Abstract
While a variety of in-vitro models have been employed to investigate the response of load-bearing tissues to hydrostatic pressure, long-term studies are limited by the need to provide for adequate gas exchange during pressurization. Applying compression in vitro may alter the equilibrium of the system and thereby disrupt the gas exchange kinetics. To address this, several sophisticated compression chamber designs have been developed. However, these systems are limited in the magnitude of pressure that can be applied and may require frequent media changes, thereby eliminating critical autocrine and paracrine signaling factors. To better isolate the cellular response to long-term compression, we created a model that features continuous gas flow through the chamber during pressurization, and a negative feedback control system to rigorously control dissolved oxygen levels. Monitoring dissolved oxygen continuously during pressurization, we find that the ensuing response exhibits characteristics of a second- or higher-order system which can be mathematically modeled using a second-order differential equation. Finally, we use the system to model chronic nerve compression injuries, such as carpal tunnel syndrome and spinal nerve root stenosis, with myelinated neuron-Schwann cell co-cultures. Cell membrane integrity assay results show that co-cultures respond differently to hydrostatic pressure, depending on the magnitude and duration of stimulation. In addition, we find that myelinated Schwann cells proliferate in response to applied hydrostatic compression.
Key words: in-vitro studies, ischemia, models of injury, peripheral nerve injury
Introduction
Hydrostatic compression is an important mechanical stimulus that directs cellular activity in several biological tissues including bone (Zhang et al., 1998), intervertebral disc (Setton and Chen, 2006), articular cartilage (Lammi et al., 2004), and the vascular system (Muller et al., 2004). As the study of mechanotransduction in vivo is very complex, various in-vitro models have been used to study tissues that experience physiological mechanical loading (Blackman et al., 2000; Halka et al., 2008; Matsuzaki et al., 2005; Reilly et al., 2003), as well as for studies of mechanically-induced injury (Bottlang et al., 2007; Gidday et al., 1999; LaPlaca et al., 2005; Pfister et al., 2004; Yang et al., 2004), and to facilitate the engineering of load-bearing tissues (Hasel et al., 2002; Park et al., 2008; Takai et al., 2004). One of the primary challenges when designing compression chambers for chronic compression studies is the necessity to provide adequate gas exchange for the cells during pressurization. This has been addressed by some groups by cycling media through the chamber during cyclic depressurization (Carver and Heath, 1999; Myers et al., 2007). However, autocrine and paracrine signaling are important to cellular function, and these signaling factors may be washed away during frequent media changes. For example, axon-derived growth factors have autocrine and paracrine actions on neurons and glial cells that direct gliogenesis and myelination (Melli and Hoke, 2007). Other systems include a gas-liquid interface at which oxygen and carbon dioxide gas exchange can occur during pressurization (Hasel et al., 2002; Yang et al., 2004). However, pressurizing a system with a gas-liquid interface will increase the solubility of gas in the media, altering the gas exchange equilibrium. Cells are sensitive to changes in dissolved oxygen and other variables. For example, neural tissues, which have a well-developed vasculature in vivo, are susceptible to transitory changes in oxygen and pH (Dean and Mulkey, 2000). Therefore, in order to isolate the cellular response to a purely mechanically-derived stimulus, it is necessary to compensate for these changes in dissolved gas levels.
In this study, we created a novel chamber for the compression of cell cultures in vitro. This compression system features a gas-liquid interface and continuous gas flow through the chamber to allow for adequate gas exchange during long-term experimentation, as well as a negative feedback control system that provides rigorous control of dissolved oxygen during pressurization. By dynamically monitoring dissolved oxygen in the system using a fiberoptic probe, we found that the compression chamber system exhibits a rapid transient increase in dissolved oxygen in response to pressurization. The reasons underlying the transient response may be complex. In general, mechanical and electrical systems that exhibit transient oscillations or overshoot are represented as second- or higher-order systems. While first-order systems can be modeled by a single “energy storage unit,” higher-order systems contain multiple independent energy storage units that exchange stored energy. Such systems cannot respond instantaneously to changes and will exhibit transient responses when subjected to inputs or disturbances. In this study, we apply standard control theory and mathematically model the compression system as a second-order system. The rise, peak, and settling times, as well as the percentage overshoot are computed using experimental data and used to identify parameters that predict transient peak dissolved oxygen, steady-state dissolved oxygen, and the associated time scales. In addition, we use the compression chamber as a model for chronic nerve compression (CNC) injuries, such as carpal tunnel syndrome and spinal nerve root stenosis, and assess the integrity of the cell membrane in neuron-Schwann cell co-cultures. Our results show that myelinated co-cultures respond differently to hydrostatic pressure depending on the magnitude and duration of stimulation.
Methods
Bioreactor design
A custom bioreactor was developed to apply sustained defined hydrostatic pressure to a myelinated dorsal root ganglion (DRG) neuron-Schwann cell co-culture system (Fig. 1). To this end, the compression chamber was designed and manufactured in-house to resemble a standard multi-well culture plate. The main structural components of the chamber were machined from biologically inert 316-L stainless steel. To enable direct microscopic visualization of the cultures during pressurization, the bottom of the chamber wells was composed of borosilicate glass slides and the top from transparent polycarbonate. The chamber was sealed using medical-grade silicone rubber gaskets. A sealed diaphragm pump (KNF Neuberger, Trenton, NJ) pumped sterile air from the incubator into a separate humidity chamber before it entered the compression chamber. An outlet flow control valve connected to the top of the chamber allowed for continuous gas flow. Media evaporation due to flow was insignificant.
