Abstract
BRCA1, a tumor suppressor, participates in DNA damage signaling and repair. Previously, we showed that BRCA1 overexpression caused inhibition of telomerase activity and telomere shortening in breast and prostate cancer cells. We now report that BRCA1 knockdown causes increased telomerase reverse transcriptase expression, telomerase activity, and telomere length; but studies utilizing a combination of BRCA1 and telomerase reverse transcriptase small interfering RNAs suggest that BRCA1 also regulates telomere length independently of telomerase. Using telomeric chromatin immunoprecipitation assays, we detected BRCA1 at the telomere and demonstrated time-dependent loss of BRCA1 from the telomere following DNA damage. Further studies suggest that BRCA1 interacts with TRF1 and TRF2 in a DNA-dependent manner and that some of the nuclear BRCA1 colocalizes with TRF1/2. Our findings further suggest that Rad50 is required to localize BRCA1 at the telomere and that the association of BRCA1 with Rad50 does not require DNA. Finally, we found that BRCA1 regulates the length of the 3′ G-rich overhang in a manner that is dependent upon Rad50. Our findings suggest that BRCA1 is recruited to the telomere in a Rad50-dependent manner and that BRCA1 may regulate telomere length and stability, in part through its presence at the telomere.
Introduction
Telomeres are hexameric guanine-rich duplex DNA repeats (TTAGGG) that function to “cap” chromosome ends and prevent them from being recognized as DNA damage (1–3). In humans, telomeres end in a single-stranded 3′ G-rich overhang of 100–300 nucleotides that gradually erodes during DNA synthesis because DNA polymerase cannot fully replicate the lagging strand (2, 4). Telomeres may also shorten due to oxidative damage or exonuclease activity. When they reach a critical length, senescence, growth arrest, or programmed cell death is triggered (3). The human telomere is composed of the “T-loop” (telomere loop), created by the telomere folding back on itself, and a “D-loop” (displacement loop), formed by intercalation of the 3′-overhang with the T-loop (5). These loops are associated with a complex of six proteins (TRF1 (telomere repeat binding factor 1), TRF2, POT1 (protector of telomeres 1), TIN2 (TRF1-interacting nuclear factor 2), TPP1, and RAP1), the first three of which bind directly to the TTAGGG repeats (6). This protein complex (“shelterin”) shapes and protects the telomere.
Telomere length is maintained primarily by telomerase, a multisubunit enzyme that synthesizes telomeres (1). The catalytic subunit of human telomerase is telomerase reverse transcriptase (hTERT),2 which catalyzes the addition of telomeric repeats via interactions with the 3′ G-rich overhang (7, 8). Telomerase uses an RNA component (hTR (TERC)) as a template corresponding to the TTAGGG repeats (9). Telomerase activity is usually absent in normal somatic cells but is present in stem cells (10). Telomerase is linked to cellular immortalization, a prerequisite for transformation. About 75% of human cancer cell lines express telomerase activity (11).
BRCA1 (breast cancer susceptibility gene 1) encodes a tumor suppressor, mutations of which confer a high risk for breast and ovarian cancers (12). The BRCA1 product is an 1863-amino acid nuclear phosphoprotein with a conserved N-terminal RING domain and an acidic C-terminal transcriptional activation domain (12, 13). The mechanisms by which BRCA1 suppresses tumorigenesis are not fully understood but may be due, in part, to its roles in DNA repair, cell cycle progression, and transcriptional regulation (14, 15). Previously, we showed that BRCA1 regulates telomerase and telomere length (16). Thus, BRCA1 overexpression inhibited hTERT expression and telomerase activity in human prostate and breast carcinoma cell lines. Inhibition of hTERT expression was due, in part, to inhibition of TERT promoter activity via the c-Myc E-box element. Furthermore, overexpression of BRCA1 (but not a tumor-associated point mutant BRCA1 protein) caused telomere shortening. Although wild-type BRCA1-transfected cell lines showed much shorter telomeres, these cells continued to proliferate and did not enter into senescence or apoptosis, suggesting that BRCA1 may contribute to telomere stabilization.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture
Human breast (T47D, MCF-7) and prostate (DU-145) carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, non-essential amino acids (100 mm), l-glutamine (5 mm), streptomycin (100 μg/ml), and penicillin (100 units/ml) (all from BioWhittaker, Walkersville, MD), as described before (16, 17).
Small Interfering RNAs (siRNAs)
Proliferating cells (at 20–30% confluence) were treated with a 100 nm concentration of the indicated siRNA for 72 h, using siPORT amine transfection reagent (Ambion, Foster City, CA). Knockdowns were confirmed by Western blotting. siRNAs used in this study were BRCA1 siRNA (two siRNAs custom synthesized by Dharmacon (Chicago, IL), CAGCUACCCUUCCAUCAUA and CUAGAAAUCUGUUGCUAUG (5′→3′)), BRCA1 control siRNA (D-001810-01 (Dharmacon)), TRF1 siRNA (sc-36722 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA)), TRF1 control siRNA (sc-37007 (Santa Cruz Biotechnology, Inc.), TRF2 siRNA (sc-38505 (Santa Cruz Biotechnology, Inc.)), TRF2 control siRNA (sc-44230 (Santa Cruz Biotechnology, Inc.)), TERT siRNA (sc-36641 (Santa Cruz Biotechnology, Inc.)), and Rad50 siRNA (sc-37398 (Santa Cruz Biotechnology, Inc.)).
Transient Transfections
Cells at 70–80% confluence were transfected overnight using a wild-type BRCA1 expression vector or empty pcDNA3 vector (17). Six μg of plasmid DNA was transfected per well in a 6-well plate utilizing Lipofectamine 2000 transfection reagent (Invitrogen).
Semiquantitative RT-PCR Analysis
Semiquantitative RT-PCR was performed as described earlier (16, 17). Total cell RNA was extracted using the RNase Easy minikit (Qiagen, Valencia, CA). Aliquots (200 μg) of total RNA were reverse-transcribed using 50 units of Superscript III reverse transcriptase (Invitrogen) in a 20-μl reaction volume. One μl of the transcribed cDNA was used for PCR amplification, using hot start Taq polymerase (Denville, South Plainfield, NJ). For each amplified product, the PCR conditions and cycle numbers were adjusted so that all reactions fell into the linear range of product amplification. The PCR primer sequences and predicted product sizes are listed in Table 1. PCR products were analyzed by electrophoresis through 0.8% agarose gels containing 0.1 mg/ml ethidium bromide, and the gels were photographed under UV light. mRNA levels were quantified by densitometry of the cDNA bands and expressed relative to the control gene (β-actin).
TABLE 1.
Primers used for RT-PCR in this study
| Gene | Direction | Primer sequence (5′→3′) | Annealing temperature | Size |
|---|---|---|---|---|
| °C | bp | |||
| BRCA1 | Forward | TTGCGGGAGGAAAATGGGTAGTTA | 50 | 292 |
| Reverse | TGTGCCAAGGGTGAATGATGAAAG | |||
| hTERT | Forward | CGGAAGAGTGTCTGGAGCAA | 52 | 146 |
| Reverse | GGATGAAGCGGAGTCTGGA | |||
| hTR | Forward | GAAGGGCGTAGGCGCCGTGCTTTTGC | 55 | 655 |
| Reverse | GTTTGCTCTAGAATGAACGGTGGAAGG | |||
| TRF1 | Forward | CTAAGGAGCAGATGGCAGAAAC | 54 | 112 |
| Reverse | GACTACGAGCTAAAGGAGACG | |||
| TRF2 | Forward | TCAGACAGTCCGGGACATCAT | 54 | 525 |
| Reverse | CCTTCCTTCTATTTGTCGGTCG | |||
| β-Actin | Forward | AAATCTGGCACCACACCTTC | 49 | 432 |
| Reverse | CCATCTCTTGCTCGAAGTCC | |||
| POT1 | Forward | TCAGTCTGTTAAACTTCATTGCCC | 61 | 614 |
| Reverse | TGCACCATCCTGAAAAATTATATCC |
Telomerase Assays
Telomerase activity was measured using the TRAPEZE-RT telomerase detection kit (Millipore, Bedford, MA). This assay quantifies telomerase activity by measuring real-time fluorescence emission using quantitative PCR. Briefly, after the indicated treatment, cells were lysed in 200 μl of CHAPS buffer. Aliquots of cell lysate (1 μg of protein/well) were assayed in a 96-well quantitative PCR plate. Wells were set aside for generation of the standard curve (TSR8 control template), negative control (no sample), and a PCR amplification efficiency control (TSK, K1). Telomerase activity (total product generated) was calculated by comparing the average Ct values from the sample wells against the standard curve generated by the TSR8 control template. Assays were performed on an ABI 7500 quantitative PCR machine (Applied Biosystems, Foster City, CA).
