Abstract
Antigen-presenting cells expressing indoleamine 2,3-dioxygenase (IDO) play a critical role in maintaining peripheral tolerance. Strategies to inhibit IDO gene expression and enhance antigen-presenting cell function might improve anti-tumour immunity. Here we have designed highly effective anti-IDO small interfering (si) RNAs that function at low concentrations. When delivered to human primary immune cells such as monocytes and dendritic cells (DCs), they totally inhibited IDO gene expression without impairing DC maturation and function. Depending on the design and chemical modifications, we show that it is possible to design either monofunctional siRNAs devoid of immunostimulation or bifunctional siRNAs with gene silencing and immunostimulatory activities. The latter are able to knockdown IDO expression and induce cytokine production through either endosomal Toll-like receptor 7/8 or cytoplasmic retinoid acid-inducible gene 1 helicase. Inhibition of IDO expression with both classes of siRNAs inhibited DC immunosuppressive function on T-cell proliferation. Immature monocyte-derived DCs that had been transfected with siRNA-bearing 5′-triphosphate activated T cells, indicating that, even in the absence of external stimuli such as tumour necrosis factor-α, those DCs were sufficiently mature to initiate T-cell activation. Collectively, our data highlight the potential therapeutic applications of this new generation of siRNAs in immunotherapy.
Keywords: bifunctional small interfering RNA; dendritic cells; immunosuppression; indoleamine 2,3-dioxygenase; small interfering RNA
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that are seeded throughout peripheral tissues to function as sentinels that process and present antigen to mount adequate immune responses. In addition to their stimulatory function, they can induce tolerance.1 Among the mechanisms that have been linked to immunosuppression by DCs, is the expression of indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme in tryptophan catabolism that is known to be involved in fetus tolerance during pregnancy.2 There are at least three mechanisms that can explain the immune inhibitory function of IDO;3 First, the depletion of tryptophan from the extracellular space leading to starvation and cell cycle arrest of T cells; second, the accumulation of toxic tryptophan-metabolites like kynurenine; and third, the induction and attraction of regulatory T (Treg) cells. Expression of IDO in monocytes and DCs is induced by a variety of agents such as prostaglandins, interferon-γ (IFN-γ) and toll-like receptor (TLR) 7/8 ligands.4 Treg cells expressing cytotoxic T lymphocyte-associated antigen-4 can induce IDO-expression in DCs.3 We have previously shown that monocytes/macrophages expressing IDO acquire an immunosuppressive phenotype capable of suppressing T-cell proliferation.4 Also, previous studies suggested that IDO-expressing host DCs found in tumour-draining lymph nodes might induce anergy in tumour-antigen-specific T cells.5
To interfere with the negative regulatory mechanisms in DCs, such as the expression of interleukin-10 (IL-10), we have used RNA interference (RNAi),6 a sequence-specific gene silencing mechanism triggered by double-stranded RNA.7 It has been shown to be a powerful tool to study gene function in a broad range of organisms. Small interfering RNAs (siRNAs), the effector of RNAi, hold great promise as a therapeutic tool.8,9 They can be used alone or in combination with other traditional therapy. We and others have shown that chemically made siRNAs can activate innate immunity when delivered to the endosomes via cationic liposomes.10–12 Both single- and double-stranded siRNAs induce cytokines and IFN expression in a sequence-specific manner and the response is mainly mediated by TLR7/8 in humans. Activation of the IFN pathway by siRNAs is thought to hamper the therapeutic use of siRNAs. However, a combinatorial strategy that blocks the expression of immunosuppressive factors and simultaneously activates innate immunity could lead to more effective cancer treatments. In this respect, we have shown recently that targeting IL-10 expression by bifunctional siRNA can block IL-10 expression and activate TLR7/8 signalling in human monocytes and DCs.6 In the same report, we also showed that cytoplasmic delivery of in vitro transcribed siRNA bearing 5′-triphosphate inhibited gene expression and induced cytokine production through probably the activation of the retinoic acid-inducible gene I (RIG-1), which recognizes a variety of RNA viruses.13 To further exploit this design for cancer immunotherapy, we report on the design and characterization of mono- and bifunctional siRNAs targeting IDO.
Materials and methods
Small interfering RNAs
Four different siRNAs targeting IDO were designed. All siRNA sequences were obtained from Eurofins MWG Operon (Ebersberg, Germany). The non-modified siRNA duplexes were purchased ready-made. To prepare the modified siRNA duplexes, complementary strands were mixed at equal concentrations, then heated at 70° for 1 min and allowed to anneal at 37° for 30 min. Successful annealing was controlled with polyacrlamide gels. In addition to chemical synthesis, siRNA-4 targeting IDO was synthesized in vitro from oligonucleotide DNA templates and the T7 RNA polymerase as described previously.6 The double-stranded DNA oligonucleotide templates used to transcribe the sense and anti-sense siRNA-4 strands were as follows:
DNA oligonucleotides for the sense strand
5′-TAATACGACTCACTATAGCCGTGAGTTTGTCCTTTCAA-3′
5′-TTGAAAGGACAAACTCACGGCTATAGTGAGACGTATTA-3′
DNA oligonucleotides for the anti-sense strand
5′-TAATACGACTCACTATAGAAAGGACAAACTCACGGACT-3′
5′-AGTCCGTGAGTTTGTCCTTTCTATAGTGAGACGTATTA-3′
Letters in bold type indicate the expected siRNA transcripts. Subsequent to transcription, sense and anti-sense siRNA strands were purified and annealed as described previously.6 The in-vitro-transcribed IDO siRNA-4 has the following sequence:
Sense strand: 5′-GCCGUGAGUUUGUCCUUUCAA-3′
Antisense strand: 5′-GAAAGGACAAACUCACGGACU-3′
Using the same conditions, sense and a siRNA targeting β-galactosidase was transcribed in vitro and used as a control siRNA for in-vitro-transcribed anti-IDO siRNA.
Sense strand: 5′-GUUGAUGUGUUUAGUCGCUAUUU-3′
Antisense strand: 5′-GUAGCGACUAAACACAUCAAAUU-3′
Cells and culture conditions
Peripheral blood mononuclear cells were isolated by density-gradient centrifugation (Lymphoprep; Fresenius Kabi Norge AS, Oslo, Norway) of buffy coats obtained from healthy adult donors. Monocytes were isolated by plastic adherence. Immature monocyte-derived DCs (immoDCs) were generated by culturing adherent monocytes in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF; 25 ng/ml, R&D, Minneapolis, MN) and IL-4 (50 ng/ml, R&D) for 4–5 days. To generate mature monocyte-derived DCs (mmoDCs), the cells were stimulated with tumour necrosis factor-α (TNF-α; 50 ng/ml, R&D) for the two following days. The cells were cultured in RPMI-1640 supplemented with 10% fetal calf serum and antibiotics.
