Abstract
Purpose:
To evaluate neuroretinal function and anatomical outcomes in patients with neovascular age-related macular degeneration (AMD) after three treatments with ranibizumab.
Design:
Observational case reports.
Methods:
We investigated visual function in three patients, one female (80 years) and two male (77 and 74 years) with neovascular AMD. Twenty healthy participants served as control group. We measured visual acuity (Bailey-Lovie charts), contrast sensitivity (Pelli-Robson) and neuroretinal function using the multifocal electroretinogram (mfERG). Central macular thickness was evaluated using optical coherence tomography (OCT). Main outcome measures were central and peripheral mfERG peak to trough (N1P1) response density amplitudes and peak (P1) implicit times. All tests were performed before the first treatment (baseline) and after each of the three treatments with intravitreal 0.3 mg ranibizumab.
Results:
Visual acuity and contrast sensitivity remained stable or improved. Central macular thickness decreased after three treatments in all three patients. We found no significant change in central and peripheral neuroretinal function in the AMD patients between pre- and post-treatments 2 and 3. Although the mfERG amplitudes in the AMD patients were not significantly reduced compared with the age-similar group at baseline, there was a statistically significant reduction in central and peripheral mfERG amplitudes after three treatments
Conclusion:
Anatomical outcomes and central visual function improved or remained stable in the three AMD patients in concordance with past reports. Further investigations of possible adverse effects of ranibizumab on the central and peripheral neuroretina in large prospective clinical trials are suggested.
Keywords: age-related macular degeneration, optical coherence tomography, OCT, multifocal electroretinogram, multifocal ERG, ranibizumab
Introduction
Targeting angiogenesis, the underlying mechanism promoting choroidal neovascularisation is a new treatment alternative in neovascular age-related macular degeneration (AMD). Of the available antivascular endothelial growth factor (anti-VEGF) drugs (Gragoudas et al 2004; Avery et al 2006; VISION 2006), ranibizumab has been demonstrated to stabilize/or improve visual acuity and anatomical outcomes in patients with neovascular (AMD), in large clinical trials (Brown et al 2006; Rosenfeld et al 2006a, 2006d). Ranibizumab is a murine, monoclonal anti-VEGF antibody given intravitreally every four weeks. Its monthly application is based upon studies in primates that showed its rapid distribution in the retina (6–24 hours) and three day half life before its clearance from all ocular compartments (Gaudreault et al 2005). While its most favourable outcomes (prevention of visual acuity loss in 95% of patients and improvement in visual acuity in 25%–30% of patients) have been described when given monthly over a period of two years (Brown et al 2006; Rosenfeld et al 2006a), new treatment strategies in combination with photodynamic therapy are being extensively investigated (Dhalla et al 2006; Heier et al 2006). Mild adverse effects after treatment with ranibizumab, such as conjunctival hyperaemia and subconjunctival haemorrhage have been reported in about 80% of patients and serious complications such as endophthalmitis and uveitis were evident in about 1%–1.3% (Rosenfeld et al 2006c). Ongoing clinical trials are currently investigating the effect of less frequent dosing regimens. These studies indicate that optical coherence tomography (OCT) is helpful in retreatment decisions (Lalwani et al 2006; Rosenfeld et al 2006b).
While studies with anti-VEGF drugs have investigated its toxicity and penetration in animals (Manzano et al 2006; Shahar et al 2006), only two studies have measured local cellular function in humans. Both studies suggest further evaluation of neuroretinal function (Maturi et al 2006; Moschos et al 2007). Maturi and colleagues (2006) showed that there was no deterioration of neuroretinal function after one intravitreal treatment with bevacizumab, another anti-VEGF drug which has been used ‘off-label’ for treatment of AMD (Rosenfeld 2006). They showed some small improvement of central neuroretinal activity compared with baseline which they attributed to variability in the results rather than a recovery of function. The authors concluded that testing over a longer term was necessary (Maturi et al 2006). Moschos et al (2007) found no improvement in retinal function as measured using the mfERG after one bevacizumab injection after three months in 56% of their patients. While there was some improvement in amplitude on average compared with baseline, they attributed this to a decrease in macular edema rather than recovery of neuroretinal function. No published study has evaluated neuroretinal function after multiple treatments with ranibizumab.