Telomere Length Assays
Telomere length was determined by Southern blotting, using the TeloTAGG telomere length assay kit (Roche Applied Sciences). Aliquots of purified genomic DNA (2 μg) were digested by incubation with HinfI (20 MU) and RsaI (20 MU) for 2 h at 37 °C. The samples were separated by electrophoresis on a 1% agarose gel. Gels were transferred to a Brightstar Plus positively charged nylon membrane (Ambion) and cross-linked by UV light for 2 min. Membranes were incubated for 2 h in a prehybridization solution at 42 °C, hybridized for 3 h under similar conditions by the addition of 2 μl of digoxigenin-labeled telomere-specific probe, washed twice at 25 °C for 15 min in washing solution and twice at 50 °C in 0.5× washing solution for 20 min, and incubated with a digoxigenin-specific antibody coupled to alkaline phosphatase. The products were visualized using a chemiluminescent substrate (alkaline phosphatase metabolizing CDP-Star) and recorded on x-ray film. The mean telomere restriction fragment (TRF) length was calculated by densitometric segmenting of the output signal into grids and calculating the signal within each grid as a function of the corresponding molecular weight. TRF length was calculated based on three independent experiments per cell line.
MTT Assays of Cell Viability
These assays are based on the ability of viable mitochondria to convert MTT, a soluble tetrazolium salt, into an insoluble formazan precipitate, which is dissolved in DMSO and quantified by spectrophotometry (18). Subconfluent proliferating cells were seeded into 96-well dishes (3000 cells/well) in growth medium, incubated for 24 h to allow attachment, and treated with adriamycin or cis-platinum (Sigma) as indicated. They were then postincubated for 24 h in fresh drug-free medium, after which cell viability was measured based on MTT dye conversion (absorbance difference at 570 and 630 nm) relative to sham-treated control cells. Cell viability was expressed as the mean ± S.E. of three independent experiments, each of which used 8 replicate wells/assay condition.
Telomeric Chromatin Immunoprecipitation (ChIP) Assays
Assays were carried out as described before (19), using a ChIP-IT kit (Active Motif, Carlsbad, CA). After the indicated cell treatments, formaldehyde was added to the culture medium to a final concentration of 1%. Fixation was carried out at 22 °C for 10 min and stopped by the addition of glycine (final concentration 0.125 m). The cells were collected by centrifugation, rinsed in PBS, resuspended in lysis buffer (ChIP-IT kit), incubated at 0 °C for 30 min, and homogenized at 0 °C to release the nuclei. The nuclei were pelleted by microcentrifuge (5000 × g for 10 min at 4 °C), resuspended in 1.0 ml of digestion buffer (ChIP-IT kit), incubated at 37 °C for 5 min, and then incubated with an enzymatic shearing mixture (200 units/ml) for 10 min to shear the chromatin to an average length of about 600 bp. The samples were incubated with EDTA (0.5 m) for 10 min at 0 °C and centrifuged (13,500 × g for 10 min at 4 °C) to collect the sheared chromatin.
Chromatin (6 μg unless otherwise specified) was incubated with the indicated antibody for 12–16 h at 4 °C. The Immunoprecipitation (IP) antibodies were BRCA1 (combination of Ab-1 plus Ab-2, Calbiochem); TRF1 (C-19, Santa Cruz Biotechnology, Inc.); TRF2 (H-300, Santa Cruz Biotechnology, Inc.); or normal mouse IgG, rabbit IgG, or goat IgG (Santa Cruz Biotechnology, Inc.). Each IP used 5 μg of IgG. Incubations were carried out in ChIP Buffer 1 containing protease inhibitor mixture (ChIP-IT kit) along with protein-G magnetic beads. The beads were washed twice with ChIP Buffer 1 and ChIP Buffer 2 and collected via a magnetic stand. The chromatin was eluted using Elution Buffer AM2 (ChIP-IT kit), reverse cross-linked with Reverse Cross-linking Buffer (ChIP-IT kit), and incubated at 94 °C for 15 min. Samples were treated with Proteinase K (1 h at 25 °C) and Proteinase K stop solution (ChIP-IT kit). The remaining DNA was transferred to a Brightstar Plus positively charged nylon membrane via dot blotting and cross-linked via UV light for 2 min. Telomeric DNA was detected using the TeloTAGG telomere length assay kit (see above), quantified by densitometry, and expressed relative to the input signal as means ± S.E. of three independent experiments.
ChIP Assays for BRCA1 Association with Alu Repeats
Assays were performed as above except using a DNA probe (5′-CGGGAAGCAGAGGTTGTAGTGAGCC-3′) to the 3′-end of the Alu repeat element modified by the addition of a 3′-digoxigenin label (Integrated DNA Technologies, San Diego, CA). The Alu sequence (Alu-C) was derived from the highly diverged 3′-end of the Alu-type repeat, as described earlier (20). Hybridization and detection of the DNA Alu probe (0.1 μg/ml) was performed using a DIG luminescence detection kit (Roche Applied Science).
Immunuprecipitation
Subconfluent proliferating cells in 150-cm2 dishes were harvested, and whole cell extracts were prepared and subjected to IP as described before (21). Each IP was performed using 5 μg of antibody or antibody combination and 500 μg of cell protein. In some experiments, 500 μg of cell extract was incubated with 100 units/ml DNase I (Qiagen) at 37 °C for 30 min prior to IP. Precipitated proteins were collected using protein-G beads, washed, eluted in boiling Laemmli sample buffer, and subjected to Western blotting. The IP antibodies were BRCA1 (combination of mouse monoclonals Ab-1 plus Ab-2, Calbiochem), TRF1 (C-19, Santa Cruz Biotechnology, Inc.), TRF2 (H-300, Santa Cruz Biotechnology, Inc.), Rad50 (sc-36641, Santa Cruz Biotechnology, Inc.), and normal (non-immune) IgG (mouse IgG, goat IgG, or rabbit IgG, Santa Cruz Biotechnology, Inc.).
In additional experiments to test if the association of TRF1 and BRCA1 required telomeric DNA, genomic DNA (5-μg aliquots) was prepared as above and incubated with Bal-31 (40 units/ml) (New England Biolabs, Ipswich, MA) for 30 min at 30 °C for varying times, followed by inactivation in 20 mm of EDTA at 65 °C for 10 min. Bal-31 is an exonuclease that degrades both 3′ and 5′ termini of duplex DNA without generating internal cuts (22). The Bal-31-digested DNA was then subjected to telomere length assays, as described above, to confirm digestion of chromosome ends. To test the effect of Bal-31 on BRCA1/TRF1 association, cell lysates were treated with Bal-31 for 120 min prior to IP-Western blotting.
Western Blotting
Western blotting was performed as described before (16). Equal aliquots of total cell protein (50 μg) or the contents of an IP were electrophoresed on a 4–20% Tris-glycine gel and transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was blocked for 1 h in blocking buffer (Sigma) and blotted using the desired primary antibody. The membranes were incubated with the appropriate species-specific horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution; Santa Cruz Biotechnology, Inc.). Blotted proteins were visualized using the ECL system (Santa Cruz Biotechnology, Inc.). Kaleidoscope prestained protein markers (Bio-Rad) were used as size standards. Primary antibodies were as follows (all from Santa Cruz Biotechnology, Inc.): BRCA1 (C-20, 1:200), Rad50 (sc-36641), TRF1 (C-19, 1:200), TRF2 (H-300, 1:200), hTERT (l-20, 1:200), and α-actin (I-19, 1:400).
