Abstract
This is the first phase III randomised trial to evaluate maintenance immunotherapy in metastatic renal cell cancer (mRCC). Patients were randomised to receive treatment with a 4-week cycle of subcutaneous low doses IL-2 + IFN in months 1, 3 and 5, and then every 3 months until the first documented disease progression (arm A, suspension), or the same regimen, with chronic maintenance of immunotherapy, regardless of tumour response, until death or intolerable toxicity (arm B, maintenance). The primary endpoint was overall survival (OS); secondary endpoints were time from first progression to death (TFPTD) and tolerability. One hundred and eighty-three patients were enrolled between January 1998 and November 2003. After a median follow-up of 53.9 months, response rate, median OS and median TFPTD were 14.7% (6.3% CR) versus 11.3% (5.5% CR), 14 versus 14 months, 6 versus 5 months, in arms A and B, respectively with no significant differences between the groups. Cox regression analysis showed that the use of chemotherapy after first progression (HR 0.54; 95% CI 0.35–0.86; p = 0.008), PS = 0 (HR 0.53; 95% CI 0.35–0.81; p = 0.001) and female gender (HR 0.63; 95% CI 0.41–0.98; p = 0.038) were significantly associated with a longer TFPTD; treatment arm was not significant (HR 0.88; 95% CI 0.60–1.31; p = 0.54). Toxicity was mainly limited to WHO grades 1 or 2. Chronic maintenance immunotherapy after disease progression is feasible, but does not significantly increase OS or the TFPTD.
Keywords: Renal cell cancer, Immunotherapy, Maintenance, Chemotherapy
Introduction
Renal cell carcinoma (RCC) is the seventh leading cause of death due to cancer and accounts for about 3% of all tumours. Its incidence has continuously increased over the last few years, with about one-third of the cases presenting distant metastases at the time of diagnosis, and about one-half of the remaining 60–70% becoming metastatic [1, 2]. The treatment of metastatic RCC (mRCC) is rapidly changing, different new biological agents proved to positively impact on progression-free survival and/or overall survival as compared to interferon-α (IFN) in first-line therapy or placebo in second line, leading to a substantial improvement in medical treatment [3–10].
At the time, this study was planned and started (January 1998), combined IL-2 and IFN-α treatment was considered an effective IT for metastatic renal carcinoma (mRCC) [11–13]. IL-2 is a 15 kD protein produced by activated T lymphocytes that was originally discovered as a growth factor for T cells. It acts by binding to a specific receptor on target cells that consists of three (α, β and γ) chains: the β and γ chains are essential for signalling, whereas the α chain increases the affinity of the complex. IL-2 induces the proliferation of activated T cells, stimulates the cytotoxicity of natural killer (NK) cells, and acts as a cofactor in T cells activating macrophages and B cells [11]. IL-2 has been studied in mRCC, since 1984, achieving RRs that range from 15 to 35% [14, 15]. The interferon’s were first described in 1957 as a family of proteins produced by cells after virus exposure, which interfere with viral replication [16], and include IFN-α derived from leukocytes, IFN-β derived from fibroblasts, and IFN-γ derived from lymphocytes. The exact mechanism of the anti-tumoural action of the IFNs is unclear, but they directly inhibit tumour cell proliferation, enhance the lytic activity of NK cells, increase the tumour cell expression of human leukocyte antibody (HLA) class I, and has anti-angiogenic effects [17].
Amongst the effective immunotherapy options, high-dose bolus i.v. interleukin-2 (IL-2), IFN and low/intermediate dose of s.c. IL-2 plus IFN have been used with evidence of anti-tumour activity, but no impact on overall survival (OS), with the sole, notable, exception of those few patients who achieved a complete response following the high-dose bolus i.v. IL-2 therapy [12, 13]. As far as low doses of s.c. IL-2, the rationale behind its use came from the observation that natural killer (NK) cells and other subpopulations of T lymphocytes can be effectively stimulated with doses as low as 500,000 IU [18]. A number of uncontrolled studies and a few randomised trials have shown that low doses s.c. IL-2 are associated with less acute toxicity and capable of inducing partial and complete remission (albeit at lower rates than those obtainable with higher doses), with a comparable effect on median survival [19, 20]. Moreover, they can be administered even for long periods of time with the aim of persistently stimulating immune cells without evidence of potential T cell exhaustion [21, 22].
