Abstract
Purpose
To evaluate the role of diffusion-weighted magnetic resonance imaging (MRI) in determining tumor necrosis and contrast-enhanced MRI using gadoxetic acid disodium (Gd-EOB-DTPA) in determining maximum tumor size measurement and tumor delineation compared with criterion-standard histologic measurements in the rabbit VX2 liver tumor model.
Materials and Methods
VX2 tumors were implanted in the livers of 13 rabbits. Magnetic resonance imaging was performed using a 1.5-T MRI scanner and an extremity coil. The imaging protocol included T2-weighted fast spin-echo images, 3-dimensional T1-weighted spoiled gradient-echo with and without fat suppression after administration of Gd-EOB-DTPA, and diffusion-weighted echo planar images. Rabbits were killed, and the tumor was harvested and sliced at 4-mm intervals in the axial plane. The MRI parameters evaluated were tumor size, tumor delineation, and tumor apparent diffusion coefficient (ADC) values. Histologic sections were evaluated to quantify tumor necrosis.
Results
On contrast-enhanced MRI (obtained from 11 rabbits), the mean tumor sizes were 20, 19, and 20 mm in the arterial, portal venous, and delayed phases, respectively. Tumor delineation was most distinguishable in the delayed phase. On diffusion-weighted MRI (acquired in 13 rabbits), the mean tumor ADC value was 1.84 × 10−3 mm2/s. The mean tumor size at pathology was 16 mm. The mean percent necrosis at the tumor’s pathologic condition was 36%. The correlation between ADC value and percent necrosis showed an R value of 0.68.
Conclusions
Contrast-enhanced MRI using Gd-EOB-DTPA may provide additional information about tumor outline in the liver. More-over, we showed a remarkable correlation between ADC values and tumor necrosis. Thus, diffusion-weighted imaging may be useful to assess tumor necrosis; nevertheless, the search for new modalities remains important.
Keywords: VX2, Gd-EOB-DTPA, diffusion-weighted MRI, tumor size, HCC, liver cancer, tumor necrosis
Evaluation of tumor response by imaging after locoregional therapy is generally based on tumor size and tumor enhancement on contrast-enhanced computed tomography or magnetic resonance imaging (MRI).1 Unfortunately, these imaging techniques may be limited in providing clinically satisfactory information about the extent of tumor necrosis, which is the main indicator of tumor cell death. Therefore, improvements of current imaging techniques play a critical role in finding the optimal strategy to determine treatment success and guide future therapy.
A recent imaging technique for assessment of tumor response is diffusion-weighted MRI. This imaging technique is used to detect the thermally induced random movement of water molecules in biologic tissues, known as Brownian motion.2 Diffusion may be affected by the biophysical properties of tissues such as cell organization and density, microstructure, and microcirculation. Viable tumor cells restrict the mobility of water, whereas necrotic tumor cells allow increased diffusion of water molecules caused by decreased cellularity and compromised cell membrane integrity, displayed as areas of high signal intensity.3 The primary application of diffusion-weighted MRI has been in brain imaging, mainly in the evaluation of acute cerebral infarcts.4 In the liver, diffusion-weighted imaging has been used to characterize focal hepatic lesions and to assess tumor response after locoregional therapy.5–8
Another approach to tumor evaluation is the use of tissue-specific contrast media. Normally, dynamic contrast-enhanced MRI is performed with the traditional extracellular gadolinium-based contrast agents. Gadolinium–ethoxybenzyl–diethylenetriamine pentaacetic acid (gadoxetic acid disodium or Gd-EOB-DTPA) is a third-generation gadolinium-based MRI contrast agent with the unique ability to combine MR perfusion imaging with hepatocyte-specific uptake.9,10 Therefore, information about lesion vascularity is obtained during the dynamic phase, and information about tumor delineation is obtained during the delayed phase of imaging because of the lack of uptake in the tumor.
Gadoxetic acid disodium has been useful in the evaluation of liver function and dysfunctional states such as hepatitis and in the detection of liver metastasis and hepatocellular carcinoma (HCC).11–13 After intravenous injection, the contrast between the lesion and the surrounding parenchyma is increased because of the positive enhancement of the normal liver tissue on T1-weighted MR images.
The aim of our animal study was to evaluate the role of diffusion-weighted MRI in determining tumor necrosis and the role of contrast-enhanced MRI using Gd-EOB-DTPA in determining maximum tumor size measurement and tumor delineation compared with criterion-standard histologic measurements in the rabbit VX2 liver tumor model.