FIG. 1.
The hydrostatic compression chamber system is designed to apply a defined magnitude of pressure to cell cultures while maintaining dissolved oxygen and pH homeostasis.
A solid-state piezoresistive pressure sensor (GE Novasensor, Billerica, MA) was connected to the compression chamber to continuously monitor the internal pressure during pressurization. These data were input into the computer using a data acquisition board (DAQPad 6015; National Instruments, Austin, TX) and recorded with custom LabView code (National Instruments). Temperature was also measured and recorded using an independent probe (Presens, Regensburg, Germany). A semi-micro pH electrode (SensorEx, Garden Grove, CA) and fiberoptic dissolved oxygen sensor (Fibox; Presens) were used to continuously monitor the conditions of the culture media during the experiments. The most commonly used Clark dissolved oxygen sensors employ a flexible membrane that distorts under pressure. Fiberoptic dissolved oxygen sensors have no membrane and thus can be used for hydrostatic pressure experiments. To compensate for fluctuations in dissolved oxygen, a custom proportional-integral-derivative (PID) feedback control system (LabView; National Instruments) was used to regulate oxygen and nitrogen flow into the incubator via two voltage-operated mass flow control valves (Clippard, Cincinnati, OH; Fig. 2). As the pH changes were less dramatic and occurred over a longer timeframe, pH homeostasis was maintained by manually altering the CO2 settings on the incubator. The entire device was placed in a 37°C incubator and all components equilibrated to the environment therein prior to experimentation.
FIG. 2.
A feedback control system maintains dissolved oxygen homeostasis during pressurization. Dissolved oxygen is measured continuously using a fiberoptic dissolved oxygen sensor and recorded with LabView software. A PID controller compares the actual and baseline dissolved oxygen values to regulated oxygen and nitrogen flow into the incubator.
Primary Schwann cell cultures
All animal procedures were approved by the university's institutional animal care and use committee. Sciatic nerves were harvested from 3-day-old neonatal rats. The Schwann cells were extracted and purified using the modified Brockes technique (Brockes and Raff, 1979). Briefly, the tissue was digested with 0.1% collagenase (Worthington Biochemical, Lakewood, NJ) followed by 0.25% trypsin (Sigma, St. Louis, MO), and triturated with a fire-polished glass pipette to disaggregate the cells. To eliminate fibroblast contamination, the cells were treated with an anti-mitotic agent, cytosine b-D arabinoside (10–5 M; Sigma), followed by anti-Thy1.1 (Serotec, Oxford, U.K.), and rabbit serum complement (Calbiochem, San Diego, CA). The Schwann cell cultures were expanded on poly-D-lysine-coated Petri dishes (BD Biosciences, San Jose, CA) in DMEM/F12 media (Millipore, Billerica, MA) supplemented with 3% FBS (Hyclone, Logan, UT), 1% penicillin-streptomycin (Millipore), 10 nM heregulin (Sigma), and 5 μM forskolin (Fisher Scientific, Pittsburgh, PA).
Primary dorsal root ganglion neuron cultures
DRGs were harvested from 14-day-old rat embryos and placed in ice-cold Liebovitz L15 media (Millipore). The cells were then digested and cultured according to a modification of previously established techniques (Banker and Goslin, 1998). Briefly, the DRGs were digested with 0.25% trypsin (Sigma) for 45 min at 37°C. The extracted cells were plated in neurobasal (NB) medium (Millipore) supplemented with 1% FBS (Hyclone), 2% B27 supplement (Millipore), 1% penicillin-streptomycin (Millipore), 1% glutamax (Millipore), and 0.4% glucose (Sigma) on type I rat tail collagen-coated (Sigma) Thermanox coverslips (Nalge Nunc, Rochester, NY). As fibroblasts are an important component of the intraneural environment and are known to respond to mechanical stimuli in a variety of other load-bearing tissues (Wang et al., 2005), they were not eliminated from the co-cultures by cycling with anti-mitotoic factors. The neurons were cultured for 1 week before 2 × 105 purified Schwann cells were added to each cover-slip. The cells were then cultured for 1 week in NB media without serum, followed by NB with 10% FBS, and 50 μg/mL vitamin C to initiate myelination. Myelination occurred within 7–8 days. While there may be some slight variations between percentage cell content and/or rate of Schwann cell proliferation in the different co-cultures, all co-cultures were harvested and cultured identically for these experiments, and thus intra-culture variability should be negligible.
Membrane integrity assay
As cell membrane damage is a primary pathophysiological change that occurs in response to mechanically-mediated neuronal injury (Serbest et al., 2005), membrane integrity was evaluated using a lactate dehydrogenase (LDH) assay to measure LDH released from the cytosol of damaged cells before and after pressurization. Cell culture medium was collected during regular medium changes and the assays were performed according to the manufacturer's specifications (Roche Applied Science, Indianapolis, IN). Colorimetric changes were calculated by measuring absorbance of the samples at 490 and 650 nm using a microplate reader (ThermoMax, Molecular Devices, Sunnyvale, CA). Background absorbance from plain culture media was subtracted from all values. Control cultures were transferred to a similarly designed chamber and kept in a separate incubator under normal culture conditions during experimentation (37°C, 100% humidity, 5% CO2, and 20% oxygen). A cell lysis reagent was applied to uncompressed co-cultures as a positive control according to the manufacturer's specifications. Eqn. (1) was used to calculate the percentage of LDH release, according to the manufacturer's instructions:
 |
(1) |
For pressure magnitude studies, cells were subjected to varying magnitudes of pressure for 24 h, and LDH was assayed at 0 h post-compression (18 mm Hg, n = 21; 37 mm Hg, n = 11; 46 mm Hg, n = 9; and 62 mm Hg, n = 6), or 24 h post-compression (37 mm Hg, n = 11; 46 mm Hg, n = 9; and 62 mm Hg, n = 8). For pressure duration studies, cells were subjected to 18 mm Hg of pressure for varying durations and LDH assayed at 0 h post-compression (1 day, n = 21; 3 days, n = 10; 4 days, n = 15; 6 days, n = 10; and 7 days, n = 10).