Transfection conditions
The human monocyte nucleofactor kit from Amaxa (Cologne, Germany) was used for siRNA delivery into human monocytes and DCs. For each electroporation 0·1–3 μg siRNA was mixed with 5 × 106 to 10 × 106 cells in 100 μl nucleofection solution according to the manufacturer’s instructions. Subsequently, they were diluted with 0·5 ml RPMI-1640 pre-warmed medium and transferred to six-well plates containing 2·5 ml RPMI-1640 medium, and incubated at 37° in 5% CO2. In addition, we used a standard electroporation technique. In this procedure, 0·1–1·5 μg (7·5–112·5 pmol) siRNA was mixed with 5 × 106 cells in 0·5 ml RPMI-1640 medium, and then the cells were transferred to 4-mm BTX cuvettes and pulsed at 500 V for 2 milliseconds. After transfection the cells were diluted in 2·5 ml pre-warmed medium and processed as above. Expression of IDO was induced by IFN-γ (40 U/ml; Sigma, St. Louis, MO) or R-848 (5 μm; Alexi’s biochemicals, Farmindale, NY).
Western blotting
Equal amounts of protein extracts were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to a Whatman Protran nitrocellulose transfer membrane by electroblotting. The membranes were blocked with 5% milk in phosphate-buffered saline (PBS)/Tween (0·1%) for 16–18 hr at 4° and subsequently incubated with the primary antibody indoleamine 2,3-dioxygenase (US Biologicals, Swampscott, MA) followed by horseradish peroxidase-conjugated rabbit anti-mouse secondary antibodies (Dako, Glostrup, Denmark) both diluted in 1% milk in PBS/Tween (0·1%). Immunoreactive proteins were visualized with autoradiography using the enhanced chemiluminescence system. The blots were stripped and reprobed with antibodies against actin (Santa Cruz Biotech., Santa Cruz, CA). The intensity of the IDO immunoreactive bands was quantified using Adobe Photoshop software (http://www.lukemiller.org/journal/2007/08/quantifying-Western-blots-without.html).
T-cell activation
To test the suppressive effects of IDO+ dendritic T cells, CD4+ T cells were prepared from peripheral blood mononuclear cells using CD4+ Dynabeads (Dynal, Oslo, Norway) according to the manufacturer’s instructions. Subsequently, they were incubated with increasing numbers of either IDO-positive or IDO-negative DCs. To prepare DCs, mmoDC were transfected with IDO siRNA-3 or control siRNA, and then stimulated with IFN-γ for 18 hr to induce the expression of IDO. Subsequently, DCs (105) were washed, irradiated (3000 cGy) and incubated with T cells (5 × 104) in round-bottomed 96-well plates along with phytohaemagglutinin (PHA) mitogen (5 μg/ml). T-cell proliferation was assessed by pulsing the cells with 1 μCi [3H]thymidine for 18 hr before harvesting on day 6. [3H]Thymidine incorporation was quantified with a β-counter. In addition, T-cell proliferation assays were set up to assess the capacity of siRNA-transfected DCs to process and present antigens to T cells using bacillus Calmette–Guérin protein lysates as described previously.14
Phenotypic analysis of IDO siRNA-transfected DCs
Mature DCs were transfected with either IDO siRNA-3 or control siRNAs, and then incubated at 37° in 5% CO2 for 2 days. Subsequently, they were stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies (mAbs) against human CD80, CD86 and human leucocyte antigen (HLA) -DR molecules. Briefly, the cells (105) were stained with the respective phycoerythrin-conjugated antibodies in PBS containing 1% fetal calf serum and 0·1% sodium azide [fluorescence-activated cell sorter (FACS) buffer] for 30 min at 4°. Negative controls were isotype-matched mAbs. After washing, the cells were resuspended in FACS buffer and surface expression was determined by flow cytometry (Becton Dickinson, San Jose, CA). Data were analysed using a FACSort flow cytometer and CellQuest software package (BD Biosciences, San Diego, CA).
Cytokine measurement
Monocytes were seeded at 2 × 105 per 200 μl in 96-well plates. In a previous study, we have shown that endosomal localization of siRNA was required for immune stimulation by chemically made siRNAs.10 Therefore, the siRNAs were delivered using the cationic liposomes N-[1-(2,3-Dioleoyloxy)-N,N,N-trimethylammonium methylsulfate (DOTAP) as described previously.10 After 18 hr of transfection, culture supernatants were collected and TNF-α contents were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s recommendations (BD Biosciences). In-vitro-transcribed siRNAs were delivered using electroporation as described previously.6 Cytokine contents were measured in culture supernatant 18 hr after transfection using a human TNF-α and IL-12 (p70) ELISA kit (BD Biosciences). In some cases, the expression of CD80, CD86 and HLA-DR molecules was analysed by flow cytometry.
Total RNA preparation and reverse transcription–polymerase chain reaction
Total RNA was prepared using Trizol (Invitrogen, Carlsbad, CA) and complementary DNA was synthesized from 1·5 μg total RNA with an oligo(dT) primer in a 15 μl reaction volume according to the manufacturer’s recommendations (GE Healthcare, Olso, Norway). Polymerase chain reaction (PCR) amplification was performed on 1/30 (50 ng total RNA) of the complementary DNA reaction (2 min at 94°), followed by 29 cycles (1 min at 94°, 1 min at 60° and 1 min at 72°) followed by a final extension of 5 min at 72°. The primer sequences used for reverse transcription-PCR were: IDO forward 5′-GGAAATAGCAGCTGCTTCTGCA-3′; IDO reverse 5′-CTCCTCAGGGAGACCAGAGCTT-3′; β-actin forward 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′; β-actin reverse 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′. The PCR products were size-separated on 1·5% agarose gels and visualized by SYBR Safe DNA gel staining (Invitrogen).