MATERIALS AND METHODS
Study Design
This study was approved by the animal care committee at our facility and performed in accordance with our institutional guidelines. A total of 13 New Zealand white rabbits were included. Each animal received tumor implantation in the left lobe of the liver. Diffusion-weighted and contrast-enhanced MRIs were performed 2 weeks after implantation in 8 animals and 3 weeks after implantation in 5 animals to reach various degrees of necrosis within the tumors. All animals were killed immediately after MRI, and their livers were explanted and submitted to a pathologist for analysis.
Tumor Implantation
Adult New Zealand white rabbits (Myrtle’s Rabbitry, Thompson’s Station, Tenn) weighing 3.5 to 4.2 kg were anesthetized with a mixture of acepromazine (2.5 mg/kg; Phoenix, St Joseph, Mo) and ketamine hydrochloride (44 mg/kg; Phoenix) administered intramuscularly. The VX2 tumor cell suspension was first injected into the hind legs of 2 carrier rabbits and grown for 2 weeks. Resultant tumors were harvested from each carrier, and a tumor suspension was prepared from each harvested tumor by dissection of viable tumor tissue and aseptic mincing. For the rabbits that were going to receive the VX2 tumor implanted in the liver, intravenous access was gained via a marginal ear vein, and 0.1 to 0.2 mL (2.5–5 mg) of sodium pentobarbital (Abbott Laboratories, Abbott Park, Ill) was given periodically to maintain anesthesia. The abdomen of each recipient rabbit was shaved and disinfected with ethanol and povidine iodine. The liver of the rabbit was exposed by a midline incision, and then an aliquot of the tumor cell suspension (0.2 mL) was injected directly using a 21-gauge angiocatheter into the left lobe of the liver to develop a solitary lesion with adequate surrounding liver parenchyma. The abdomen was closed in 2 layers. The tumor was allowed to grow in the rabbit livers for 14 to 21 days. Because of the fast-growing and aggressive nature of the VX2 tumors, various degrees of necrosis are obtained at different time points after implantation.
Magnetic Resonance Imaging
All 13 rabbits underwent MRI immediately before they were killed. Diffusion-weighted and contrast-enhanced MRIs were performed 2 weeks after implantation in 8 animals and 3 weeks after implantation in 5 animals. Magnetic resonance imaging was performed by using a 1.5-T MRI scanner (CV/i; General Electric Medical Systems, Milwaukee, Wis) and dedicated phased-array body coils. Precontrast axial acquisitions included a T2-weighted fast spin echo with fat suppression, breath-hold diffusion-weighted echo planar images (matrix, 128 × 128; slice thickness, 8 mm; interslice gap, 2 mm; B values, 0 and 500; repetition time, 5000–6500 milliseconds; echo time, 110 milliseconds; and receiver bandwidth, 64 kHz), and 3-dimensional T1-weighted spoiled gradient-echo with and without fat suppression. All animals received a 0.025-mmol dose of 0.25-mol/L Gd-EOB-DTPA per kilogram of body weight (Schering AG, Berlin, Germany) intravenously at a speed of 2 mL/s. The line was flushed with 5 mL of 0.9% saline. Dynamic imaging was performed in the arterial (2–5 seconds after injection) and portal venous phases (20 seconds after injection) using the dynamic 3-dimensional T1-weighted GRE sequence without fat suppression. In addition, delayed T1-weighted GREs with fat suppression were performed 10 minutes after contrast administration.
Image Analysis
Magnetic resonance image processing and apparent diffusion coefficient (ADC) maps were generated using a commercially available Advantage Windows workstation (General Electric Medical Systems). Images were interpreted through the consensus of 2 experienced MR radiologists. Apparent diffusion coefficient maps were generated from the diffusion-weighted images, and the values were recorded by placing a region of interest (ROI) over the entire lesion, as seen on the image with the largest lesion size. Separate ADC maps were generated by placing the ROI on the viable and necrotic regions of each tumor. Histologic slides were used to determine the position of the necrotic and viable regions. Other parameters evaluated were tumor size and delineation in arterial, portal venous phase, and 10 minutes post injection. Tumor size was measured in all 3 phases of contrast enhancement, using electronic calipers. Tumor delineation was scored using the following 5-grade scale: grade 1 indicates no distinguishable outline of the tumor, grade 2 is defined as tumor outline seen in 90 degrees along the tumor border, grade 3 is defined as outline seen in 180 degrees along the tumor border, grade 4 is defined as outline seen in 270 degrees along the tumor border, and grade 5 indicates a definite distinguishable tumor seen all along the tumor border (360 degrees).