Schwann cell proliferation
As the cultures contain several cell types, for BrdU labeling experiments the co-cultures were double immuno-labeled with a pan-specific Schwann cell marker, S-100. Nuclear incorporation of a thymidine analog, bromodeoxyuridine (BrdU) was used to evaluate Schwann cell proliferation. At 24 h following compression BrdU was added to the cells and incubated for 1.5 h to allow for incorporation into the DNA of proliferating cells. The cells were fixed, and fluorescein-conjugated BrdU labeling was performed according to the manufacturer's specifications (Roche Applied Science). Nonspecific immunoreactivity was blocked with 4% normal goat serum (diluted in PBS with 0.25% Triton-X) for 1 h, and the cultures were then immunostained with antibodies against S-100 (rabbit anti-S100, 1:300; Dako Cytometrics, Glostrup, Denmark) at 4°C overnight, followed by AlexaFluor 546-conjugated secondary antibody (goat anti-rabbit, 1:1000) for 1 h. An Olympus IX-71 fluorescence microscope and Visiopharm software (Hørsholm, Denmark) were used to perform an unbiased, uniformly random estimate of the total number of BrdU-labeled Schwann cells and total Schwann cell nuclei per cover-slip. Data are presented as the percentage of proliferating Schwann cells relative to the total number of Schwann cells on the cover-slip for experimental (n = 4) and control (n = 4) cultures. Only cells double-labeled with BrdU and S-100 were counted for the proliferation assay.
Statistical analysis
Statistical analysis was performed using Microsoft Excel software. Data are presented as means and standard error of the mean (SEM). Comparisons were done using one-way analysis of variance (ANOVA), and significance was tested by the Student's t-test. p Values ≤ 0.05 were considered to be significant.
Results
Hydrostatic compression chamber design
The hydrostatic compression chamber is designed to apply a defined magnitude of static pressure to neural cell cultures (Fig. 1). It is composed of biologically inert 316-L stainless steel which, unlike many polymers and resins, can be repeatedly sterilized without leaching toxic degradation products into the media. The cells are placed in individual culture wells within the chamber and covered with media. A piezoresistive pressure sensor is used to verify the magnitude of applied pressure within the chamber during experimentation. This allows us to ensure that there are no significant pressure fluctuations due to turbulence of air within the chamber during pressurization. A micro-pH electrode and fiberoptic dissolved oxygen sensor are used to monitor the condition of the cell culture media continuously during pressurization. A small volume of air was allowed to leak out of the chamber continuously through a flow control valve, allowing for gas exchange during long-term experimentation.
Hydrostatic compression chamber characterization
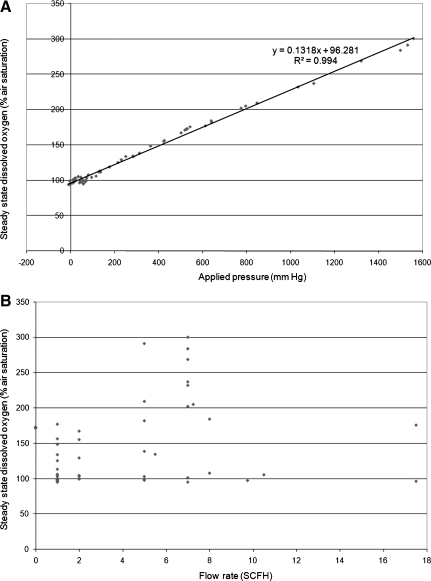
Dissolved oxygen was measured and recorded as a function of time for various applied pressures and flow rates (through the flow control valve). In both the transient and steady states, there was no correlation between the flow rate and the dissolved oxygen, indicating that neither response is related to the rate of flow (Figs. 3A and B). In the steady state, the system exhibited a linear relationship between pressure and dissolved oxygen (Fig. 3A). This is consistent with Henry's law, which describes the steady-state concentration of gas (C) dissolved in media under pressure (P), and is shown in Eqn. (2),
 |
(2) |
FIG. 3.
(A) The steady-state dissolved oxygen is directly proportional to the applied hydrostatic pressure. (B) Flow rate is not correlated with steady-state dissolved oxygen standard cubic feet per hour (SCFH).
where kH = Henry's constant for a given gas, solute, and temperature.
Using linear regression analysis to find the line of best fit relating pressure to concentration of dissolved oxygen, the calculated Henry's constant for oxygen in culture media at 37°C is 0.847 mm Hg/μM. This the same as the known Henry's constant value for oxygen in water at 37°C (kH = 0.847 mm Hg/μM).
In the transient state, the peak of the transient dissolved oxygen is directly proportional to the pressure change (Fig. 4A). We find that the compression chamber system with a gas-liquid interface exhibits characteristics of a damped second-order or higher system. The simplest model may be described by the second-order differential equation, Eqn. (3):
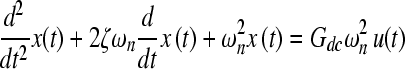 |
(3) |
FIG. 4.