Confocal Microscope Analysis
Cells cultured in 4-well culture slide chambers (BD Biosciences) were fixed for 8 min at 37 °C with 2.0% paraformaldehyde in fixation buffer (274 mm NaCl, 10 mm PIPES, 4 mm EGTA, 11 mm glucose). After three rinses with PBS, the cells were permeabilized with 0.5% Triton X-100 at 25 °C in fixation buffer for 20 min, washed three times with PBS, blocked with 10% donkey serum in PBS at 25 °C (90 min), incubated overnight with primary antibody at 4 °C, incubated with blocking solution at 37 °C for 20 min, rinsed, and incubated with secondary antibody. Cells were then washed twice with PBS, and coverslips were applied using Prolong Gold Anti-Fade plus DAPI mounting medium (Invitrogen). Primary antibodies were BRCA1 (Ab-1, Calbiochem), TRF1 (C-19, Santa Cruz Biotechnology, Inc.), and TRF2 (H-300, Santa Cruz Biotechnology, Inc.). Secondary antibodies were donkey anti-mouse IgG conjugated to Alexa Fluor 488 (green), donkey anti-goat IgG conjugated to Alexa Fluor 594 (red), and donkey anti-rabbit IgG conjugated to Alexa Fluor 594 (Invitrogen).
The stained cells were examined with an Olympus confocal laser fluorescence microscope with a ×60 objective (LSM 410, Carl Zeiss, Oberkochen, Germany) using simultaneous lasers with excitation wavelengths of 594 (red), 546 (Cy3), 488 (green), and 364 nm (DAPI). Detection was carried out using red and green narrow band filters. Images were collected every 2 μm in the vertical plane, overlaid to generate focus composite images, and stored as TIFF files.
Quantitative colocalization analysis was performed using Metamorph version 6.2 software (Molecular Devices, Sunnyvale, CA). Randomly chosen confocal images were analyzed to establish a positive threshold for each filter. The threshold for positive slides was adjusted to exclude obvious background or nonspecific staining. Green and red images were merged by the Metamorph colocalization module (23). Positive fluorescence (yellow) was collected as the number of pixels and automatically converted to percentage colocalization. Manual counting of colocalization was done on randomly chosen cells (n = 3) by counting yellow granules as a percentage of the total number of telomere granules (yellow plus red) in the same area. Values were expressed as the means ± S.E. of three independent experiments.
Teli-FISH Assays
Combined telomere DNA staining (FISH probe) and immunostaining (BRCA1 or TRF1) was performed as described before (24). Cells cultured in 4-well Culture Slide Chambers (BD Biosciences) were fixed for 8 min at 37 °C with 2.0% paraformaldehyde and rinsed three times with PBS. Fixed samples were placed in deionized water followed by deionized water plus 0.2% Tween 20 (Sigma). Samples then underwent antigen retrieval in citrate buffer (Vector Laboratories, Burlingame, CA), followed by a 5-min 95% ethanol treatment and air drying. Samples were hybridized with a Cy3-labeled telomere-specific peptide nucleic acid (PNA) probe with nucleotide sequence 5′-CCCTAACCCTAACCCTAA-3′ (Dako, Carpinteria, CA) (0.3 μg/ml PNA in 70% formamide, 10 mm Tris, pH 7.5, 0.5% blocking reagent). Slides were washed twice in PNA wash solution (70% formamide, 10 mm Tris, pH 7.5, 0.1% albumin) (Sigma), washed three times in PBS plus Tween 20, blocked with 10% donkey serum in PBS at 25 °C for 90 min, incubated overnight with primary antibody at 4 °C, blocked at 37 °C for 20 min, rinsed, and incubated with secondary antibody conjugated to Alexa Fluor dye. Cells were then washed twice with PBS, and coverslips were applied as above. The primary and secondary antibodies were the same as above. Three independent experiments were performed per assay type per cell line.
Assays of 3′ G-rich (G-strand) Overhang Length
Hybridization Protection Assay
This assay uses an acridinium ester (AE)-labeled probe that is hybridized to the 3′ overhang (25). The mislabeled or unbound AE is inactivated by hydrolysis, but the hybridized AE probe is protected from hydrolysis and measured by chemiluminescence. Aliquots of genomic DNA (5 μg) were incubated with an AE-labeled telomere probe (5′-CCCTAACCCTAACC*CTAACCCTAACCCTA-3′, where the asterisk represents AE position) in hybridization buffer (0.1 m lithium succinate buffer, pH 4.7, containing 200 g/liter lithium lauryl sulfate, 1.2 m lithium chloride, 20 mm EDTA, 20 mm EGTA) at 60 °C for 20 min. AE labeling of telomere probe was performed by Integrated DNA Technologies. Hydrolysis of the unbound AE was carried out by incubation in hydrolysis buffer (0.6 m sodium tetraborate buffer, pH 8.5, containing 0.5% Triton X-100) at 60 °C for 10 min. After cooling at 0 °C for 1 min, luminescence was measured for 5 s with a luminometer and Gen-Probe detection reagent kit (Gen-Probe, Inc., San Diego, CA). The luminescence was normalized to AluDNA. To detect AluDNA, the Alu probe (5′-TGTAATCCCA*GCACTTTGGGAGGC-3′, where the asterisk represents AE position) was hybridized to genomic DNA (5 μg), as described before (25). As a negative control, genomic DNA was treated with Exo I (0.2 unit/μg DNA) (New England Biolabs) in exonuclease buffer (67 mm glycine-KOH, 6.7 mm MgCl2, 10 mm 2-mercaptoethanol) at 37 °C for 2 h and heat-inactivated at 80 °C for 20 min before G-strands were assayed. Exo I removes nucleotides from single-stranded DNA in the 3′–5′ direction. G-strand overhang was also measured after incubation of genomic DNA with T7 exonuclease (1 unit/μg DNA) (New England Biolabs) in NE-Buffer 4 at 25 °C for 60 s. T7 exonuclease acts in the 5′–3′ direction to remove 5′ nucleotides from duplex DNA. The reaction was stopped by the addition of 20 mm EDTA at 65 °C for 10 min.
Double-stranded Nuclease (DSN) Assay
This assay uses a nuclease (DSN) that digests double-stranded DNA while preserving single-stranded DNA (26). The G-strand overhang remains intact after incubation with DSN and is measured by hybridization to a C-rich digoxigenin-labeled probe and Southern blotting. Genomic DNA (5 μg) was digested with DSN (2 units/μg DNA) (Axxora, San Diego, CA) at 65 °C for 20 min. The digestion was stopped by adding 5 μl of 2× DSN stop solution as per the manufacturer's instructions. As a control, Exo I (0.2 units/μg DNA) was added to genomic DNA prior to DSN treatment and incubated at 37 °C for 2 h to digest 3′ overhangs. After an equal volume of formamide (Sigma) was added, samples were heated at 65 °C for 5 min, loaded on a 6% denaturing polyacrylamide gel containing 8 m urea, and run in 0.5× Tris borate-EDTA (TBE) buffer. DNA was then transferred to a Brightstar positively charged membrane (Ambion) for 90 min in 0.5× TBE. The membrane was air-dried, UV-cross-linked and hybridized to a C-rich digoxigenin-labeled probe (CCCTAACCCTAACCCTAA) and processed using the TeloTAGG telomere length assay kit (Roche Applied Science). The mean sizes of overhangs were calculated as described for the telomere length assays.
Statistical Methods
Data are presented as means ± S.E. from at least three independent experiments. Comparisons were made using the two-tailed Student's t tests, where appropriate.