Histologic Analysis
All animals were euthanized under deep anesthesia by slow injection of a lethal dose (100 mg/5 mL) of sodium pentobarbital intravenously after the completion of MRI. Immediately after euthanasia, the livers of the rabbits were carefully removed and subsequently placed in 10% formaldehyde for fixation. After fixation, the liver was examined, and the liver tumor was dissected out the nontumorous liver tissue. Tumors were sliced at 3- to 4-mm intervals in the axial plane to correspond to the plane of the MR images and placed in standard cassettes. To maintain proper orientation, the dorsal and medial sides of each slice were stained with different colors. All sections were submitted to a pathologist for histologic preparation. The tissue slices were embedded in paraffin, and 2 sections of each paraffin block were stained with hematoxylin-eosin. A Nikon SMZ800 microscope (Nikon Instruments Inc, Melville, NY) was coupled with a Nikon digital sight DS-U1 camera (resolution, 1376 × 1032). The images of the tumors were captured at a magnification of ×10. Digital images were first converted to jpeg format and then imported into ImageJ. The ImageJ 1.37v software (National Institutes of Health, Bethesda, Md) was used to estimate the percentage of necrosis within the tumor. For each liver tumor, the 3 axial slides representing the most central part of the tumor were selected. For each slide, an ROI was delineated around the entire tumor, and another ROI was delineated around the necrotic part of the tumor. The mean ratio of the necrotic part to the total tumor was calculated for each liver tumor. To assess tumor size, the axial slide representing the center of the tumor was selected. The tumor size was recorded as the maximum diameter in millimeter along the same plane and axis used in MRI.
Statistical Analysis
The collected data were entered into a Microsoft Excel spreadsheet (Microsoft, Redmond, WA). Tumor size, tumor enhancement during contrast-enhanced MRI, and ADC values were correlated with pathologic findings using Pearson product moment correlation coefficient (r). The estimates of fractions of viable cells obtained with MRI and histopathology were compared using Student t test. P < 0.05 was considered to indicate significant difference at 95% confidence interval.
RESULTS
All implantations were successful, and in all 13 rabbits, a tumor developed. All rabbits underwent MRI. Two rabbits died after diffusion-weighted imaging but before the injection of contrast medium.
The results of experiments are presented in Table 1. The mean (SD) duration between implantation and MRI was 16 (3.3) days. All tumors appeared heterogeneous on T2-weighted images (Fig. 1A). On contrast-enhanced MRI (n = 11), the mean tumor sizes were 20 mm (range, 12–36 mm), 19 mm (range, 12–33 mm), and 20 mm (range, 13–36 mm) in the arterial, portal venous, and delayed phases, respectively. Tumor delineation as scored on the 5-grade scale was most distinguish-able in the delayed phase for all tumors, with scores from 3 to 5. The portal venous phase was equal or superior to the arterial phase for the distinction of tumor outline in 82% of the lesions.
TABLE 1.
Imaging and Pathologic Variables for All 13 Rabbits
| Time Between Implantation and MRI, d |
Tumor size, mm | Delineation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor ADC Value, × 10−3 mm2/s |
% Necrosis on Pathology |
|||||||||
| ID | Arterial | PV | Delayed | Pathology | Arterial | PV | Delayed | |||
| 1 | 13 | 16.1 | 15.2 | 14.6 | 14.5 | 1.45 | 19 | 2 | 3 | 5 |
| 2 | 13 | 13.3 | 11.5 | 13.0 | 7.8 | 1.97 | 17 | 1 | 2 | 3 |
| 3 | 13 | 19.5 | 17.2 | 17.6 | 12 | 1.63 | 22 | 2 | 1 | 4 |
| 4 | 14 | 14.0 | 13.7 | 14.9 | 10.8 | 1.69 | 20 | 1 | 2 | 4 |
| 5 | 14 | 14.4 | 15.4 | 16.4 | 15 | 1.65 | 22 | 1 | 1 | 3 |
| 6 | 14 | NA | NA | NA | 11 | 0.80 | 11 | NA | NA | NA |
| 7 | 15 | 12.1 | 12.7 | 13.5 | 13.3 | 1.61 | 32 | 1 | 2 | 3 |
| 8 | 15 | 12.5 | 12.6 | 13.4 | 10.8 | 1.56 | 26 | 2 | 3 | 5 |
| 9 | 17 | 23.6 | 18.5 | 21.1 | 21 | 1.31 | 38 | 2 | 1 | 4 |
| 10 | 18 | 20.5 | 21.2 | 21.6 | 13.5 | 2.93 | 40 | 1 | 1 | 4 |
| 11 | 21 | 34.7 | 33.0 | 35.7 | 26 | 2.84 | 72 | 1 | 2 | 3 |
| 12 | 21 | 35.7 | 32.9 | 33.5 | 27 | 2.11 | 69 | 1 | 2 | 4 |
| 13 | 22 | NA | NA | NA | 20.5 | 2.34 | 80 | NA | NA | NA |
PV indicates portal venous; NA, not applicable.