In the small series of neovascular AMD patients considered here, we extend the information about neuroretinal function after anti-VEGF therapy by monitoring patients who received several treatments with ranibizumab. The follow up of three treatments in this study is based on large clinical trials that currently test the drug efficacy after an induction period of three monthly injections followed by quarterly injections (Rosenfeld et al 2006d). Besides well established visual acuity measures and OCT for monitoring treatment effects, we investigated contrast sensitivity and objectively documented central and peripheral neuroretinal function (up to 25 degree in diameter) with the multifocal electroretinogram (mfERG). A functional documentation of central and peripheral retinal areas is necessary to estimate a patient’s reading and mobility performance. So far, large clinical trials investigating anti-VEGF therapy have only reported visual acuity as a functional outcome measure. Visual acuity, however, reflects function of only about the central 1 degree of the retina.
In addition to neuroretinal activity, the mfERG reflects hypoxic effects on retinal function (Feigl et al 2007), which are likely to occur when there is choroidal atrophy. The latter has been described in relation to deprivation of VEGF from the choroid as it may occur during anti-VEGF therapy (McLeod et al 2002; Marneros et al 2005).
Methods
Participants
We investigated three patients (1 female, 2 male) aged 80 y (AMD 1), 77 y (AMD 2) and 74 y (AMD 3), respectively. All patients had a diagnosis of neovascular AMD as assessed ophthalmoscopically and by fluorescein angiography. Patients were treated with 0.3 mg ranibizumab intravitreally by an ophthalmologist (AG) according to the recommended protocol (Rosenfeld et al 2006a). Participants were in good general health although one patient (AMD 3) had primary chronic open angle glaucoma which was well controlled by topical medication. Two of the participants (AMD 2 and AMD 3) had a new choroidal neovascular membrane (CNV) and were treated with first line therapy ranibizumab whereas patient 1 (AMD 1) had a recurrent CNV following three preceding photodynamic therapies before she received ranibizumab. Twenty participants (aged between 58 and 77 years, mean 69 ± 5) contributed to the healthy control group.
All patients gave written informed consent and the study was conducted in accordance with the tenets of the Declaration of Helsinki and the requirements of the Queensland University of Technology Human Research Ethics Committee.
Tests
All tests were performed under the instructions of the same researcher (BF). Visual acuity (VA) was assessed with Bailey Lovie charts (Bailey and Lovie 1976) at a starting distance of 6 m and contrast sensitivity with Pelli Robson charts at 3 m (Pelli et al 1988). An improvement in visual acuity was defined as an increase of three lines (15 letters) (Rosenfeld et al 2006d) and improvement in contrast sensitivity as an increase of 6 letters (Rubin et al 2002). Macular thickness was measured using optical coherence tomography (OCT, Stratus III, Zeiss, Germany) (Hee et al 1995).
To evaluate neuroretinal activity we used the mfERG (VERIS, EDI, Redwood City, USA). The mfERG gives a topographical map of local neuroretinal function of the posterior pole. The waveform of the conventional fast flicker application is thought to reflect photoreceptor function and primarily ON and OFF bipolar cell function (Hood et al 2002). In contrast to the full-field ERG which gives a global retinal response, the mfERG allows the derivation of many local areas of the central and peripheral retina between 25 to 50 degrees in diameter. The mfERG has been shown to be particularly useful in documenting treatment effects in the central and peripheral retina in AMD and diabetes (Greenstein et al 2000; Moschos et al 2001; Feigl et al 2005; Maturi et al 2006).
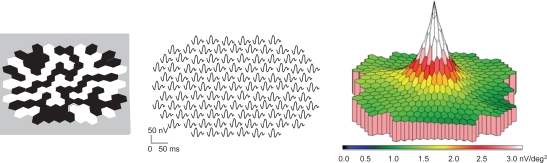
The visual stimulus of the mfERG consisted of 103-scaled black and white hexagons displayed on a calibrated 7-inch CRT monitor. Figure 1 demonstrates the stimulus array extending to 25 degrees in diameter (left), the local 103 waveform responses corresponding with each of the stimulated retinal areas (middle), and a three dimensional response density map of the retinal responses. The responses are higher centrally and decrease with increasing eccentricity according to the topographical distribution of cone photoreceptors and their associated postreceptoral pathways (Sutter and Tran 1992).
