–3608, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 19: 717–797, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CH, Matthews DR, Pringle PJ, Brook CG. A sexually dimorphic pattern of growth hormone secretion in the elderly. J Clin Endocrinol Metab 84: 2679–2685, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Keenan DM, Chattopadhyay S, Veldhuis JD. Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236: 242–255, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Keenan DM, Roelfsema F, Biermasz N, Veldhuis JD. Physiological control of pituitary hormone secretory-burst mass, frequency, and waveform: a statistical formulation and analysis. Am J Physiol Regul Integr Comp Physiol 285: R664–R673, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kraicer J, Cowan JS, Sheppard MS, Lussier B, Moor BC. Effect of somatostatin withdrawal and growth hormone (GH)-releasing factor on GH release in vitro: amount available for release after disinhibition. Endocrinology 119: 2047–2051, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Lanzi R, Tannenbaum GS. Time-dependent reduction and potentiation of growth hormone (GH) responsiveness to GH-releasing factor induced by exogenous GH: role of somatostatin. Endocrinology 130: 1822–1828, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, Leong GM, Ross RJ, Ho KK. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci USA 100: 1016–1021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107: 1571–1580, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merimee TJ, Bergess JA, Rabinowitz D. Sex-determined variation in serum insulin and growth hormone response to amino acid stimulation. J Clin Endocrinol Metab 26: 791–793, 1966. [DOI] [PubMed] [Google Scholar]
- 26.Miki N, Ono M, Shizume K. Withdrawal of endogenous somatostatin induces secretion of growth hormone-releasing factor in rats. J Endocrinol 117: 245–252, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Kato Y, Kabayama Y, Inoue T, Koshiyama H, Imura H. Involvement of hypothalamic growth hormone (GH)-releasing factor in GH secretion induced by intracerebroventricular injection of somatostatin in rats. Endocrinology 120: 311–316, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Ovesen P, Vahl N, Fisker S, Veldhuis JD, Christiansen JS, Jørgensen JO. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal women. J Clin Endocrinol Metab 83: 1662–1667, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci 16: 8140–8148, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus SM, Gevers E, Robinson IC, van den Berg G, Roelfsema F, Hartman ML, Veldhuis JD. Females secrete growth hormone with more process irregularity than males in both human and rat. Am J Physiol Endocrinol Metab 270: E107–E115, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Pincus SM, Hartman ML, Roelfsema F, Thorner MO, Veldhuis JD. Hormone pulsatility discrimination via coarse and short time sampling. Am J Physiol Endocrinol Metab 277: E948–E957, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Pontiroli AE, Lanzi R, Pozza G. Inhibition of the growth hormone (GH) response to GH-releasing hormone by constant met-GH infusions. J Clin Endocrinol Metab 68: 956–959, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frolich M, Stokvis-Brantsma WH, Wit JM. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab 86: 2459–2464, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal SM, Kaplan SL, Grumbach MM. Short term continuous intravenous infusion of growth hormone (GH) inhibits GH-releasing hormone-induced GH secretion: a time-dependent effect. J Clin Endocrinol Metab 68: 1101–1105, 1989. [DOI] [PubMed] [Google Scholar]
- 35.Sato M, Chihara K, Kita T, Kashio Y, Okimura Y, Kitajima N, Fujita T. Physiological role of somatostatin-mediated autofeedback regulation for growth hormone: importance of growth hormone in triggering somatostatin release during a trough period of pulsatile growth hormone release in conscious male rats. Neuroendocrinology 50: 139–151, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz O, Porksen N, Nyholm B, Skjaerback C, Butler PC, Veldhuis JD, Pincus SM. Disorderly and nonstationary insulin secretion in relatives of patients with NIDDM. Am J Physiol Endocrinol Metab 272: E218–E226, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Sheppard MC, Kronheim S, Pimstone BL. Stimulation by growth hormone of somatostatin release from the rat hypothalamus in vitro. Clin Endocrinol (Oxf) 9: 583–586, 1978. [DOI] [PubMed] [Google Scholar]
- 38.Sugihara H, Minami S, Wakabayashi I. Post-somatostatin rebound secretion of growth hormone is dependent on growth hormone-releasing factor in unrestrained female rats. J Endocrinol 122: 583–591, 1989. [DOI] [PubMed] [Google Scholar]
- 39.Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin, somatostatin and growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 144: 967–974, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Vale WW, Vaughan J, Yamamoto G, Spiess J, Rivier J. Effects of synthetic human pancreatic (tumor) GH releasing factor and somatostatin, triiodothyronine and dexamethasone on GH secretion in vitro. Endocrinology 112: 1553–1555, 1983. [DOI] [PubMed] [Google Scholar]
- 41.Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab 90: 6006–6013, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Veldhuis JD, Evans WS, Bowers CY. Estradiol supplementation enhances submaximal feedforward drive of growth hormone (GH) secretion by recombinant human GH-releasing hormone-1,44-amide in a putatively somatostatin-withdrawn milieu. J Clin Endocrinol Metab 88: 5484–5489, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Veldhuis JD, Fletcher TP, Gatford KL, Egan AR, Clarke IJ. Hypophyseal-portal somatostatin (SIRF) and jugular venous growth hormone secretion in the conscious unrestrained ewe. Neuroendocrinology 75: 83–91, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Veldhuis JD, Frystyk J, Iranmanesh A, Orskov H. Testosterone and estradiol regulate free IGF-I, IGFBP-I and dimeric IGF-I/IGFBP-I concentrations. J Clin Endocrinol Metab 90: 2941–2947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veldhuis JD, Iranmanesh A, Mielke K, Miles JM, Carpenter PC, Bowers CY. Ghrelin potentiates growth hormone secretion driven by putative somatostatin withdrawal and resists inhibition by human corticotropin-releasing hormone. J Clin Endocrinol Metab 91: 2441–2446, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29: 823–864, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veldhuis JD, Mielke KL, Cosma M, Soares-Welch C, Paulo R, Miles JM, Bowers CY. Aromatase and 5-alpha-reductase inhibition during an exogenous testosterone clamp unveils selective sex-steroid modulation of somatostatin and growth-hormone secretagogue actions in healthy older men. J Clin Endocrinol Metab 94: 973–981, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev 27: 101–140, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe CA, Barkan A, Johnson ML, Pincus SM. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol Regul Integr Comp Physiol 280: R721–R729, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Yen SS, Vela P, Rankin J, Littell AS. Hormonal relationships during the menstrual cycle. JAMA 211: 1513–1517, 1970. [PubMed] [Google Scholar]
- 51.Zar JH Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1996.
- 52.Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME, van der Ploeg LH, Schaeffer JM, Leng G, Smith RG. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol 11: 1709–1717, 1997. [DOI] [PubMed] [Google Scholar]