In a phase II trial, we found that a low-dose chronic regimen of IL-2 + IFN had a persistent immunological effect and led to a response rate and survival duration that were similar to those obtained with regimens based on higher doses, without any relevant toxic effects [23]. However, no randomised trial has yet demonstrated the beneficial effect of chronic maintenance immunotherapy in patients with progressive disease, and so a controlled study is needed to assess whether it has any real value.
The aim of this randomised, multicentre trial was to test whether chronically administering a maintenance regimen of low doses IL-2 + IFN in patients progressing on immunotherapy can prolong the time from first progression to death (TFPTD) and overall survival (OS).
Methods
Patient selection
Eligibility criteria included histological diagnosis of RCC, ECOG performance status of 0–2, no prior immunotherapy and a life expectancy of more than 3 months. Laboratory requirements included total bilirubin and AST or ALT levels of <1.5 times the institutional upper normal limit (UNL), with serum creatinine levels below the UNL or a calculated creatinine clearance of at least 50 mL/min. Patients were excluded if they had brain metastasis, signs or symptoms of cardiac failure, arrhythmias, autoimmune diseases, liver metastases involving more than 50% of the organ, an ongoing need for systemic corticosteroid therapy, pregnancy and breastfeeding: the women and men of reproductive potential had to agree to use an effective contraceptive method. The study was approved by the Ethics Committees of the participating institutions, and all of the participants gave their written informed consent.
Study design and treatment
The study design is shown in the consort diagram (Fig. 1). Each patient received immunotherapy for 5 days a week, usually from Monday to Friday, no therapy on Saturday and Sunday. This was repeated for four consecutive weeks on months 1, 3, 5 and then every 3 months until progression in arm A (suspension) or indefinitely in arm B (maintenance). Treatment consisted of subcutaneous (s.c.) IL-2 (5 days/week, 1 million UI/m2 bid on days 1 and 2, and 1 million UI/m2 × 1 on days 3, 4 and 5) + IFN (1.8 million UI/m2 on days 3 and 5).
Fig. 1.
CONSORT diagram. IT immunotherapy, PD progression of disease. Asterisks indicate number of patients who received at least one cycle of maintenance immunotherapy after progression. Nine patients in arm A received maintenance after progression, thus violating the protocol, because no maintenance was scheduled in this arm. Dagger symbol indicates number of patients who did not receive at least one cycle of maintenance immunotherapy after progression. Thirty-four (47%) of the patients in arm B did not receive maintenance for various reasons: rapid disease progression, lost to follow-up, non-compliance with the protocol
In the case of disease progression, the patients randomised to arm A (suspension group) had to stop treatment with IL2 + IFN permanently, but could switch to a different type of treatment (chemotherapy, radiotherapy or others therapy) in accordance with their physician’s judgement, whereas the patients in arm B (maintenance group) continued immunotherapy (except in the case of serious adverse events, intolerance or refusal) and were allowed to add any other type of therapy (chemotherapy, radiotherapy or others therapy).
Oral acetaminophen 500–1,000 mg was administered before IFN to attenuate therapy-related pyretic reactions. A 50% dose reduction in both immunotherapeutic drugs was allowed following toxicity of grade 3 or more.
Baseline and follow-up evaluation, and response criteria
Before randomisation, all of the patients underwent a physical examination, performance status assessment, laboratory tests and tumour evaluation with instrumental examinations: brain, chest and abdomen CT scans, and radionuclide bone scan with X-rays of suspected sites.
Therapeutic response was evaluated after the first three cycles or earlier in the case clinically suspected progressive disease, using the Response Evaluation Criteria in Solid Tumours (RECIST) [24]. Toxicity was assessed using the WHO criteria. After the first assessment, the tumour evaluation was performed every 3 months (or earlier in the case clinically suspected progressive disease) using the same instrumental examinations as those used for the previous evaluations. Moreover, every 3 months, the patients underwent: physical examination hemochrome with leukocytic formula, blood chemistry tests, follow-up of any unresolved adverse event, survival evaluation.