(A) The transient-state dissolved oxygen is directly proportional to the applied hydrostatic pressure. (B) Flow rate is not correlated with transient-state dissolved oxygen standard cubic feet per hour (SCFH).
where x(t) = response of the system, u(t) = input to the system, ζ = damping ratio, ωn = undamped natural frequency, and Gdc = gain of the system.
Using the characteristics of the transient response (Table 1), we can calculate the parameters for the differential equation (333; = 0.72, ωn = 0.82 min−1). Since 0 < ζ < 1, this is an underdamped system. Assuming that the application of pressure is a step-function input, we can solve the differential equation to predict the system response as a function of time, using Eqn. (4):
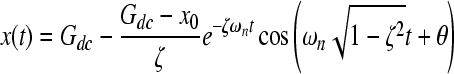 |
(4) |
Table 1.
Parameters Used To Generate a Second-Order Mathematical Model of the Dissolved Oxygen Response
| Value | Standard deviation | |
|---|---|---|
| Percent Overshoot (from transient to steady state) | 3.0% | 1.8% |
| Normalized rise time (to rise past the steady-state value) | 3.2 min | 2.9 min |
| Peak time (from initial value to transient peak) | 9.1 min | 5.9 min |
| Settling time (to settle within 2% of the steady-state value) | 14.1 min | 7.2 min |
Using the dissolved oxygen response data recorded with the fiberoptic sensor during pressurization, the percent overshoot, normalized rise time, peak time, and settling time were calculated. These parameters were used to generate a second-order mathematical model of the dissolved oxygen response.
where 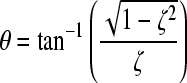 and
and 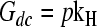
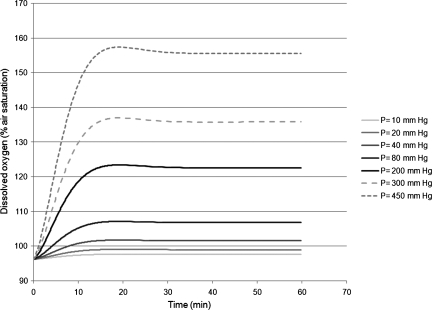
Figure 5 shows the predicted dissolved oxygen response as a function of time calculated using Eqn. (4) at various applied pressures.
FIG. 5.
Using the solution calculated from the second-order differential equation [Eqn. (4)], the dissolved oxygen is graphed as a function of time for different applied pressures.
Hydrostatic compression chamber dissolved oxygen control
To compensate for transient and steady-state increases in dissolved oxygen, we used a PID feedback control system (Fig. 2). Piezoresistive pressure-sensor data and fiberoptic dissolved-oxygen-sensor data are read into the computer and recorded using LabView software. To maintain dissolved oxygen and pH homeostasis, a negative feedback PID control system was used to regulate dissolved oxygen within the chamber during pressurization. During pressurization, the oxygen dissolved in the cell culture media was monitored continuously using a fiberoptic dissolved-oxygen sensor. The recorded value was compared with the ideal baseline dissolved oxygen level that was measured prior to pressurization. When the dissolved oxygen deviated significantly from the baseline value, a voltage was generated by the data acquisition (DAQ) board to transiently open either the oxygen or nitrogen flow control valve. The resulting change in dissolved oxygen was recorded and fed back into the control system. With this control system, we are able to maintain a steady dissolved oxygen value that was within 2% of the baseline uncompressed dissolved oxygen level (Fig. 6).
FIG. 6.
The chamber was pressurized to 21 mm Hg with continuous gas flow through the chamber during pressurization of 0.5 standard cubic feet per hour. Using the feedback control system, we maintained the dissolved oxygen response within 2% of the baseline value before pressurization.
Hydrostatic compression model of chronic nerve compression injury
Neuron-Schwann cell-fibroblast co-cultures were used as an in-vitro model of the peripheral nerve to study CNC injuries. The cells maintained morphological integrity after 1 week of being cultured in the compression chamber under normal culture conditions (37°C, pH 7.1–7.3, and dissolved oxygen 95–105%) with no applied pressure. There was no morphological indication of axonal degeneration, as evidenced by loss of axon number, loss of myelinated axons, a large number of myelin ovoids, or paranodal swellings.
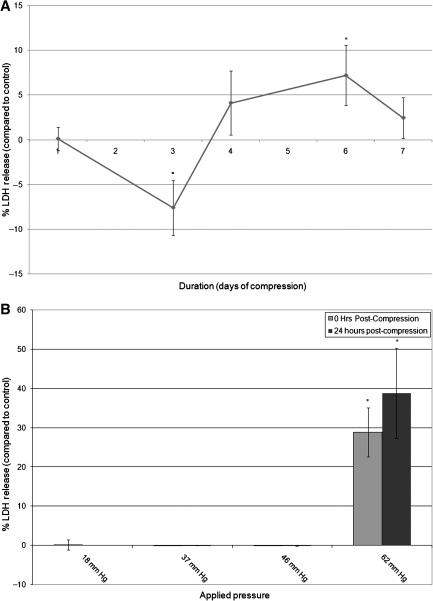
Using the fiberoptic dissolved oxygen sensor and dissolved oxygen control system to maintain rigorous control of dissolved oxygen, we pressurized neuron Schwann cell co-cultures with different magnitudes and durations of stimuli. The magnitudes and durations of pressure were selected to remain within a reasonable physiologic range without causing immediate LDH release from the cells (following 24 h of compression). Cell membrane assay results showed that there was no significant production of LDH by the cultures at low levels of pressure (18–46 mm Hg) for 24 h (Fig. 7B). A pressure of 62 mm Hg was required to elicit a response from the cells. However, when a low level of pressure (18 mm Hg) was applied for 7 consecutive days, the LDH levels increased (Fig. 7A). These data appear to indicate that chronic compression has a different effect on the nerve cells than acute compression. Interestingly, the LDH level decreased significantly after 3 days of compression, possibly indicating a protective response. While LDH release was insignificant in the co-cultures with 24 h of compression (18 mm Hg), BrdU S-100 labeling showed a significant (2.3-fold) increase in Schwann cell proliferation at 24 h post-compression (21 mm Hg, 24 h) (Fig. 8).