FIGURE 1.
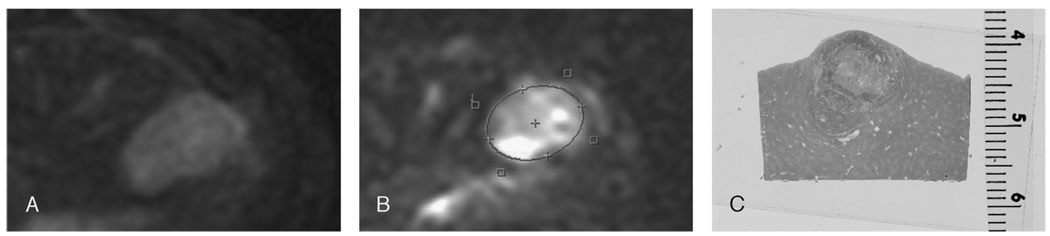
A, Typical example of a rabbit VX2 tumor in the left lateral lobe of the liver appearing heterogeneously bright on T2-weighted images. B, Diffusion-weighted MR image of the same lesion. After placing an ROI on the lesion, mean ADC value was calculated to be 2.93 × 10−3 mm2/s. C, The same tumor after hematoxylin-eosin staining consisting of necrotic (central) and viable (peripheral) areas.
On diffusion-weighted MRI, all 13 lesions were markedly hyperintense compared with the surrounding liver parenchyma. The mean ADC value was 1.84 × 10−3 mm2/s (Fig. 1B).
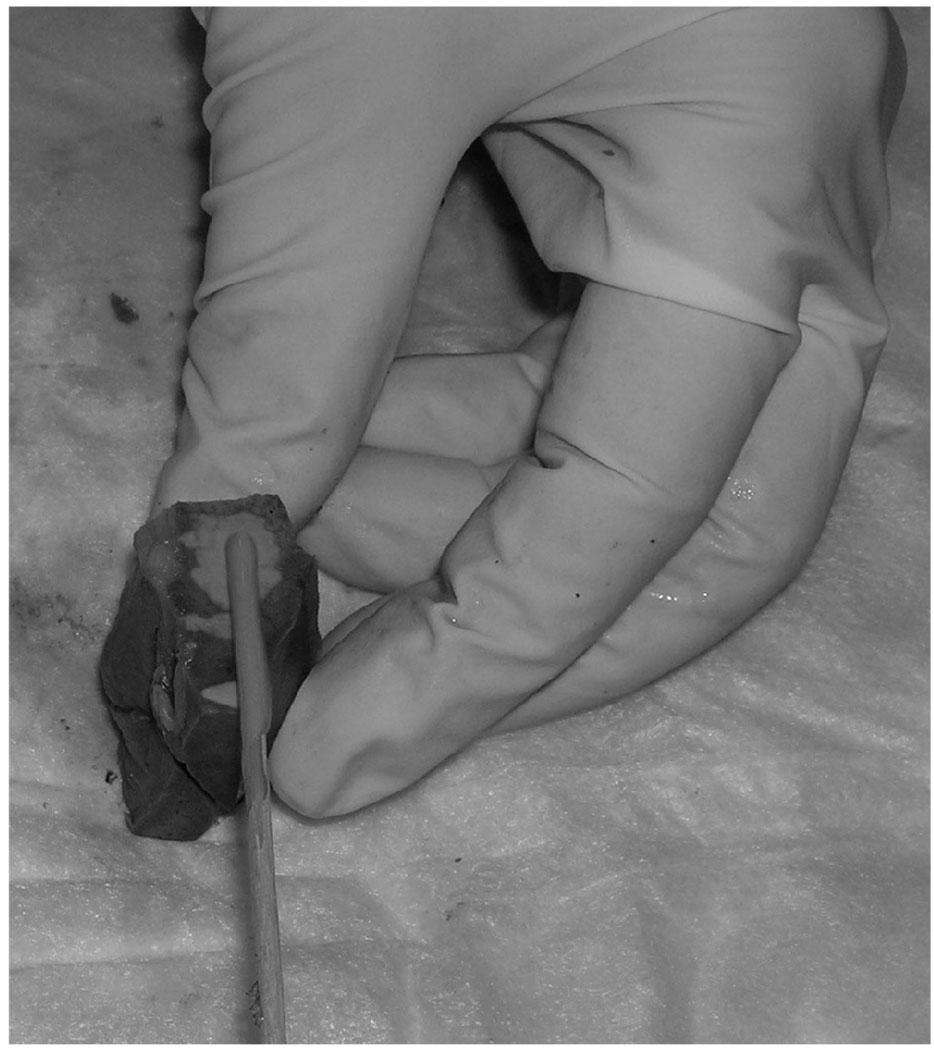
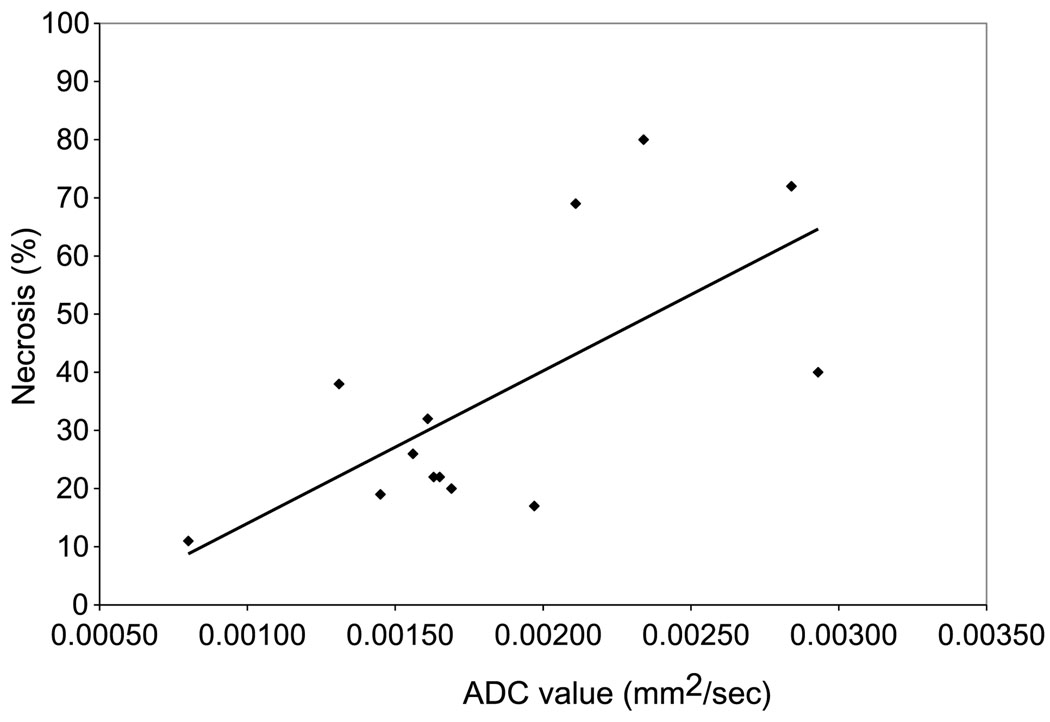
All tumors were sliced in the axial plane to correspond to the plane of the MR images and placed in standard cassettes. Proper orientation was maintained by staining the dorsal and medial sides of each slice with different colors (Fig. 2). At pathologic analysis, all tumors were undifferentiated and consisted of necrotic and viable cells (Fig. 1C). The mean tumor size at pathology was 16 mm (range, 8–27 mm). The mean percent necrosis at the tumor’s pathologic condition was 36% (range, 11%–80%). The correlation between ADC value and percent necrosis showed an R value of 0.68 (Fig. 3). In addition, the tumor size measured at pathology and percent necrosis showed a strong correlation, with an R value of 0.91. Compared with peripheral viable areas, the central necrotic areas of the tumor showed a significantly higher ADC value of 2.25 × 10−3 mm2/s compared with 1.39 × 10−3 mm2/s (P = 0.02).
FIGURE 2.
Method of staining of the pathologic specimen to maintain proper anatomical orientation.