Statistical analysis
Randomisation was centralised at the data centre of the Italian Oncology Group for Clinical Research (GOIRC) in Parma (Italy). The patients were stratified on the basis of their performance status (0 vs. 1–2) and oncology centre of enrolment. The primary objective of this phase III study was to determine whether maintenance immunotherapy in patients with progressive mRCC was superior to no immunotherapy in terms of overall survival (OS) and time from first progression to death (TFPTD). On the basis of our previous findings [21–23], the planned sample size of 180 patients was based on a power of 80% to detect a 20% improvement in 5-year OS using a two-sided stratified log-rank test at an overall 0.05 level of significance, with 140 expected events. Data were analysed according to the intention-to-treat principle. The χ 2 statistic was used to test for univariate associations. Time-to-event variables were summarised using Kaplan–Meier curves, with a log-rank test being used to compare treatment arms. The survival endpoints were defined from the date of randomisation to the date of documented progressive disease for time-to-progression (TTP), or documented death for OS, and from the date of documented progressive disease to the date of death from any cause for TFPTD, or were censored on the date of the last follow-up visit.
Cox proportional hazards regression models (backward stepwise method) were used to estimate hazard ratios. Two-sided p values were reported for all of the analyses, which were made using SAS 8.2 software (SAS Institute Inc, Cary, NC).
Results
Patient characteristics
One hundred and eighty-three patients were enrolled between January 1998 and November 2003: 95 in arm A and 88 in arm B. Four patients were judged ineligible: three because they had a PS of 3 at the time of randomisation, and one because of renal failure, but, in accordance with the intention-to-treat principle, all were included in the analyses.
At the time of analysis, the median follow-up was 53.9 months (range 17.0–88.1). There were no significant differences in the characteristics of the patients in the two arms. About 15% in both arms did not undergo nephrectomy before treatment because this was not indicated at the time the study started; their other characteristics are shown in Table 1.
Table 1.
Patients’ characteristics
| Arm A suspension N = 95 (100%) | Arm B maintenance N = 88 (100%) | p value | |
|---|---|---|---|
| Age, years, median (range) | 63 (33–79) | 63 (35–81) | 0.39 |
| Sex | |||
| Male | 65 (68%) | 65 (74%) | 0.42 |
| Female | 30 (32%) | 23 (26%) | |
| ECOG performance status | |||
| 0 | 49 (52%) | 49 (56%) | 0.84 |
| 1 | 34 (36%) | 26 (29%) | |
| 2 | 10 (10%) | 11 (13%) | |
| 3 | 2 (2%) | 2 (2%) | |
| Histology | |||
| Clear cell | 80 (84%) | 72 (82%) | 0.70 |
| Papillary | 5 (6%) | 3 (3%) | |
| Sarcomatoida | 6 (6%) | 7 (8%) | |
| Other | 4 (4%) | 6 (7%) | |
| Grading | |||
| 1 | 5 (6%) | 3 (4%) | 0.82 |
| 2 | 27 (28%) | 26 (30%) | |
| 3 | 27 (28%) | 30 (33%) | |
| 4 | 8 (9%) | 7 (8%) | |
| Unknown | 28 (29%) | 22 (25%) | |
| No. of metastatic sites | |||
| 1 | 50 (53%) | 44 (50%) | 0.67 |
| 2 | 28 (29%) | 30 (34%) | |
| ≥3 | 17 (18%) | 14 (16%) | |
| Liver metastasis | |||
| Yes | 11 (12%) | 15 (17%) | 0.29 |
| No | 84 (88%) | 73 (83%) | |
| Prior nephrectomy | |||
| No | 15 (16%) | 13 (15%) | 0.55 |
| Yes | 80 (84%) | 75 (85%) | |
| Prior treatment | |||
| Chemotherapy | 1 (1%) | 1 (1%) | 0.73 |
| Medroxyprogesterone acetate | 1 (1%) | 2 (2%) | |
| Surgery for metastases | 2 (2%) | 1 (1%) | |
aAt least 25% of sarcomatoid features
Treatment administration and patient disposition
Nine patients randomised to arm A (suspension group) actually received immunotherapy after the first documented progression of disease, thus, violating the protocol, due to the lack of alternatives perceived as active by treating physicians (Fig. 1).
Of progressing patients randomized to maintenance arm, only 38 (53%) really received at least one cycle of maintenance according to the protocol. Furthermore, the median number of maintenance cycles per patient was 1 (range 1–11). As a whole, the low percentage of patients treated with maintenance immunotherapy in the maintenance arm, as well as the low median number of maintenance cycles received were mainly due to early deaths or deterioration of PS in progressing patients.