FIG. 7.
Myelinated neuron-Schwann cell co-cultures were pressurized at 18 mm Hg for 1–7 days (A), or 18–62 mm Hg for 24 h (B). Cell membrane integrity, as measured by LDH release into the culture medium at 0 and 24 h post-compression, depends on the magnitude and duration of applied pressure (p ≤ 0.05).
FIG. 8.
Schwann cells were subjected to 24 h of hydrostatic compression at 21 mm Hg. The percentage of Schwann cells undergoing proliferation at 24 h post-compression increased significantly (*p ≤ 0.05) when compared with uncompressed control cultures.
Discussion
Several injuries involve chronically elevated levels of hydrostatic compression, including chronic nerve compression injury, glaucoma, traumatic brain injury, and spinal cord injury (Hernandez et al., 2008; Rempel and Diao, 2004; Shreiber et al., 1999; Sparrey et al., 2009). One way to model these injuries is to introduce excess hydrostatic pressure in a non-elastic chamber. The need to provide gas exchange for long-term experimentation has been addressed in several different ways, including cycling media through the chamber during depressurization (Carver and Heath, 1999; Myers et al., 2007), and providing a gaseous headspace for continuous gas exchange during pressurization (Hasel et al., 2002; Yang et al., 2004). However, these models are limited in their ability to completely isolate the mechanical response of the cells from other non-mechanical effects.
In this study, we have designed an in-vitro model that allows for the study of chronic hydrostatic compression by rigorously controlling the loading parameters, such as dissolved oxygen concentration and pH, as well as the magnitude of stimulation. This is accomplished via a novel chamber that uses feedback to control the dissolved oxygen level. By measuring oxygen levels continuously during pressurization, we found that while the physiologic levels of pressure used for many experiments are too low to result in a significant steady-state increase in dissolved oxygen or pH as previously reported (Hasel et al., 2002), there was a rapid and transient increase in the dissolved oxygen immediately following pressurization of the chamber. We hypothesized that this transient response may be due to the cell culture medium acting as a dampening agent in the system, “storing” the transient increase in dissolved oxygen caused by pressurization. Based on this hypothesis and constants calculated from the experimental parameters (Table 1), we were able to adequately model this response using a second-order differential equation [Eqn. (3)]. Finally, using a PID feedback-control system to modulate dissolved oxygen during pressurization, we could compensate for the transient and steady-state response of the system (Fig. 4). This in-vitro model is not limited to a range of low pressures that do not affect steady-state dissolved gas levels, and thus may be used for many different cell types, including bone and cartilage (≈300 kPa) (Ozawa et al., 1990). In future studies, the system may be modified to allow for application of both static and dynamic pressurization regimes by the addition of a voltage-controlled flow outlet valve.
Next, we used the in-vitro model to study the effect of chronic hydrostatic compression on neuron-Schwann cell co-cultures. Several factors contribute to CNC injury, including mechanical stimulus, ischemia, and the immune response (Gupta et al., 2005). This is the first study, to our knowledge, that uses an in-vitro model of CNC injury that isolates the effects of direct mechanical stimulus on myelinated neurons, while maintaining the reciprocal relationship between neurons and glial cells that is critical to their function (Fu and Gordon, 1997). We found that the cellular response to sustained hydrostatic compression varies based on both the magnitude and duration of stimulation. Low pressures had no effect on LDH release from the co-cultures, while a pressure of 62 mm Hg elicited a significant response. Hydrostatic pressures of magnitudes less than 100 MPa (750,000 mm Hg) generally do not affect cell viability (Frey et al., 2004), and other mechanosensitive cells, such as chondrocytes, can experience pressures as high as 18 MPa in vivo during normal physical activity (Hodge et al., 1986). These data suggest that neural cells may be particularly sensitive to elevated hydrostatic pressures. The normal ambient and pathological levels of pressure that these cells experience in vivo remain unknown. While several studies have shown an average increase in pressure in the carpal tunnel, from 8.3–26.8 mm Hg in normal patients (Werner et al., 1997) to 18–159 mm Hg in patients with carpal tunnel syndrome (Okutsu et al., 2001), these values may not correlate with the levels seen at a cellular level. Peripheral nerves are heterogeneous composite structures comprised of multiple layers of connective tissue, and these layers likely either contribute to the ambient pressure exerted on the cells, or aid in absorbing some of the force from compression. As such, the pressures measured in the carpal tunnel may not necessarily correlate with the pressures exerted on the cells. In addition to external compressive forces exerted on the nerve (Ko and Brown, 2007), low levels of pressure may cause microvascular trauma in the epineurial sheath that surrounds the nerve (Lundborg et al., 1983). Due to limited lymphatic clearance of endoneurial fluid edema may result, causing increased intra-neural fluid pressures (Mizisin et al., 1990). For these studies, pressures were chosen from within the physiologic range for the carpal tunnel, at the lower end of those seen in patients with carpal tunnel syndrome, that would induce a physiological response (as assessed by a cell proliferation assay), but not cause immediate traumatic injury to the cells (as assessed by LDH release). Future studies that more accurately determine the pressure exerted on cells within the nerve with CNC injury would aid in mechanical modeling of CNC injuries in the future.
