Abstract
Nuclear factor of activated T cells (NFAT), a family of transcription factors, has been implicated in many cellular processes, including some cancers. Here, we characterize, for the first time, the role of NFAT3 in doxorubicin (DOX)-mediated apoptosis, migration, and invasion in SNB19 and U87 glioma cells. This study demonstrates that the specific knockdown of NFAT3 results in a dramatic inhibition of the apoptotic effect induced by DOX and favors cell survival. Inhibition of NFAT3 activation by shNFAT3 (shNF3) significantly downregulated tumor necrosis factor (TNF)-α induction, its receptor TNFR1, caspase 10, caspase 3, and poly (ADP-ribose) polymerase, abrogating DOX-mediated apoptosis in glioma cells. DOX treatment resulted in NFAT3 translocation to the nucleus. Similarly, shNF3 treatment in SNB19 and U87 cells reversed DOX-induced inhibition of cell migration and invasion, as determined by wound healing and matrigel invasion assays. Taken together, these results indicate that NFAT3 is a prerequisite for the induction of DOX-mediated apoptosis in glioma cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-009-0157-5) contains supplementary material, which is available to authorized users.
Keywords: Doxorubicin and apoptosis, Glioma, NFAT3, SNB19, U87
Introduction
Despite aggressive surgery, radiotherapy, and chemotherapy, malignant brain tumors remain a therapeutic challenge [1]. An inherent resistance to apoptosis may account for the low success rate of glioma treatments [2, 3]. However, because the effector arm of the apoptotic pathway often remains intact, these cells can be induced to undergo apoptosis by direct stimulation of the cell death receptors in the tumor necrosis factor receptor (TNFR) superfamily [4].
Nuclear factor of activated T cells (NFAT) is a family of transcription factors initially discovered in T cells that plays a crucial role in immunity. More recently, however, the NFAT family has been implicated in many cellular processes, including malignancies [5]. NFAT signaling has been reported to be involved in the regulation of cell growth and development in a wide variety of different tissues and cell types [6, 7]. There are currently five known isoforms of this family of transcription factors, all of which are known to be involved in activity-dependent gene expression, linking surface receptor stimulation to nuclear events and ultimately altering the fate of the cell. Unlike NFAT1, -2 or -4, NFAT3 is expressed in non-immune tissues, including the heart, brain, and breast [8, 9], where it is thought to influence such varied processes as cardiac hypertrophy, learning and memory, adipocyte differentiation, and breast cancer development [10–15].
NFAT-1, -2, -3, and -4 are cytosolic proteins, constitutively expressed in resting cells, and their activation is regulated by calcium and the Ca2+/calmodulin-dependent serine–phosphatase, calcineurin [16]. Upon stimulation, NFATs are dephosphorylated by calcinuerin, and they are then translocated to the nucleus where they bind to consensus DNA sites and control the expression of target genes, including interleukin (IL)-2, IL-4, IL-5, IL-13, interferon (IFN)-gamma, TNF-α, and COX-2 [17–21]. Activation of NFAT is sensitive to calcineurin inhibitors and immunosuppressive agents, such as cyclosporin A and FK506 [16, 22].
Doxorubicin (DOX) is an anti-tumor drug that is being widely used in the treatment of a broad spectrum of cancers [23, 24]. The pro-apoptotic effects of DOX are well established: it can induce cell death via DNA damage through topoisomerase II inhibition, by inducing the expression of the tumor suppressor protein, p53, and by free radical generation through the redox reaction [25–27]. DOX has shown a marked cytotoxic effect against malignant glioma cells in vitro [28]. Niiya et al. [29] reported that DOX induced upregulation of TNF-α, uPA, IL-8, and monocyte chemotactic protein (MCP-1) in a number of lung cancer cells. A highly enhanced anti-tumor effect has also been reported with systemic administration of TNF-α in combination with DOX for lung tumors [30, 31]. Kalivendi et al. [31] documented that DOX activated the transcription factor NFAT, leading to the upregulation of Fas/FasL-dependent apoptosis through a calcium/calcineurin-signaling pathway in cardiac cells.
To date, there has been no comprehensive study published on the role of DOX in NFAT activation in cancer cells. Given the importance of cell cycle, apoptosis, and angiogenesis phenomena in the development of cell malignancies, it is of considerable interest to understand the mechanisms by which NFAT affects cell growth, differentiation, and function. In the investigation reported here, we studied the role of NFAT3 in DOX-mediated apoptosis in SNB19 and U87 glioma cell lines. The hypothesis that DOX causes apoptosis in these cells was tested by fluorescence-activated cell sorter (FACS) analysis and DNA fragmentation. We demonstrate, for the first time, the role of the NFAT3 isoform in SNB19 and U87 glioma cell lines in DOX-mediated cellular apoptosis.
Materials and methods
Construction of a vector expressing shRNA for NFAT3
pSilencerCMV3.1 (Ambion, Applied Biosciences, Foster City, CA) was used to construct a vector expressing shRNA for NFAT3 downstream of the cytomegalovirus promoter (Fig. 1a). Exons 4, 5, and 6 were targeted simultaneously by including 21 bases corresponding to each exon. The following was used for the NFAT sequence: aatggatccGCCACTGACCCTACAGATGTTCAAGAGACATCTGTAGGGTCAGTGGCTTCAAGAGATTCAAGAGAGGGTGAGACGG-ACATCGGGTTCAAGAGACCCGATGTCCGTCTCACCCTTCAAGAGATTCAAGAGACTGGTACTGACTGGCTCCATTCAAGAGAT-GGAGCCAGTCAGTACCAGaagcttccg. It is 93 bases in length, with BamHI and HindIII sites incorporated at the ends, and has a nine-base loop region between each 21-base molecule. The oligo was self-annealed in 6× SSC using standard protocols and ligated onto the BamHI and HindIII site of a pSilencerCMV3.1 vector. This yielded a shRNA expression plasmid for NFAT3, which was designated shNF3. The BGH poly-A terminator served as a stop signal for RNA synthesis.
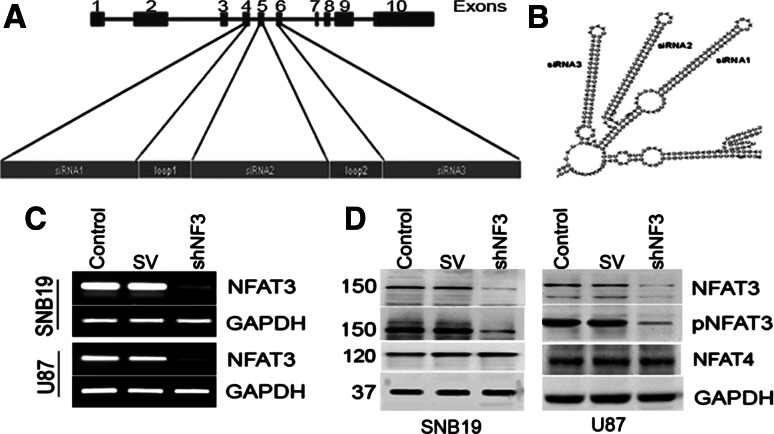
Fig. 1.