The therapeutic option most frequently chosen after disease progression was chemotherapy, which was used in 37 patients, mainly the combination of gemcitabine plus 5-fluorouracil (5-FU), or vinblastine alone or in combination with other chemotherapeutic agents. There was a clear imbalance in the distribution of post-progression treatment: 28 patients (34%) in the suspension group underwent chemotherapy and only 9 (12%) in maintenance group (p = 0.001). This was due to the fact that the physicians in some centres continued immunotherapy alone in the patients in the maintenance arm, without introducing chemotherapy for progressing patients, a choice that could have influenced TFPTD.
Responses and efficacy
The data refer to all of the randomised patients. There were a total of 11 CRs (6.0%), 13 PRs (7.2%), and 36 SD (19.7%), for an overall response rate of 13.2% and a disease control rate of 32.9% (Table 2). No significant differences in response rates were recorded between the treatment arms. Median TTP was 6.8 months: 7.0 months in arm A and 6.4 months in arm B, without any significant difference between them. These data confirm the efficacy of low doses of IL-2 plus IFN in mRCC, as previously described in a phase II study [23].
Table 2.
Tumour response data by treatment arm
| Response | ARM A suspension N = 95 (100%) | ARM B maintenance N = 88 (100%) | Overall N = 183 (100%) |
|---|---|---|---|
| CR | 6 (6.3) | 5 (5.6) | 11 (6.0) |
| PR | 8 (8.4) | 5 (5.7) | 13 (7.2) |
| SD | 18 (18.9) | 18 (20.4) | 36 (19.7) |
| PD | 54 (56.8) | 50 (56.8) | 104 (56.8) |
| NV | 9 (9.4) | 10 (11.3) | 19 (10.4) |
All randomised patients were considered in accordance with the intention-to-treat principle
CR complete response, PR partial response, SD stable disease, PD progression disease, NV not evaluable
Survival data
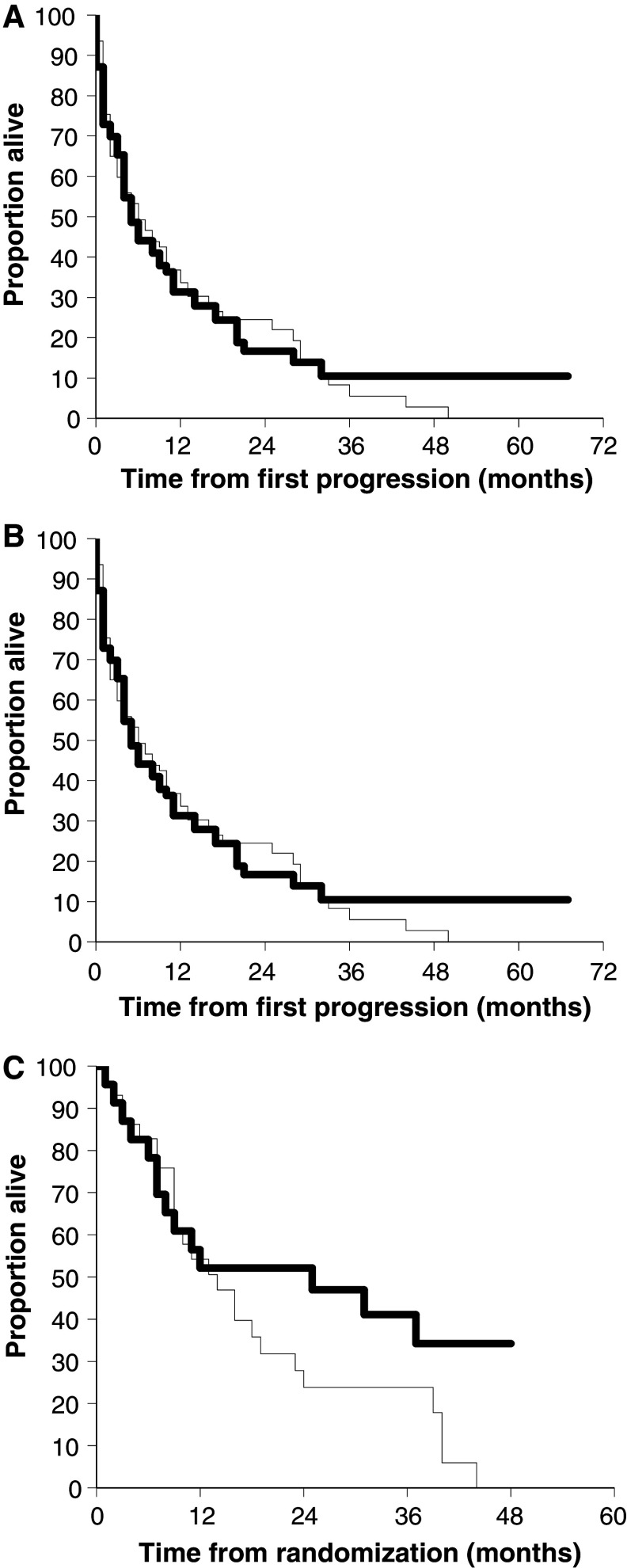
After a median follow-up of 53.9 months, 136 patients had died: 70 (74%) in group A (suspension) and 66 (75%) in group B (maintenance). There were no differences between the two arms in terms of median OS (15.5 months in arm A and 14.5 months in arm B) or median TFPTD (5.5 months in arm A and 4.7 months in arm B), but there was a non-significant trend towards a slight increase in long-term survival in the maintenance arm: actuarial 5-year OS 20 versus 10%, and TFPTD 10 versus 0% (Fig. 2a, b). Furthermore, no OS or TFPTD events were recorded in the maintenance arm after 3 years of follow-up. There was also a non-significant trend in favour of the maintenance arm amongst the female patients, which suggests that hormone status may influence the efficacy of immunotherapy (Fig. 2c).
Fig. 2.
a Overall survival amongst 183 patients randomly assigned to suspension of immunotherapy (n = 95 thin line) or maintenance of immunotherapy (n = 88 thick line). p = 0.91 by log-rank test. b Time from first progression to death amongst the 148 patients who experienced progression to immunotherapy and randomly assigned to suspension of immunotherapy after progression (n = 78 thin line) or maintenance of immunotherapy (n = 70 thick line). p = 0.98 by log-rank test. c Overall survival amongst 53 female patients randomly assigned to suspension of immunotherapy after progression (n = 30 thin line) or maintenance of immunotherapy (n = 23 thick line). p = 0.09 by log-rank test