Figure 1.
The mfERG uses black and white hexagons presented on a monitor (left) that flicker according to a pseudorandom m-sequence. Each waveform represents the electrical response from a hexagon which stimulates a focal retinal area. The waveforms can be plotted as a trace array (middle) or as a three dimensional response density (amplitudes per unit retinal area) plot (right). The latter reflects the distribution of the cone-mediated (photoreceptor and bipolar) cellular responses.
Participants were instructed to fixate on a large central cross at the center of a CRT monitor and were corrected for the test distance using an eye monitor/refraction unit. Pupils were dilated using tropicamide 0.5% and phenylephrine 2.5%. The black and white hexagons flickered according to a pseudorandom m-sequence where the CRT monitor screen was refreshed every 13.33 ms (75 Hz frame rate) (Zele and Vingrys 2005). The mfERG was recorded monocularly with DTL (Dawson-Trick-Litzkow) thread electrodes that were placed across the bulbar conjunctiva. The reference Ag-AgCl cup electrodes were placed on the temple close to the testing eye and the ground was placed on the forehead. Retinal signals were band pass filtered (10–300 Hz), sampled every 0.83 ms and amplified (50,000x, Grass amplifier). Recordings were made under ambient room light conditions. An eye camera and the on-line signal were used for monitoring fixation and blinks; small eye movements and artefacts detected during the recording caused signal segments to be rejected and re-recorded.
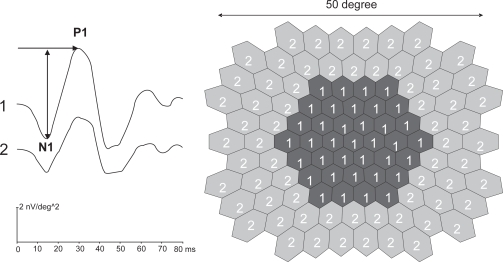
We analysed N1P1 peak to trough response densities (amplitudes per unit retinal area, in nV/deg2) and peak P1 implicit times (time from the onset of the stimulus to the first positive peak, in ms) as shown in Figure 2 (left). Before analysing the first order kernel (mean local responses to all stimuli in a stimulus cycle) amplitudes and implicit times, one spatial averaging procedure (ratio = 6) and one noise reduction procedure were performed on the raw mfERG data (see Sutter and Tran [1992]).
Figure 2.
The stimulus array (right) and how the waveform responses were averaged into a central (1) and peripheral (2) area are shown. We analyzed N1P1 trough to peak amplitude response densities and P1 peak implicit times (arrows).
Statistical analysis
Main outcome variables were means of N1P1 amplitudes and peak P1 implicit times. For better signal to noise ratio, N1P1 amplitude and P1 implicit time waveform responses were averaged into a central (∼13.5°) and peripheral area (∼13.5°−25°) in diameter (Figure 2, right).
For statistical analysis, central and peripheral N1P1 amplitude response densities and P1 implicit time averages of each AMD patient were compared with responses of the healthy age-similar group before and after three treatments. The central and peripheral N1P1 amplitudes and P1 implicit times were also compared within each AMD patient’s pre-and second and third treatment. A repeated measures ANOVA (SPSS-14) was used to identify a significant (main) effect between the treatments and retinal locations (central and peripheral) between each AMD patient and the healthy control group before and after three treatments and within each AMD patient’s treatments.
Results
Visual acuity, contrast sensitivity, optical coherence tomography (OCT), and the mfERG were performed before (a week or less) and three to four weeks after each of the three treatments. One patient (AMD 3) was not able to perform a mfERG after the first treatment because of illness unrelated to the treatment. For uniform presentation of mfERG data, the results are reported before treatment, and after the second and third treatment in all participants.
