Abstract
Horizontal cells (HCs) are involved in establishing the center-surround receptive field organization of photoreceptor and bipolar cells. In many species, HCs respond differentially to colors and may play a role in color vision. An earlier study from our lab suggested that four types of HCs exist in the zebrafish retina: three cone HCs (H1, H2 and H3) and one rod HC. In this study, we describe their photoreceptor connections. Cones are arranged in a mosaic where rows of alternating blue- (B) and ultraviolet-sensitive (UV) single cones alternate with rows of red- (R) and green-sensitive (G) double cones; the G cones are adjacent to UV cones and B cones adjacent to R cones. Two small-field (H1 and H2) and two large-field (H3 and rod HC) cells were observed. The cone HC dendritic terminals connected to cones with single boutons, doublets, or rosettes, whereas the rod HCs connected to rods with single boutons. The single boutons/doublets/rosettes of cone HCs were arranged in double rows separated by single rows for H1 cells, pairs and singles for H2 cells, and in a rectilinear pattern for H3 cells. These connectivity patterns suggest that H1 cells contact R, G and B cones, H2 cells G, B and UV cones, and H3 cells B and UV cones. These predictions were confirmed by applying the DiI method to SWS1-GFP retinas whose UV cones express green fluorescent protein. Each rod HC was adjacent to the soma or axon of a DiI-labeled cone HC and connected to 50–200 rods.
Keywords: DiI, photoreceptors, horizontal cells, retina, connectivity, zebrafish
Introduction
Horizontal cells are inhibitory second-order neurons in the retina. They receive input from the photoreceptors and modify the signal from photoreceptors to bipolar cells. Horizontal cells respond to light with sustained, graded potentials and form the antagonistic surrounds of bipolar cell receptive fields and thus aid in detecting light contrasts and edges (Dowling, 1987). They may also play a role in color vision, particularly in non-mammalian species, as they respond to different colors of light with different combinations of hyperpolarizing and depolarizing potentials which contribute, presumably, to the generation of color opponency in more proximal neurons (Twig et al., 2003; Vanleeuwen et al., 2007). To understand better the mechanisms by which horizontal cells contribute to center/surround formation and color vision, two fundamental questions need to be answered: How many types of horizontal cells are there in the retina? And, to which photoreceptors do they connect?
These two questions have been asked in many studies using retinas of different vertebrates such as fish (Connaughton et al., 2004; Djamgoz and Downing, 1988; Djamgoz and Greenstreet, 1996; Kamermans et al., 1991; Kraaij et al., 1998; Song et al., 2008; Stell et al., 1982; Stell and Lightfoot, 1975; Wagner et al., 1982), turtle (Ammermüller and Kolb, 1996; Leeper, 1978a; b; Ohtsuka and Kouyama, 1986), chicken (Fischer et al., 2007), cat (Fisher and Boycott, 1974; Kolb, 1974; 1977; Wässle et al., 1978) and primates (Ahnelt and Kolb, 1994; Dacey et al., 1996). Different vertebrates have multiple types of photoreceptors as well as multiple types of horizontal cells. The connectivity pattern between the horizontal cells and photoreceptors varies between different vertebrates and overall the connectivity has been suggested to be either specificor unselective.
Zebrafish (Danio rerio) are highly visual animals with a rich cone system. In the zebrafish retina, there are five types of photoreceptors: double cones, consisting of a red-sensitive (R) principal member (long-double cone) and a green-sensitive (G) accessory member (short-double cone), blue-sensitive (B) long-single cones, ultraviolet-sensitive (UV) short-single cones and rods. The position of each cone subtype is arranged relative to the others, forming a highly ordered mosaic where rows of alternating B and UV cones alternate with rows of R and G cones; G cones are adjacent to UV cones, and B cones adjacent to R cones (Robinson et al., 1993). The terminals and inner segments of rods also maintain a topographically organized pattern (Fadool, 2003).
In a previous study using the adult zebrafish, single DiI crystals were inserted into the retinas to visualize horizontal cells (Song et al., 2008). Although horizontal cells were labeled in only one out of forty insertions; four types of horizontal cells (H1, H2, H3 and rod horizontal cells) were suggested, though the H1 and H2 horizontal cells could not be clearly differentiated due to their overlapping dendritic and somal areas. Rod horizontal cells also were not satisfactorily visualized. No specific cone connectivity was observed for the H1/2 cells, but H3 cells appeared to connect to either B cones or UV cones as their dendritic terminals corresponded to the rectilinear pattern of those single cones.
In this study, we modified the DiI insertion method so that the horizontal cells were labeled more consistently. We observed DiI-labeled cells surrounding nearly every DiI insertion site. Different types of individual horizontal cells as well as several neighboring horizontal cells of the same type were labeled. Therefore, we could readily compare the dendritic terminal distribution patterns of the different types of horizontal cells and visualize the collective dendritic terminal distribution pattern of the same type of horizontal cell, all in the same region of the retina. We found that the dendritic terminal distribution patterns were unique to each type of horizontal cell, indicating that the photoreceptor-horizontal cell connections are specific and can be used to differentiate different types of horizontal cells. By referring to the cone mosaic and using the SWS1-GFP transgenic zebrafish whose UV cones are GFP-positive (Takechi et al., 2003), we could identify the locations of the four types of cones in the zebrafish retina so that the specific photoreceptor-horizontal cell connections could be identified. We found that H1 cells connected to R, G and B cones; H2 cells connected to G, B and UV cones; H3 cells connected to B and UV cones; and rod horizontal cells connected to rods.