Construction of the shRNA molecule for nuclear factor of activated T cells-3 (NFAT3) and downregulation of NFAT3 in SNB19 and U87 using shNF3. a Schematic representation of the NFAT3 gene with its exons. Exons 4, 5, and 6 were targeted for the construction of short hairpin (sh) RNA for NFAT3. b Tertiary structure of the proposed shRNA showing the folds of small interfering (si) RNA 1, 2 and 3 deduced from M-fold software. c Reverse transcription (RT)-PCR analysis of glioma cells with NFAT3 primers and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers. SV Sindbis virus. d Immunoblot analysis of cell lysates from SNB19 and U87 cells. Whole cell lysates (50-μg aliquots) from control SNB19 and U87 cells and cells transfected with SV and shNFAT3 (shNF3) for 48 h were probed with anti-NFAT3, phospho-specific anti-NFAT3 (pNFAT), and anti-NFAT4 antibodies
Cell culture and transfection of short hairpin RNA vectors
For this study, we used the established human glioblastoma cell lines SNB19 and U87. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 1% glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, and 10% fetal bovine serum (pH 7.2–7.4) at 37°C in a humidified atmosphere containing 5% CO2. The cells were grown in 100-mm dishes or six-well plate for all treatment conditions and on eight-well chamber slides for immunocytochemistry and tunel assays. The cells were sub-cultured every 3–5 days
Scrambled vector (pSV) and short hairpin (sh)NF3 vector were transfected into SNB19 and U87 cells independently by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions. Briefly, the cells were cultured in a 100-mm dish to 85–90% confluence and then 21 μl of lipofectamine (diluted in 100 μl of serum-free medium) was added dropwise to 7 μg of plasmid DNA (in 100 μl of serum-free medium). This mixture was incubated for 30 min and then used to transfect each plate in the absence of serum. After 6 h, the medium was replaced with Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, and the cells were incubated for another 12–24 h. DOX (1 μM/l) was added to these cells in the absence of serum and incubated for an additional 24 h. To study the role of TNF-α in DOX-induced NFAT3-mediated apoptosis, cells were also treated with TNF-α protein (200 U/ml) for 24 h.
Reverse transcripton-PCR analysis
A total of 2 × 105 of SNB19 and U87 cells were independently cultured in each well of six-well plates to 80% confluence. After transfection with either shNF3 or pSV and subsequent treatment with DOX, the cells were extracted with Trizol reagent (Invitrogen), and the total RNA was isolated as per the manufacturer’s instructions. One microgram of total RNA was reverse-transcribed (Superscript II; Invitrogen) and the cDNA was subjected to PCR amplification targeting NFAT3 (forward primer 5′-AGGCCTACAGCCCCAGTG-3′ and reverse primer 5′-CGCCCATTGGAGACATAAAA-3′) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward primer 5′-AGCCACATCGCTCAGACACC-3′ and reverse primer 5′-GTACTCAGCGGCCAGCATCG-3′). The PCR cycling conditions were: 30 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 45 s and a final extension of 72°C for 10 min.
Western blot analysis
SNB19 and U87 cells were washed with ice-cold Dulbecco's phosphate buffered saline (DPBS) 48 h after treatment and resuspended in 150 μl of radioimmune precipitation assay buffer [20 mmol/l Tris–HCl (pH 7.4), 2.5 mmol/l EDTA, 1% Triton-X 100, 1% sodium deoxycholate, 1% sodium dodecyl sulfate (SDS), 100 mmol/l NaCl, and 100 mmol/l sodium fluoride] containing 1 mmol/l sodium vanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Cells were then homogenized using five 1-s ultrasonic pulses. The lysate was centrifuged at 12,000 g for 10 min at 4°C to remove cellular debris.
Equal amounts of protein were separated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The membranes were probed overnight with antibodies against NFAT3, phospho-NFAT3, NFAT4, FADD, p21, p53, AIF, Bcl2, TNF-α, cyclin E, cyclin D1, pCDK2, caspase 10, caspase 3, PARP [poly (ADP-ribose) polymerase], and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were subsequently washed three times with PBS to remove excess primary antibodies, incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies and then developed according to enhanced chemiluminescence protocol (Amersham, Arlington Heights, IL, USA).
NFAT3 translocation assay by immunofluorescence
The expression and localization of NFAT were observed using an immunocytochemistry assay according to published standard protocols. Human glioma cells SNB19 and U87 grown in eight-well chamber slides were treated as described previously, fixed (4% paraformaldehyde, 10 min at room temperature), permeabilized (cold methanol for 2 min), and rehydrated (PBS for 10 min). The PBS-Tween 20 (PBST) solution containing 2% bovine serum albumin (BSA) was used for blocking the cells for 1 h, followed by a 2-h incubation with anti-NFAT3 antibodies (Santa Cruz Biotechnology) at a dilution of 1:300 in PBST containing 2% BSA and 1.5% horse serum, followed by a final incubation with Texas Red conjugated secondary antibody (1:1,000 in PBS/2% bovine serum albumin, 0.5% tween 20) for 1 h. NFAT expression was visualized by fluorescence microscopy (Olympus IX71; Olympus Optical Co, Tokyo, Japan) and photographed.
DNA fragmentation assay
SNB19 and U87 cells were cultured in six-well culture plates at a concentration of 2 × 105 cells/well. After a 24-h incubation period, cells were transfected with the shNF3 vector or pSV. Untreated cells were also cultured under similar conditions and served as the control. Genomic DNA was extracted following the protocol described by Huang et al. [32]. In brief, the cells were resuspended in 50 μl PBS, fixed in 1 ml of ice cold 70% ethanol, and stored at –20°C overnight. After fixation, the mixture was centrifuged at 6000 rpm at 4°C for 5 min, and the pellet was resuspended in 50 μl phosphate–citrate buffer (192 parts of 0.2 M Na2HPO4 and 8 parts of 0.1 M citric acid, pH 7.8) and then left at room temperature for 30 min. After another centrifugation at 10,000 rpm for 5 min, the supernatant was transferred to 0.5-ml Eppendorf tubes and incubated at 37°C with 3 μl of NP40 (0.23%) and 3 μl of RNase (1 mg/ml). The solution was further incubated for 30 min after adding 3 μl proteinase K (1 mg/ml). The DNA content in the mixture was quantified using a spectrophotometer and 10 μg of DNA was electrophoresed on a 2% agarose gel using TAE buffer. DNA fragmentation was visualized under UV light after staining with ethidium bromide.
Matrigel invasion assay
SNB19 and U87 cells were transfected with either shNF3 or scrambled vector and treated with DOX as described above. After treatment, cells were trypsinized, and 2 × 105 cells were placed into matrigel-coated transwell inserts with an 8-μm pore size. Cells were allowed to migrate through the matrigel for 24 h. The cells in the upper chamber were removed with a cotton swab, while those cells that adhered to the outer surface of the transwell (i.e., the cells which had invaded the matrigel) were fixed, stained using the Hema-3 staining kit, and counted under a light microscope as described previously [33].
In situ terminal-deoxytransferase mediated dUTP nick endlabeling assay
The terminal-deoxytransferase mediated dUTP nick endlabeling (TUNEL) assay was carried out to detect apoptotic cells after the above-described treatments. SNB19 and U87 cells were cultured on eight-well chamber slides at a density of 2 × 103 per well. After 24 h, the cells were transfected with either shNFTA3 vector or scrambled vector in serum-free medium. Twenty-four hours later, the cells were treated with DOX and incubated for another 24 h. The cells were fixed after termination in 10% phosphate-buffered formalin for 15 min. TUNEL staining for the detection of apoptotic cells was carried out using the TUNEL Apoptotic Detection kit (Roche Molecular Biochemicals, Indianapolis, IN) as per the manufacturer’s instructions. Briefly, the fixed cells were washed three times in PBS (5 min/wash), incubated with 0.05% Tween 20 in PBS containing 0.2% BSA for 15 min at room temperature, washed twice in PBS, and finally incubated with 50 μl terminal deoxynucleotidyl transferase end-labeling cocktail for 60 min at room temperature. The reaction then was terminated, and the slides were washed three times in PBS and blocked with blocking buffer for 20 min at room temperature. The slides were then incubated with 50 μl avidin-Texas red in the dark for 30 min at room temperature, washed three times in PBS, and mounted with anti-fading gel mount (Biomeda, Foster City, CA, USA). Slides were allowed to dry in the dark, observed under a fluorescent microscope (model Olympus IX71; Olympus Optical Co), and photographed.