While lower non-noxious levels of compression (18–46 mm Hg) applied for 24 h did not induce significant release of LDH from the cultures, applying a low pressure (18 mm Hg) for 6 consecutive days did (Fig. 7). Peripheral nerves normally experience fluctuations of pressure during normal daily activities without causing cellular injury (Rempel et al., 1994). However, in the case of CNC injuries, chronic application of physiologic levels of pressure can alter the cellular response and lead to injury. Interestingly, integrity of the cell membrane decreased significantly with 3 days of hydrostatic compression. This may be due to an overall decrease in the total number of Schwann cells in the culture. However, this is an unlikely explanation, as cell death was minimal at 24 h post-injury. As cell death is measured compared with uncompressed control cultures, the apparent decrease in cell death could also be due to an increased rate of proliferation in the control cultures compared with the experimental cultures, increasing the overall cell number. A third possibility is that the decrease in cell death indicates the activation of neuroprotective mechanisms. In normal patients, peripheral nerve cells likely experience an ambient level of pressure in vivo. These pressures could contribute to the normal functioning of the cells, and thus culturing neural cells at atmospheric pressures may induce a low level of cellular stress. Further study is needed to investigate a possible neuroprotective response to hydrostatic compression.
Following peripheral nerve injury, Schwann cells in the distal nerve stump proliferate and undergo a phenotypic switch to facilitate axonal regeneration. This process involves producing cytokines to recruit macrophages to the site of injury and clearing axonal and myelin debris (Banner and Patterson, 1994; Bolin et al., 1995; Siebert et al., 2000; Toews et al., 1998; Tofaris et al., 2002), increasing synthesis of neural cell adhesion molecules and basement membrane components (Araki and Milbrandt, 1996; Kleitman et al., 1988; Martini, 1994), and producing trophic factors that promote the survival of their associated neurons (Boyd and Gordon, 2003; Chan et al., 2004; Cosgaya et al., 2002; Peng et al., 2003). Previous studies have shown that with CNC injury Schwann cells exhibit robust proliferation early in the disease process (Gupta and Steward, 2003). Consistent with this, we found a significant 2.3-fold increase in Schwann cell proliferation in the co-cultures following 24 h of compression. The reason for this dramatic proliferation following injury remains unclear. It has been suggested that an excessive number of Schwann cells are needed to form the abnormally short internodes associated with remyelinated nerve fibers. However, inhibiting Schwann cell proliferation has no apparent affect on remyelination post-injury (Yang et al., 2008). Future studies will focus on defining the role that chronic mechanical stimulation plays in the pathology associated with CNC injury.
Paracrine and autocrine signals play an important role in cell survival and response to injury. Following injury to the peripheral nervous system, neurons and glial cells depend on each other for survival and regeneration, mediated primarily by neurotrophic factors. For example glial cell line–derived neutrophilic factor (GDNF), which is rapidly expressed in Schwann cells following injury, promotes nerve regeneration (Hoke et al., 2002). Neurotrophic factors may also be involved in triggering programmed cell death and demyelination following injury. For example, NT3 inhibits myelination when added exogenously to co-cultures in vitro (Chan et al., 2001), and induction of NO expression by Schwann cells promotes neuron cell death in culture (Lee et al., 2007). Since the hydrostatic compression chamber system does not require media cycling, it has been specifically designed for the study of complex co-cultures that involve paracrine signaling (i.e., myelinated co-cultures), and may be used to further elucidate the role of these signaling factors in the cellular response to mechanical stimuli.
Acknowledgments
Funding sources include NIH/NINDS grant 5R01 NS049203 (R.G.) and the American Society for Surgery of the Hand Basic Science Research Grant (R.G. and L.R.F.). We would like to thank Winnie Palispis and Dr. Hermann Frieboes for their assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Araki T. Milbrandt J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. doi: 10.1016/s0896-6273(00)80166-x. [DOI] [PubMed] [Google Scholar]
- Banker G. Goslin K. Culturing Nerve Cells. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Banner L.R. Patterson P.H. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman B.R. Barbee K.A. Thibault L.E. In vitro cell shearing device to investigate the dynamic response of cells in a controlled hydrodynamic environment. Ann. Biomed. Eng. 2000;28:363–372. doi: 10.1114/1.286. [DOI] [PubMed] [Google Scholar]
- Bolin L.M. Verity A.N. Silver J.E. Shooter E.M. Abrams J.S. Interleukin-6 production by Schwann cells and induction in sciatic nerve injury. J. Neurochem. 1995;64:850–858. doi: 10.1046/j.1471-4159.1995.64020850.x. [DOI] [PubMed] [Google Scholar]