Results
Silencing IDO expression in monocytes and DCs
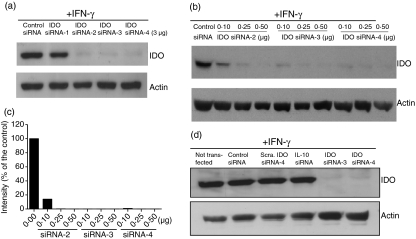
Most of the reports on siRNAs have used published design rules to design functional siRNAs.15,16 Although these programmes identify possible messenger RNA (mRNA) target sequences, they are not efficient tools with which to predict siRNAs that can silence gene expression at low concentrations and simultaneously activate innate immunity. To identify effective siRNAs against IDO, we visually examined the sequence of IDO mRNA and selected four targeting sites (Table 1). The targeting sites are located in unstructured parts of the mRNA secondary structure as detected by the Mfold software (http://bioinfo.hku.hk/Pise/mfold.html) for RNA folding.17 The silencing potency of the designed siRNAs was tested in freshly isolated human monocytes (Fig. 1a). Three of the four selected siRNAs (siRNA-2, -3, and -4) exhibited a high silencing potency. Dose–response experiments showed that both siRNA-3 and siRNA-4 were very effective (Fig. 1b,c). Within a range of 0·1–0·5 μg (7·5–37·5 pmol) per transfection, silencing efficiency was between 95 and 99%. To achieve gene silencing using the nucleofection technique, other investigators have used around 0·5–12 μg (37·5–900 pmol) per transfection.18–21, significantly higher concentrations than those used in this study. To further check for specificity, we tested a scrambled IDO siRNA-4 and an additional siRNA targeting human IL-10. As shown in Fig. 1(d), none of the tested siRNAs inhibited IDO gene expression when compared with untransfected cells. In contrast, IDO expression was completely inhibited by anti-IDO siRNA-4 or siRNA-3.
Table 1.
Small interfering (si) RNA sequences
| siRNA | Sequence |
|---|---|
| IDO siRNA-1 | S: 5′-GCCUCCUAUUUUGGUUUAUTT-3′ A: 5′-AUAAACCAAAAUAGGAGGCTT-3′ |
| IDO siRNA-2 | S: 5′-GCAGCGUCUUUCAGUGCUUTT-3′ A: 5′-AAGCAUUGAAAGACGCUGCTT-3′ |
| IDO siRNA-3 | S: 5′-CCCUUCAAGUGUUUCACCATT-3′ A: 5′-UGGUGAAACACUUGAAGGGTT-3′ |
| IDO siRNA-4 | S: 5′-CCGUGAGUUUGUCCUUUCATT-3′ A: 5′-UGAAAGGACAAACUCACGGTT-3′ |
| Scrambled IDO siRNA-4 | S: 5′-GAUUUCGUACUCUGUGCUCTT-3′ A: 5′-GAGCACGGAGUACGAAAUCTT-3′ |
| Control siRNA β-galactosidase | S: 5′-UUGAUGUGUUUAGUCGCUATT-3′ A: 5′-UAGCGACUAAACACAUCAATT-3′ |
| Interleukin-10 siRNA | S: 5′-AGGAUCAGCUGGACAACUUTT-3′ A: 5′-AAGUUGUCCAGCUGAUCCUTT-3′ |
A, anti-sense strand; IDO, indoleamine 2,3-dioxygenase; S, Sense strand (target site).
Figure 1.
Silencing potency of indoleamine 2,3-dioxygenase (IDO) small interfering (si) RNAs in human monocytes. (a) Down-regulation of IDO gene expression by siRNAs. Freshly isolated monocytes were transfected with anti-IDO siRNAs (3 μg/225 pmol each) or control siRNA targeting β-galatosidase (3 μg/225 pmol) using nucleofection. Subsequently, IDO expression was induced with interferon-γ (IFN-γ; 500 U/ml) for 18 hr and IDO protein levels were determined by Western blots. (b) Concentration-dependent down-regulation of IDO gene expression by siRNAs. Freshly isolated monocytes were transfected with various concentrations of IDO siRNAs and then processed as in (a). (c) Quantification of the IDO signals shown in (b). The control intensity is defined as 100%. (d) Anti-IDO siRNAs are specific. Monocytes were transfected with the indicated siRNA duplexes (1 μg/75 pmol) and processed as in (a). Sca = a scrambled IDO siRNA-4. Not transfected but IFN-γ-stimulated cells were included in these experiments.
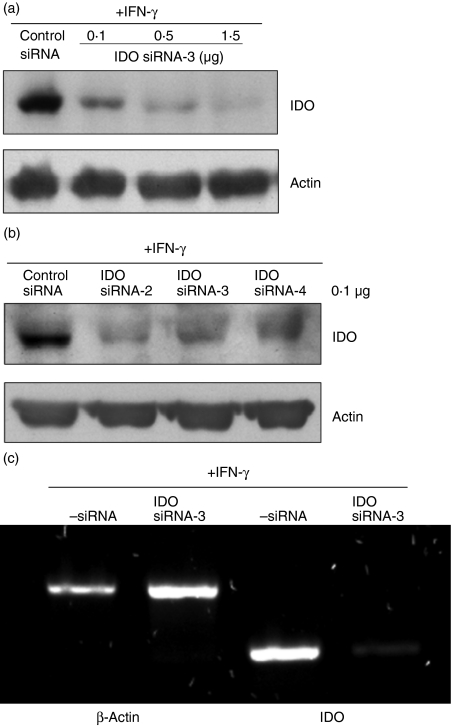
Having demonstrated that IDO siRNAs are effective in monocytes, we assessed their potency in monocyte-derived DCs. To obtain immoDC, human monocytes were incubated with optimized concentrations of GM-CSF and IL-4 for 4–5 days. Subsequently, TNF-α was added to the culture for 2 days to obtain mmoDC as described previously.4 The cells were then washed and transfected with IDO siRNA using nucleofection, and IDO gene expression was induced by IFN-γ for 18 hr. As shown in Fig. 2(a,b), the designed siRNAs were able to knockdown IDO expression in both immoDC and mmoDC. Within a range of 0·1–1·5 μg (7·5–112·5 pmol)/transfection, knockdown efficiency was between 95 and 99% in DCs when compared with the cells transfected with control siRNA. In accordance with the protein levels, DC transfected with, for example, siRNA-3 had a very low IDO mRNA signal, if any, when compared with control (Fig. 2c). The expression of the actin gene served as an internal control.
Figure 2.
Silencing potency of indoleamine 2,3-dioxygenase (IDO) small interfering (si) RNAs in human dendritic cells (DCs). (a) Down-regulation of IDO gene expression by siRNAs in immature monocyte-derived (immo) DCs. To generate immoDCs, monocytes were treated with granulocyte–macrophage colony-stimulating factor (GM-CSF; 25 ng/ml) and interleukin-4 (IL-4; 50 ng/ml) for 5 days. Then the cells were transfected with various concentrations of IDO siRNA-3 (0·1–1·5 μg/7·5–112·5 pmol) or control siRNA (1·5 μg/112·5 pmol). Subsequently, IDO gene expression was induced by interferon-γ (IFN-γ; 500 U/ml) for 18 hr and IDO protein levels were determined by Western blots. (b) Down-regulation of IDO gene expression by siRNAs in mature monocyte-derived (mmo) DCs. To generate mmoDCs, immoDCs were stimulated with tumour necrosis factor-α (TNF-α; 50 ng/ml) for 2 days, and then they were washed, transfected with various IDO siRNAs (0·1 μg/7·5 pmol each) and processed as in (a). (c) Reverse transcription–polymerase chain reaction (RT-PCR) analysis of IDO mRNA levels. ImmoDCs were transfected with siRNA-3 (0·4 μg/30 pmol) and then IDO expression was induced with IFN-γ for 18 hr. Subsequently, total RNA was prepared and IDO and β-actin messenger RNA levels were analysed by RT-PCR as described in Materials and methods.