Fluorescence-activated cell sorter analysis
The induction of apoptosis in response to shRNA-induced downregulation of NFAT3 and DOX individually and in combination was tested using FACS analysis. The FACS analysis was performed according to the manufacturer’s instructions, and 10,000 cells were recorded for each transfection. Cells were treated as described earlier, trypsinized, washed with 1× PBS, and incubated for 30 min with 1 ml propidium iodide in the dark. After the incubation period, the cells in propidium iodide were analyzed using a FACS Calibur flow cytometer (BD BioSciences, San Jose, CA, USA) with an excitation wavelength of 488 nm and emission wavelength of 530 nm.
Wound healing assay
To study cell migration and cell interactions, we seeded the cells at a density of 2 × 106 in a six-well plate and treated then as described earlier with shNF3 and DOX. After the transfection and the DOX treatments were completed, a straight scratch was made in individual wells with a 200-μl pipette tip. This point was considered as the 0 h, and the width of the wound was photographed under the microscope. After 18–24 h, the cells were checked for the wound healing and again photographed under the microscope. Wound healing was measured by calculating the reduction in the width of the wound after incubation.
Isolation of nuclear and cytoplasmic cell fractions
SNB19 and U87 cells cultured in 100-mm Petri dishes were treated with shNF3 and DOX as described earlier. Harvested cells were washed once with 1× PBS, and the cell pellet was resuspended in 300 μl cytoplasmic extraction buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.3 mM sodium vanadate, 1 mM PMSF, 10 μg/ml Aprotinin, and 10 μg/ml leupeptin). This mixture was incubated for 10 min at 4°C and then centrifuged at 6000 rpm at 4°C for 10 min. The supernatant (cytoplasmic extract) was collected, and the remaining pellet was suspended in 250 μl nuclear extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 0.2 mM EDTA, 0.3 mM sodium vanadate, 1 mM PMSF, 10 μg/ml Aprotinin, and 10 μg/ml leupeptin). The fluid was incubated for 30 min on ice and then centrifuged at 13,000 rpm for 5 min at 4°C, following which the supernatant (nuclear extract) was collected and used for western blot analysis.
Results
Knockdown of NFAT3 expression upon RNA interference treatment
We transfected SNB19 and U87 cells with either pSV and/or shNF3. RT-PCR and immunoblot analysis showed a significant decrease in NFAT3 expression in shNF3-treated cells compared to the controls (Fig. 1c, d). GAPDH activity was analyzed at both the mRNA and protein levels to serve as a loading control (results confirmed equal loading). Taken together, these results demonstrate the efficient downregulation of NFAT3 with the shNF3 plasmid. To determine the specificity of the shNF3 construct, we immunoblotted the proteins after treatment for other NFATs. Figure 1d shows the unaltered NFAT4 expression after shNF3 treatment.
DOX-induced apoptosis in glioma cells requires NFAT3
The mechanism of DOX-induced apoptosis via distinct pathways has been well elucidated in different cancers, including gliomas. To investigate the potential role of NFAT3 in DOX-mediated apoptosis, we transfected SNB19 and U87 cells with the NFAT3 shRNA expression vector or pSV (negative control) and then with DOX. We found that the DOX treatment was able to induce apoptosis in glioma cells. Importantly, however, suppression of endogenous NFAT3 enhanced cell survival with shRNA alone or shRNA and DOX treatment together as compared to the DOX treatment alone (Fig. 2a).
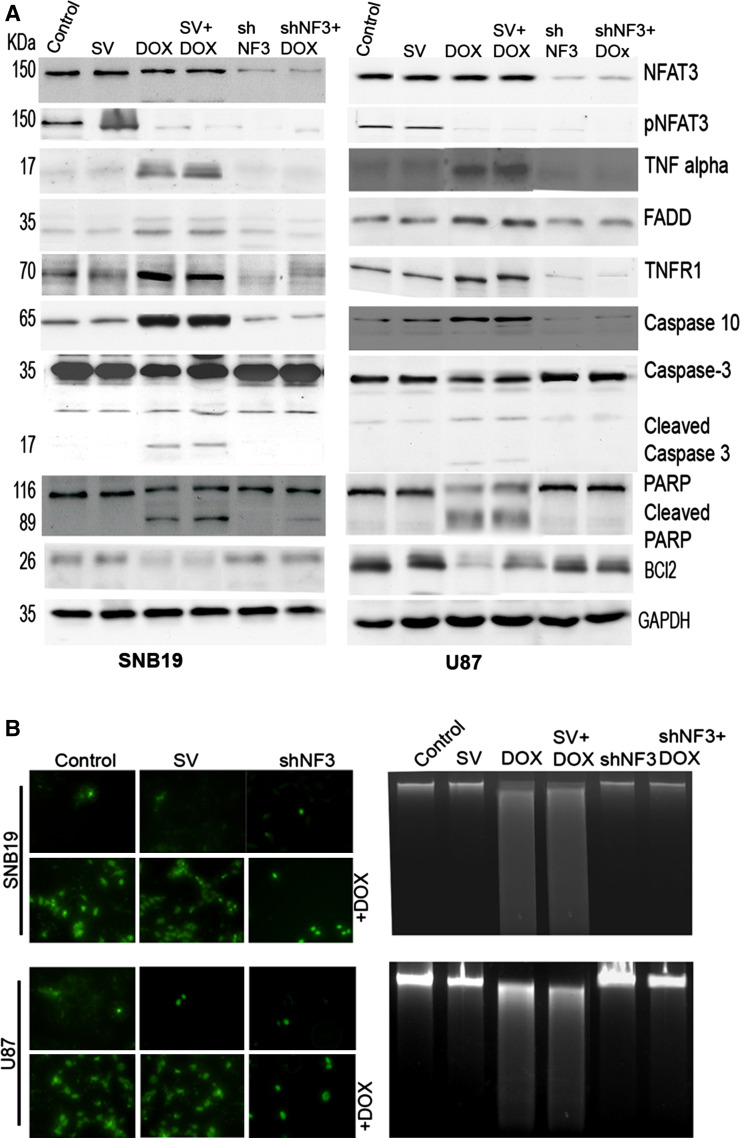
Fig. 2.
Apoptosis induced by the doxorubicin (DOX) treatment is halted in the absence of NFAT3 expression. a Cell lysates were collected from SNB19 and U87 after transfection with SV or shNF3, with or without DOX. Western blot analysis of 50 μg of total cell lysates was performed to check the expression of NFAT3, pNFAT3, Fas-associated protein with Death Domain (FADD), tumor necrosis factor alpha (TNF-α), TNF receptor 1 (TNFR1), Bcl2, caspase 3, caspase 10, and poly (ADP-ribose) polymerase (PARP). GAPDH was used as a loading control. b SNB19 and U87 cells were transfected with SV or shNF3, incubated for 16 h, treated with DOX (1 μM), and incubated for another 24 h. Apoptosis was detected using the terminal-deoxytransferase-mediated dUTP nick endlabeling (TUNEL) assay as per the manufacturer’s instructions. Cells were photographed under a light or fluorescent microscope. c Agarose gel electrophoresis pattern of DNA obtained from SNB19 and U87 cells after transfection with either SV or shNF3 and treatment with DOX, respectively
Further studies showed that the role of NFAT3 in DOX-induced apoptosis was mediated by upregulation of its target gene, TNF-α. This conclusion was based on the findings that inhibition of NFAT3 activation by shNF3 dramatically downregulated the induction of TNF-α, its receptor TNFR1, caspase 10, caspase 3, and PARP, sequentially, and thus led to the abrogation of DOX-mediated apoptosis in glioma cells. DOX treatment led to increased expression of the aforementioned molecules, whereas shNF3 treatment alone or in combination with DOX showed decreased expression in both cell lines (Fig. 2a), which indicates that NFAT3 is required for DOX-mediated apoptosis. Moreover, caspases 3 and 10 and PARP (molecules that mediate the apoptotic mechanism) were cleaved and activated by DOX, as shown by western blot analysis of cell lysates (Fig. 2a). Cells treated with shNF3 showed no indication of activated caspases or cleaved PARP. shNF3-inhibited apoptosis was further confirmed by the increased expression of the anti-apoptotic molecule, Bcl2. Certain molecules, such as AIF, p53, and cleaved BID, were unchanged with any of the treatments (Supplementary Fig. 1). The addition of TNF-α protein increased the expression of caspase 10 and PARP irrespective of the treatments (Supplementary Fig. 2).