Materials and Methods
Wild-type AB-strain and SWS1-GFP transgenic (line 5.5A) (Takechi et al., 2003) zebrafish were maintained in the Harvard University Zebrafish Facility. All protocols were approved by the Harvard University Institutional Animal Care and Use Committee and conform to National Institute of Health animal care guidelines.
Fish were euthanized using 0.02% tricaine (Sigma Chemical Company, St. Louis, MO) in Tris buffer (pH 7.4) for 10 minutes and their spinal cord transected behind the head with a razor blade. The retinas were kept constantly hydrated with phosphate-buffered saline (PBS, pH 7.4, Sigma) to avoid shrinkage of retinal tissue through desiccation. Eyes were enucleated from the fish. The anterior segment was cut off and discarded. The eyecups were fixed in fresh 4% paraformaldehyde (Fisher Scientific, Fair Lawn, NJ) at room temperature for 1 hour or at 4°C overnight. Following a 3×10 minute wash in PBS, the sclera was peeled away and the pigment epithelium sometimes removed. The residual vitreous was carefully removed under the dissecting microscope to help flatten the retina. Four radial cuts were made to allow for further flattening. The retinas were mounted onto glass slides with the ganglion cell side facing up.
For DiI staining, a 1% CellTracker™ CM-DiI (Invitrogen, Carlsbad, CA) ethanol solution was spread over a glass slide and air-dried. By scratching over the DiI layer on the slide, dry DiI powder was loaded onto the outer surface of pulled tips (2–6 μm) from 1.5 mm diameter borosilicate glass pipettes (World Precision Instruments, Inc., Sarasota, FL). One or two tips were inserted perpendicularly into the middle one third of each quadrant of the retina through the vitreous side and broken off. Retinas were kept in PBS at 4°C for three to seven days. The retinas were whole-mounted with the ganglion cell layer side up on glass slides and cover-slipped using No. 0 cover glass (VWR Scientific, Media Park, PA) in VECTASHIELD® mounting medium (Vector Labs, Burlingame, CA). Pieces of No. 1 cover glass were placed around the retinas to prevent them from being crushed during imaging.
Horizontal cells were optically sectioned at optimal thickness (0.41 μm for a 63× lens and 0.49 μm for a 40× lens) using an upright LSM 510 Meta Axioplan 2 Imaging confocal microscope (Carl Zeiss MicroImaging Inc., Thornwood, NY). 488nm FluoArc argon laser light and a band-pass filter (505–530 nm) were used for GFP and 543 nm laser light and a long wavelength filter (560 nm) for rhodamine. Four objectives were used to collect images including: a 10× Plan-NEOFLUAR objective (0.30); a 25× Plan-NEOFLUAR oil-immersion corrected objective (0.80); a 40× Plan-NEOFLUAR Oil DIC objective (1.30); and a 63× Plan-APOCHROMAT Oil DIC objective (1.40).
The optical serial images of horizontal cells were analyzed using the Zeiss LSM Image Browser program (Carl Zeiss MicroImaging Inc.) either as is or flattened by projecting the maximum pixel values onto a single plane, which helped visualize the overall morphology (Song et al., 2008). Figures were created in Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA) and no enhancement other than contrast and brightness was made.
Results
In a previous study, individual DiI crystals were inserted into adult zebrafish retinas with pulled glass pipette tips (Song et al., 2008). Only 2–3 percent of the insertions resulted in a successful horizontal cell “bloom” and only 20–30 horizontal cells were labeled in a bloom (Song et al., 2008). In the current study, pipette tips were loaded with dry DiI powder and broken off inside the retina. Nearly every insertion resulted in a horizontal cell bloom in which fifty to well over a hundred cells were labeled with DiI (Fig. 1).
Figure 1.

Confocal image of DiI-labeled horizontal cells. Many horizontal cells are labeled up to several hundreds of micrometers away from the DiI insertion site.
Cone Horizontal Cells
Four types of horizontal cells were observed: two small-field cells (H1 and H2) and two large-field cells (H3 and rod horizontal cells). The morphology of the DiI-labeled cone horizontal cells is summarized in Figure 2. The H1 cells had axons, a round soma, and short dendrites when compared to the other types of horizontal cells (Fig. 2A). Their dendritic terminals appeared as clustered “rosettes”, as partial rosettes or as individual boutons (Fig. 2D). The rosettes were usually located in the center of the dendritic spread of the cell. Together with the partial rosettes, the dendrites were arranged in two rows, separated by single rows of rosettes/partial rosettes/single boutons. The distance between two neighboring rosettes/partial rosettes/single boutons within the single rows was approximately double that of the double rows.
Figure 2.
The morphology of cone horizontal cells in the adult zebrafish retina. (A–C) The z-axis projections of serial confocal images of horizontal cells; (D–F) the single-plane images of these horizontal cells at their dendritic terminals. The H1 cell has a round soma, short dendrites, and dendritic terminal clusters that are localized in the center of the cell. The H2 cell has an irregular soma, more elongated dendrites and dendritic terminal clusters spread out throughout its dendritic field. The H3 cell is larger than the H1 and H2 cells and has long dendrites. The dendritic terminals of H3 cells consist of small clusters that form two overlaying rhombic patterns. H1, H2 and H3 cells all have an axon. The scale bar applies to all panels in the figure.
The H2 cells also had axons but more irregularly shaped somata and more elongated dendrites than the H1 cells (Fig. 2B). Although the dendritic terminals of the H2 cells also appeared as clustered rosettes, partial rosettes, or as individual boutons, the rosettes were distributed over the entire dendritic field (Fig. 2E). These rosettes were arranged in pairs or individually. The pairs were in roughly the same orientation and located in one row or parallel rows; those individual rosettes were located outside of these rows and mirrored each other across these rows.