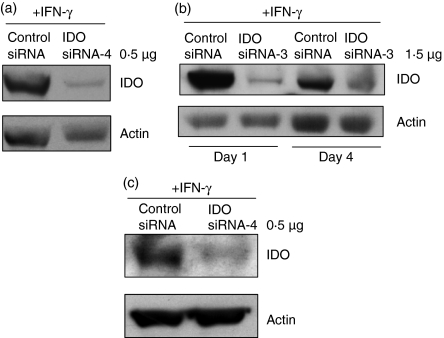
In addition to the nucleofection, we also evaluated standard electroporation on siRNA delivery to DCs. Electroporation is a useful tool to transfect siRNA in vitro into cell lines that are refractory to lipid-based transfection strategies. It was employed to test the sensing of siRNA by innate immune receptors in human monocytes and DCs.10 Also, the technique has been used by several investigators to load DCs with mRNA encoding tumour antigens.22,23 Using the BTX instrument and 0·5 μg (37·5 pmol) of siRNA-4 (pulsed conditions: 500 V for 2 milliseconds), we were able to almost abolish IDO gene expression in DCs (Fig. 3a,c). Under our experimental conditions, the transfection efficiency was up to 95%, consistent with the high percentage of IDO knockdown. Transfected cells showed that 75–85% of total cells are viable cells as assessed by trypan blue exclusion test. Using Gene-Pulser II (pulsed conditions: 300 V, 150 μF, 100 Ω), and 5 μg siRNA (375 pmol), Prechtel et al.,24 achieved around 60% reduction of lamin AC expression in DCs.
Figure 3.
Efficient small interfering (si) RNA gene silencing in dendritic cells (DCs) using the BTX electroporation method. (a) Transfection of indoleamine 2,3-dioxygenase (IDO) siRNAs with BTX electroporation in immature monocyte-derived (immo) DCs. Around 5 × 106 cells were transfected with IDO siRNA-4 (0·5 μg/37·5 pmol) or control siRNA (0·5 μg/37·5 pmol) and then IDO expression was induced with interferon-γ (IFN-γ; 500 U/ml) for 18 hr. Subsequently, IDO and actin protein levels were determined by Western blots. (b) IDO siRNA remains functional during DC maturation. The immoDCs were transfected with IDO siRNA- 3 (1·5 μg/112·5 pmol) or control siRNA (1·5 μg/112·5 pmol) and then the cells were cultured in the presence of interleukin-4 (IL-4), granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumour necrosis factor-α (TNF-α; 50 ng/ml) to induce maturation. The expression of IDO following 18 hr stimulation with IFN-γ was analysed by Western blots at day 1 or 4 subsequent to transfection (day 0). (c) IDO siRNA remains functional after DC freezing and thawing. The immoDCs were transfected with IDO siRNA-4 (0·5 μg/37·5 pmol) or control siRNA (0·5 μg/37·5 pmol) and then they were cultured in the presence of IL-4, GM-CSF and TNF-α (50 ng/ml) for 2 days to induce maturation. Subsequently, the cells were frozen in liquid nitrogen. On day 2 after freezing, the cells were thawed, cultured in complete medium supplemented with IFN-γ (500 U/ml) for 18 hr, and then IDO protein levels were determined by Western blots.
To assess the effect of DC maturation on siRNA silencing potency and the duration of gene silencing, we transfected immoDCs with siRNA-3 and cultured them with TNF-α to induce maturation. The cells were harvested after 1 or 4 days of TNF-α stimulation. To induce IDO expression, the cells were stimulated with IFN-γ for 18 hr before harvesting. As shown in Fig. 3(b), the siRNA give efficient knockdown on both days 1 and 4 when compared with control siRNA.
In view of the clinical use of DCs transfected ex vivo in cancer vaccines, we asked the question whether freezing and thawing of siRNA-transfected DCs would affect siRNA silencing potency. In these experiments, immoDCs were transfected with IDO siRNA and stimulated with TNF-α for 2 days to induce maturation into mmoDCs. The matured DCs were subsequently frozen and stored in liquid nitrogen. At day 2, the cells were thawed, cultured in RPMI-1640 medium and then treated with IFN-γ for 18 hr to induce IDO expression. Interestingly, even after freezing and thawing, the tested siRNA maintained its silencing activity (Fig. 3c).
Constitutively expressed IDO was silenced by siRNAs
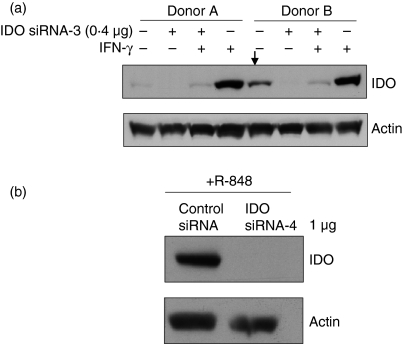
Although a number of mechanisms have been implicated in IDO gene expression, the signals that regulate its expression in specific cells or tissues are not exactly known. In response to inflammatory mediators such as IFN-γ, DCs up-regulated the expression of IDO.3 In human monocytes, both TLR7/8 ligands and cytomegalovirus infection induced IDO expression.4 Prostaglandin E2 also induced IDO expression in monocyte-derived DCs. Although freshly isolated monocytes and monocyte-derived DCs from most donors did not constitutively express IDO, we have noted that cells from a few donors did. Although the expression level was low when compared with that of induced cells, this constitutive IDO expression might be triggered by factors other than IFN-γ. Therefore, we assessed whether the designed siRNAs could block the constitutive expression of IDO. As shown in Fig. 4(a), constitutively expressed and IFN-γ-induced IDO were effectively silenced by siRNA-3 in immoDCs. In addition, R-848-induced IDO in monocytes was also efficiently silenced (Fig. 4b). R848 is a synthetic ligand for TLR7/8. Therefore, regardless of the mechanism of IDO expression, the designed siRNAs are very effective.
Figure 4.
Effect of small interfering (si) RNA on constitutively and induced indoleamine 2,3-dioxygenase (IDO) protein. (a) Immature monocyte derived dendritic cells (immoDCs) were transfected with IDO siRNA-3 (0·4 μg/30 pmol) and one half of the cells was incubated for 18 hr with interferon-γ (IFN-γ; 500 U/ml) to induce IDO expression and the other half was not stimulated. Subsequently, IDO protein levels were determined by Western blots. Data are from two donors. The cells from donor B constitutively expressed IDO as indicated by the arrow. (b) IDO expression in monocytes following siRNA transfection was induced by R-848 (5 μm), a specific Toll-like receptor 7/8 ligand, for 18 hr and IDO expression was assessed as in (a).