FIGURE 3.
Correlation between ADC value and percent necrosis. R = 0.68.
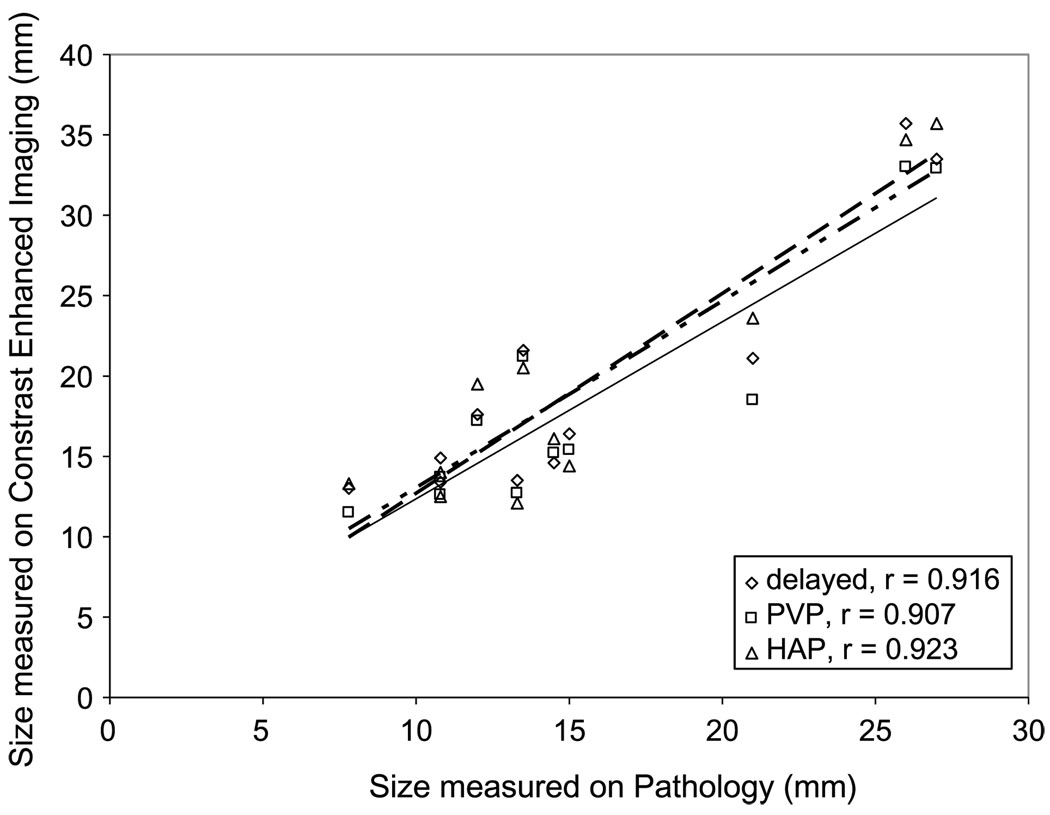
The tumor size on all phases of contrast-enhanced MRI showed a strong correlation with the tumor size at pathology (Fig. 4), with the strongest correlation in the portal venous phase. The mean tumor size of all 3 obtained phases on contrast-enhanced MRI was 28% (0%–62%) larger than the pathologic tumor size for 10 lesions. One lesion was slightly (4%) smaller on contrast-enhanced MRI than on its actual pathologic appearance. The remaining 2 lesions were not measured because contrast was not administered owing to the animal’s death after the diffusion-weighted images were acquired. This difference in tumor size showed no correlation with tumor size (R = 0.12) or tumor necrosis (R = 0.014).
FIGURE 4.
Correlation between tumor size on contrast-enhanced MRI and tumor size on pathology.
DISCUSSION
In the clinical setting, it is of crucial importance to determine tumor delineation and accurately differentiate between viable tumor and necrotic tumor, especially in treatment planning and the evaluation of treatment success. The aim of our animal study was to evaluate the role of diffusion-weighted MRI in determining tumor necrosis and contrast-enhanced MRI using Gd-EOB-DTPA in determining maximum tumor size measurement and tumor delineation using pathologic correlation. Our results indicate that diffusion-weighted MRI may be useful in quantifying tumor necrosis. Gadoxetic acid disodium, however, did not provide additional information on tumor necrosis but was valuable in determining tumor outline.