The H3 cells also had axons (Fig. 2C). The dendrites of the H3 cells were long and spread over a greater area than those of the H1 and H2 cells. The dendritic terminals of H3 cells were arranged as pairs of partial rosettes or single boutons (Fig. 2F). The distances between the two partial rosettes/single boutons in each pair varied between different H3 cells. If two closely-opposed boutons in a pair were treated as one unit, the boutons of the H3 cells were arranged in a single rectilinear pattern as previously reported (Song et al., 2008). However, the two partial rosettes/single boutons in each pair were often sufficiently separated to be considered as individual rosettes/boutons. Thus, these rosettes/boutons were arranged in two overlapping rectilinear patterns (Fig. 2F).
Rod Horizontal Cells
The DiI insertion method requires an axon to take up DiI. Thus, only about 30 rod horizontal cells were clearly visualized and all of them were axonless. Most of them were adjacent to the soma of a brightly DiI-stained cone horizontal cell (data not shown), or to a well-stained axon (Fig. 3A). Stained axons sometimes wrapped around the rod horizontal cell (Fig. 3B), or some spines from the axon extended to the soma of the rod horizontal cell (data not shown). Their dendrites were thicker than those of cone horizontal cells when imaged in the flat-mount preparations. The dendritic terminals of rod horizontal cells were numerous individual spheres, about 1 μm in diameter and 50–200 in number, forming single rows but with seams between the single rows (Fig. 3C). This pattern matches the rod mosaic in zebrafish described by Fadool (2003).
Figure 3.

A rod horizontal cell in the retina of a 2-year-old zebrafish. (A) The z-axis projection of serial confocal images of the rod horizontal cell. A brightly DiI-stained horizontal cell axon (arrow) ends by the soma of this rod horizontal cell. (B) The axon (arrow) wraps around the soma of the rod horizontal cell (arrowheads). (C) The rod horizontal cell has about 160 individual spherical dendritic terminals, which arrange into single rows with seams between these single rows.
Possible Cone Horizontal Cell-Photoreceptor Connections
Since many horizontal cells were labeled with our modified DiI insertion method, more than one type of horizontal cell, as well as clusters of several horizontal cells of the same type, could be observed in the same visual field (Figs. 4A, 5A, 6A).
Figure 4.
The cone connections of H1 cells. Selected images from a single confocal z-stack series. (A) Several H1 cells are visualized in a cluster. (B) The dendritic terminal rosettes of these H1 cells are organized into alternating double and single rows pattern. (C) Diagram of the idealized cone photoreceptor mosaic. The double cones (R and G) form the double rows and the alternating short- (UV) and long-single (B) cones form the single rows; the double rows alternate with the single rows; the neighboring single cones form a rhombus pattern (white rhombus). R, red-sensitive cones; G, green-sensitive cones; B, blue-sensitive cones; and UV, ultraviolet-sensitive cones. (D) The neighboring rosettes in the single rows of the H1 cell terminals form a rhombus pattern (blue rhombus). A magenta-green version of this figure is available as Supplemental Figure 1.
Figure 5.
The cone connections of a H3 cell. Selected images from a single confocal z-stack series. (A) A single H3 cell and several H1 cells (H1s) are shown in this field. (B) The dendritic terminals of the H3 cell are organized into single rows that parallel the orientation of the double and single rows formed by the dendritic terminal rosettes of the H1 cells. (C) Diagram of the idealized cone photoreceptor mosaic. The neighboring single cones form overlapping rhombic patterns (white and brown rhombi). R, red-sensitive cones; G, green-sensitive cones; B, blue-sensitive cones; and UV, ultraviolet-sensitive cones. (D) The neighboring terminals of the H3 cell form overlapping rhombic patterns, marked in blue and purple. A magenta-green version of this figure is available as Supplemental Figure 2.
Figure 6.
The cone connections of a H2 cell. (A) The z-axis projection of serial confocal images of a H2 cell and a H3 cell. (B) The dendritic terminals of the H2 and H3 cells. Some of the H2 cell dendritic terminal rosettes (in pairs) coincide with the H3 dendritic terminal pattern. (C) Diagram of the idealized cone photoreceptor mosaic. R, red-sensitive cones; G, green-sensitive cones; B, blue-sensitive cones; and UV, ultraviolet-sensitive cones. (D) The H2 cell appears to connect to G, B and UV cones (one of the two possible scenarios). The mosaic here is less regular than suggested in C; nevertheless, it is clear that UV cones (purple dots) are most often closer to G cones (green dots, blue lines) and B cones (blue dots) to the presumed R cones (open circles, open yellow lines). A magenta-green version of this figure is available as Supplemental Figure 3.
The dendritic terminals of clustered H1 cells were arranged into rows of rosettes in a “2+1” pattern where double rows of rosettes alternated with single rows of rosettes (Fig. 4B). The distance between two adjacent rosettes in the single rows was about twice of that in each of the double rows (Fig. 4B). Every four nearest-neighbor rosettes from two neighboring single rows formed a rhombus (marked in blue in Fig. 4D).