Table 3 shows the results of the multivariate analysis of the factors influencing OS and TFPTD, which indicates no differences in favour of maintenance treatment in either case. The variables associated with a longer OS were a PS of 0 versus ≥1 (HR 0.44; 95% CI 0.31–0.63; p < 0.0001), having undergone nephrectomy (HR 0.58; 95% CI 0.37–0.92; p = 0.02), and a median age of ≥63 years (median value) versus <63 years (HR 0.68; 95% CI 0.48–0.95; p = 0.02); there was also an effect related to the centre in which the patients were enrolled and treated (HR 1.22; 95% CI 1.08–1.38; p = 0.002). The variables associated with a longer TFPTD were a PS of 0 versus ≥1 (HR 0.53; 95% CI 0.35–0.81, p < 0.001), the use of chemotherapy after disease progression (HR 0.54; 95% CI 0.35–0.86, p < 0.008), female gender (HR 0.63; 95% CI 0.41–0.98, p < 0.038), and the centre of enrolment (HR 1.17; 95% CI 1.02–1.34, p = 0.002).
Table 3.
Proportional hazards models
| Analysis | Factor | HR | 95% CI | p |
|---|---|---|---|---|
| Overall survival, univariate | Treatment (maintenance) | 0.98 | 0.70–1.37 | 0.91 |
| Overall survival, multivariate | Treatment (maintenance) | 0.89 | 0.63–1.28 | 0.55 |
| Gender (female) | 0.92 | 0.62–1.37 | 0.69 | |
| Performance status (0 vs. ≥1) | 0.44 | 0.31–0.63 | <0.0001 | |
| Age ≥63 years (median) | 0.68 | 0.48–0.95 | 0.02 | |
| Nephrectomy (yes vs. no) | 0.58 | 0.37–0.92 | 0.02 | |
| Centres | 1.22 | 1.08–1.38 | 0.001 | |
| Survival following first progression, univariate | Treatment (maintenance) | 1.00 | 0.70–1.44 | 0.98 |
| Survival following first progression, multivariate | Treatment (maintenance) | 0.88 | 0.60–1.31 | 0.55 |
| Gender (female) | 0.63 | 0.41–0.98 | 0.04 | |
| Performance status (0 vs. ≥1) | 0.53 | 0.35–0.81 | 0.002 | |
| Chemotherapy (yes vs. no) | 0.54 | 0.35–0.86 | 0.008 | |
| Nephrectomy (yes vs. no) | 0.72 | 0.42–1.26 | 0.25 | |
| Centres | 1.17 | 1.02–1.34 | 0.002 |
Toxicity
Table 4 shows WHO toxicity. In the vast majority of patients, toxicity was limited to grades 1 or 2 (rarely grade 3), and, therefore, did not require any in-patient treatment; no grade 4 toxicity was recorded. Most of the patients reported constitutional symptoms, including fever, chills, fatigue and malaise, all of which disappeared during the days of the week on which the therapy was not administered, and were completely reversible at the end of each treatment cycle; most of them also reported skin manifestations such as erythema and hardening at the injection site. Incidence of side effects was similar in both arms, and tended to diminish with the continuation of treatment. Thirty (16%) of the 183 patients stopped treatment before any signs of disease progression had been documented, 12 (13%) in arm A and 18 (20%) in arm B: 10 stopped because of a rapid worsening in their clinical conditions due to the disease, seven died, two because of the occurrence of a second primary tumour, six were lost to follow-up, and five spontaneously decided to stop taking the treatment following adverse events (constitutional symptoms and severe asthenia).