- Bottlang M. Sommers M.B. Lusardi T.A. Miesch J.J. Simon R.P. Xiong Z.G. Modeling neural injury in organotypic cultures by application of inertia-driven shear strain. J. Neurotrauma. 2007;24:1068–1077. doi: 10.1089/neu.2006.3772. [DOI] [PubMed] [Google Scholar]
- Boyd J.G. Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Brockes J.P. Raff M.C. Studies on cultured rat Schwann cells. II. Comparison with a rat Schwann cell line. In Vitro. 1979;15:772–778. doi: 10.1007/BF02618303. [DOI] [PubMed] [Google Scholar]
- Carver S.E. Heath C.A. Semi-continuous perfusion system for delivering intermittent physiological pressure to regenerating cartilage. Tissue Eng. 1999;5:1–11. doi: 10.1089/ten.1999.5.1. [DOI] [PubMed] [Google Scholar]
- Chan J.R. Cosgaya J.M. Wu Y.J. Shooter E.M. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.R. Watkins T.A. Cosgaya J.M. Zhang C. Chen L. Reichardt L.F. Shooter E.M. Barres B.A. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgaya J.M. Chan J.R. Shooter E.M. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Dean J.B. Mulkey D.K. Continuous intracellular recording from mammalian neurons exposed to hyperbaric helium, oxygen, or air. J. Appl. Physiol. 2000;89:807–822. doi: 10.1152/jappl.2000.89.2.807. [DOI] [PubMed] [Google Scholar]
- Frey B. Franz S. Sheriff A. Korn A. Bluemelhuber G. Gaipl U.S. Voll R.E. Meyer-Pittroff R. Herrmann M. Hydrostatic pressure induced death of mammalian cells engages pathways related to apoptosis or necrosis. Cell Mol. Biol. (Noisy-le-grand) 2004;50:459–467. [PubMed] [Google Scholar]
- Fu S.Y. Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gidday J.M. Beetsch J.W. Park T.S. Endogenous glutathione protects cerebral endothelial cells from traumatic injury. J. Neurotrauma. 1999;16:27–36. doi: 10.1089/neu.1999.16.27. [DOI] [PubMed] [Google Scholar]
- Gupta R. Steward O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J. Comp. Neurol. 2003;461:174–186. doi: 10.1002/cne.10692. [DOI] [PubMed] [Google Scholar]
- Gupta R. Rummler L. Steward O. Understanding the biology of compressive neuropathies. Clin. Orthop. Relat. Res. 2005:251–260. doi: 10.1097/01.blo.0000164354.61677.f5. [DOI] [PubMed] [Google Scholar]
- Halka A.T. Turner N.J. Carter A. Ghosh J. Murphy M.O. Kirton J.P. Kielty C.M. Walker M.G. The effects of stretch on vascular smooth muscle cell phenotype in vitro. Cardiovasc. Pathol. 2008;17:98–102. doi: 10.1016/j.carpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Hasel C. Durr S. Bruderlein S. Melzner I. Moller P. A cell-culture system for long-term maintenance of elevated hydrostatic pressure with the option of additional tension. J. Biomech. 2002;35:579–584. doi: 10.1016/s0021-9290(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Hernandez M.R. Miao H. Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- Hodge W.A. Fijan R.S. Carlson K.L. Burgess R.G. Harris W.H. Mann R.W. Contact pressures in the human hip joint measured in vivo. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A. Gordon T. Zochodne D.W. Sulaiman O.A. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Wood P. Johnson M.I. Bunge R.P. Schwann cell surfaces but not extracellular matrix organized by Schwann cells support neurite outgrowth from embryonic rat retina. J. Neurosci. 1988;8:653–663. doi: 10.1523/JNEUROSCI.08-02-00653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. Brown T.D. A fluid-immersed multi-body contact finite element formulation for median nerve stress in the carpal tunnel. Comput. Methods Biomech. Biomed. Engin. 2007;10:343–349. doi: 10.1080/10255840701430480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi M.J. Elo M.A. Sironen R.K. Karjalainen H.M. Kaarniranta K. Helminen H.J. Hydrostatic pressure-induced changes in cellular protein synthesis. Biorheology. 2004;41:309–313. [PubMed] [Google Scholar]
- LaPlaca M.C. Cullen D.K. McLoughlin J.J. Cargill R.S., 2nd High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 2005;38:1093–1105. doi: 10.1016/j.jbiomech.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Lee H. Park C. Cho I.H. Kim H.Y. Jo E.K. Lee S. Kho H.S. Choi S.Y. Oh S.B. Park K. Kim J.S. Lee S.J. Double-stranded RNA induces iNOS gene expression in Schwann cells, sensory neuronal death, and peripheral nerve demyelination. Glia. 2007;55:712–722. doi: 10.1002/glia.20493. [DOI] [PubMed] [Google Scholar]
- Lundborg G. Myers R. Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome.”. J. Neurol. Neurosurg. Psychiatry. 1983;46:1119–1124. doi: 10.1136/jnnp.46.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R. Expression and functional roles of neural cell surface molecules and extracellular matrix components during development and regeneration of peripheral nerves. J. Neurocytol. 1994;23:1–28. doi: 10.1007/BF01189813. [DOI] [PubMed] [Google Scholar]
- Matsuzaki I. Chatterjee S. Debolt K. Manevich Y. Zhang Q. Fisher A.B. Membrane depolarization and NADPH oxidase activation in aortic endothelium during ischemia reflect altered mechanotransduction. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H336–H343. doi: 10.1152/ajpheart.00025.2004. [DOI] [PubMed] [Google Scholar]
- Melli G. Hoke A. Canadian Association of Neurosciences review: regulation of myelination by trophic factors and neuron-glial signaling. Can. J. Neurol. Sci. 2007;34:288–295. doi: 10.1017/s0317167100006703. [DOI] [PubMed] [Google Scholar]