RESULTS
BRCA1 Knockdown Causes Increased hTERT Expression, Telomerase Activity, and Telomere Length
BRCA1 siRNA caused a 1.4-fold increase in hTERT mRNA levels in T47D cells (Fig. 1A), which was significant compared with control siRNA (p < 0.05) when quantified with densitometry. Similarly, BRCA1 siRNA caused a significant (1.45-fold) increase in hTERT protein, determined by Western blotting (p < 0.05) (Fig. 1B). Here, BRCA1 siRNA reduced the BRCA1 mRNA and protein levels to about 25% of control. We next tested the effect of BRCA1 siRNA on telomerase activity, using the TRAPEZE-RT assay, a commercial fluorescence detection assay that utilizes quantitative PCR. BRCA1 siRNA caused a 45% increase in telomerase activity in T47D, compared with either control siRNA or vehicle-treated cells (p < 0.05) (Fig. 1C). The efficacy of the BRCA1 knockdown is shown in the right panel. The TSK control is an internal control for the PCR efficiency, and the negative control is a no sample PCR control. Similar results were observed using the standard telomerase repeat amplification protocol (data not shown), suggesting that BRCA1 siRNA consistently increases telomerase activity. Similar to T47D, BRCA1 siRNA caused comparable increases in hTERT expression and telomerase activity in MCF-7 and DU-145 cells (p < 0.05) (supplemental Fig. 1, A–D).
FIGURE 1.
BRCA1 knockdown causes increased hTERT expression, telomerase activity, and telomere length. A and B, subconfluent proliferating T47D cells were treated with BRCA1 siRNA (100 nm), control siRNA (100 nm), or vehicle for 72 h and harvested for semiquantitative RT-PCR assays (A) or Western blotting (B) for hTERT, BRCA1, and actin (control gene). The PCR product and protein bands were quantified by densitometry, expressed relative to actin, and normalized to the control (vehicle-treated cells). C, T47D cells treated with siRNAs were tested for telomerase activity using the TRAPEZE assay. A Western blot to confirm the BRCA1 knockdown is included. Telomerase activity was calculated as total product-generated units and normalized to the vehicle control. Note that TSK is an internal control for standardization of the assay, and a no sample negative control is included. D, MCF-7 or T47D cells were treated with BRCA1 siRNA, control (CON) siRNA, or vehicle over a period of 21 days with replating in fresh medium plus siRNA every 3 days. The cells were then assayed to determine telomere length by Southern blotting. Telomere length was quantitated as the mean telomere repeat fragment (TRF) length. All values shown in A–D are means ± S.E. of three independent experiments. *, p < 0.05 (two-tailed t test).
To test the effect of BRCA1 on telomere length, cells were treated with BRCA1 siRNA (versus control siRNA or vehicle) over 21 days, with harvesting and replating in fresh medium with fresh siRNA every 3 days. They were then assayed for telomere length by Southern blotting. BRCA1 siRNA caused significant increases in telomere length (quantified as TRF) (p < 0.05) in T47D, MCF-7, and DU-145 cells (Fig. 1D and supplemental Fig. 1E). In T47D, the mean TRF length increased from 4.8 kbp (vehicle-treated cells) to 8.1 kbp (BRCA1 siRNA-treated cells). The corresponding increases in telomere length were from 4.3 to 7.9 kbp in MCF-7 and from 5.1 to 7.6 kbp in DU-145 cells. Western blotting after 3, 9, and 21 days revealed that BRCA1 protein levels remained decreased and hTERT levels remained increased in BRCA1 siRNA-treated cells during the experiment.
In contrast to hTERT, BRCA1 siRNA had little or no effect on the mRNA or protein levels of TRF1 and TRF2 in T47D (supplemental Figs. 2, A and B). Similarly, BRCA1 siRNA had little or no effect on the levels of hTR or POT1 mRNA (supplemental Fig. 2, C and D) in T47D cells. Similar results were observed in MCF-7 cells (supplemental Fig. 3). Thus, BRCA1 knockdown confers higher levels of the catalytic subunit hTERT but not the RNA component or several subunits of shelterin.
BRCA1 siRNA Causes Telomere Lengthening in the Setting of hTERT Knockdown
We performed an experiment similar to that shown in Fig. 1D, except that BRCA1 siRNA and TERT siRNA were tested, alone and in combination. After passage 6 (and to a lesser extent passage 3), TERT siRNA alone caused a reduction in telomere length from 4.8 to 1.8 kbp (Fig. 2, A and B). The addition of BRCA1 siRNA to TERT siRNA completely blocked this reduction in telomere length, and BRCA1 siRNA alone caused an increase in telomere length to 8 kbp. The telomerase activity (Fig. 2C) and TERT protein levels (Fig. 2D) were similar in cells treated with TERT siRNA alone versus TERT siRNA combined with BRCA1 siRNA. Taken together, these findings suggest that BRCA1 knockdown caused increased telomere length by both TERT-dependent and TERT-independent mechanisms (see “Discussion”).
FIGURE 2.
BRCA1 siRNA blocks telomere shortening due to TERT knockdown. A and B, T47D cells were treated with BRCA1 siRNA (100 nm), TERT siRNA (100 nm), BRCA1 siRNA plus TERT siRNA (100 nm each), or control (CON) siRNA (200 nm). The total siRNA concentration was kept constant at 200 nm by the addition of control siRNA. Cells were passaged every 3 days into fresh medium containing fresh siRNA. Cells were assayed at the indicated passage for telomere length by Southern blotting as in Fig. 1. Values plotted are means ± ranges of two independent experiments. C, cells were assayed for telomerase activity after passage 6 (see Fig. 1). Values are means ± S.E. of three experiments. D, Western blots are provided to show the effects of siRNA treatments on BRCA1 and TERT levels after passage 6.
BRCA1 Is Present at the Telomere and Is Lost following DNA Damage
To test whether any BRCA1 is located at telomeres, we used a telomeric ChIP assay in which protein-associated telomeric DNA is detected by hybridization using a digoxigenin-labeled telomere-specific probe, visualized by dot blots, and quantified by densitometry. Telomeric DNA was detected in BRCA1 IPs of T47D cells, but a control IP carried out using the same quantity of non-immune IgG yielded no telomeric DNA (Fig. 3A). The graph on the right shows the relationship between the telomeric DNA signal/input ratio for the BRCA1 IP and quantity of chromatin used. Based on these results, we chose 6 μg of chromatin, which was within the linear region of the curve, for subsequent assays. We also tested the ability of BRCA1 to associate with AluDNA repeat elements by ChIP assays using a digoxigenin-labeled Alu probe (20). The signal/input ratio for the BRCA1 IP using the Alu probe was less than 10% of that using the telomere probe, suggesting that BRCA1 association with telomeric DNA is not a nonspecific finding. MCF-7 cells gave an association of BRCA1 with telomeric DNA similar to that of T47D cells (data not shown).
FIGURE 3.
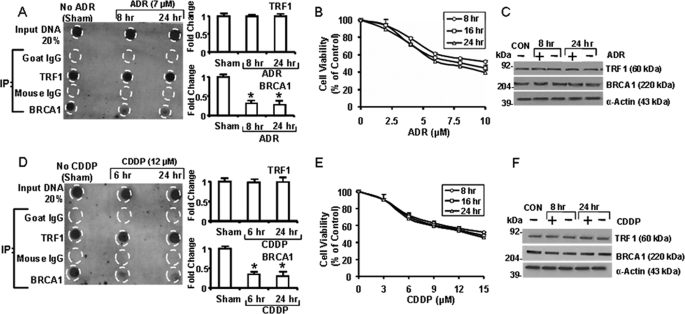
Telomeric ChIP assays to detect telomeric BRCA1 protein in T47D cells. A, cells were subjected to ChIP assays using anti-BRCA1 as the IP antibody and different amounts of chromatin. The precipitated DNA was hybridized with a telomeric probe or an Alu probe. Dot blots are shown using anti-BRCA1 or normal mouse IgG as the IP antibody. The graph on the right shows the signal/input ratio from the BRCA1 IPs as a function of the quantity of chromatin used in the assay. B and D, dot blots and densitometry quantification for telomeric ChIP assays after treatment of cells with adriamycin (ADR; 7 μm) (B) or cis-platinum (CDDP; 12 μm) (D) for the indicated times. Assays were performed using 6 μg of chromatin with IP antibodies against BRCA1, TRF1, or the corresponding normal IgG types. Graphs on the right show quantification of the telomeric TRF1 and BRCA1 content. C and E, Western blots for TRF1 and BRCA1 for cells treated with or without ADR or CDDP. All values plotted in A, B, and D are means ± S.E. of three independent experiments (*, p < 0.05, relative to sham-treated control).