DOX is known to induce cellular apoptosis by intercalating into the DNA. The results of the TUNEL assay showed increased DNA damage and fragmentation in glioma cells treated with DOX as compared to controls and cells transfected with pSV (Fig. 2b). Knockdown of NFAT3 reduced the DNA damage caused by DOX. We were also able to observe DNA fragmentation after purifying total nuclear DNA, which was electrophoretically separated on an agarose gel. Cells treated with DOX underwent DNA degradation, which indicates apoptosis, whereas shNF3 treatment in combination with DOX showed no degradation of DNA (Fig. 2c).
shNF3 treatment reversed DOX-mediated inhibition of cell invasion through matrigel
SNB19 and U87 cells transfected with shNF3 or shNF3 and DOX treatment invaded matrigel-coated filters to a greater extent than did the cells treated with DOX alone (Fig. 3a). The percentage of invasive cells after the treatment with shNF3 or the shNF3/DOX combination to the lower side of the membrane at 48 h was 95 (SNB19), 82 (U87), 90 (SNB19), and 80% (U87), respectively. Only 18 and 15% of the DOX-alone-treated SNB19 and U87 cells invaded the lower side of the membrane during the same period of time (Fig. 3b). Respective controls showed 85–100% of cell invasion.
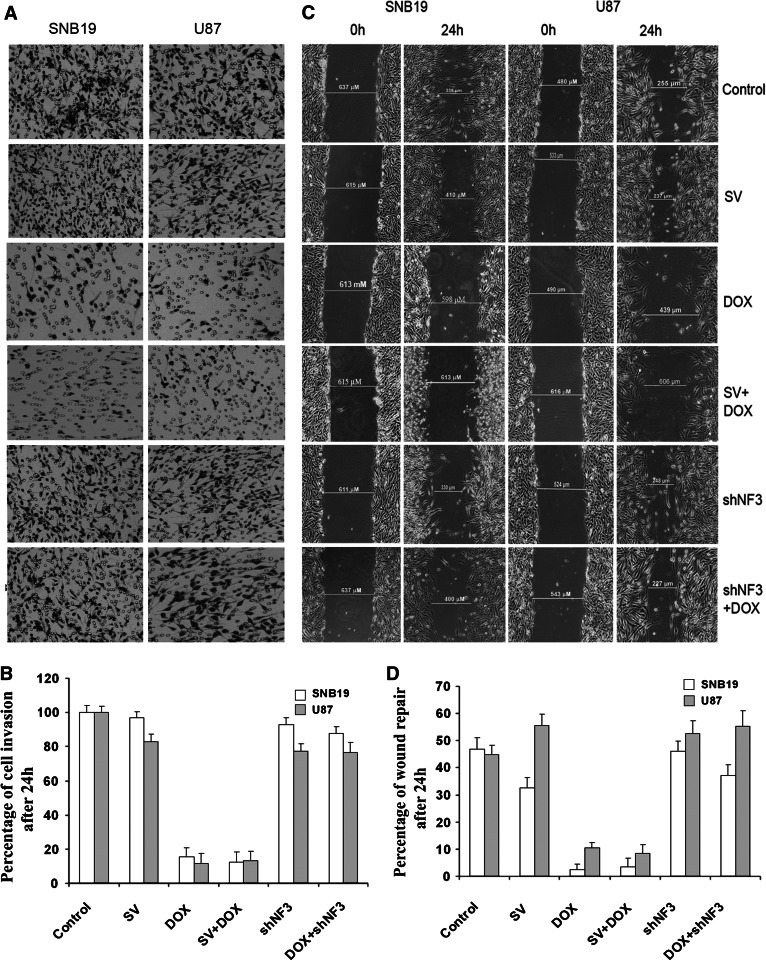
Fig. 3.
shNF3 treatment reversed DOX-mediated inhibition of cell invasion and wound healing. a Phase micrographs showing invading SNB19 and U87 cells. After transfection with SV or shNF3, with or without DOX, cells were trypsinized, and 2 × 105 cells were placed into matrigel-coated transwell inserts with an 8-μm pore size. After 24 h, the cells that adhered to the outer surface of the transwell (i.e., the cells which had invaded the matrigel) were fixed, stained using the Hema-3 staining kit, and counted under a light microscope. b Number of cells from ten different fields was counted for each sample, and the percentage of cells that invaded after transfection with SV or shNF3, with or without DOX, were analyzed and compared with untreated control cells. The graph represents quantification of cell invasion through the matrigel and shows the mean of ten randomly selected different fields. c SNB19 and U87 cells were cultured in six-well plate at a concentration of 2 × 106 and transfected with SV or shNF3, with or without DOX. Untreated cells were also maintained simultaneously. After the transfection and the DOX treatment were complete, a straight scratch was made in individual wells with a 200-μl pipette tip. This point was considered as the 0 h, and the width of the wound was photographed under the microscope. After 24 h, the cells were checked for wound healing and again photographed under the microscope. d Quantification of cell wound healing after cells were transfected with SV or shNF3 and/or DOX. Shown are the mean values of ten randomly selected different fields
shNF3 treatment reversed DOX-mediated inhibition of cell migration in wound healing assay
shNF3 treatment in SNB19 and U87 cells also reversed the DOX-induced inhibition of cell migration, as determined by the wound healing assay wherein a wound was made in a sub-confluent cell monolayer and cells were allowed to migrate into the cell-free area. The distance moved by the cells in treated and untreated wells, respectively, were compared (Fig. 3c). The percentage of wound repaired by cell motility of shNF3 and the combination of shNF3 and DOX treatment was 45 (SNB19), 52 (U87), 35 (SNB19), and 52% (U87), respectively. In contrast, the percentage of wound repair in the DOX-alone treatment was 5 (SNB19) and 10% (U87) (Fig. 3d). These results indicate that after knockdown of NFAT3 and treatment with DOX, the cells were able to divide and survive, suggesting that NFAT3 plays an important role in DOX-mediated cell migration.