The cones in the adult zebrafish retina are arranged in a highly ordered mosaic (Robinson et al., 1993). Single rows of alternating blue-sensitive (B) and ultraviolet-sensitive (UV) cones alternate with double-rows of alternating red-sensitive (R) and green-sensitive (G) cones and the parallel rows are aligned such that the G cones flank the UV cones, and the B cones are nearer to the R cones (Robinson et al., 1993) (Fig. 4C). Every four nearest-neighbor B cones form a rhombus (marked in white in Fig. 4C); every four nearest-neighbor UV cones form another overlapping rhombus (not drawn). The double rows of the dendritic terminal rosettes of the H1 cells match the double rows of alternating R and G cones; the single rows of the dendritic terminal rosettes of the H1 cells match the rows of alternating B and UV cones. Therefore, the H1 cells most likely connect to either the R, G and B cones or to the R, G and UV cones.
A single H3 cell and several H1 cells are shown in Figure 5A. The H3 cell’s dendritic terminals formed two overlapping rhombi (marked in blue and purple in Fig. 5D, respectively). One side of both the blue and the purple rhombi paralleled the orientation of the double and single rows formed by the dendritic terminal rosettes of the H1 cells and matched the periodic position of the single rows of alternating B and UV cones (Fig. 5B). Therefore, the H3 cell(s) probably connect to both the B and UV cones.
One H2 cell and one H3 cell are clearly shown in Figure 6A. The dendritic terminal rosettes of the H2 cell were arranged individually or in pairs, some of which coincided with those of the H3 cell, indicating these pairs connected to B and UV cones (Fig. 6B). If the dendritic terminals were overlaid with the cone mosaic (Fig. 6C), the other dendritic terminal rosettes would match with one member of the double-cones, either R or G cones, but not both. One possible scenario is shown in Figure 6D. Therefore, the H2 cells may connect selectively either to the R, B and UV cones or to the G, B and UV cones.
Specific Horizontal Cell-Photoreceptor Connections
The uncertainty of H1 and H2 cone connections described above arises because it is not known which member of a double cone is the G cone, or which one is a B cone in the row of the single cones. If the location of any one of the four types of cones in the double/single cone rows is known, then we can derive the location of the other three types of cones in the ideal mosaic where the G cones flank the UV cones and that the R cones are adjacent to the B cones (Robinson et al., 1993). Therefore, we inserted DiI into the retinas of SWS1-GFP transgenic zebrafish whose UV cones are GFP-positive(Takechi et al., 2003).
A H1 cell is shown in Figure 7A. Double rows of the H1 cell dendritic terminal rosettes lie between the GFP-positive UV cones (Figs. 7B, C). Thus, the double rows of the dendritic terminal rosettes of the H1 cell connect to the double cones. The single GFP-positive cones lie between the double cone rows (Fig. 7C). No H1 cell dendritic terminal rosettes were found in the GFP-positive UV cone pedicles (Fig. 7C′); rather, they were only found between the GFP-positive UV cone pedicles, i.e., in B cones (Fig. 7C). Thus, H1 cells connect to the double cones (R + G cones) and to the B cones.
Figure 7.
Confirming the cone connections of H1, H2 and H3 cells with SWS1-GFP zebrafish retinas. (A –C, and C′) A H1 cell; (D–F, and F′) a H2 cell; and (G–I, and I′) a H3 cell. (A, D and G) The z-axis projection of serial confocal images of the horizontal cell; (B, E and H) the dendritic terminals; (C, F and I) the overlay image of both the red (the horizontal cell dendritic terminals) and the green (GFP-positive UV cones) channels at the dendritic terminal level of the horizontal cell; (C′, F′ and I′) the enlarged view of the boxed area of a UV cone pedicle and the surrounding horizontal cell terminals in C, F and I, respectively. A magenta-green version of this figure is available as Supplemental Figure 4. The scale bar in panel A applies to panels A–I; the scale bar in panel C′ applies to panels C′, F′ and I′.
A H2 cell is shown in Figure 7D. Its dendritic terminal rosettes are in pairs along one side of the rhombus formed by the GFP-positive cones, and one member of a pair overlies a GFP-positive UV cone and the other fits between the two neighboring UV cones, i.e., a B cone (Figs. 7E, F). The individual dendritic terminal rosettes that mirror each other across a single cone row fall between the single cone rows and are most often adjacent to the GFP-positive UV cones, the location of G cones (Figs. 7F, F′). We conclude that this H2 cell connects to the G, B and UV cones.
H3 cells have dendritic terminals that are partial rosettes and single boutons arranged in pairs (Fig. 7H). The distance separating a pair varies and it is either smaller or larger than the size of a UV cone pedicle (Figs. 7H, I). However, for both cases, one member of a pair overlies the GFP-positive UV cone, the other is outside of the UV cone (Figs. 7I, I′). Therefore, H3 cells connect to both the B and the UV cones.
133 cone horizontal cells were imaged and analyzed using the SWS1-GFP zebrafish retinas (Fig. 8). Out of the 53 H1 cells identified, 48 cells (91%) connected to the R, G and B cones; the other 5 cells (9%) may have connected to the UV cones as well because there was some red color (the DiI channel) in the green-colored UV cone pedicles (the green channel). Out of the 34 H2 cells identified, 23 cells (68%) were clearly connected to the G, B and UV cones; 10 cells (29%) connected to either R or G cones besides the B and UV cones because the individual rosettes were at similar distance to the UV and B cones so that their connection partner could not be definitively determined; and 1 cell (3%) connected possibly to the R cones besides the B and UV cones as the individual rosettes appeared to be closer to the B cones than to the UV cones. Out of the 46 H3 cells identified, 44 cells (96%) were unambiguously connected to both the B and UV cones; 1 cell (2%) connected to the R and/or G cones besides the B and UV cones as some of its dendritic terminals were outside of the rhombus pattern; and 1 cell (2%) connected to the UV cones and ambiguously to the B cones as its dendritic boutons outside of the UV pedicles seemed to touch the UV pedicles.