To test the effect of DNA damage on telomeric BRCA1, T47D cells were sham-treated or treated with adriamycin (ADR; 7 μm) (a topoisomerase IIα inhibitor) for 2–8 h (Fig. 3B), and ChIP assays were performed using IP antibodies against BRCA1 or TRF1, with normal IgG as controls. There was no change in the telomeric TRF1 for up to 8 h, and whereas the telomeric BRCA1 content was unchanged for up to 6 h, the BRCA1 content was decreased at the 8 h time point. It is unlikely that the loss of BRCA1 at 8 h was due to a general reduction in BRCA1 protein levels, because the whole cell BRCA1 (determined by Western blotting) was unchanged (Fig. 3C). Similar results were obtained for MCF-7 (supplemental Fig. 4). Control IPs using goat IgG (for TRF1) or mouse IgG (for BRCA1) yielded no telomeric DNA.
In parallel studies using cis-platinum (CDDP), a DNA-cross-linking agent, exposure of T47D cells to CDDP (12 μm) for 2–4 h did not alter the telomeric BRCA1 content, but after 6 h, there was significantly less BRCA1 at the telomere (p < 0.05) (Fig. 3D). Again, CDDP did not cause any change in telomeric TRF1 content or in the total cell content of BRCA1 or TRF1 (Fig. 3E).
Fig. 4A shows telomeric ChIP assays of T47D cells exposed to ADR for 8 and 24 h. Consistent with previous experiments, telomeric BRCA1 content was decreased after 8 h and remained reduced at 24 h (p < 0.05). In contrast, there was no reduction of telomeric TRF1 at 8 or 24 h. Fig. 4B shows survival curves for T47D cells treated with different doses of ADR for different times, based on MTT assays 24 h after the end of exposure to ADR. These data show cell survival of 45–56% for cells treated with 7 μm ADR for 8–24 h. ADR exposure for 8 or 24 h did not cause any obvious change in total cell BRCA1 or TRF1 (Fig. 4C). Like ADR, exposure of T47D cells to CDDP for 6–24 h resulted in continued reduced telomeric BRCA1 (p < 0.05) with no changes in telomeric TRF1 (Fig. 4D) and with cell survival rates of 55–60% (Fig. 4E) and no obvious changes in total cellular BRCA1 or TRF1 (Fig. 4F). Similar results were obtained for MCF-7 (supplemental Fig. 5), and neither an 8-h nor a 24-h exposure of T47D cells to ADR caused a change in total cell hTERT levels (supplemental Fig. 6).
FIGURE 4.
Adriamycin and cis-platinum cause loss of telomeric BRCA1 in T47D cells. Cells were sham-treated or exposed to ADR or CDDP for the indicated times and harvested for telomeric ChIP assays to detect TRF1 and BRCA1 (A and D). As negative controls, ChIP assays were performed using normal goat IgG (control for TRF1 IP) or mouse IgG (control for BRCA1 IP). Dot blots were quantified using densitometry, normalized to the input, and expressed relative to sham-treated controls, as means ± S.E. of three independent experiments (*, p < 0.05 relative to controls). B and E show MTT assays for cells treated with ADR or CDDP for 8, 16, or 24 h and assayed 24 after treatment. C and F provide Western blots to show the effects of ADR or CDDP on TRF1, BRCA1, and α-actin levels.
Next, T47D cells were exposed to ADR for 8 h to deplete telomeric BRCA1 and allowed to recover in fresh medium for 48–96 h prior to ChIP assays. Telomeric BRCA1 content was restored to control levels by 48 h of recovery and remained at these levels for at least 96 h, whereas the TRF1 content was unchanged (supplemental Fig. 7A). Western blots revealed no changes in total cellular hTERT or BRCA1 during ADR exposure or recovery (supplemental Fig. 7B). An IP-Western blot for BRCA1 showed no changes in the amount of BRCA1 immunoprecipitated under treatment conditions (supplemental Fig. 7C), ruling out the possibility that post-translational modification of BRCA1 to a form not recognized by the IP antibody could explain the loss of telomeric BRCA1 during ADR exposure or its reappearance after recovery. Supplemental Fig. 7, D–F shows a similar experiment, except that the cells were sham-treated rather than exposed to ADR. As expected, there were no changes in telomeric BRCA1 or TRF1, total cellular hTERT or BRCA1, or immunoprecipitatable BRCA1 protein.
TRF1 and TRF2 Are Required to Localize BRCA1 at the Telomere
To determine if TRF1 or TRF2 is required to localize BRCA1 to the telomere, T47D cells were treated with TRF1 siRNA or TRF2 siRNA prior to telomeric ChIP assays. Here, TRF1 siRNA caused a significant loss of both TRF1 (p < 0.05) and BRCA1 (p < 0.05) from the telomere (Fig. 5A). In contrast, TRF1 siRNA did not alter the total cell BRCA1 content (Fig. 5B). Likewise, TRF2 siRNA caused loss of telomeric BRCA1 without a reduction in total cell BRCA1 content (Fig. 5, C and D). Similarly, knockdown of TRF1 or TRF2 in MCF-7 cells caused a significant reduction in the telomeric content of BRCA1 (supplemental Fig. 8). Conversely, we tested if BRCA1 siRNA could alter the telomeric TRF1 or TRF2. Here, BRCA1 siRNA caused a significant reduction in telomeric BRCA1 (p < 0.05) but had little or no effect on the telomeric content of TRF1 or TRF2 in T47D cells (Fig. 5E). Although BRCA1 siRNA effectively knocked down total cell BRCA1 levels, it had little or no effect on the total cell levels of TRF1 or TRF2 in T47D (Fig. 5F) or MCF-7 cells (data not shown). These findings suggest that TRF1 and TRF2 are each required for telomere localization of BRCA1, but BRCA1 is not required for telomere localization of TRF1 or TRF2.
FIGURE 5.
Knockdown of TRF1 or TRF2 causes loss of telomeric BRCA1 (but not vice versa) in T47D cells. Cells were treated with TRF1 siRNA (A and B), TRF2 siRNA (C and D), BRCA1 siRNA (E and F), control siRNA (100 nm), or vehicle for 72 h and harvested for telomeric ChIP assays using antibodies against TRF1, TRF2, and BRCA1 or using normal IgG as a control (goat IgG (TRF1), rabbit IgG (TRF2), mouse IgG (BRCA1)). In A, C, and E, the left panels show representative ChIP dot blots, whereas the right panels show densitometry quantification based on three independent experiments (*, p < 0.05). B, D, and E, Western blots to document the TRF1, TRF2, and BRCA1 knockdowns.