Visual acuity improved by 6 lines and contrast sensitivity by 10 letters in one patient (AMD 3) and remained stable in AMD 1 and AMD 2 after three treatments with ranibizumab (Table 1). Decreased central macular thickness was evident in all patients after three treatments compared with pre-treatment values. Patient AMD 1 still showed cystoid intraretinal fluid formation after three treatments.
Table 1.
The patient’s visual acuity (VA), contrast sensitivity (Contrast) and OCT
| VA | Contrast (letters) | OCT (microns-central 1 mm) | |
|---|---|---|---|
| AMD 1 (left eye) | |||
| Before | 6/30 | 21 | 319 |
| After 2 | 6/30 | 21 | 200 |
| After 3 | 6/48 | 24 | 223 |
| AMD 2 (left eye) | |||
| Before | 6/9.5 | 34 | 388 |
| After 2 | 6/9.5 | 34 | 194 |
| After 3 | 6/9.5 | 34 | 201 |
| AMD 3 (right eye) | |||
| Before | 6/30 | 23 | 279 |
| After 2 | 6/15 | 26 | 237 |
| After 3 | 6/7.5 | 33 | 245 |
Comparison between AMD patients and healthy control group before and after three treatments
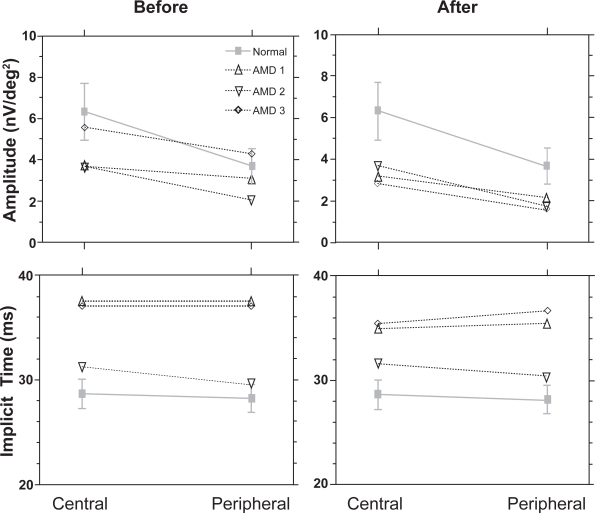
Before treatment (baseline) there was no statistically significant difference between central and peripheral N1P1 amplitudes of the healthy group and AMD patients (AMD 1: F1,18 = 2.0, p = 0.2, AMD 2: F1,18 = 3.4, p = 0.1, AMD 3, F1,18 = 0.002, p = 0.9). After the third treatment central and peripheral N1P1 amplitudes significantly decreased in AMD 1 (F1,18 =4.3, p ≤ 0.05), AMD 2 (F1,18 = 3.9, p ≤ 0.05) and AMD 3 (F1,18 F = 6.0, p = 0.03) when compared with the healthy control group (Figure 3, upper panel). Central and peripheral P1 implicit times were significantly delayed before treatment in AMD 1 (F1,18 = 33.1, p < 0.001) and AMD 3 (F1,18 = 43.4, p < 0.001) and were not significantly different in AMD 2 (F1,18 = 1.8, p = 0.2) compared with the healthy group, and this did not change after three treatments (Figure 3, lower panel).
Figure 3.
The N1P1 amplitudes ± SD (A) and peak implicit times ± SD (B) for the central and peripheral rings for each ARM patient are shown compared with a healthy control group. While P1 implicit times remained stable, N1P1 amplitudes were significantly reduced after the third treatment.
Comparison within pre-treatment and post-treatment values in AMD patients
Table 2 demonstrates the central and peripheral N1P1 response densities and P1 implicit times before and after treatments 2 and 3 for each AMD patient. There was no statistically significant change in central and peripheral N1P1 amplitudes for AMD 1 (F1,2 = 17, p = 0.2), AMD 2 (F1,2 = 55.6, p = 0.1) and and AMD 3 (F1,2 = 112, p = 0.1) before and after treatments 2 and 3 when compared with pre-treatment. Also, central and peripheral P1 implicit times were not significantly different to pre-treatment values in all patients (AMD 1: F1,2 = 8.2, p = 0.2, AMD 2: F1,2 = 2.5, p = 0.4 and AMD 3: F1,2 = 11.7, p = 0.2) after treatments 2 and 3.