Figure 8.

The frequency of the cone connections that were seen in the SWS1-GFP zebrafish retinas. R, red-sensitive cones; G, green-sensitive cones; B, blue-sensitive cones; and UV, ultraviolet-sensitive cones. Out of the H1 cells identified, 91% connected to the R, G and B cones; 9% may have connected to the UV cones as well. Out of the H2 cells identified, 68% connected to the G, B and UV cones; 29% connected to either R or G cones besides the B and UV cones; and 3% connected possibly to the R cones besides the B and UV cones. Out of the H3 cells identified, 96% connected to both the B and UV cones; 2% connected to the R and/or G cones besides the B and UV cones; and 2% connected to the UV cones and ambiguously to the B cones.
Discussion
Horizontal cell labeling by DiI insertion
Horizontal cells in fish are located in the outermost layer of the inner nuclear layer (INL) and their axons extend into the INL. An earlier study showed that zebrafish horizontal cells could be retrogradely labeled by DiI through their axons (Song et al., 2008). The individual DiI crystals were placed into the retina using pulled glass pipette tips but few DiI crystals successfully localized to the INL and resulted in robust horizontal cell staining. Most of the DiI crystals labeled ganglion rather than horizontal cells and so only a few of the DiI insertions were deemed successful. We modified the method by scratching the pulled tips of glass pipettes over a dried DiI surface so that many tiny DiI crystals stuck onto each pipette tip. When the pipette tip was inserted perpendicularly into a retina and broken off, the DiI crystals were accessible to the whole thickness of the retina including the INL. Nearly every insertion resulted in a horizontal cell bloom, and fifty to well over a hundred horizontal cells were typically labeled in each bloom. Some ganglion cells, photoreceptors and bipolar cells were also labeled with the DiI. However, except for those within the fluorescence flare, the somata of the DiI-labeled horizontal cells were far enough away from the DiI fluorescence flare to be identifiable (Fig. 1).
Differentiation of H1 and H2 cells
The dimensions of the specific retinal cells of the same type varies depending on their location in the retina (Boycott and Wässle, 1974). Moreover, the cell dimensions of H1 and H2 horizontal cells in zebrafish overlap so that H1 and H2 cells could not be differentiated in our earlier study (Song et al., 2008). Whereas the type specimen cells we identified as H1 cells in the earlier study had rounder cell bodies and shorter, thinner dendrites than did the type specimen H2 cells, a quantitative analysis did not support differentiating the cells into two distinct groups based only on these criteria. In this study, we found that dendritic terminal patterning and, in turn, cone connectivity clearly differentiated the two cell types, and these differences correlated with the type specimen cells we identified in the earlier study, confirming the existence of two distinct small field horizontal cells in zebrafish. The dendritic terminal rosettes of H1 cells organize into alternating single and double rows, connecting to R, G and B cones, whereas those of H2 cells organize into individual and paired rosettes, connecting usually to G, B and UV cones. In a minority of cases however, it was not possible to determine unequivocally whether a H2 cell connected to a G or R cone, because the H2 terminal rosettes were equidistant between the two cone types.
H3 cell-cone connections
The distance between the two members of a pair of dendritic terminal rosettes, partial rosettes, or single boutons of H3 cells varied, being either smaller or larger than the size of a UV cone pedicle (Fig. 7I). Song and colleagues (2008) also observed this variation and suggested that each pair connected to either the B or the UV cones. However, for both cases, only one member of each pair of dendritic terminal rosettes, partial rosettes, or single boutons aligned with the GFP-positive UV cones; the other was observed between the UV cones. As the GFP fluorescence proteins thoroughly filled the UV cones (Takechi et al., 2003), it is unlikely that the other member of each pair of dendritic terminal rosettes, partial rosettes, or single boutons also connected with a UV cone. Therefore, we conclude that the H3 cells connect to both B and UV cones.
Selective photoreceptor-horizontal cell connectivity
We demonstrate here that the H1, H2 and H3 cells connect selectively to different combinations of cones H1 cells connect to R, G and B cones, H2 cells mainly to G, B and UV cones, and H3 cells to B and UV cones. Together with the rod horizontal cells data, all of the four types of horizontal cells in the zebrafish retina connect selectively to specific photoreceptors.
This has also been suggested for other cyprinid fish. For example, the goldfish (Carassius auratus) retina also has four types of horizontal cells–3 cone types (H1, H2 and H3) and rod horizontal cell (Stell, 1975). Light microscopic analysis of serial semi-thin sections through Golgi stained goldfish cone horizontal cells revealed that H1 cells contacted all cones within their dendritic field; H2 cells selectively contacted the long members of double cones and some long single cones; and H3 cells contacted selectively only the short and miniature single cones (Stell and Lightfoot, 1975). The UV-sensitive cones had not been characterized at that time so that the miniature single cones in the goldfish retina were believed to be blue-sensitive rather than UV-sensitive cones. Therefore, the color-coding of cone inputs to the horizontal cells in the goldfish retina was suggested as follows: H1 cells connect to R, G and B cones; H2 cells connect to G and B cones; and H3 cells connect to B cones (Stell and Lightfoot, 1975). Later, the miniature single cones were recognized as UV cones in the goldfish retina (Hisatomi et al., 1996; Tokunaga et al., 1999), at which point the color-coding of cone inputs to the horizontal cells could be reinterpreted as: H1 cells connect to R, G, B and UV cones; H2 cells connect to G and B cones; and H3 cells connect to B and UV cones.