NFAT3 is translocated into the nucleus by DOX
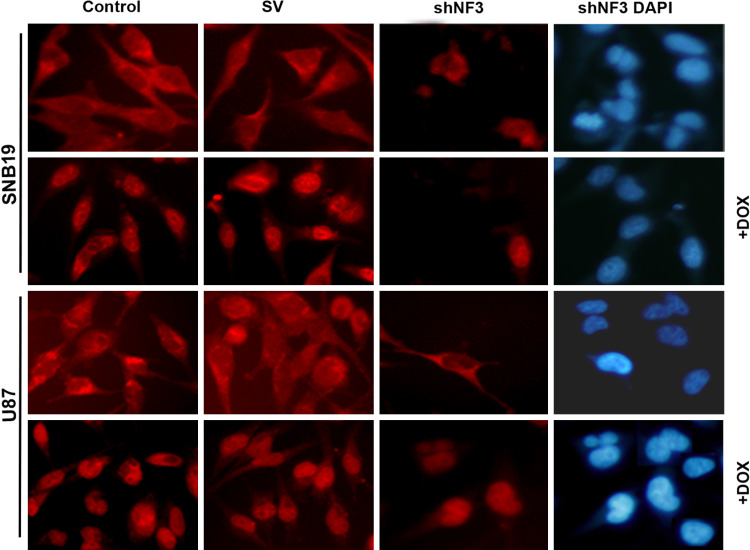
NFATs in the phosphorylated form are known to reside in the cytoplasm and those in the dephosphorylated form in the nucleus. Immunocytochemistry assays showed that the shNF3 treatment of cells led to the reduction of NFAT3 in the cytosol as well as in nucleus 48 h after treatment. DOX treatment resulted in the translocation of NFAT3 into the nucleus, and treatment with the combination of shNF3 and DOX resulted in very little or no NFAT3 in the cytoplasm or nucleus. In the control cells and the scrambled vector treatment, NFAT3 was present only in the cytoplasm (Fig. 4). This was also proven by western blot analysis of nuclear and cytoplasmic extracts from these cells. As shown in Supplementary Fig. 3, when nuclear extracts were probed with anti-NFAT3 antibody, the DOX treatment showed increased expression relative to the control and no expression with the shNF3 treatment. Similarly, the cytoplasmic extracts, when probed with phospho-NFAT3, showed a decreased expression of NFAT3 relative to the control and less or no expression in shNF3 treatment. These results indicate that DOX translocates cytosolic NFAT3 into the nucleus, thereby leading to apoptosis.
Fig. 4.
DOX treatment leads to NFAT3 activation, based on its nuclear translocation. Cytosolic and nuclear presence of NFAT3 in SNB19 and U87 cells that were treated with SV or shNF3 and/or DOX. After treatment, the cells were fixed and checked for the expression of NFAT3 by the immunofluorescence assay using anti-NFAT3 primary antibody followed by fluorescein isothiocyanate-conjugated anti-rabbit secondary antibody. Cells were observed under a fluorescent microscope. DOX stimulates the NFAT3 translocation into the nucleus and the downregulation of NFAT3 resulted in the decreased expression of NFAT3 in both the cytoplasm and nucleus
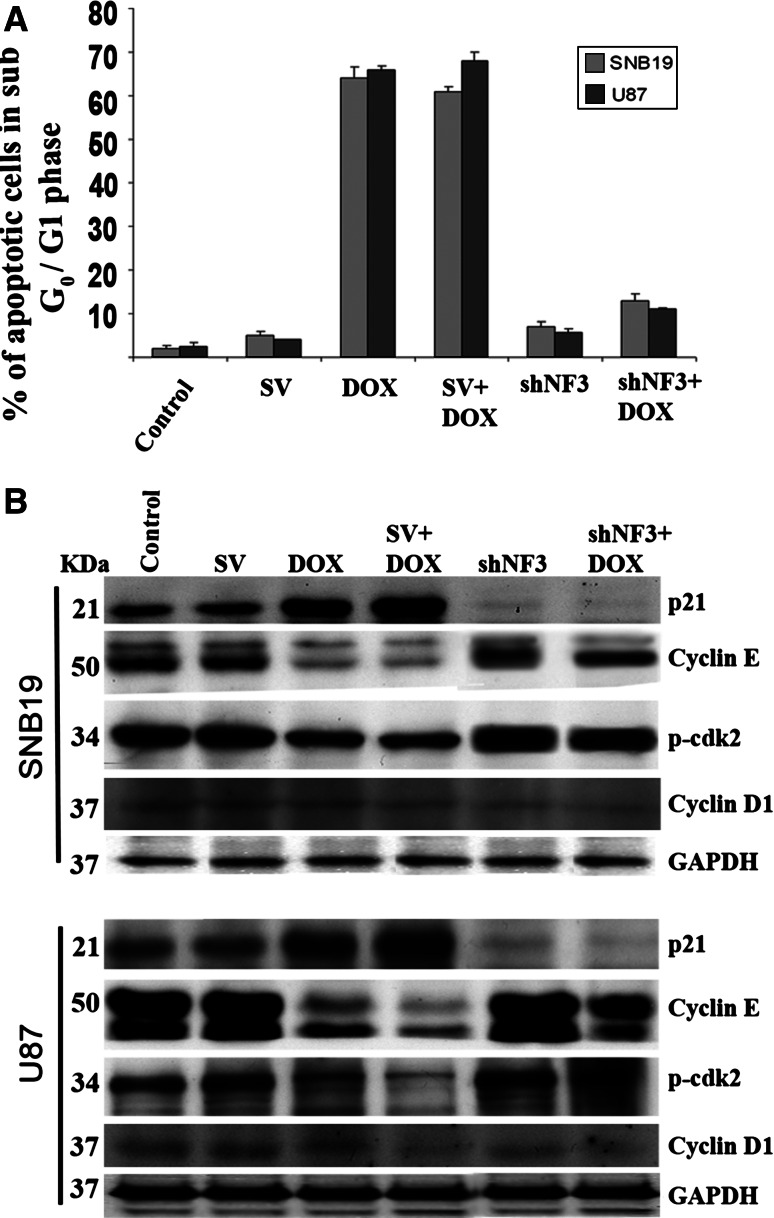
Downregulation of NFAT3 by shNF3 reverses DOX-induced inhibition of cell cycle progression
The FACS analysis of SNB19 and U87 cells treated with DOX resulted in apoptosis. The percentage of cells in the sub G0/G1 phase was as high as 61–64 and 66–68% in the cells treated with DOX alone in SNB19 and U87, respectively. In comparison, only 2–13% of cells in the sub G0/G1 phase was found in the controls and shNF3-treated cells, with or without the DOX treatment (Fig. 5a). Cell cycle progression into the S phase requires the enzyme cdk2, which is inhibited by p21. In our study, we observed that the amount of p21 expression was lower after treatment with shNF3 or shNF3 + DOX, than in the treatment with DOX alone. Similarly, increased expression of cyclin E and pcdk2 was observed, which indicates the role of NFAT3 in cell cycle progression and DOX-mediated apoptosis. The expression of cyclin D1 was unchanged (Fig. 5b). Collectively, these results provide direct evidence for the important role of NFAT3 activation in DOX-induced apoptosis by upregulation of TNF-α expression in glioma cells.
Fig. 5.
shNF3 transfection reverses DOX-induced inhibition of cell cycle progression. a DNA content of SNB19 and U87 cells were measured by flow cytometric analysis to determine the fate of the cell cycle after transfection with SV or shNF3, with or without DOX. DOX treatment alone increased the number of apoptotic cells in the sub G0/G1 phase. Relatively fewer cells treated with shNF3 alone or with a combination of shNF3 + DOX were in the sub G0/G1 phase. The graph shows the percentage of apoptotic cells in the sub G0/G1 phase after treating SNB19 and U87 cells with SV or shNF3 and/or DOX. Values are mean ± standard deviation (SD) from three different experiments (P < 0.001). b After 48 h, total cell lysates collected from the control and transfected cells with or without DOX treatment were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and transferred onto a nitrocellular membrane. The membranes were probed for p21, p-cdk2, cyclin E, and cyclin D1 using the respective antibodies. GAPDH was used as the loading control
Discussion
Programed cell death occurs in virtually all cell types and is precisely regulated at the cellular and molecular levels. NFAT proteins have been shown to bind to promoter regions and upregulate the expression of some effectors of apoptosis, such as TNF-α and FasL [16]. Most NFATs perform their function by partnering with other transcription factors and are known for their controversial properties, such as tumor suppression and pro-apoptotic activity. Yan et al. [34] demonstrated that the inhibition of COX-2 expression with diminishing endogenous NFAT3 blocked the transformation of Cl41 cells when treated with TNF-α. Similarly, Li et al. [35] also documented the role of NFAT3 in the regulation of iNOS and in the suppression of cell transformation, suggesting a tumor suppressor activity of NFAT3. In contrast, tumor development via angiogenesis and apoptotic resistance with the upregulation of COX-2 has also been documented in a variety of human cancer cells [36–38]. These actions could be due to the inability of NFATs to partner with other transcription factors. Studies by Ranger et al. [39] revealed that the deletion of NFAT1 in cartilage cells leads to uncontrolled cell proliferation and the presence of cancer phenotypes both in vitro and in vivo, suggesting a potential tumor suppressor role of NFAT1 in this special cell lineage. NFAT3 has also been found to enhance estrogen receptor transcriptional activity in breast cancer cells and to play an important role in the regulation of breast cancer cell growth [15, 40]. These conflicting results highlight the versatility of NFAT proteins in cell fate determination, depending on their functional modulations under certain conditions [6, 41]. Zhang et al. [42] demonstrated that NFAT3, as a new kind of cofactor, displays dual transcription modulation modes dependent on tissue types: in breast cancer cells, NFAT3 acted as a transcription activator of estrogen receptors and inhibited their transcription activities in all cell types derived from kidney tissue. Recently, NFAT transcriptional activity has been reported to regulate the acetylcholinesterase promoter during ionophore-induced apoptosis in HeLa cells [43].