Table 2.
The central and peripheral N1P1 response density (above) and P1 implicit time
| N1P1 in nV/deg2 | Before | After 2 | After 3 | |||
|---|---|---|---|---|---|---|
| Area | central | peripheral | central | peripheral | central | peripheral |
| AMD 1 | 3.7 | 3.1 | 2.8 | 2.1 | 3.2 | 2.1 |
| AMD 2 | 3.7 | 2.2 | 5.2 | 2.6 | 3.7 | 1.8 |
| AMD 3 | 5.6 | 4.3 | 3.1 | 2.0 | 2.9 | 1.6 |
| P1 in ms | Before | After 2 | After 3 | |||
|---|---|---|---|---|---|---|
| Area | central | peripheral | central | peripheral | central | peripheral |
| AMD 1 | 36.6 | 35.4 | 37.5 | 37.5 | 35.0 | 35.4 |
| AMD 2 | 31.3 | 29.2 | 31.3 | 29.6 | 31.7 | 30.4 |
| AMD 3 | 37.1 | 37.1 | 31.7 | 31.7 | 35.5 | 36.7 |
Note: (below) values before and after treatment 2 and 3 for each AMD patient.
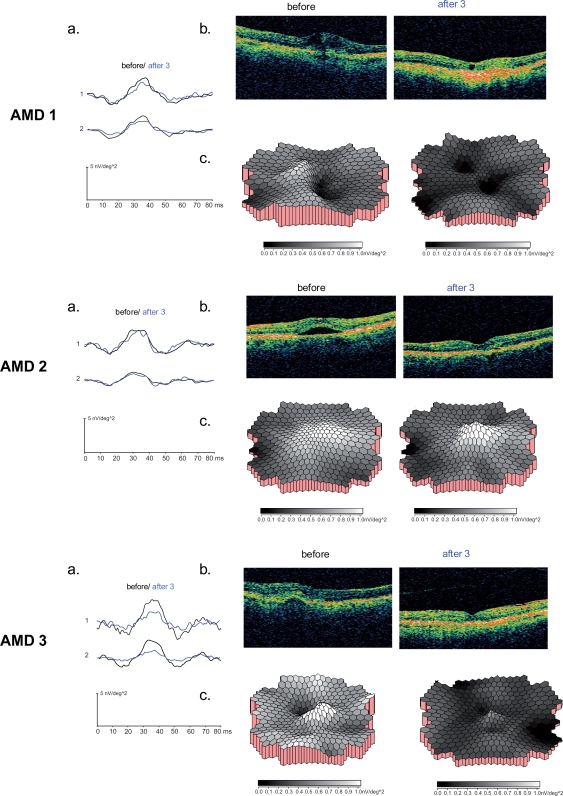
Figures 4 shows the mfERG averaged central and peripheral ring responses (a) and OCT (b) before and after three treatments with ranibizumab from AMD patient 1, AMD patient 2, and AMD patient 3. The three dimensional amplitude response density plots (c) for each patient demonstrate lower responses (as indicated in darker gray scale areas) after three treatments compared with the healthy control group.
Figure 4.
The mfERG and OCT results for the AMD 1 (A), AMD 2 (B) and AMD 3 (C) are demonstrated. The mfERG central and peripheral area waveform averages (a) and OCT images (b) before and after three treatments are shown for each patient. In c. the three dimensional response density plots of each patient compared with the age-similar control group (n = 20) are outlined before and after three treatments with ranibizumab. Note that darker gray scales indicate poorer response densities which were evident in all patients after the third treatment. These were not only reduced in the central area but throughout the whole field up to 25° (see also waveform averages in a).
Discussion
After three treatments with ranibizumab, all AMD patients had stable or improved visual acuity and contrast sensitivity and a decrease in central macular thickness compared with pretreatment values (Table 1). While neuroretinal function remained stable within the treatments (Table 2), there was a significant decrease in central and peripheral mfERG amplitudes for all patients compared with a healthy age-similar group after three treatments (Figure 3). Our findings of no significant change compared with baseline values are in concordance with other studies investigating neuroretinal function after one treatment with anti-VEGF (Maturi et al 2006; Moschos et al 2007). However, these studies did not include a control group. We extend this observation to show that AMD patients have a significant reduction in amplitudes compared with an age-similar control group after three treatments.