Horizontal cell connectivity was also shown to be selective in the roach (Rutilus rutilus) retina: H1 cells connect to the R and G cones and to a few B cones; H2 cells connect to the G and B cones; and H3 cells connect to the B cones (Djamgoz and Downing, 1988; Djamgoz and Greenstreet, 1996; Downing and Djamgoz, 1989). Surprisingly, however, the H3 cells in the carp (Cyprinus carpio) retina were reported to be unselective in their connections, i.e., they contact all spectral classes of cones (Wagner et al., 1982; Weiler, 1978); and an electrophysiological study of carp (Ctenopharyngodon idella) horizontal cells also suggested spectrally non-selective cone contacts (Kamermans et al., 1991).
Horizontal cells in the turtle have also been studied (Leeper, 1978a; b; Ohtsuka and Kouyama, 1986). Four types of horizontal cells (H1, H2, H3 and H4 cells) were identified and selectively connect to photoreceptors: the H1 cell dendrites connect to both the R and G members of the double cones and the R and G single cones and the H1 cell axon terminals connect to the R member of the double cones, R single cones and rods; the H2 cells connect to the G, B single cones, and possibly UV cones; the H3 cells connect to the B single cones, and possibly UV cones; the H4 cells were suggested to connect to the G member of the double cones, but this is still being debated (Ammermüller and Kolb, 1996; Leeper, 1978b).
Mammalian retinas, such as rabbit, cat, appear to have only two types of horizontal cells. The A-type (or H1 cell) has a larger dendritic field, connects exclusively to cones and is often axonless. The B-type (or H2 cell) is more numerous and also connects to cones, with a smaller dendritic field and an axon whose terminals end in rod spherules (Kolb, 1974). Although neither of the two horizontal cell types selectively connect to cones, there may be cone connection variability among individual cells in the ratios of their R and G cone connections and in their B cone connections (Boycott, 1988).
In the primate retina, two or three types of horizontal cells have been identified where the H1 cells connect to the R and G cones, and sparsely to the B cones; the H2 cells connect diffusely to the R and G cones, and heavily to the B cones; a possible third type, the H3 cells, connect to only the R and G cones (Ahnelt and Kolb, 1994; Dacey et al., 1996).
Morphological-physiological correlations
How might the connections between horizontal and photoreceptor cells correlate with the responses of horizontal cells? In goldfish, horizontal cells are classified physiologically into four types according to their spectral response patterns. Three types of horizontal cells receive input from cones (L-type or monophasic, C1-type or biphasic, and C2-type or triphasic) and one type from rods (Stell et al., 1982). Specifically, the L-type cells respond to light with hyperpolarizing potentials regardless of wavelength; the C1 biphasic cells respond by hyperpolarizing to green light and depolarizing to red light; the C2 triphasic cells respond by hyperpolarizing to blue and red light, and depolarizing to green or yellow light. The rod cells, like H1 cells, respond only with hyperpolarizing potentials; however, the rod responses are slower in time course, but more sensitive to light than the cone responses (Stell et al., 1982). Based on their light and electron microscopic observations and physiological data from others, Stell and colleagues (1975) proposed a model of goldfish horizontal cells in which the H1 cells generated monophasic responses, the H2 cells biphasic responses, the H3 cells triphasic responses, and the R, G and B cones each acted mainly upon one horizontal cell type (the R cones upon the H1 cells, the G cones upon the H2 cells, and the B cones upon the H3 cells, respectively) through excitatory or sign-conserving synapses. The horizontal cells, they suggested, send feedback signals to cones through inhibitory or sign-inverting synapses (the H1 cells to the G cones, and the H2 cells to the B cones) (Stell et al., 1975; Stell and Lightfoot, 1975). At that time, UV cones had not yet been identified in the goldfish retina, and few today would agree with their suggestion that the horizontal processes lateral to the synaptic ribbon are exclusively the feedback elements whereas the central processes are the horizontal cell feedforward elements. Nonetheless, the Stell model of feedforward and feedback interactions between the photoreceptor and horizontal cells underlying biphasic and triphasic horizontal cell responses has gained general acceptance.
Connaughton and Nelson have recently identified six types of chromatic horizontal cell responses in zebrafish–L1- and L2-type monophasic cells, C-type biphasic cells, C-type blue and C-type UV triphasic cells, and C-type blue tetraphasic cells (Connaughton and Nelson, 2007; Nelson and Connaughton, 2008). Their recordings show that the L1 cells receive hyperpolarizing input equally from R and G cones, whereas the L2 cells receive their input mainly from R cones. Their biphasic cells receive hyperpolarizing input equally from B and G cones and depolarizing input from R cones. The blue triphasic cells receive hyperpolarizing input from B and R cones and depolarizing input from G cones, whereas the UV triphasic cells receive hyperpolarizing input from UV and R cones, and depolarizing input from G cones. Finally, the tetraphasic cells receive hyperpolarizing input from B and R cones and depolarizing input from G and UV cones.
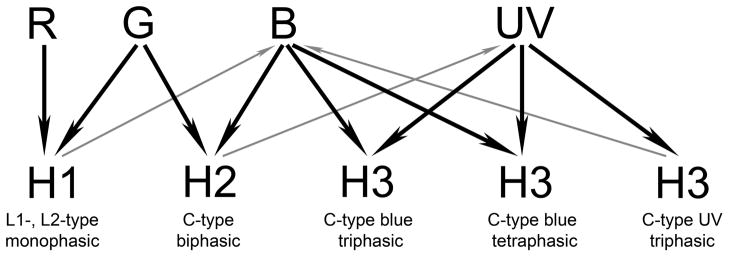
If we extend the model of Stell and colleagues to the zebrafish and include the UV cones into the cone-horizontal cell circuitry, our morphological data can begin to explain Nelson and Connaughton’s observed spectral responses (Fig. 9). The H1 cells connect to the R, G and B cones, receiving direct hyperpolarizing input mainly from the R and G cones while providing feedback to the B cone terminals. Thus the H1 cells are their L1-and L2-type cells; the L2 cells receiving more R cone input than the L1 cells. The H2 cells, on the other hand, connect to the G, B and UV cones, receiving input mainly from the G and B cones while providing feedback to the UV cones. These cells depolarize to red light because of the sign-reversing feedback input from L2 horizontal cells to the B cone terminals; the H2 cells would be, therefore, the C-type biphasic cell.