IDO silencing in DCs enhanced T-cell response
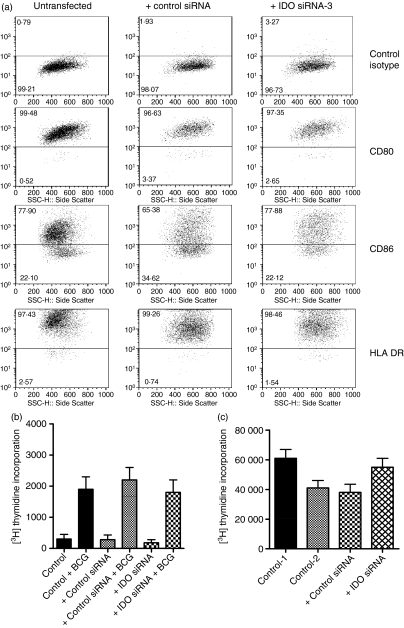
The effect of IDO gene silencing on the ability of DCs to stimulate T cells was examined. First, we studied the impact of IDO siRNA on DC maturation by analysing the expression of certain cell surface molecules. In these experiments, mmoDCs were transfected with siRNA-3 and cultured for 48 hr. Subsequently, surface expression of HLA-DR and co-stimulatory molecules was analysed by flow cytometry (Fig. 5a). Transfection of the cells did not affect the expression of the investigated cell surface molecules compared with either untransfected or control siRNA-transfected cells. Also, transfection of immoDC with siRNAs and subsequent maturation with TNF-α did not affect the expression of CD80, CD86 and HLA-DR molecules (data not shown). In the next experiments, we have tested whether the inhibition of IDO expression can affect the ability of DCs to activate CD4+ T cells to recall antigens such as bacillus Calmette–Guérin.14 The stimulatory potential of anti-IDO siRNA-transfected DCs is comparable to that of control DCs (Fig. 5b), indicating that antigen processing and presentation to T cells were not altered.
Figure 5.
Effect of indoleamine 2,3-dioxygenase (IDO) small interfering (si) RNA on dendritic cell (DC) function, costimulatory and human leucocyte antigen (HLA)-DR expression. (a) Expression of costimulatory and HLA-DR molecules in siRNA-transfected DCs. Immature DCs were transfected with IDO siRNA-3 (1·5 μg/112·5 pmol) or control siRNA (1·5 μg/112·5 pmol) and then they were stimulated with tumour necrosis factor-α (TNF-α) for 2 days to induce maturation. Subsequently, the cells were washed and the expression of the indicated cell surface markers was analysed by flow cytometry using specific monoclonal antibodies. The percentages of positive cells for this representative experiment are shown. (b) siRNA-transfected DCs are capable of processing and presenting soluble antigens to autologous T cells. Irradiated control DCs, control-siRNA-transfected DCs and IDO-siRNA-transfected DCs (5 × 104) were incubated with autologous T cells (105) in the absence or presence of bacillus Calmette–Guérin (BCG) antigens (5 μg/ml) for 5 days. T-cell proliferation was measured by [3H]thymidine incorporation. Samples were assayed in triplicate and the results represent the mean ± SD of two independent experiments. (c) Anti-IDO siRNA inhibits the suppressive effect of IDO-positive DCs on T-cell proliferation. Control DCs, control-siRNA-transfected DCs and IDO-siRNA-transfected DCs were stimulated for 18 hr with IFN-γ to induce IDO expression. Subsequently, they were washed, irradiated and 105 cells were incubated with autologous T cells (104) for 3 days in the presence of phytohaemagglutinin (5 μg/ml). Cell proliferation was measured by [3H]thymidine incorporation. Control 1 = not transfected and IFN-γ-stimulated (IDO-negative DCs), Control 2 = not transfected but IFN-γ-stimulated (IDO-positive DCs), Control siRNA = transfected with control siRNA and IFN-γ-stimulated, IDO siRNA = transfected with IDO siRNA and IFN-γ-stimulated. Samples were assayed in triplicates and the results represent the mean ± SD of two independent experiments.
One of the aims of inhibiting the expression of immunosuppressive factors such as IDO is to facilitate the development of effective DC cancer vaccines. To investigate the effect of IDO gene silencing on T-cell proliferation, we have tested the suppressive potential of DC on PHA-stimulated T cells. In these experiments, DCs were transfected with IDO siRNA-3 or control siRNA and IDO expression was induced by IFN-γ for 18 hr. Subsequently, transfected and control DCs were washed, irradiated and then incubated with autologous CD4+ T cells. As shown in Fig. 5(c), IDO-positive DCs reduced activated T-cell proliferation in response to PHA (P< 0·05).
Effects of uridine substitution on IDO siRNA gene silencing
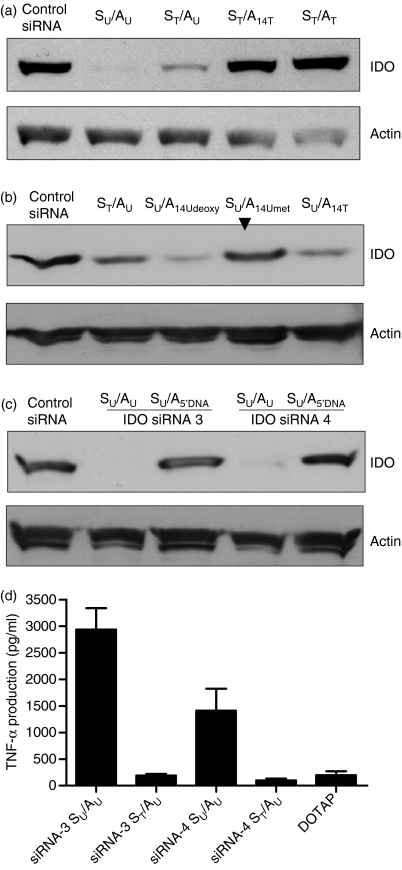
We and others have previously shown that chemically made siRNAs can activate innate immunity through endosomal TLRs.10–12 Because of the significant role of TLRs in innate immunity and DC function, the sense strands of the designed siRNAs were selected to contain a high number of 2′-hydroxyl uridines that have been found to be critical for recognition by TLRs.25,26 Also, we have shown that immunostimulation can be prevented by 2′-modifications of uridines.27 Based in these findings, we investigated the effect of uridine replacement by thymidines on siRNA silencing potency. It should be noted that the most critical issue in developing chemically modified siRNAs is the placement of the modification in a siRNA duplex because chemical modifications have been known to affect siRNA silencing potency. As shown in Fig. 6(a) (lane ST/AU), substitution of all uridines with their DNA counterparts in the sense strand (ST) did not significantly affect IDO siRNA-4 gene silencing when compared with the unmodified siRNA-4 (lane SU/AU). However, when the uridines in both the sense and the anti-sense strands were replaced with their thymidine counterparts, the silencing activity was almost abolished (Fig. 6a, lane ST/AT). These results are surprising given the low uridine contents in the anti-sense strand (see Table 1).