The mfERG can measure function in localized retinal areas and in our study, areas of approximately 13.5° (central) and 13.5°–25° (peripheral) in diameter. In contrast visual acuity only reflects the function of the foveal area which is less than one degree of visual angle. The mfERG therefore better represents areas covered by the neovascularisation membrane than visual acuity. In addition although anti-VEGF targets new vessel growth, it is not clear if there are adverse effects on the healthy peripheral retina. Our findings of impaired central and peripheral neuroretinal function compared with a healthy control group after three treatments, suggest a more widespread effect of the anti-VEFG therapy on the retina than the clinically defined area affected by AMD.
In concordance with other studies we found stable or improved visual acuity. Anti-VEGF drugs reduce vascular permeability (Witmer et al 2003) and a reduction in macular edema alone, could have resulted in recovery or stabilization of foveal visual acuity (Costa et al 2006). However, a reduced edema might not recover neuroretinal function. Recently Moschos and colleagues (2007) reported a discrepancy between functional (visual acuity and electrophysiology) and anatomical outcomes (OCT) of patients after one bevacizumab treatment. Functional outcomes did not improve with the decrease in macular thickness after three months. They suggested that “macular edema is only a parameter that may affect visual acuity and electrophysiological responses in the beginning of the disease” (Moschos et al 2007). This implies disruption of delicate intraretinal mechanisms subserving psychophysical and electrophysiological responses that do not resolve after resolution of the edema.
We are aware that patients were still undergoing treatment that was not yet complete and the recovery of neuroretinal function might occur over a longer time course. In particular amplitude recovery might take more than three treatments given that a decrease in amplitude reflects widespread cellular alteration (Gerth et al 2003). However, adverse effects of anti-VEGF interfering with central and peripheral neuroretinal activity may have been evident in our mfERG results. While a lack of improvement of central neuroretinal function could have indicated the natural course of progression in AMD (Jurklies et al 2002), a central and peripheral functional deficit, as detected in this study, may suggest a broader, possibly adverse effect. It is well established that VEGF is essential for choriocapillaris development (Marneros et al 2005). In addition VEGF has a neuroprotective role (Robinson et al 2001; Storkebaum et al 2004) and essential role in physiological angiogenesis such as the regulation of embryonic and postnatal processes (Gerber et al 1999), the regulation of vascular and nervous networks in the heart and brain (Galvan et al 2006; Maulik 2006), wound healing (Schlingemann 2004) and the regulation of antithrombotic processes (Manley et al 2002). Inhibition or manipulation of VEGF could be harmful in the developing retina, but also in hypoxic retinal tissue (Robinson et al 2001). Hypoxia and ischemia have been discussed in AMD (Pauleikhoff et al 1990; Grunwald et al 1998, 2005; Arden et al 2005; Feigl et al 2006) and while a normal retina might tolerate successive treatment with anti-VEGF, a hypoxic “pre-injured” retina might not.
This is the first report of the effect of multiple treatments with ranibizumab on neuroretinal function. Although we have described this effect in only a small number of patients, we want to raise awareness of possible adverse effects on the central and peripheral neuroretina. Our results should encourage large controlled trials to include vision measures other than visual acuity and OCT in documenting treatment effects of anti-VEGF therapy. Ideally these should give measures of central and peripheral neuroretinal function, as well as measures reflecting choroidal perfusion.
Acknowledgments
This work was supported by an Institute of Health and Biomedical Innovation (IHBI), Queensland University of Technology postdoctoral fellowship grant. We have benefited from discussions with Andrew J Zele.