Association of BRCA1 with TRF1/2
We tested if BRCA1 associates with TRF1 or TRF2 by IP-Western blotting. In T47D cells, IP of BRCA1 co-precipitated TRF1 and TRF2, whereas IP of TRF1 or TRF2 co-precipitated BRCA1, and control IPs using non-immune IgG did not yield BRCA1, TRF1, or TRF2 (Fig. 6, A and B). Similar results were observed in MCF-7 cells (supplemental Fig. 9). Although it is difficult to draw quantitative conclusions, these data suggest that at least a fraction of the BRCA1 and TRF1/2 are associated in vivo. To test whether the association of BRCA1 with TRF1 or TRF2 is DNA-dependent, T47D cell lysates were treated with or without DNase I prior to IP-Western blotting. DNase I treatment did not alter the ability to immunoprecipitate BRCA1, TRF1, or TRF2, but it blocked the association of BRCA1 with TRF1 and TRF2 (Fig. 6, C and D). DNase I treatment had no effect on the levels of BRCA1, TRF1, or TRF2 in the input lanes showing non-precipitated total cell protein (Fig. 6E). Fig. 6F shows the effect of DNase I treatment on the DNA present in a T47D whole cell lysate after electrophoresis on a 1% agarose gel.
FIGURE 6.
BRCA1 association with TRF1 and TRF2 is mediated by DNA. Cells were subjected to reciprocal IP-Western blotting for BRCA1/TRF1 (A and C) or BRCA1/TRF2 (B and D). In C and D, IPs were carried out using lysates pretreated with or without DNase I (100 units/ml for 30 min at 37 °C). For each IP, a control IP using the same quantity of normal IgG was performed, and an input lane is provided corresponding to 10% of the amount of protein used for IP. E, Western blots of unprecipitated lysates treated with or without DNase I; F, electrophoresis of lysates pretreated with or without DNase I on a 1% agarose gel.
Bal-31 is an exonuclease that digests linear DNA from both ends. As shown in supplemental Fig. 10A, Bal-31 progressively digested telomeric DNA until it reached a size of <0.9 kbp by 120 min. When T47D lysates were digested with Bal-31 for 120 min, the association of BRCA1 with TRF1, determined by IP-Western blotting, was disrupted (supplemental Fig. 10, B and C). Supplemental Fig. 10D shows that although a 120-min exposure to Bal-31 degraded telomeres, a significant quantity of high molecular weight DNA was still present. These findings suggest that the interaction of BRCA1 with TRF1/2 is DNA-dependent, and the association of BRCA1 with TRF1 is probably dependent upon telomeric DNA.
Colocalization of BRCA1 with TRF1/2
T47D cells were sham-treated or exposed to ADR for 8 h and analyzed by confocal microscopy. Nuclei were detected by DAPI staining (blue), whereas BRCA1 (green), TRF1 (red), and TRF2 (red) were detected by immunostaining. In sham-treated cells, a fraction of the BRCA1 and TRF1 colocalized, indicated by yellow granules in the merged image (Fig. 7A, top row). The extent of colocalization was reduced in ADR-treated T47D cells (Fig. 7A, bottom). In the graph (right), the percentage of colocalization was quantified using Metamorph software. ADR caused a reduction in colocalization of BRCA1 with TRF1 from 50 to 12%. Similar results were observed for BRCA1 colocalization with TRF2 (Fig. 7B). Similar colocalization of BRCA1 with TRF1/2 was observed in MCF-7 (supplemental Fig. 11). These findings suggest colocalization of a portion of the nuclear pool of BRCA1 with TRF1 and TRF2 and disruption of this colocalization due to DNA damage. As a test of the confocal microscopy methodology, we confirmed that knockdown of BRCA1, TRF1, and TRF2 each sharply reduced the immunofluorescence of the target protein (supplemental Fig. 12).
FIGURE 7.
Colocalization of BRCA1 with TRF1 and TRF2. Subconfluent proliferating T47D cells were sham-treated or exposed to ADR (7 μm) for 8 h and processed for confocal microscopy. Images are shown of DAPI stain (nuclei), BRCA1 immunostaining (green), TRF1 or TRF2 immunostaining (red), and merged BRCA1 plus TRF1 (A) or BRCA1 plus TRF2 (B) images. Yellow granules in the merged images indicate regions of colocalization. Quantitation of colocalization of BRCA1/TRF1 and BRCA1/TRF2 was carried out using Metamorph version 6.2 software (n = 25 cells).
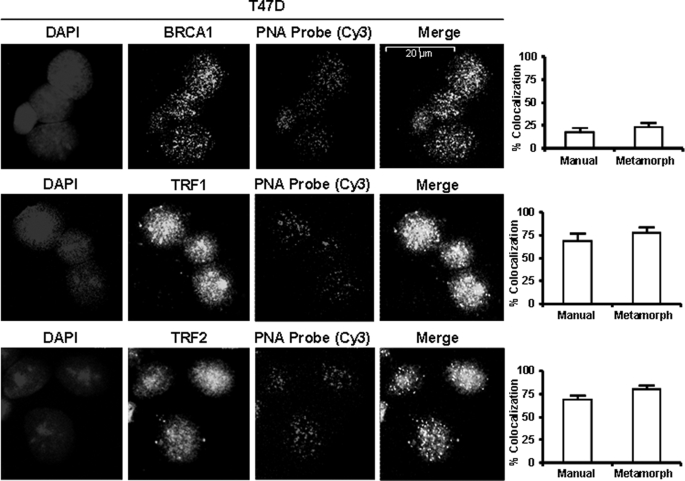
Colocalization of BRCA1 with Telomeres
To visualize BRCA1 at telomeres in intact cells, T47D cells were subjected to combined staining of telomeric DNA (using a Cy3-labeled telomere-specific PNA probe) and immunostaining for BRCA1 (or TRF1/2 as positive controls). A fraction of the BRCA1 reproducibly colocalized with the telomeric probe (yellow granules in Fig. 8 (top right)). The extent of colocalization (quantified by manual counting of yellow granules as a percentage of the total number of telomere granules (yellow plus red) or by the Metamorph software) ranged from 20 to 25% for T47D (Fig. 8) and MCF-7 (supplemental Fig. 13) cells. By comparison, TRF1 and TRF2 showed colocalization with the telomeric probe in the range of 65–80% (Fig. 8 and supplemental Fig. 13). Although not all of the TRF1/2 localized to telomeres, a large majority of telomeres had immunodetectable TRF1/2.
FIGURE 8.
Teli-FISH of BRCA1 colocalization with telomeric probe. Subconfluent proliferating T47D cells on slides were fixed and hybridized to a Cy3-labeled telomere-specific PNA probe. The slides were immunostained with BRCA1, TRF1, or TRF2 primary antibody and secondary antibody conjugated to Alexa Fluor dye and processed for confocal imaging. Images are shown of DAPI stain (blue), PNA probe (red), BRCA1 (green), TRF1 (green), or TRF2 (green). Quantification of colocalization of BRCA1, TRF1, or TRF2 with the PNA probe (yellow granules in merged images) was carried out using Metamorph version 6.2 software (n = 28) or by manual counting of the percentage of total telomeric granules (yellow + red) that is colocalized (yellow) with BRCA1, TRF1, or TRF2 (n = 3 cells).
Rad50 Is Required for Telomeric Localization of BRCA1
Rad50 is a component of the “MRN” complex (Mre11-Rad50-NBS1) that both interacts with BRCA1 (27) and appears to play significant functional roles at the telomere (28–30). To test whether Rad50 is required for recruitment of BRCA1 to telomeres, T47D cells were treated with Rad50 siRNA and then subjected to telomeric ChIP assays. Here, Rad50 knockdown caused a sharp reduction in the telomeric content of both Rad50 and BRCA1 (Fig. 9A). In contrast, BRCA1 knockdown had little or no effect on the telomeric Rad50 content (Fig. 9B). Neither Rad50 knockdown nor BRCA1 knockdown had a significant effect on the total cellular content of the other protein (Fig. 9C). Treatment of cells with ADR caused a loss of telomeric Rad50 with a time course similar to that observed for loss of telomeric BRCA1 (Fig. 9, D and E), but ADR had no obvious effect on the total cellular Rad50 or BRCA1 abundance. In contrast to TRF1/2, treatment of cell lysates with DNase I did not disrupt the association of Rad50 and BRCA1 (supplemental Fig. 14). Taken together, these findings suggest that Rad50 may be required for the recruitment of BRCA1 to telomeres.
FIGURE 9.