Figure 6.
Effect of uridine substitution on small interfering (si) RNA silencing potency. (a) Freshly isolated monocytes were transfected with unmodified and modified indoleamine 2,3-dioxygenase (IDO) siRNA-4 or control siRNA and then stimulated for 18 hr with interferon-γ (IFN-γ; 500 U/ml) to induce IDO expression and IDO protein levels were determined by Western blots. SU/AU = unmodified siRNA, ST/AU = the uridines in the sense strand were replaced by thymidines, ST/A14U = the uridines in the sense strand were replaced by thymidines and only uridine at position 14 in the anti-sense strand was replaced by a thymidine, ST/ST = the uridines in the sense and the anti-sense strands were replaced by thymidines. (b) Effects of site-specific modification at position 14 of the anti-sense strand on siRNA-4 silencing potency. Experimental conditions are as in (a). Modified anti-sense strand carrying either 2′-deoxyuridine, 2′-O-methyl uridine, or thymidine at position 14 was combined with unmodified sense strand to form siRNA duplexes that were tested. As indicated by the arrow, the siRNA with the 2′-O-methyl uridine exhibited no significant silencing effect when compared with the other molecules. (c) Modified IDO siRNAs with a DNA seed sequence were inactive. Anti-IDO siRNA-3 and siRNA-4 with 5′-DNA seed sequences (nucleotides 1–7) were designed and tested in human monocytes. Experimental conditions are as in (a). (d) Effect of thymidines in the sense strand on tumour necrosis factor-α (TNF-α) expression. Unmodified or chemically made siRNAs carrying thymidine only in the sense strand were complexed with the cationic liposome DOTAP (1 μg) and delivered to monocytes. After 18 hr of incubation, TNF-α levels in culture supernatants were analysed by enzyme-linked immunosorbent assay. The final concentration of each siRNA was 1·5 μg (112·5 pmol)/ml. Samples were assayed in triplicates and the results represent the mean ± SD of two independent experiments.
The anti-sense strand of siRNA-4 contains two uridines, one at position 1 and the other at position 14 (Table 1). To identify which uridine is responsible for the inhibition of siRNA silencing activity, the uridine in position 14 was replaced with thymidine. Interestingly, this single modification inhibited gene silencing (Fig. 6a, lane ST/A14T). Given that thymidine has a methyl group at the 5′ position, we thought it might affect the interaction with the RNA-induced silencing complex (RISC). Therefore, two additional modifications, 2′-deoxy- and 2′-O-methyl uridines were incorporated at position 14, and tested. Both modifications inhibited gene silencing (data not shown). However, when unmodified sense strand (all RNA) was combined with the anti-sense strand with different modifications in position 14, only 2′-O-methyl uridine inhibited gene silencing (Fig. 6b, lane SU/A14Umet). It should be noted that the methyl group abolishes hydrogen bonding of the 2′-OH group as a donor and it reduces the likelihood that it acts as an H-bond acceptor.
With the aim of lowering siRNA cost, we also tested the possibility of substituting the 5′-end (nucleotides 1–7) or the 3′-end proximal (nucleotides 14–21) sequence in the anti-sense strand with DNA. Replacing the 5′ proximal RNA sequence with its DNA counterpart abolished gene silencing for both siRNAs tested (Fig. 6c, lanes SU/A5DNA). These modified anti-sense strands were combined with unmodified sense strands. Replacing the 3′ proximal sequence (nucleotides 14–21) of the anti-sense strand with DNA showed very low silencing effect compared with that of the unmodified siRNA (data not shown).
Having demonstrated that the replacement of all uridines with thymidines in the sense strand does not affect gene silencing, next we investigated the immunostimulatory potential of the modified IDO siRNAs. In these experiments, cells were transfected with siRNAs for 18 hr, and then TNF-α production was measured in culture supernatants using ELISA. As shown in Fig. 6(d), unmodified IDO siRNA-3 and siRNA-4 administered using DOTAP induced TNF-α production while their thymidine-modified versions did not. It should be noted that cytoplasmic delivery of chemically made siRNAs via electroporation did not induce cytokine production (data not shown), confirming the requirement of endosomes as demonstrated elsewhere.10
Effects of in-vitro-transcribed anti-IDO siRNAs on DC function and maturation
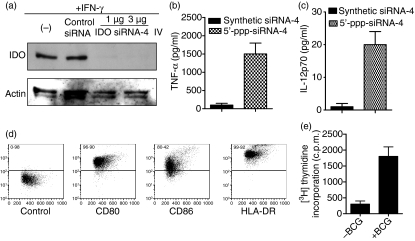
In a previous study we have shown that cytoplasmic delivery of in-vitro-transcribed siRNA bearing 5′-triphosphate inhibited IL-10 gene expression and induced cytokine production through probably the activation of RIG-1.6,28 This finding prompted us to test whether in-vitro-transcribed anti-IDO siRNA would silence its expression and activate cytokine production in DCs. The in-vitro-transcribed anti-IDO siRNA-4 effectively inhibited IDO expression and simultaneously induced TNF-α and IL-12 production (Fig. 7a–c, respectively). In accordance with our early work, cytoplasmic delivery of chemically made siRNA did not induce cytokine production because they lack the 5′-triphosphate signature.
Figure 7.
Effects of bifunctional anti-indoleamine 2,3-dioxygenase (IDO) small interfering (si) RNAs on IDO expression, cytokine production, and dendritic cell (DC) function and maturation. (a) Gene silencing using in-vitro-transcribed anti-IDO siRNA-4. Immature monocyte-derived (immo) DCs were transfected with in-vitro-transcribed siRNA-4 (1 or 3 μg/75 or 225 pmol) using the BTX electroporation method and then they were stimulated with interferon-γ (IFN-γ) to induce IDO expression. After 18 hr of transfection, IDO and actin expression were analysed by Western blots. (b and c) Effects of in-γvitro-transcribed siRNA-4 on tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12) expression. ImmoDC were transfected with in-vitro-transcribed siRNA-4 (3 μg/225 pmol) as in (a). After 18 hr of transfection, TNF-α and IL-12 levels in culture supernatants were analysed by enzyme-linked immunosorbent assay. Samples were assayed in triplicates and the results represent the mean ± SD of two independent experiments. (d) The expression of costimulatory molecules and human leucocyte antigen (HLA) -DR in the transfected immoDCs was determined by flow cytometry after 48 hr of transfection. The percentages of cells for this representative experiment are indicated. (e) The capacity of the 5′-triphosphate siRNA-4-transfected immoDCs to activate T cells was tested using bacillus Calmette–Guérin (BCG) protein lysates.12 Irradiated DCs (5 × 104) were incubated with autologous T cells (105) in the presence or absence of BCG protein extracts (5 μg/ml) for 5 days. Cell proliferation was measured by [3H]thymidine incorporation. Samples were analysed in triplicates and the results represent the mean ± SD of three independent experiments.