References
- Arden G, Sidman R, Arap W, Schlingemann R. Spare the rod and spoil the eye. Br J Ophthalmol. 2005;89:764–69. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery R, Pieramici D, Rabena M, Castellarin A, Nasir M, Guist M. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Bailey I, Lovie J. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–5. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- Brown D, Kaiser P, Michels M, Soubrane G, Heier J, Kim R, Sy J, Schneider S, for the ANCHOR Study Group Ranibizumab versus verteporfin for neovascular Age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Costa R, Jorge R, Calucci D, Cardillo J, Melo LAJ, Scott I. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- Dhalla M, Shah G, Blinder K, Ryan EJ, Mittra R, Tewari A. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006;29:988–93. doi: 10.1097/01.iae.0000247164.70376.91. [DOI] [PubMed] [Google Scholar]
- Feigl B, Brown B, Lovie-Kitchin J, Lee L. Dynamics of retinal function after multiple photodynamic therapies in age-related macular degeneration: a report of cases. Doc Ophthalmol. 2005;111:135–48. doi: 10.1007/s10633-005-5319-7. [DOI] [PubMed] [Google Scholar]
- Feigl B, Brown B, Lovie-Kitchin J, Swann P. Functional loss in early age-related maculopathy: the ischaemia postreceptoral hypothesis. Eye. 2007;21:689–96. doi: 10.1038/sj.eye.6702389. [DOI] [PubMed] [Google Scholar]
- Feigl B, Stewart I, Brown B. Experimental hypoxia in human eyes: implications for ischaemic disease. Clin Neurophysiol. 2007;118:887–95. doi: 10.1016/j.clinph.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Galvan V, Greenberg D, Jin K. The role of vascular endothelial growth factor in neurogenesis in adult brain. Mini Rev Med Chem. 2006;6:667–69. doi: 10.2174/138955706777435742. [DOI] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (fhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- Gerber H, Hillan K, Ryan A, Kowalski J, Keller G, Rangell L, Wright B, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–59. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Gerth C, Hause D, Delahunt P, Morse L, Werner J. Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics with large drusen. Arch Ophthalmol. 2003;121:1404–14. doi: 10.1001/archopht.121.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gragoudas E, Adamis A, Cunningham E, Feinsod M, Guyer D, for the VEGF Inhibition Study in Ocular Neovascularisation Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Greenstein V, Chen H, Hood D, Seiple W, Carr R. Retinal function in diabetic macular edema after focal laser photocoagulation. Invest Ophthalmol Vis Sci. 2000;41:3655–64. [PubMed] [Google Scholar]
- Grunwald J, Hariprasad S, DuPont J, Maguire M, Fine S, Brucker A, Maguire A, Ho A. Foveal choroidal blood flow in age-related macular degeneration (AMD) Invest Ophthalmol Vis Sci. 1998;39:385–90. [PubMed] [Google Scholar]
- Grunwald J, Metelitsina T, DuPont J, Ying G-S, Maguire M. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–38. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- Hee M, Izatt J, Swanson E, Huang D, Schuman J, Lin C, Puliafito C, Fuji-moto J. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- Heier J, Boyer D, Ciulla T, Ferrone P, Jumper J, Gentile R, Kotlovker D, Chung C, Kim R. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS Study. Arch Ophthalmol. 2006;124:1532–42. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- Hood D, Frishman L, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG. Implication for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–85. [PubMed] [Google Scholar]
- Jurklies B, Weismann M, Sutter E, Bornfeld N. Monitoring retinal function in neovascular maculopathy using multifocal electroretinography-early and long-term correlation with clinical findings. Graefe’s Arch Clin Exp Ophthalmol. 2002;240:244–64. doi: 10.1007/s00417-002-0439-1. [DOI] [PubMed] [Google Scholar]
- Lalwani G, Fung A, Michels S, Venkatraman A, Puliafito C, Rosenfeld P.2006Optical coherence tomography findings in a variable-dosing regimen with ranibizumab (Lucentis): The PrONTO Study Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO)E-abstract 2147 [Google Scholar]
- Manley P, Martiny-Baron G, Schlaeppi J, Wood J. Therapies directed at vascular endothelial growth factor. Expert Opin Investig Drugs. 2002;11:1715–36. doi: 10.1517/13543784.11.12.1715. [DOI] [PubMed] [Google Scholar]