In our study, we demonstrated that the treatment of SNB19 and U87 glioma cell lines with shNF3 resulted in a downregulation of NFAT3. DOX treatment of these cells resulted in apoptosis in a dose-dependent manner (Supplementary Fig. 4). We further investigated the influence of NFAT3 on DOX-mediated apoptosis by NFAT3 downregulation in SNB19 and U87 cells. Western blot analysis showed a decrease in expression of TNF-α, a downstream element of NFAT3, which is known for its roles in immunity and cellular remodeling as well as for its effect on apoptosis and cell survival [44]. Data from many different studies have underlined the importance of TNF-α activation in human cancers. DOX-induced TNF-α, uPA, IL-8, and MCP-1 in a number of lung cancer cells with different levels of expression has been reported by Niiya et al. [29]. An enhanced anti-tumor effect was also reported with systemic administration of TNF-α in combination with DOX for lung tumors [30]. Cells lacking NFAT1 showed a decreased expression of TNF-α [45]. In our study, decreased expression of TNF-α lowered the expression of TNFR1, caspase 10, caspase 3, and PARP. Similar results were obtained when shNF3 was used in combination with DOX. Moreover, DOX treatment alone increased the level of the above-mentioned molecules. The addition of TNF-α also increased the expression of caspase-10 and PARP. These results show that NFAT3 has a pro-apoptotic role in glioma cells. Thus, we anticipate that NFAT3 represents a putative tumor-suppressing mechanism and that the downregulation of NFAT3 reverses the apoptotic mechanism induced by DOX.
Certain molecules (e.g., p53, apoptosis-inducing factor, and cleaved BID) have been found to be unchanged with either treatment. p53-mediated apoptosis is common in tumor cells treated with DOX and is dependent on the Apaf-I/caspase 9 pathway involving cytochrome c release from mitochondria [46]. The p53-mediated activation of calcineurin in lung, renal, colon, and ovarian carcinoma cells and the subsequent activation of NFAT to induce apoptosis have been reported as well [47]. However, in our study, we did not observe any change in the expression of p53 or in cleaved BID.
Under basal conditions, NFAT3 proteins are present in the cytoplasm in its phosphorylated state and, upon increase in the intracellular calcium, it translocates to the nucleus by dephosphorylation at multiple serine residues [48–50]. We found that NFAT3 translocated to the nucleus with the DOX treatment alone and that there was little or no NFAT3 in the nucleus of either the control cells or the cells treated with shNF3 alone or in combination of DOX. This result could be due to an increase in the calcium levels of the cells when treated with DOX. Similar results have been documented in rat cardiac cells: DOX activated the transcription factor NFAT, leading to the upregulation of Fas/FasL-dependent apoptosis through a calcium/calcineurin-signaling pathway [31]. Yaghi and Sims [51] reported NFAT3 nuclear translocation in smooth muscle cells treated with phenylephrine or 20-hydroxyeicosatetraenoic acid.
The NFAT family of proteins plays an important role in the regulation of genes that control cell cycle progression, cell development and differentiation, angiogenesis and, possibly, tumorigenesis [17, 52–55]. Consequently, we also studied the effect of shNF3 on cell cycle progression and found that DOX treatment led the cells to apoptosis. Interestingly, shNF3 treatment with or without DOX resulted in only 7–13% of the cells being apoptotic, suggesting that the decreased expression of NFAT3 in the cells will lead to survival, and vice versa. It also suggests the important role played by NFAT3 in DOX-mediated apoptosis. This hypothesis was verified by the results of the western blot analysis for cell cycle-controlling genes, such as phospho-cdK2 and cyclin E, which showed increased expression with DOX treatment and decreased expression with shNF3 alone or with a combination of shNF3 and DOX. These results suggest that NFAT3 is required for DOX to inhibit cell cycle progression and that it has a central role in cell cycle control. Baksh et al. [56] demonstrated that the calcineurin/NFAT1 pathway negatively regulates the expression of cyclin-dependent kinase 4 (CDK4) in Jurkat cells. NFATs need not always be repressors of cell cycle-controlling genes. Accumulating evidence suggests that the phosphatase calcineurin plays a major role in the regulation of cell cycle progression by acting during the early stages of the G1 phase [57–61]. In addition, it has also been demonstrated that overexpression of NFAT2 in pre-adipocyte 3T3-L1 cells promotes cell cycle progression even under low serum concentrations and induces the altered expression of cell cycle-related genes, such as cyclin D1, cyclin D2, pRB, and c-Myc [62].
Infiltrative and destructive growth patterns of malignant gliomas are characterized by their migratory and invasive properties. Infiltrative growth prevents complete tumor resection and therefore causes significant neurological morbidity and mortality [63]. Jauliac et al. [64] reported that there are two dominant negative NFATs: one deleted in the carboxyl terminus of the sequence necessary for DNA-binding12, and the VIVIT peptide13 that blocks the ability of calcineurin to activate NFAT-inhibited carcinoma invasion when transfected into MDA-MB-435. In our study, we found that downregulation of NFAT3 alone or in combination with DOX increased the migration and invasion of carcinoma when compared to DOX-alone-treated cells. Impaired migration in DOX-treated cells could be due to active apoptosis caused by DOX, as seen in the Tunel assay. Nevertheless, these data implicate conventional NFATs in carcinoma migration and invasion.
NFAT3-induced, TNF-α-mediated apoptosis by the DOX treatment include multiple signal molecules, as described earlier. The conflicting role of TNF-α in cell cycle control [30, 44] necessitates further investigation of whether the role of NFAT3 in cell cycle control is through TNF-α or whether it is mediated by other molecule. These issues are currently being investigated in our laboratory. Since downregulation of NFAT3 reverses DOX-induced apoptosis and DOX treatment alone induces apoptosis with the available basal NFAT3, upregulation of NFAT3 may not be required for cells to undergo apoptosis in the presence of DOX.