Rad50 knockdown causes loss of telomeric BRCA1 in T47D cells. A and B, cells were treated with Rad50 siRNA (A), BRCA1 siRNA (B), control siRNA (100 nm), or vehicle for 72 h and harvested for telomeric ChIP assays using IP antibodies against Rad50, BRCA1, and TRF1 and normal IgGs as controls (goat IgG (TRF1), mouse IgG (Rad50 and BRCA1)). Typical dot blots and densitometry quantitation based on three independent experiments are shown (*, p < 0.05). C, Western blots to document the Rad50 and BRCA1 knockdowns. D–F, cells were sham-treated or exposed to ADR for the indicated time intervals and then harvested for telomeric ChIP assays to detect Rad50, BRCA1, and TRF1 (D and E). Representative dot blots and densitometric quantification based on three experiments are shown (*, p < 0.05). F, Western blots to show the effects of ADR on whole cell Rad50, BRCA1, and α-actin protein levels.
BRCA1 Regulates 3′ G-strand Overhang Length
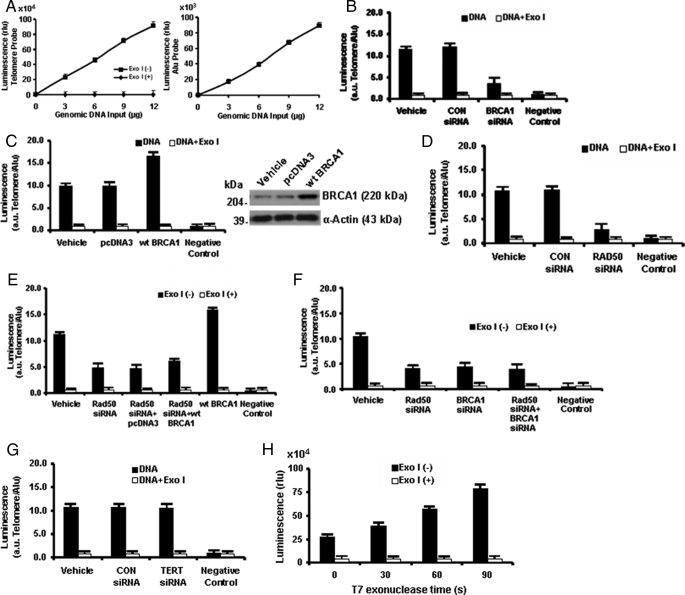
The hybridization protection assay employs an acridinium ester-labeled telomeric probe to obtain a relative measure of 3′ G-strand overhang length (25). An AE-labeled AluDNA probe was used to normalize the assay. The luminescence signal for the telomeric probe is an integrated reflection of the number of telomeres and the length of each telomere. Over the range of genomic DNA tested (3–12 μg), the telomeric and Alu probes each gave a linear signal (Fig. 10A), and 5-μg aliquots of genomic DNA were used in subsequent experiments. Compared with vehicle or control siRNA, T47D cells treated with BRCA1 siRNA showed a large reduction in overhang length (p < 0.05) (Fig. 10B). Conversely, transfection of a wild-type BRCA1 expression vector caused an increase in the signal (p < 0.05) (Fig. 10C), whereas Rad50 siRNA caused a similar reduction in signal intensity to BRCA1 siRNA (p < 0.05) (Fig. 10D). The combination (Rad50 siRNA + wild-type BRCA1) yielded only a modest increase in signal intensity, compared with Rad50 siRNA alone, whereas Rad50 siRNA + BRCA1 siRNA gave results similar to Rad50 siRNA alone (Fig. 10, E and F). Knockdown of TERT yielded no change in telomere overhang signal (Fig. 10G). In additional experiments, knockdown of TRF2 caused a marked reduction in the telomere overhang signal, whereas knockdown of TRF1 had little or no effect on the signal (data not shown). Finally, Fig. 10H provides a positive control using T7 exonuclease, which removes nucleotides from duplex DNA in a 5′ to 3′ direction and thus increases the overhang signal.
FIGURE 10.
BRCA1 regulates 3′ G-strand overhang length; hybridization protection assay. A, standard curves of luminescence (relative units) versus genomic DNA input were obtained using an AE-labeled telomeric probe (left) or an AE-labeled AluDNA probe (right). Data are shown for DNA treated with or without Exo I, which removes single-stranded DNA. B–G, cells were treated with the indicated siRNAs and/or transfected overnight with wild-type (wt) BRCA1 or empty pcDNA3 vector, and genomic DNA (5 μg) was assayed to determine the ratio of luminescence (arbitrary units (a.u.)) obtained using the telomeric and Alu probes. Controls using Exo I and, in some cases, negative controls (no DNA) are provided. C, a Western blot to document overexpression of BRCA1 in cells transfected with wild-type BRCA1. H, the telomeric probe signal for genomic DNA (5 μg) treated with T7 exonuclease (which digests duplex DNA, but not single-stranded DNA, in a 5′ to 3′ direction) for different time intervals. All data are means ± S.E. of three independent experiments.
We used DSN assays (26) to confirm some of the hybridization protection assay results. This assay uses DSN to digest away duplex DNA, leaving the 3′ overhang, which is detected using a C-rich digoxigenin-labeled probe. Compared with control siRNA or vehicle, T47D cells treated with BRCA1 siRNA showed a loss of longer 3′ G-strand overhangs (Fig. 11A, left), with a reduction in mean overhang length (p < 0.05) (Fig. 11A, right). Conversely, wBRCA1-transfected cells showed loss of shorter overhangs and increased mean overhang length (p < 0.05) (Fig. 11B). Like BRCA1 siRNA, Rad50 siRNA caused a decrease in overhang length (p < 0.05) (Fig. 11C). As in hybridization protection assays, treatment of DNA with Exo I, an enzyme that excises nucleotides from single-stranded DNA in the 3′ to 5′ direction, effectively degraded the 3′ G-strand overhangs. Fig. 11D shows that DSN digests duplex genomic DNA to fragments ≤20 bp in length. Taken together, our findings suggest that BRCA1 regulates telomere overhang length. The significance of the data will be considered under “Discussion.”
FIGURE 11.
BRCA1 regulates G-strand overhang length; DSN assays. A–C, T47D cells were treated with the indicated siRNAs or transfected with wild-type BRCA1 or empty pcDNA3 vector, and the genomic DNA was isolated. Aliquots of genomic DNA (5 μg) were treated with DSN, incubated with or without Exo I, electrophoresed on a denaturing polyacrylamide gel containing 8 m urea, transferred to a Brightstar membrane, hybridized to a C-rich digoxigenin-labeled probe, and processed to calculate the mean sizes of overhangs as in the telomere length assays. Values are means ± S.E. of three independent experiments. D, genomic DNA (5 μg) was incubated with or without DSN for 7 min at 65 °C. Digested products were then electrophoresed on a 4% agarose gel and visualized via ethidium bromide staining.
Finally, we tested the effect of ADR treatment on overall telomere length and 3′ G-strand overhang length in T47D cells. As shown in supplemental Fig. 15, A–C, treatment of cells with ADR for 24–72 h either in the absence or presence of BRCA1 siRNA had little or no effect on telomere length. This finding may reflect the fact that changes in overall telomere length related to loss of BRCA1 require a number of passages (population doublings) to occur (see Figs. 1D and 2, A and B). However, we observed a modest but significant reduction in G-strand overhang length after 8 h of ADR treatment (p < 0.05) (supplemental Fig. 15D), which is the minimum time required for loss of telomeric BRCA1. These findings suggest a correlation between loss of telomeric BRCA1 and shortening of the 3′ overhang.