- Mizisin A.P. Kalichman M.W. Myers R.R. Powell H.C. Role of the blood-nerve barrier in experimental nerve edema. Toxicol. Pathol. 1990;18:170–185. doi: 10.1177/019262339001800123. [DOI] [PubMed] [Google Scholar]
- Muller S. Labrador V. Da Isla N. Dumas D. Sun R. Wang X. Wei L. Fawzi-Grancher S. Yang W. Traore M. Boura C. Bensoussan D. Eljaafari A. Stoltz J.F. From hemorheology to vascular mechanobiology: An overview. Clin. Hemorheol. Microcirc. 2004;30:185–200. [PubMed] [Google Scholar]
- Myers K.A. Shrive N.G. Hart D.A. A novel apparatus applying long term intermittent cyclic hydrostatic pressure to in vitro cell cultures. J. Biosci. Bioeng. 2007;103:578–581. doi: 10.1263/jbb.103.578. [DOI] [PubMed] [Google Scholar]
- Okutsu I. Hamanaka I. Chiyokura Y. Miyauchi Y. Sugiyama K. Intraneural median nerve pressure in carpal tunnel syndrome. J. Hand Surg. Br. 2001;26:155–156. doi: 10.1054/jhsb.2000.0534. [DOI] [PubMed] [Google Scholar]
- Ozawa H. Imamura K. Abe E. Takahashi N. Hiraide T. Shibasaki Y. Fukuhara T. Suda T. Effect of a continuously applied compressive pressure on mouse osteoblast-like cells (MC3T3-E1) in vitro. J. Cell Physiol. 1990;142:177–185. doi: 10.1002/jcp.1041420122. [DOI] [PubMed] [Google Scholar]
- Park S. Nicoll S.B. Mauck R.L. Ateshian G.A. Cartilage mechanical response under dynamic compression at physiological stress levels following collagenase digestion. Ann. Biomed. Eng. 2008;36:425–434. doi: 10.1007/s10439-007-9431-6. [DOI] [PubMed] [Google Scholar]
- Peng H.B. Yang J.F. Dai Z. Lee C.W. Hung H.W. Feng Z.H. Ko C.P. Differential effects of neurotrophins and schwann cell-derived signals on neuronal survival/growth and synaptogenesis. J. Neurosci. 2003;23:5050–5060. doi: 10.1523/JNEUROSCI.23-12-05050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister B.J. Iwata A. Meaney D.F. Smith D.H. Extreme stretch growth of integrated axons. J. Neurosci. 2004;24:7978–7983. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly G.C. Haut T.R. Yellowley C.E. Donahue H.J. Jacobs C.R. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40:591–603. [PubMed] [Google Scholar]
- Rempel D.M. Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. J. Electromyogr. Kinesiol. 2004;14:71–75. doi: 10.1016/j.jelekin.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Rempel D. Manojlovic R. Levinsohn D.G. Bloom T. Gordon L. The effect of wearing a flexible wrist splint on carpal tunnel pressure during repetitive hand activity. J. Hand Surg. [Am.] 1994;19:106–110. doi: 10.1016/0363-5023(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Serbest G. Horwitz J. Barbee K. The effect of poloxamer-188 on neuronal cell recovery from mechanical injury. J. Neurotrauma. 2005;22:119–132. doi: 10.1089/neu.2005.22.119. [DOI] [PubMed] [Google Scholar]
- Setton L.A. Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. Am. 2006;88(Suppl. 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- Shreiber D.I. Smith D.H. Meaney D.F. Immediate in vivo response of the cortex and the blood-brain barrier following dynamic cortical deformation in the rat. Neurosci. Lett. 1999;259:5–8. doi: 10.1016/s0304-3940(98)00853-2. [DOI] [PubMed] [Google Scholar]
- Siebert H. Sachse A. Kuziel W.A. Maeda N. Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J. Neuroimmunol. 2000;110:177–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- Sparrey C.J. Manley G.T. Keaveny T.M. Effects of white, grey, and pia mater properties on tissue level stresses and strains in the compressed spinal cord. J Neurotrauma. 2009;26:585–595. doi: 10.1089/neu.2008.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai E. Mauck R.L. Hung C.T. Guo X.E. Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure. J. Bone Miner. Res. 2004;19:1403–1410. doi: 10.1359/JBMR.040516. [DOI] [PubMed] [Google Scholar]
- Toews A.D. Barrett C. Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J. Neurosci. Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tofaris G.K. Patterson P.H. Jessen K.R. Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.H. Yang G. Li Z. Controlling cell responses to cyclic mechanical stretching. Ann. Biomed. Eng. 2005;33:337–342. doi: 10.1007/s10439-005-1736-8. [DOI] [PubMed] [Google Scholar]
- Werner R. Armstrong T.J. Bir C. Aylard M.K. Intracarpal canal pressures: the role of finger, hand, wrist and forearm position. Clin. Biomech. (Bristol, Avon) 1997;12:44–51. doi: 10.1016/s0268-0033(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Yang D.P. Zhang D.P. Mak K.S. Bonder D.E. Pomeroy S.L. Kim H.A. Schwann cell proliferation during wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: axon-dependent removal of newly generated Schwann cells by apoptosis. Mol. Cell Neurosci. 2008;38:80–88. doi: 10.1016/j.mcn.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. Agapova O. Parker A. Shannon W. Pecen P. Duncan J. Salvador-Silva M. Hernandez M.R. DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol. Genomics. 2004;17:157–169. doi: 10.1152/physiolgenomics.00182.2003. [DOI] [PubMed] [Google Scholar]
- Zhang D. Weinbaum S. Cowin S.C. Estimates of the peak pressures in bone pore water. J. Biomech. Eng. 1998;120:697–703. doi: 10.1115/1.2834881. [DOI] [PubMed] [Google Scholar]