In summary, NFAT transcription factors are known for their versatile functions in cell cycle progression, cell development, and differentiation. We have demonstrated the importance of NFAT3 by siRNA-mediated downregulation of NFAT3, which led to cell survival and subsequent inhibition of DOX-mediated apoptosis in SNB19 and U87. Western blot analysis showed the influence of NFAT3 downregulation on apoptotic molecules in both the DOX treatment alone and the DOX + shNF3 treatment. These findings imply that NFAT3 possesses anti-tumorigenic properties and may play an important role in DOX-mediated apoptosis in SNB19 and U87 glioma cell lines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1. Western blot analysis of AIF, p53, and cleaved BID. Immunoblot analysis of total protein from SNB19 and U87 cells after transfection with pSV or shNF3 and/or DOX. Proteins were probed for AIF, p53, and cleaved BID. The expression of these molecules was unaffected with the treatments Supplementary material 1 (TIFF 1509 kb) (TIFF 1509 kb)
Supplementary Fig. 2. TNF-α induces apoptosis by upregulating caspase 10 expression and PARP cleavage. Cell lysates were collected from SNB19 and U87 after transfection with SV or shNF3, and treatment with or without TNF-α. Western blot analysis of 50 μg of total cell lysates was performed to check the expression of caspase 10 and PARP. GAPDH was used as a loading control. TNF-α treatment increased the expression of these molecules irrespective of other treatments
Supplementary Fig. 3. DOX treatment leads to NFAT3 activation. Cytoplasmic (Cy) and nuclear extracts (Nu) from SNB19 and U87 cells transfected with SV or shNF3 and treated with or without DOX were immunoblotted and probed with anti-phospho NFAT3 antibody and anti-NFAT3 antibody, respectively. When nuclear extracts were probed with anti-NFAT3 antibody, the DOX treatment showed increased expression as compared to the control and no expression with the shNF3 treatment. Similarly, the cytoplasmic extracts, when probed with phospho-NFAT3, showed decreased expression of NFAT3 as compared to the control and less or no expression in the shNF3 treatment
Supplementary Fig. 4. DOX induces apoptosis in a dose-dependent manner. SNB 19 and U87 cells were treated with various concentrations of DOX (0.25, 0.5, 0.75, 1.0 μM). DNA content of control and DOX-treated SNB19 and U87 cells were measured by flow cytometric analysis to determine the cell cycle progression and apoptosis
Acknowledgments
This research was supported by National Cancer Institute Grants CA75557, CA116708, CA138409 (to JSR). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We thank Shellee Abraham for helping in manuscript preparation, and Diana Meister and Sushma Jasti for manuscript review.
References
- 1.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JW, Pollack IF, Martinez AJ, Shultz B. High-grade astrocytomas in children: radiologically complete resection is associated with an excellent long-term prognosis. Neurosurgery. 1996;38:258–264. doi: 10.1097/00006123-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767–775. doi: 10.3171/jns.1993.78.5.0767. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 6.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 7.Horsley V, Pavlath GK. NFAT: ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NFAT gene family and functional characterization of the NFAT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 9.Lyakh L, Ghosh P, Rice NR. Expression of NFAT-family proteins in normal human T cells. Mol Cell Biol. 1997;17:2475–2484. doi: 10.1128/mcb.17.5.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedito AB, Lehtinen M, Massol R, Lopes UG, Kirchhausen T, Rao A, Bonni A. The transcription factor NFAT3 mediates neuronal survival. J Biol Chem. 2005;280:2818–2825. doi: 10.1074/jbc.M408741200. [DOI] [PubMed] [Google Scholar]
- 11.Bushdid PB, Osinska H, Waclaw RR, Molkentin JD, Yutzey KE. NFATc3 and NFATc4 are required for cardiac development and mitochondrial function. Circ Res. 2003;92:1305–1313. doi: 10.1161/01.RES.0000077045.84609.9F. [DOI] [PubMed] [Google Scholar]
- 12.Groth RD, Mermelstein PG. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci. 2003;23:8125–8134. doi: 10.1523/JNEUROSCI.23-22-08125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HB, Kong M, Kim TM, Suh YH, Kim WH, Lim JH, Song JH, Jung MH. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes. 2006;55:1342–1352. doi: 10.2337/db05-1507. [DOI] [PubMed] [Google Scholar]
- 14.Mathew S, Mascareno E, Siddiqui MA. A ternary complex of transcription factors, Nished and NFATc4, and co-activator p300 bound to an intronic sequence, intronic regulatory element, is pivotal for the up-regulation of myosin light chain-2v gene in cardiac hypertrophy. J Biol Chem. 2004;279:41018–41027. doi: 10.1074/jbc.M403578200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Xie X, Zhu X, Zhu J, Hao C, Lu Q, Ding L, Liu Y, Zhou L, Liu Y, Huang C, Wen C, Ye Q. Stimulatory cross-talk between NFAT3 and estrogen receptor in breast cancer cells. J Biol Chem. 2005;280:43188–43197. doi: 10.1074/jbc.M506598200. [DOI] [PubMed] [Google Scholar]
- 16.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 17.Baksh S, Widlund HR, Frazer-Abel AA, Du J, Fosmire S, Fisher DE, DeCaprio JA, Modiano JF, Burakoff SJ. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol Cell. 2002;10:1071–1081. doi: 10.1016/S1097-2765(02)00701-3. [DOI] [PubMed] [Google Scholar]
- 18.Cockerill PN, Shannon MF, Bert AG, Ryan GR, Vadas MA. The granulocyte–macrophage colony-stimulating factor/interleukin 3 locus is regulated by an inducible cyclosporin A-sensitive enhancer. Proc Natl Acad Sci USA. 1993;90:2466–2470. doi: 10.1073/pnas.90.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iniguez MA, Martinez-Martinez S, Punzon C, Redondo JM, Fresno M. An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem. 2000;275:23627–23635. doi: 10.1074/jbc.M001381200. [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey PG, Goldfeld AE, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-alpha gene transcription. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- 21.Shannon MF, Coles LS, Vadas MA, Cockerill PN. Signals for activation of the GM-CSF promoter and enhancer in T cells. Crit Rev Immunol. 1997;17:301–323. doi: 10.1615/critrevimmunol.v17.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 22.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–S79. doi: 10.1016/S0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 23.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 24.Tan C, Etcubanas E, Wollner N, Rosen G, Gilladoga A, Showel J, Murphy ML, Krakoff IH. Adriamycin—an antitumor antibiotic in the treatment of neoplastic diseases. Cancer. 1973;32:9–17. doi: 10.1002/1097-0142(197307)32:1<9::AID-CNCR2820320102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 26.Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- 27.Jung K, Reszka R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv Drug Deliv Rev. 2001;49:87–105. doi: 10.1016/S0169-409X(01)00128-4. [DOI] [PubMed] [Google Scholar]
- 28.Wolff JE, Trilling T, Molenkamp G, Egeler RM, Jurgens H. Chemosensitivity of glioma cells in vitro: a meta-analysis. J Cancer Res Clin Oncol. 1999;125:481–486. doi: 10.1007/s004320050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niiya M, Niiya K, Kiguchi T, Shibakura M, Asaumi N, Shinagawa K, Ishimaru F, Kiura K, Ikeda K, Ueoka H, Tanimoto M. Induction of TNF-alpha, uPA, IL-8 and MCP-1 by doxorubicin in human lung carcinoma cells. Cancer Chemother Pharmacol. 2003;52:391–398. doi: 10.1007/s00280-003-0665-1. [DOI] [PubMed] [Google Scholar]
- 30.Cao W, Chi WH, Wang J, Tang JJ, Lu YJ. TNF-alpha promotes doxorubicin-induced cell apoptosis and anti-cancer effect through downregulation of p21 in p53-deficient tumor cells. Biochem Biophys Res Commun. 2005;330:1034–1040. doi: 10.1016/j.bbrc.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 31.Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Adams MD, Zhou H, Kerlavage AR. A tool for analyzing and annotating genomic sequences. Genomics. 1997;46:37–45. doi: 10.1006/geno.1997.4984. [DOI] [PubMed] [Google Scholar]