In the next experiment, we tested whether 5′-triphosphate anti-IDO siRNA-4 can promote DC maturation. ImmoDCs were electroporated with the 5′-triphosphate siRNA-4 and then they were cultured for 48 hr without the addition of external maturation cytokines such as TNF-α. Subsequently, the cells were characterized for the expression of CD80, CD86 and HLA-DR molecules by flow cytometry (Fig. 7d). The data show high expression levels of the investigated markers. Subsequently, we tested the stimulatory potential of 5′-triphosphate siRNA-transfected immoDC on T-cell proliferation. Even in the absence of external maturation cytokines, these DCs are able to activate T cells (Fig. 7e). Consequently, the possibility of blocking IDO expression and simultaneously activating cytokine production and DC maturation using in-vitro-transcribed siRNAs is a novel strategy that should facilitate the design of effective cancer vaccines.
Discussion
Although DC-based vaccines hold an exciting promise, several obstacles such as the efficacy of DCs to activate T cells and immunosuppression mechanisms are yet to be addressed. Among the immunosuppressive factors, IDO plays an essential role and it has been shown to inhibit lymphocyte proliferation.29,30. In this study, we designed two highly effective anti-IDO siRNAs (siRNA-3, siRNA-4) and showed that they can effectively block IDO gene expression in human primary cells such as monocytes and DCs. Owing to their design, anti-IDO bifunctional siRNAs were able to knockdown IDO expression in DCs and activate cytokine secretion, particularly IL-12, which is important for the T helper type 1 (Th1) response. Furthermore, both classes of siRNAs reversed the suppressive effects of IDO-positive DCs on T-cell proliferation in vitro.
Notably, one of the key experimental strategies to elucidate the function of a gene in vitro and in vivo is the specific ablation of its expression. To check for specificity, most siRNA suppliers recommend the use of negative siRNA controls with sequences that do not show sequence similarity to the human sequences such as the siRNA targeting β-galactosidase used in this study. Also, it is recommended to test multiple negative siRNA controls to identify those that do not induce non-specific effects on gene expression. In addition, we think that it is central to use multiple siRNAs targeting different sites of the gene transcript being investigated. These siRNAs should first be analysed for effective reduction of target gene expression. Different siRNAs to the same target and with similar gene silencing efficiency should induce similar phenotypic effects. Any changes induced by one siRNA and not the other(s) could be attributed to bystander effects (e.g. off-target effects) and these siRNAs should not be used in therapy, particularly when bystander effects are not desired (see below). In our study, we have systematically tested at least two active siRNAs (siRNA-3 and siRNA-4) targeting IDO and obtained the same results on IDO expression and DC functions such as processing and presenting antigens to T cells. Furthermore, we show that the expression of CD80, CD86 and HLA-DR molecules by DCs are not affected by IDO siRNAs, indicating that any bystander effects related to the electroporation method and anti-IDO siRNAs used in this study are relatively low.
In most clinical studies to date, DC maturation was induced by a cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and prostaglandin E2.31 Although the generated DCs exhibited a mature phenotype and activated T cells, these DCs are poor producers of IL-12 and so are less effective in inducing the Th1 type response that is essential for a productive anti-tumour cytotoxic T-lymphocyte response.32 In addition, in a separate study we found that all five tested DC preparations from patients undergoing cancer vaccine administration at our institution constitutively expressed high levels of IDO protein (unpublished data). Unfortunately, such IDO-positive DCs are expected to induce immune tolerance instead of immunity. Therefore, there is a need to include anti-IDO siRNAs during the transfection of DC with mRNA-encoding tumour antigens. We are confident that clinical researchers will implant RNAi technology in DC-based vaccination to interfere with the negative regulatory mechanisms (e.g. IDO, IL-10) used by DCs to induce peripheral tolerance.
To improve the quality of DC used in cancer vaccines, additional activation of DCs with, for example, TLR ligands has resulted in more effective anti-tumoral immunity.33 The TLRs are transmembrane proteins expressed by the cells of the innate immune system that recognize invading microbes and are important for immediate microbe protection, as well as for the activation of adaptive immunity via inflammatory cytokines.34 Viral nucleic acids are recognized by intracellular TLRs that are located on the intracellular endosome membranes. Certain siRNA sequences are recognized by endomosal TLR7/8, leading to rapid production of pro-inflammatory cytokines and IFNs, therefore mimicking a viral infection. In addition to TLRs, immune cells also use RIG-1 to recognize viral RNAs and in-vitro-transcribed siRNA-bearing 5′-triphosphate. 28,35
Previously, we have shown that it is possible to combine immunostimulation and gene-silencing in one single siRNA molecule.6 In addition to liposomal delivery, we have shown that in-vitro-transcribed anti-IL-10 siRNA can induce cytokine production and block IL-10 expression when delivered via electroporation.6 In the present study, we also developed anti-IDO siRNAs that simultaneously silenced gene expression and activated either endosomal TLR or cytoplasmic RIG-1 protein. The developed 5′-triphosphate anti-IDO siRNA not only silenced IDO expression but also activated RIG-1, leading to better DCs, as evidenced by the secretion of IL-12. In addition, immoDCs that had been transfected with 5′-triphosphate siRNAs were able to express costimulatory molecules and activate T cells. This finding is important because it serves to open up a new method for the generation of mature DCs without the addition of external cytokines.
To improve siRNA stability and pharmacokinetics, a variety of chemical modifications have been used.36 They can be used alone or in combination with a number of modified nucleotides. The modifications also differ in how they are tolerated by the RISC. Some modifications can be introduced at most or all bases of both strands, whereas other modifications cannot be placed at some positions. In the cases where immunostimulation is not needed, replacement of uridine residues with their 2′-modified counterparts such as 2′-deoxyuridines abrogated immune stimulation without affecting siRNA silencing.24 Hence, it is possible to separate immunostimulation from RNAi. In this study, the substitution of uridines by their 2′-deoxy counterparts in IDO siRNA led to the design of non-stimulatory siRNAs. Although further work is needed, an important observation that has emerged from the chemical modifications is that a single modification at position 14 of the anti-sense strand can have a severe negative effect on gene silencing.