Necrosis is the most common morphologic alteration found in tumors and the surrounding tissue after radiotherapy and chemotherapy. In view of the emergence of new anticancer therapies, which are based on stabilizing disease rather than causing tumor disappearance, tumor necrosis has become the most important indicator of successful therapy.14,15 Moreover, the degree of therapy-induced necrosis has been shown to be associated with long-term prognosis in some tumors.16 Thus, although reduction in tumor size is the conventional method to assess tumor response, the percentage of tumor necrosis after therapy may be more informative.17
Gadoxetic acid disodium is a more specific and sensitive cellular marker for hepatocyte function than are current techniques such as gadolinium MRI and biphasic computed tomographic imaging.18 The uptake and accumulation of Gd-EOB-DTPA is caused by a lipophilic ethyl-oxybenzyl group resulting in selective enhancement of the normally functioning liver. Gadoxetic acid disodium enters into the hepatocytes through the organic anion-transporting polypeptide 1, which is involved in the hepatocellular uptake of bilirubin. Hereby, the possibility to detect tumors within the liver is increased because undifferentiated neoplastic cells, which lack anion transport and phagocytic functions, cannot extract Gd-EOB-DTPA from the blood. Thus, the healthy liver parenchyma is enhanced, whereas the tumors do not possess normally functioning hepatocytes and therefore lack accumulation, appearing as hypointense lesions.19 Our results confirmed the specific uptake of Gd-EOB-DTPA by a healthy liver tissue in the delayed phase. However, parts of the tumor that were viable on pathologic analysis showed similar absence of the uptake of contrast compared with parts of the tumor that were necrotic on pathology. Therefore, Gd-EOB-DTPA did not provide valuable information on tumor necrosis but was useful in determining tumor outline.
Diffusion-weighted MRI and ADC values represent the cellular integrity and motion of water molecules in biologic tissues and are thereby able to provide insight into tumor microstructure.20 The motion includes not only molecular diffusion of water but also microcirculation of blood (micro-perfusion). In the liver, diffusion-weighted imaging has been used to characterize focal hepatic lesions and to assess tumor response.21,22 Viable tumors are high in cellularity. These cells have an intact cell membrane that restricts the mobility of water molecules and causes a relatively low ADC value. Conversely, cellular necrosis causes increased membranous permeability, which allows water molecules to move freely and thus causes a relative increase in the ADC value. In our animal study, we demonstrated that ADC values are strongly correlated to the percentage of tumor necrosis on pathologic analysis (r = 0.68). These results reinforce the notion that diffusion-weighted MRI is useful in assessing tumor necrosis. Moreover, because pathologic correlation in the clinical setting is rarely possible, for obvious ethical reasons, these results add evidence to the use of diffusion-weighted MRI in evaluating tumor necrosis.
Although the tumor sizes measured on contrast-enhanced MRI and on their actual pathologic appearance were strongly correlated, we found a significant difference between radiologic and pathologic tumor sizes. This difference had poor positive correlation with tumor necrosis (R = 0.014), showing the same tendency in both viable and necrotic tumors. Moreover, this difference between contrast-enhanced MRI and pathologic findings was seen in both small and large tumors. One possible explanation for these findings in large necrotic tumors is the partial shrinkage of these tumors during liver fixation. It is possible that small viable tumors decrease in size at pathology because of the cessation of blood flow. Our findings are similar to those of a previous report, concluding that there is no adequate correlation between the radiologic and histologic sizes of HCC.23
This study has several limitations. First, our study group was relatively small, so further studies with a larger sample size are needed to confirm our conclusions. Second, the VX2 tumor used in our study is of nonhepatic origin. However, it has been proven to be convenient to study liver cancer in the animal because of the similarities in blood supply, genotype, and metabolism to advanced human HCC.24 Last, because it is difficult to use breath-holding or respiratory gating techniques in rabbits, respiratory movement during abdominal MRI might degrade image quality to some extent and cause variations in measurements.
In conclusion, contrast-enhanced MRI using Gd-EOB-DTPA may provide additional information about tumor outline in the liver. Moreover, we showed a remarkable correlation between ADC values and tumor necrosis. Thus, diffusion-weighted imaging may be useful to assess tumor necrosis; nevertheless, the search for new modalities remains important.
Acknowledgments
The study is funded in part by a research grant from Bayer Healthcare Pharmaceutical.