Table 4.
Toxicity in all randomized patients
| Toxicity | Events | WHO grade | ||
|---|---|---|---|---|
| N (%) | 1 N (%) | 2 N (%) | 3 N (%) | |
| Fever | 174 (95) | 92 (50) | 55 (30) | 27 (15) |
| Fatigue | 128 (70) | 90 (49) | 29 (16) | 9 (5) |
| Skin toxicitya | 107 (58) | 82 (45) | 18 (10) | 7 (4) |
| Arthralgias/myalgias | 119 (65) | 95 (52) | 15 (8) | 9 (5) |
| Chills | 84 (46) | 71 (39) | 13 (7) | 0 |
| Anorexia | 66 (36) | 46 (25) | 20 (11) | 0 |
| Pirosys | 44 (24) | 18 (10) | 26 (14) | 0 |
| Transaminase increase | 42 (23) | 37 (20) | 5 (3) | 0 |
| Weight loss | 35 (19) | 26 (14) | 9 (5) | 0 |
| Nausea/vomiting | 11 (6) | 4 (2) | 7 (4) | 0 |
| Diarrhoea | 9 (5) | 5 (3) | 4 (2) | 0 |
| Cardiac arrhythmias | 4 (2) | 0 | 4 (2) | 0 |
aIncluding manifestations such as erythema and hardening at the injection site
Discussion
This is the first randomised study testing the efficacy of chronic maintenance immunotherapy in mRCC patients. After a median follow-up of 53.9 months, the results do not indicate any significant benefit in terms of OS and TFPTD, although there does seem to be a small, non-significant increase in long-term survival. A longer follow-up may clarify whether there is real benefit for a small percentage of patients especially due to the finding that after 3 years of follow-up no more events were registered in the maintenance arm, either considering OS or TFPTD.
Long-term maintenance immunotherapy combines two therapeutic approaches: the use of chronic immunotherapy after the achievement of a clinical response or disease stabilisation, and its continuation after disease progression [25]. We decided to test this strategy on the basis of the results of our previous phase II study of chronic maintenance treatment with low doses of IL-2 + IFN, which showed a 36-month survival probability of 47%, persistent stimulation of T lymphocytes and NK cells, and even two cases of metastasis regression after a period of disease progression [23, 26].
Maintenance immunotherapy has been tested in many tumours, but the vast majority of trials were not randomised, included only responding or stable patients, and led to inconclusive results [27–29]. In a French cooperative group phase II sequential study of maintenance treatment with IL-2 + IFN, 128 mRCC patients received induction treatment for 12 weeks, after which the 58 who responded or achieved stable disease were randomised to maintenance or consolidation, but the trial was closed when no benefit was found at the twelfth sequential analysis [30]. However, the trial design and the number of enrolled patients made it almost impossible to show any benefit.