Given that RNA nucleotides can be replaced with their DNA counterparts, we also tested the possibility of replacing either the proximal 5′ (seed sequence) or 3′ end sequence within the anti-sense strand with DNA. The data showed that when the 5′ seed sequence was replaced with DNA sequence, the resulting siRNA did not exhibit silencing potency. Recently, Ui-Tei et al.37 reported that siRNAs with a modified DNA seed arm are active in mammalian cells. However, neither of the two tested siRNAs in the present study showed any activity even at high concentrations. Although the sequences of our siRNAs seem to satisfy the three criteria indicated by Ui-Tei et al. (A/U at the 5′ terminus of the guide strand; G/C at the 5′ terminus of the passenger strand; at least four A/U residues in the 5′ terminal 7 base pairs of the guide strand), replacement of the seed sequence with DNA in anti-IDO siRNA-3 and -4 totally inhibited gene silencing. It is unclear why our results differ from those of Ui-Tei et al. However, regardless of the exact nature of the differences, we believe that the replacement of the 5′ seed sequence with DNA is not a straightforward strategy and further optimization is needed. Data obtained with siRNAs carrying 3′-DNA sequences (nucleotides 14–21) showed that these siRNAs can induce gene silencing when combined with unmodified sense strands, although the silencing effect was relatively very low and further analysis is also needed.
In conclusion, the data presented here throw some light on the potential therapeutic applications of anti-IDO siRNAs. The inhibition of IDO expression and deliberate induction of cytokines with a single anti-IDO siRNA in immune cells should provide an important new immunotherapeutic option. Interestingly, immoDCs that had been transfected with 5′-triphosphate expressed TNF-α and were able to activate T cells, which opens the possibility of generating mature DCs without the addition of external cytokines. This strategy of DC maturation may be extended into a therapeutic vaccination setting.
Acknowledgments
This work was assisted by support from the Gene therapy program at the Norwegian Radium Hospital to M. Sioud. We thank Anne Mobergslien for technical assistance and Dr Anne Dybwad for editing the manuscript.
Acknowledgments
The authors have no financial conflict of interest.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Mellor AL. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 4.Furset G, Floisand Y, Sioud M. Impaired expression of indoleamine 2, 3-dioxygenase in monocyte-derived dendritic cells in response to Toll-like receptor-7/8 ligands. Immunology. 2008;123:263–71. doi: 10.1111/j.1365-2567.2007.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–90. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furset G, Sioud M. Design of bifunctional siRNAs: combining immunostimulation and gene-silencing in one single siRNA molecule. Biochem Biophys Res Commun. 2007;352:642–9. doi: 10.1016/j.bbrc.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 8.Sioud M. Therapeutic siRNAs. Trends Pharmacol Sci. 2004;25:22–8. doi: 10.1016/j.tips.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore IR, Fox SP, Hollins AJ, Sohail M, Akhtar S. The design and exogenous delivery of siRNA for post-transcriptional gene silencing. J Drug Target. 2004;12:315–40. doi: 10.1080/10611860400006257. [DOI] [PubMed] [Google Scholar]
- 10.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–90. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–70. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 12.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–62. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–24. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 14.Sioud M, Kjeldsen-Kragh J, Quayle AJ, Wiker HG, Sorskaar D, Natvig JB, Forre O. Immune responses to 18.6 and 30-kDa mycobacterial antigens in rheumatoid patients, and V beta usage by specific synovial T-cell lines and fresh T cells. Scand J Immunol. 1991;34:803–12. doi: 10.1111/j.1365-3083.1991.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–30. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 16.Saetrom P, Snove O., Jr A comparison of siRNA efficacy predictors. Biochem Biophys Res Commun. 2004;321:247–53. doi: 10.1016/j.bbrc.2004.06.116. [DOI] [PubMed] [Google Scholar]
- 17.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–15. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantei A, Rutz S, Janke M, et al. siRNA stabilization prolongs gene knockdown in primary T lymphocytes. Eur J Immunol. 2008;38:2616–25. doi: 10.1002/eji.200738075. [DOI] [PubMed] [Google Scholar]
- 19.Hagemann C, Meyer C, Stojic J, Eicker S, Gerngras S, Kuhnel S, Roosen K, Vince GH. High efficiency transfection of glioma cell lines and primary cells for overexpression and RNAi experiments. J Neurosci Methods. 2006;156:194–202. doi: 10.1016/j.jneumeth.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Geletu M, Balkhi MY, Peer Zada AA, Christopeit M, Pulikkan JA, Trivedi AK, Tenen DG, Behre G. Target proteins of C/EBPalphap30 in AML: C/EBPalphap30 enhances sumoylation of C/EBPalphap42 via up-regulation of Ubc9. Blood. 2007;110:3301–9. doi: 10.1182/blood-2007-01-071035. [DOI] [PubMed] [Google Scholar]
- 21.Merkerova M, Klamova H, Brdicka R, Bruchova H. Targeting of gene expression by siRNA in CML primary cells. Mol Biol Rep. 2007;34:27–33. doi: 10.1007/s11033-006-9006-x. [DOI] [PubMed] [Google Scholar]
- 22.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Michiels A, Tuyaerts S, Bonehill A, et al. Electroporation of immature and mature dendritic cells: implications for dendritic cell-based vaccines. Gene Ther. 2005;12:772–82. doi: 10.1038/sj.gt.3302471. [DOI] [PubMed] [Google Scholar]
- 24.Prechtel AT, Turza NM, Theodoridis AA, Kummer M, Steinkasserer A. Small interfering RNA (siRNA) delivery into monocyte-derived dendritic cells by electroporation. J Immunol Methods. 2006;311:139–52. doi: 10.1016/j.jim.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Sioud M. Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol. 2006;36:1222–30. doi: 10.1002/eji.200535708. [DOI] [PubMed] [Google Scholar]
- 26.Forsbach A, Nemorin JG, Montino C, et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J Immunol. 2008;180:3729–38. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- 27.Cekaite L, Furset G, Hovig E, Sioud M. Gene expression analysis in blood cells in response to unmodified and 2′-modified siRNAs reveals TLR-dependent and independent effects. J Mol Biol. 2007;365:90–108. doi: 10.1016/j.jmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 29.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuler-Thurner B, Schultz ES, Berger TG, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–88. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 33.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 35.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 36.Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Invest. 2007;117:3615–22. doi: 10.1172/JCI33483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ui-Tei K, Naito Y, Zenno S, Nishi K, Yamato K, Takahashi F, Juni A, Saigo K. Functional dissection of siRNA sequence by systematic DNA substitution: modified siRNA with a DNA seed arm is a powerful tool for mammalian gene silencing with significantly reduced off-target effect. Nucleic Acids Res. 2008;36:2136–51. doi: 10.1093/nar/gkn042. [DOI] [PMC free article] [PubMed] [Google Scholar]