- Manzano R, Peyman G, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26:257–61. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- Marneros A, Fan J, Yokoyama Y, Gerber H, Ferrara N, Crouch R, Olsen B. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–9. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturi R, Bleau L, Wilson D. Electrophysiologic findings after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:270–4. doi: 10.1097/00006982-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Maulik N. Reactive oxygen species drives myocardial angiogenesis. Antioxid Redox Signal. 2006;8:2161–8. doi: 10.1089/ars.2006.8.2161. [DOI] [PubMed] [Google Scholar]
- McLeod D, Taomoto M, Otsuji T, Green W, Sunness J, Lutty G. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43:1986–93. [PubMed] [Google Scholar]
- Moschos M, Apostolopoulos M, Ladas I, Theodossiadis P, Malias J, Moschos M, Theodossiadis G. Assessment of macular function by multifocal electroretinogram before and after epimacular membrane surgery. Retina. 2001;21:590–5. doi: 10.1097/00006982-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Moschos M, Brouzas D, Apostolopoulos M, Koutsandrea C, Loukianou E, Moschos M. Intravitreal use of bevacizumab (Avastin) for choroidal neovascularization due to ARMD: a preliminary multifocal-ERG and OCT study: Multifocal-ERG after use of bevacizumab in ARMD. Doc Ophthalmol. 2007;114:37–44. doi: 10.1007/s10633-006-9036-7. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D, Chen J, Chisholm I, Bird A. Choroidal perfusion abnormality with age-related Bruch’s membrane change. Am J Ophthalmol. 1990;109:211–7. doi: 10.1016/s0002-9394(14)75989-6. [DOI] [PubMed] [Google Scholar]
- Pelli D, Robson J, Wilkins A. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–99. [Google Scholar]
- Robinson G, Ju M, Shih SC, Xu X, McMahon G, Caldwell R, Smith L. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J. 2001;15:1215–7. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. Intravitreal avastin: the low cost alternative to lucentis? Am J Ophthalmol. 2006;142:141–3. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Brown D, Heier J, Boyer D, Kaiser P, Chung C, Kim R, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Fung A, Lalwani G, Michels S, Venkatraman A, Puliafito C.2006Visual acuity outcomes following a variable-dosing regimen for ranibizumab (lucentis) in neovascular AMD: The PrONTO Study Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO)ARVO Abstract 2958 [Google Scholar]
- Rosenfeld P, Heier J, Hantsbarger G, Shams N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:632–e631. doi: 10.1016/j.ophtha.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Rich R, Lalwani G. Ranibizumab: Phase III Clinical Trial Results. Ophthalmol Clin North Am. 2006;19:361–72. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Rubin G, Bressler N, the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Effects of Verteporfin therapy on contrast sensitivity. Results from the Treatment of Age-Related Macular Degeneration With Photodynamic Therapy (TAP) investigation-TAP report No. 4. Retina. 2002;22:536–44. doi: 10.1097/00006982-200210000-00002. [DOI] [PubMed] [Google Scholar]
- Sarks J, Sarks S, Killingworth M. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552–77. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- Sarks S. Ageing and degeneration in the macular region: a clinicopathological study. Br J Ophthalmol. 1976;60:324–41. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlingemann R. Role of growth factors and the wound healing response in age-related macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- Shahar J, Avery R, Heilweil G, Barak A, Zemel E, Lewis G, Johnson P, Fisher S, Perlman I, Loewenstein A. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin) Retina. 2006;26:262–9. doi: 10.1097/00006982-200603000-00002. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26:943–54. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- Sutter E, Tran D. The field topography of ERG components in man-I. the photopic luminance response. Vision Res. 1992;32:433–46. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- VEGF Inhibition Study In Ocular Neovascularization (V.I.S.I.O.N) Clinical Trial Group Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113:1508.e1–25. doi: 10.1016/j.ophtha.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Witmer A, Vrensen G, Van Noorden C, Schlingemann R. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Ret Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Zele AJ, Vingrys AJ. Cathode-ray-tube monitor artefacts in neurophysiology. J Neurosci Meth. 2005;141:1–7. doi: 10.1016/j.jneumeth.2004.05.005. [DOI] [PubMed] [Google Scholar]