A number of reasons may explain the lack of efficacy of maintenance immunotherapy in our study. First of all, the sample size was probably insufficient because the patients were randomised at the beginning of immunotherapy rather than at the time of documented disease progression, whereas most published studies of maintenance therapy in various tumours randomised the patients at the end of the induction treatment [25]. We chose not to do so in an attempt to avoid the selection bias of including only responders or long-term survivors. However, about 30–40% of the patients rapidly progressed or died within a few months of randomisation and before starting maintenance treatment, thus, making no contribution to the trial endpoints. Other possible reasons that could explain the lack of efficacy of maintenance IT are the low number of patients who effectively received a maintenance after progression in arm B (53%) and the discrepancy between the two arms in terms of number of patients who received chemotherapy after progression (34 vs. 12% in the suspension and maintenance arm, respectively); this imbalance was due in part to physician practice that was different amongst the centres and also due in part to the fact that the protocol did not specify that the type of therapy that could be administered after the progression of diseases, in association or less with the immunotherapy (according to the arm), had to be equal or similar in the two arms.
Some recent randomised studies comparing high-dose i.v. boluses of IL-2 with low-dose i.v. boluses or low-dose s.c. injections [19, 20] have shown that high-dose IL-2 is more effective than lower doses of IL-2 or IL-2 and IFN in terms of response rates and response quality, but not overall survival. This multicentre, randomised study confirms previous data indicating the efficacy and tolerability of very low-dose immunotherapy: the response rate was similar to that observed in other non-randomised phase II trials of low-dose immunotherapy, and the median survival of 15 months was also within the expected range. Currently, we know well that the immunotherapy proposed in this study is not sufficient as a first-line therapy and should therefore not be offered to mRCC patients. At the time when this study was designed and started, IL-2-based immunotherapy was one of the best possible therapeutic option for mRCC. It is now clear that tyrosine kinase (TK) inhibitors have replaced immunotherapy as the treatment of choice for the vast majority of naive mRCC patients; this fact clearly limits the practical applicability of the findings of our study. However, because some subgroups of patients (e.g. those with high CAIX expression) could nevertheless benefit from IL-2-based treatments and a better definition of the real importance of maintaining a sustained immune stimulation in this cancer is warranted, we consider this protocol worth of the efforts made.
As this study was planned in 1998, we did not collect sufficient baseline data to divide the patients using the prognostic classifications more recently suggested by some authors [31], and therefore cannot draw any conclusions concerning the possible positive effect of maintenance in some patient subsets. However, a proportional hazards model showed a significant prolongation of TFPTD in patients with a good PS, female patients, in those treated with chemotherapy after progression, and in those who were treated in some centres. These results confirm that PS is the main factor influencing survival in mRCC, but the positive effect of maintenance treatment on females is surprising and we cannot explain it biologically. Another interesting point is the possible role of chemotherapy. The chemotherapeutic agents so far tested in mRCC have led to disappointing results [32], but our multivariate analysis showed that the use of chemotherapy after disease progression on immunotherapy is beneficial for some patients, regardless of other risk factors. This suggests that a chemotherapeutic option should be considered in selected mRCC patients when other possibilities have failed, although the results of all the subset analyses should only be seen as generating hypotheses that will need to be confirmed in future trials. It should also be remembered that the use of anti-angiogenetic agents and mTOR-inhibitors are the best option for mRCC, and so the possible efficacy of chemotherapy should be now evaluated in the light of this.
The multivariate analysis both for OS and for TFPTD show a highly significant effect due to the centre of enrolment; the reason for this might be due to the fact that the centres were very different amongst them: some have enrolled many patients and others few (range 29–68), some habitually treated with chemotherapy and/or immunotherapy mRCC patients and others only occasionally; most centres were oncology unit but one centre was a nephrology unit (that enrolled 34 patients). We think that the selection of the centres were not optimal in this study. Moreover, this was an independent not economically supported study and so it was difficult to do a strict monitoring of all the centres.
In conclusion, our results do not support the use of maintenance treatment with low doses of IL-2 and IFN in mRCC patients progressing on first-line immunotherapy, although a longer follow-up with more events are necessary for a conclusive evaluation. However, the limited, but definite efficacy and good tolerability of this regimen make it possible to hypothesise future clinical trials designed to evaluate the combination of new targeted molecular agents and a safe immunotherapy regimen (such as ours) to obtain an additive or synergistic effect against mRCC.
Footnotes
From the GOIRC (Italian Oncology Group for Clinical Research).