Figure 9.
A model describing the cone horizontal cell pathways in zebrafish. Black arrows indicate major sign-conserving feedforward synapses whereas gray arrows indicate sign-inverting feedback synapses. R, red-sensitive cones; G, green-sensitive cones; B, blue-sensitive cones; and UV, ultraviolet-sensitive cones. Details in text.
The H3 cells connect to the B and UV cones and some receive their input mainly from the B cones and provide feedback to the UV cones; they would be the C-type blue triphasic cells. Other H3 cells receive their input mainly from the UV cones, and provide feedback to the B cones and be the C-type UV triphasic cells. The depolarizing green response of these cells, as well as the red hyperpolarizing response reflects the sign-reversing input from the biphasic H2 cells to the UV terminals.
Finally, other H3 cells could be the tetraphasic C-type cells, receiving primary input from the B cones and sign-inverting input from the H2 (biphasic) cells and the other H3 (UV-preferring triphasic) cells via the UV cone terminals.
Since the feedback synapses from the horizontal cells to photoreceptor terminals have not been identified morphologically, the above scheme is highly hypothetical. Further, it assumes that a dendritic terminal of a horizontal cell can be primarily postsynaptic, presynaptic or a mix of both. It presents only the major pathways and undoubtedly other connections exist. For example, Kraaij et al. (1998) have provided evidence that there is feedback from horizontal cells to all cone types in goldfish and not all of these connections are shown here. Indeed, the results of Kraaij and coworkers would appear to negate the Stell et al. (1975) model, but our findings that there is clear specificity in the connections of the three types of cone horizontal cells to the cones in zebrafish is clearly at odds with their view that all horizontal cells receive input from all cone types. Clearly additional experiments are needed to clarify these discrepancies. Our results fit better the Stell model and extend it by including both the UV cones and the tetrachromatic responses recorded from horizontal cells by Connaughton and Nelson (2007 and 2008).
The electrophysiological recordings by Connaughton and Nelson do raise another interesting issue–that there appear to be more physiological types of horizontal cells than morphological types. So, for example, there may be two physiological subtypes of H1 cells, the L1 and L2 cells, and we propose that there are three physiological subtypes of H3 cells, the two triphasic cells and the tetraphasic cell type. It is generally believed that cells of the same morphological type are electrically coupled to one another which would, of course, eliminate or significantly smudge the spectral selectivity of subtypes of H1 or H3 cells. This discrepancy of there being a greater diversity of recorded C-type horizontal cells than morphological types of horizontal cells in various species has been raised before (Twig et al., 2003). Clearly further electrophysiological and morphological studies are needed to examine these issues as well. But perhaps the bigger challenge is to determine how the various types of horizontal cells and their responses contribute to color vision processing in fish.
Supplementary Material
Acknowledgments
The authors thank Drs. Shoji Kawamura and Pamela Raymond for providing the SWS1-GFP zebrafish eggs, Drs. Ralph Nelson, Victoria Connaughton and Joshua Cameron for critically reading the manuscript, Drs. Farida Emran and Ishara Mills-Henry for helpful discussions. The authors also thank Jessica Miller, Steve Zimmerman and Karen Hurley in the Harvard Zebrafish Facility for their help in rearing and maintaining the fish. This work was funded by NIH Grant RO1 EY00811 (J.E.D.).
Grant support: NIH Grant EY00811 to J.E.D.