REFERENCES
- 1.Vossen JA, Buijs M, Kamel IR. Assessment of tumor response on MR imaging after locoregional therapy. Tech Vasc Interv Radiol. 2006;9:125–132. doi: 10.1053/j.tvir.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste H, Hedlund LW, Johnson GA. Mechanism of detection of acute cerebral ischemia in rats by diffusion-weighted magnetic resonance microscopy. Stroke. 1992;23:746–754. doi: 10.1161/01.str.23.5.746. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000;217:331–345. doi: 10.1148/radiology.217.2.r00nv24331. [DOI] [PubMed] [Google Scholar]
- 5.Buijs M, Kamel IR, Vossen JA, et al. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol. 2007;18:957–963. doi: 10.1016/j.jvir.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Taouli B, Vilgrain V, Dumont E, et al. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71–78. doi: 10.1148/radiol.2261011904. [DOI] [PubMed] [Google Scholar]
- 7.Deng J, Rhee TK, Sato KT, et al. In vivo diffusion-weighted imaging of liver tumor necrosis in the VX2 rabbit model at 1.5 Tesla. Invest Radiol. 2006;41:410–414. doi: 10.1097/01.rli.0000201232.14903.da. [DOI] [PubMed] [Google Scholar]
- 8.Deng J, Virmani S, Young J, et al. Diffusion-weighted PROPELLER MRI for quantitative assessment of liver tumor necrotic fraction and viable tumor volume in VX2 rabbits. J Magn Reson Imaging. 2008;27:1069–1076. doi: 10.1002/jmri.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamm B, Staks T, Muhler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995;195:785–792. doi: 10.1148/radiology.195.3.7754011. [DOI] [PubMed] [Google Scholar]
- 10.Reimer P, Rummeny EJ, Shamsi K, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996;199:177–183. doi: 10.1148/radiology.199.1.8633143. [DOI] [PubMed] [Google Scholar]
- 11.Huppertz A, Balzer T, Blakeborough A, et al. Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid–enhanced MR images with intraoperative findings. Radiology. 2004;230:266–275. doi: 10.1148/radiol.2301020269. [DOI] [PubMed] [Google Scholar]
- 12.Tsuda N, Kato N, Murayama C, et al. Potential for differential diagnosis with gadolinium–ethoxybenzyl–diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in experimental hepatic tumors. Invest Radiol. 2004;39:80–88. doi: 10.1097/01.rli.0000105331.11373.c0. [DOI] [PubMed] [Google Scholar]
- 13.Zizka J, Klzo L, Ferda J, et al. Dynamic and delayed contrast enhancement in upper abdominal MRI studies: comparison of gadoxetic acid and gadobutrol. Eur J Radiol. 2007;62:186–191. doi: 10.1016/j.ejrad.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 14.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 16.Yan K, Chen MH, Yang W, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Halavaara J, Breuer J, Ayuso C, et al. Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT-a multicenter trial. J Comput Assist Tomogr. 2006;30:345–354. doi: 10.1097/00004728-200605000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Saito K, Kotake F, Ito N, et al. Gd-EOB-DTPA enhanced MRI for hepatocellular carcinoma: quantitative evaluation of tumor enhancement in hepatobiliary phase. Magn Reson Med Sci. 2005;4:1–9. doi: 10.2463/mrms.4.1. [DOI] [PubMed] [Google Scholar]
- 20.Nasu K, Kuroki Y, Nawano S, et al. Hepatic metastases: diffusion-weighted sensitivity-encoding versus SPIO-enhanced MR imaging. Radiology. 2006;239:122–130. doi: 10.1148/radiol.2383041384. [DOI] [PubMed] [Google Scholar]
- 21.Bruegel M, Holzapfel K, Gaa J, et al. Characterization of focal liver lesions by ADC measurements using a respiratory triggered diffusion-weighted single-shot echo-planar MR imaging technique. Eur Radiol. 2008;18:477–485. doi: 10.1007/s00330-007-0785-9. [DOI] [PubMed] [Google Scholar]
- 22.Kamel IR, Reyes DK, Liapi E, et al. Functional MR imaging assessment of tumor response after 90Y microsphere treatment in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2007;18:49–56. doi: 10.1016/j.jvir.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Mejia GA, Gomez MA, Serrano J, et al. Correlation between the radiologic and histologic size of hepatocellular carcinoma in patients eligible for liver transplantation. Transplant Proc. 2006;38:1394–1395. doi: 10.1016/j.transproceed.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 24.Geschwind JF, Artemov D, Abraham S, et al. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol. 2000;11:1245–1255. doi: 10.1016/s1051-0443(07)61299-8. [DOI] [PubMed] [Google Scholar]