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/S0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. doi: 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed]
- 10.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 11.Passalacqua R, Buti S, Tomasello G, et al. Immunotherapy options in metastatic renal cell cancer: where we are and where we are going. Expert Rev Anticancer Ther. 2006;6:1459–1472. doi: 10.1586/14737140.6.10.1459. [DOI] [PubMed] [Google Scholar]
- 12.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alpha-2a, or both in metastatic renal-cell carcinoma. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 13.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–785. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lotze MT, Chang AE, Seipp CA, et al. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer: responses, treatment-related morbility and hystologic findings. JAMA. 1986;256:3117–3124. doi: 10.1001/jama.256.22.3117. [DOI] [PubMed] [Google Scholar]
- 16.Isaacs A, Lindenman J. Virus interference. I. The interferon. By A. Isaacs and J. Lindenmann, 1957. J Interferon Res. 1987;7:429–438. doi: 10.1089/jir.1987.7.429. [DOI] [PubMed] [Google Scholar]
- 17.Quesada JR, Rios A, Swanson D, et al. Antitumor activity of recombinant-derived interferon alpha in metastatic renal cell carcinoma. J Clin Oncol. 1985;3:1522–1528. doi: 10.1200/JCO.1985.3.11.1522. [DOI] [PubMed] [Google Scholar]
- 18.Caligiuri MA. Low-dose recombinant interleukin-2 therapy: rationale and potential clinical applications. Semin Oncol. 1983;20:3–10. [PubMed] [Google Scholar]
- 19.Yang JC, Sherry RM, Steinberg SM, et al. A randomised study of high-dose and low-dose interleukin-2 in patients with metastatic renal cell cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott DF, Regan MM, Clark JI, et al. Randomised phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 21.Buzio C, De Palma G, Passalacqua R, et al. Effectiveness of very low doses of immunotherapy in advanced renal cell cancer. Br J Cancer. 1997;76:541–544. doi: 10.1038/bjc.1997.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaglio A, Alberici F, Maggiore U, et al. Chronically administered immunotherapy with low-dose IL-2 and IFN-alpha in metastatic renal cell carcinoma: a feasible option for patients with a good prognostic profile. Oncology. 2009;76:69–76. doi: 10.1159/000178810. [DOI] [PubMed] [Google Scholar]
- 23.Buzio C, Andrulli S, Santi R, et al. Long-term immunotherapy with low-dose interleukin-2 and interferon-alpha in the treatment of patients with advanced renal cell carcinoma. Cancer. 2001;92:2286–2296. doi: 10.1002/1097-0142(20011101)92:9<2286::AID-CNCR1575>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Porta C. Maintenance biotherapy with interleukin-2 and interferon for metastatic renal cell cancer. Expert Rev Anticancer Ther. 2006;6:141–152. doi: 10.1586/14737140.6.1.141. [DOI] [PubMed] [Google Scholar]
- 26.Giacosa R, Santi R, Vaglio A, et al. “Late” regressions of metastases from renal cancer after a period of disease progression continuing the same intermittent low dose immunotherapy regimen. Acta Biomed. 2004;75:126–130. [PubMed] [Google Scholar]
- 27.Lissoni P, Bordin V, Vaghi M, et al. Ten-year survival results in metastatic renal cell cancer patients treated with mono immunotherapy with subcutaneous low-dose interleukin-2. Anticancer Res. 2002;22:1061–1064. [PubMed] [Google Scholar]
- 28.Mantovani G, Macciò A, Madeddu C, et al. Phase II study of subcutaneously administered interleukin-2 in combination with medroxyprogesterone acetate and antioxidant agents as maintenance treatment in advanced cancer responders to previous chemotherapy. Oncol Rep. 2002;9:887–896. [PubMed] [Google Scholar]
- 29.Recchia F, Saggio G, Cesta A, et al. Phase II randomised study of interleukin-2 with or without 13-cis retinoic acid as maintenance therapy in patients with advanced cancer responsive to chemotherapy. Anticancer Res. 2005;25:3149–3157. [PubMed] [Google Scholar]
- 30.Tourani JM, Pfister C, Tubiana N, et al. Subcutaneous interleukin-2 and interferon alfa administration in patients with metastatic renal cell carcinoma: final results of SCAPP III, a large, multicenter, phase II, nonrandomised study with sequential analysis design-the Subcutaneous Administration Proleukin Program Cooperative Group. J Clin Oncol. 2003;21:3987–3994. doi: 10.1200/JCO.2003.02.073. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 32.Lilleby W, Fossa SD. Chemotherapy in metastatic renal cell cancer. World J Urol. 2005;23:175–179. doi: 10.1007/s00345-004-0469-x. [DOI] [PubMed] [Google Scholar]