References
- Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-light microscopic study of spectral connectivity. J Comp Neurol. 1994;343(3):387–405. doi: 10.1002/cne.903430305. [DOI] [PubMed] [Google Scholar]
- Ammermüller J, Kolb H. Functional architecture of the turtle retina. Progress in Retinal and Eye Research. 1996;15(2):393–433. [Google Scholar]
- Boycott BB. Horizontal cells of mammalian retinae. Neurosci Res Suppl. 1988;8:S97–111. doi: 10.1016/0921-8696(88)90010-2. [DOI] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol. 1974;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. J Comp Neurol. 2004;477(4):371–385. doi: 10.1002/cne.20261. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Light Responses From Presumed Horizontal and Amacrine Cells in Zebrafish Retina. Invest Ophthalmol Vis Sci. 2007;48(5):5957. [Google Scholar]
- Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271(5249):656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Downing JE. A horizontal cell selectively contacts blue-sensitive cones in cyprinid fish retina: intracellular staining with horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1988;235(1280):281–287. doi: 10.1098/rspb.1988.0076. [DOI] [PubMed] [Google Scholar]
- Djamgoz MB, Greenstreet EH. Quantitative analysis of triphasic (H3) horizontal cell-cone connectivity in the cyprinid fish (roach) retina. Vision Res. 1996;36(24):4007–4014. doi: 10.1016/s0042-6989(96)00144-7. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The retina: an approachable part of the brain. xii. Cambridge, Mass: Belknap Press of Harvard University Press; 1987. p. 282. [284] of plates p. [Google Scholar]
- Downing JE, Djamgoz MB. Quantitative analysis of cone photoreceptor-horizontal cell connectivity patterns in the retina of a cyprinid fish: electron microscopy of functionally identified and HRP-labelled horizontal cells. J Comp Neurol. 1989;289(4):537–553. doi: 10.1002/cne.902890402. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258(2):277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol. 2007;500(6):1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Boycott BB. Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci. 1974;186(1085):317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- Hisatomi O, Satoh T, Barthel LK, Stenkamp DL, Raymond PA, Tokunaga F. Molecular cloning and characterization of the putative ultraviolet-sensitive visual pigment of goldfish. Vision Res. 1996;36(7):933–939. doi: 10.1016/0042-6989(95)00189-1. [DOI] [PubMed] [Google Scholar]
- Kamermans M, van Dijk BW, Spekreijse H. Color opponency in cone-driven horizontal cells in carp retina. Aspecific pathways between cones and horizontal cells. J Gen Physiol. 1991;97(4):819–843. doi: 10.1085/jgp.97.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol. 1974;155(1):1–14. doi: 10.1002/cne.901550102. [DOI] [PubMed] [Google Scholar]
- Kolb H. The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1977;6(2):131–153. doi: 10.1007/BF01261502. [DOI] [PubMed] [Google Scholar]
- Kraaij DA, Kamermans M, Spekreijse H. Spectral sensitivity of the feedback signal from horizontal cells to cones in goldfish retina. Vis Neurosci. 1998;15(5):799–808. doi: 10.1017/s0952523898154184. [DOI] [PubMed] [Google Scholar]
- Leeper HF. Horizontal cells of the turtle retina. I. Light microscopy of Golgi preparations. J Comp Neurol. 1978a;182(4 Pt 2):777–793. doi: 10.1002/cne.901820503. [DOI] [PubMed] [Google Scholar]
- Leeper HF. Horizontal cells of the turtle retina. II. Analysis of interconnections between photoreceptor cells and horizontal cells by light microscopy. J Comp Neurol. 1978b;182(4 Pt 2):795–809. doi: 10.1002/cne.901820504. [DOI] [PubMed] [Google Scholar]
- Nelson RF, Connaughton VP. Morphology of L- and C-Type Horizontal Cells in Zebrafish Retina. Invest Ophthalmol Vis Sci. 2008;49(5):5793. [Google Scholar]
- Ohtsuka T, Kouyama N. Electron microscopic study of synaptic contacts between photoreceptors and HRP-filled horizontal cells in the turtle retina. J Comp Neurol. 1986;250(2):141–156. doi: 10.1002/cne.902500202. [DOI] [PubMed] [Google Scholar]
- Robinson J, Schmitt EA, Harosi FI, Reece RJ, Dowling JE. Zebrafish ultraviolet visual pigment: absorption spectrum, sequence, and localization. Proc Natl Acad Sci U S A. 1993;90(13):6009–6012. doi: 10.1073/pnas.90.13.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song PI, Matsui JI, Dowling JE. Morphological types and connectivity of horizontal cells found in the adult zebrafish (Danio rerio) retina. J Comp Neurol. 2008;506(2):328–338. doi: 10.1002/cne.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell WK. Horizontal cell axons and axon terminals in goldfish retina. J Comp Neurol. 1975;159(4):503–520. doi: 10.1002/cne.901590405. [DOI] [PubMed] [Google Scholar]
- Stell WK, Kretz R, Lightfoot DO. Horizontal cell connectivity in goldfish. Prog Clin Biol Res. 1982;13:51–75. [PubMed] [Google Scholar]
- Stell WK, Lightfood DO, Wheeler TG, Leeper HF. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975;190(4218):989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Stell WK, Lightfoot DO. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- Takechi M, Hamaoka T, Kawamura S. Fluorescence visualization of ultraviolet-sensitive cone photoreceptor development in living zebrafish. FEBS Lett. 2003;553(1–2):90–94. doi: 10.1016/s0014-5793(03)00977-3. [DOI] [PubMed] [Google Scholar]
- Tokunaga F, Hisatomi O, Satoh T, Taniguchi Y, Matsuda S, Imanishi Y, Honkawa H, Takahashi Y, Kobayashi Y, Yoshida M, Tsukahara Y. Evolution of visual pigments and related molecules. Novartis Found Symp. 1999;224:44–52. doi: 10.1002/9780470515693.ch4. discussion 52–43. [DOI] [PubMed] [Google Scholar]
- Twig G, Levy H, Perlman I. Color opponency in horizontal cells of the vertebrate retina. Prog Retin Eye Res. 2003;22(1):31–68. doi: 10.1016/s1350-9462(02)00045-9. [DOI] [PubMed] [Google Scholar]
- Vanleeuwen MT, Joselevitch C, Fahrenfort I, Kamermans M. The contribution of the outer retina to color constancy: a general model for color constancy synthesized from primate and fish data. Vis Neurosci. 2007;24(3):277–290. doi: 10.1017/S0952523807070058. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Speck PT, Weiler R. Computer reconstruction of HRP-injected horizontal cells reveals new connectivity patterns in fish retina. Naturwissenschaften. 1982;69(3):143–145. doi: 10.1007/BF00376722. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB, Peichl L. Receptor contacts of horizontal cells in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978;203(1152):247–267. doi: 10.1098/rspb.1978.0104. [DOI] [PubMed] [Google Scholar]
- Weiler R. Horizontal cells of the carp retina: Golgi impregnation and Procion-Yellow injection. Cell Tissue Res. 1978;195(3):515–526. doi: 10.1007/BF00233893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.