- 33.Lakka SS, Rajan M, Gondi CS, Yanamandra N, Chandrasekar N, Jasti SL, Adachi Y, Siddique K, Gujrati M, Olivero W, Dinh DH, Kouraklis G, Kyritsis AP, Rao JS. Adenovirus-mediated expression of antisense MMP-9 in glioma cells inhibits tumor growth and invasion. Oncogene. 2002;21:8011–8019. doi: 10.1038/sj.onc.1205894. [DOI] [PubMed] [Google Scholar]
- 34.Yan Y, Li J, Ouyang W, Ma Q, Hu Y, Zhang D, Ding J, Qu Q, Subbaramaiah K, Huang C. NFAT3 is specifically required for TNF-alpha-induced cyclooxygenase-2 (COX-2) expression and transformation of Cl41 cells. J Cell Sci. 2006;119:2985–2994. doi: 10.1242/jcs.03014. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Song L, Zhang D, Wei L, Huang C. Knockdown of NFAT3 blocked TPA-induced COX-2 and iNOS expression, and enhanced cell transformation in Cl41 cells. J Cell Biochem. 2006;99:1010–1020. doi: 10.1002/jcb.20834. [DOI] [PubMed] [Google Scholar]
- 36.Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–310. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- 37.Mann M, Sheng H, Shao J, Williams CS, Pisacane PI, Sliwkowski MX, DuBois RN. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713–1719. doi: 10.1053/gast.2001.24844. [DOI] [PubMed] [Google Scholar]
- 38.Sheng H, Shao J, DuBois RN. K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves activation of the protein kinase B1. Cancer Res. 2001;61:2670–2675. [PubMed] [Google Scholar]
- 39.Ranger AM, Gerstenfeld LC, Wang J, Kon T, Bae H, Gravallese EM, Glimcher MJ, Glimcher LH. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med. 2000;191:9–22. doi: 10.1084/jem.191.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22:3892–3904. doi: 10.1128/MCB.22.11.3892-3904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viola JP, Carvalho LD, Fonseca BP, Teixeira LK. NFAT transcription factors: from cell cycle to tumor development. Braz J Med Biol Res. 2005;38:335–344. doi: 10.1590/S0100-879X2005000300003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Wang X, Li J, Zhu J, Xie X, Yuan B, Yang Z, Zeng M, Jiang Z, Li J, Huang C, Ye Q. Tissue type-specific modulation of ER transcriptional activity by NFAT3. Biochem Biophys Res Commun. 2007;353:576–581. doi: 10.1016/j.bbrc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H, Gao W, Jiang H, Wu J, Shi YF, Zhang XJ. Calcineurin mediates acetylcholinesterase expression during calcium ionophore A23187-induced HeLa cell apoptosis. Biochim Biophys Acta. 2007;1773:593–602. doi: 10.1016/j.bbamcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–573. doi: 10.1016/S1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 45.Chuvpilo S, Jankevics E, Tyrsin D, Akimzhanov A, Moroz D, Jha MK, Schulze-Luehrmann J, Santner-Nanan B, Feoktistova E, Konig T, Avots A, Schmitt E, Berberich-Siebelt F, Schimpl A, Serfling E. Autoregulation of NFATc1/A expression facilitates effector T cells to escape from rapid apoptosis. Immunity. 2002;16:881–895. doi: 10.1016/S1074-7613(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 46.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 47.Rivera A, Maxwell SA. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 2005;280:29346–29354. doi: 10.1074/jbc.M504852200. [DOI] [PubMed] [Google Scholar]
- 48.Liao W, Wang S, Han C, Zhang Y. 14-3-3 proteins regulate glycogen synthase 3beta phosphorylation and inhibit cardiomyocyte hypertrophy. FEBS J. 2005;272:1845–1854. doi: 10.1111/j.1742-4658.2005.04614.x. [DOI] [PubMed] [Google Scholar]
- 49.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- 51.Yaghi A, Sims SM. Constrictor-induced translocation of NFAT3 in human and rat pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1061–L1074. doi: 10.1152/ajplung.00096.2005. [DOI] [PubMed] [Google Scholar]
- 52.Caetano MS, Vieira-de-Abreu A, Teixeira LK, Werneck MB, Barcinski MA, Viola JP. NFATC2 transcription factor regulates cell cycle progression during lymphocyte activation: evidence of its involvement in the control of cyclin gene expression. FASEB J. 2002;16:1940–1942. doi: 10.1096/fj.02-0282fje. [DOI] [PubMed] [Google Scholar]
- 53.Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/S0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez GL, Volpert OV, Iniguez MA, Lorenzo E, Martinez-Martinez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607–620. doi: 10.1084/jem.193.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004;199:1513–1522. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baksh S, DeCaprio JA, Burakoff SJ. Calcineurin regulation of the mammalian G0/G1 checkpoint element, cyclin dependent kinase 4. Oncogene. 2000;19:2820–2827. doi: 10.1038/sj.onc.1203585. [DOI] [PubMed] [Google Scholar]
- 57.Anraku Y, Ohya Y, Iida H. Cell cycle control by calcium and calmodulin in Saccharomyces cerevisiae. Biochim Biophys Acta. 1991;1093:169–177. doi: 10.1016/0167-4889(91)90119-I. [DOI] [PubMed] [Google Scholar]
- 58.Lipskaia L, Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Terada N, Lucas JJ, Gelfand EW. Differential regulation of the tumor suppressor molecules, retinoblastoma susceptibility gene product (Rb) and p53, during cell cycle progression of normal human T cells. J Immunol. 1991;147:698–704. [PubMed] [Google Scholar]
- 60.Tomono M, Toyoshima K, Ito M, Amano H. Calcineurin is essential for DNA synthesis in Swiss 3T3 fibroblasts. Biochem J. 1996;317(Pt 3):675–680. doi: 10.1042/bj3170675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomono M, Toyoshima K, Ito M, Amano H, Kiss Z. Inhibitors of calcineurin block expression of cyclins A and E induced by fibroblast growth factor in Swiss 3T3 fibroblasts. Arch Biochem Biophys. 1998;353:374–378. doi: 10.1006/abbi.1998.0667. [DOI] [PubMed] [Google Scholar]
- 62.Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278:17246–17254. doi: 10.1074/jbc.M300528200. [DOI] [PubMed] [Google Scholar]
- 63.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 64.Jauliac S, Lopez-Rodriguez C, Shaw LM, Brown LF, Rao A, Toker A. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–544. doi: 10.1038/ncb816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Western blot analysis of AIF, p53, and cleaved BID. Immunoblot analysis of total protein from SNB19 and U87 cells after transfection with pSV or shNF3 and/or DOX. Proteins were probed for AIF, p53, and cleaved BID. The expression of these molecules was unaffected with the treatments Supplementary material 1 (TIFF 1509 kb) (TIFF 1509 kb)
Supplementary Fig. 2. TNF-α induces apoptosis by upregulating caspase 10 expression and PARP cleavage. Cell lysates were collected from SNB19 and U87 after transfection with SV or shNF3, and treatment with or without TNF-α. Western blot analysis of 50 μg of total cell lysates was performed to check the expression of caspase 10 and PARP. GAPDH was used as a loading control. TNF-α treatment increased the expression of these molecules irrespective of other treatments
Supplementary Fig. 3. DOX treatment leads to NFAT3 activation. Cytoplasmic (Cy) and nuclear extracts (Nu) from SNB19 and U87 cells transfected with SV or shNF3 and treated with or without DOX were immunoblotted and probed with anti-phospho NFAT3 antibody and anti-NFAT3 antibody, respectively. When nuclear extracts were probed with anti-NFAT3 antibody, the DOX treatment showed increased expression as compared to the control and no expression with the shNF3 treatment. Similarly, the cytoplasmic extracts, when probed with phospho-NFAT3, showed decreased expression of NFAT3 as compared to the control and less or no expression in the shNF3 treatment
Supplementary Fig. 4. DOX induces apoptosis in a dose-dependent manner. SNB 19 and U87 cells were treated with various concentrations of DOX (0.25, 0.5, 0.75, 1.0 μM). DNA content of control and DOX-treated SNB19 and U87 cells were measured by flow cytometric analysis to determine the cell cycle progression and apoptosis