DISCUSSION
We showed that knockdown of BRCA1 in several breast and prostate carcinoma cell lines caused increased expression of hTERT coupled to increases in telomerase activity and telomere length. However, BRCA1 knockdown had no effect on expression of the hTR or proteins that bind directly to telomere DNA (TRF1, TRF2, and POT1) (6). Taken together with a prior finding that BRCA1 overexpression causes telomere shortening (16), this suggests that BRCA1 regulates telomere length over a wide range of expression levels. It is unlikely that BRCA1-related changes in telomere length were due to alterations in cell proliferation, because in our hands, neither overexpression (31) nor underexpression (32) of BRCA1 caused significant changes in growth of these cell lines. Interestingly, our findings suggest that BRCA1 regulates telomere length by both telomerase-dependent and telomerase-independent mechanisms, because BRCA1 knockdown increased telomere length even in cells in which hTERT was knocked down. Thus, BRCA1 may not only regulate telomere synthesis but may also regulate telomere degradation, a process that is not well understood at present.
A novel finding of this study is that some BRCA1 is localized at the telomere, documented by telomeric ChIP assays. In contrast, ChIP assays using an Alu probe indicate that relatively little BRCA1 is associated with Alu repeat element DNA, suggesting that association of BRCA1 with telomeres is not due to nonspecific binding. Based on knockdown studies, TRF1 and TRF2 were both required for telomeric localization of BRCA1. We also found that a fraction of the nuclear BRCA1 associates and colocalizes with TRF1 and TRF2, based on reciprocal IP-Western blotting and confocal imaging. In contrast, BRCA1 knockdown did not cause loss of TRF1/2 from telomeres. Interestingly, digestion of DNA present in cell lysates by two different methods disrupted the association of BRCA1 with TRF1/2, suggesting that this association is mediated by DNA and may require an intact telomere structure. Although BRCA1 recruitment to telomeres may be mediated indirectly by TRF1/2, our findings suggest a more direct role of Rad50, a component of the MRN complex, a protein complex involved in DNA repair that is also present and functional at telomeres (28–30). Thus, Rad50 knockdown caused a reduction of telomeric (but not whole cell) BRCA1 content, whereas BRCA1 knockdown had no effect on telomeric Rad50 content. Unlike TRF1/2, the association of BRCA1 with Rad50 was not DNA-dependent.
Simultaneous confocal imaging of a telomeric probe and BRCA1 revealed that a fraction of the BRCA1 localizes to telomeres. We estimate that 20–25% of telomeres in T47D or MCF-7 cells contain BRCA1, whereas about 65–80% of telomeres contain TRF1/2. To the extent that the Telo-FISH procedure may alter protein antigenicity, these percentages may be underestimates. Nevertheless, it is likely that not all of the telomeres contain BRCA1. Alternatively, the number of BRCA1 molecules present at some telomeres may be too small to detect. Our findings also suggest that BRCA1 is not an integral component of the shelterin complex but that its presence at telomeres changes due to external conditions (see below).
BRCA1 was lost from the telomere after DNA damage due to ADR (which causes DNA strand breaks) or CDDP (which cross-links DNA). This loss of BRCA1 occurred without changes in total cellular BRCA1, TRF1, or TRF2 content or telomeric TRF1 content. Loss of telomeric BRCA1 occurred after 8 h of ADR or 6 h of CDDP treatment. Consistent with these findings, ADR also reduced the extent of colocalization of BRCA1 with TRF1/2. When cells were exposed to ADR for 8 h (to deplete telomeric BRCA1) and the ADR was washed out, BRCA1 reappeared at the telomere by 48 h later, suggesting that telomeric BRCA1 can decrease or increase in response to environmental changes. Here, loss of telomeric BRCA1 may be due to DNA damage-induced cell cycle arrest or another mechanism that requires hours to activate. The kinetics of ADR-induced BRCA1 loss from telomeres were similar to the kinetics for loss of telomeric Rad50, consistent with a model in which Rad50 is required to localize BRCA1 to telomeres.
A structural feature of telomeres, the 3′ G-rich single-stranded overhang, appears to be critical for T-loop formation and telomere stability (2, 4). We found that BRCA1 positively regulates 3′ overhang length based on two different assay systems. Consistent with an earlier report (30), we also found that Rad50 regulates overhang length. These results raise the possibility that regulation of overhang length by Rad50 is dependent upon BRCA1 present at telomeres. Consistent with this idea, overexpression of BRCA1 by itself increased overhang length but did not effectively rescue the reduction in overhang length due to Rad50 knockdown, and combined knockdown of Rad50 and BRCA1 did not reduce overhang length below that due to Rad50 siRNA alone. Our findings are consistent with previous reports that cells with no functional BRCA1 show chromosomal anomalies suggestive of telomere dysfunction (33–35). It was also reported that disruption of BRCA1 function (via a dominant negative mutant BRCA1) caused appearance of anaphase bridges and increased telomere length in a telomerase-positive cell line (36). This report is consistent with our finding that BRCA1 knockdown both increases telomere length and reduces 3′ G-strand overhang length, which contributes to telomere stability. Although we did not observe anaphase bridges in BRCA1 siRNA-treated cells,3 BRCA1 siRNA only reduced BRCA1 levels to 10–25% of control, which may not be enough to visualize genomic instability.
It was reported that BRCA1 is also present in the ALT-associated promyelocytic leukemia body of ALT-positive/telomerase-negative GM847 cells. Here, BRCA1 colocalized with TRF1 and other ALT-associated promyelocytic leukemia body proteins, particularly during the late S-G2 phase of the cell cycle (37). The occurrence of other proteins associated with double-stranded break repair and homologous recombination in the ALT-associated promyelocytic leukemia body (MRN complex and Rad51/Rad52) suggests a role for BRCA1 in telomere maintenance via recombination in ALT cells. BRCA1 also colocalizes with the MRN complex in radiation-induced nuclear foci (27), and this complex may facilitate the process of homology-directed DNA repair (38). Although all cell types in our study are telomerase-positive and ALT-negative, these findings are of interest because ALT-positive/telomerase-negative cells also contain 3′G-strand overhangs, and our studies indicate that BRCA1 regulates telomeres both by telomerase-dependent and telomerase-independent mechanisms.
In summary, our findings suggest that BRCA1 may regulate telomeres by multiple mechanisms, including regulation of telomerase activity and additional telomerase-independent mechanisms related to 3′ G-strand overhang length and possibly also to telomere degradation. Aside from its inactivation by mutation in the relatively small subset of hereditary breast cancers, BRCA1 expression is often absent or significantly decreased in the larger group of sporadic breast cancers (39, 40). It would be interesting to know if these “BRCA1-deficient” cancers, which have not been studied in great detail, show telomere lengthening and whether a telomeric function of BRCA1 contributes to their pathogenesis.
Acknowledgments
We are grateful to A. K. Meeker and C. Heaphy for help with the Teli-FISH assays.
This work was supported, in whole or in part, by National Institutes of Health United States Public Health Service Grants RO1-CA80000, RO1-CA82599, and RO1-CA104546 (to E. M. R.). This work was also supported by a predoctoral award from the American Federation for Aging Research (to R. D. B.) and the Cosmos Scholars Foundation Henry H. Work Science Award (to R. D. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–15.
R. D. Ballal, T. Saha, S. Fan, B. R. Haddad, and E. M. Rosen, unpublished results.
- hTERT
- human telomerase reverse transcriptase
- ADR
- adriamycin
- ALT
- alternative lengthening of telomeres
- CDDP
- cis-diaminodichloroplatinum (cis-platinum)
- ChIP
- chromatin immunoprecipitation
- FISH
- fluorescence in situ hybridization
- hTR
- human telomerase RNA component
- IP
- immunoprecipitation
- siRNA
- small interfering RNA
- RT
- reverse transcription
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PBS
- phosphate-buffered saline
- PIPES
- 1,4-piperazinediethanesulfonic acid
- DAPI
- 4′,6-diamidino-2-phenylindole
- PNA
- peptide nucleic acid
- AE
- acridinium ester
- Exo I
- exonuclease I
- DSN
- double-stranded nuclease
- TRF
- telomere restriction fragment.
REFERENCES
- 1.Morin G. B. (1989) Cell 59, 521–529 [DOI] [PubMed] [Google Scholar]
- 2.Blackburn E. H. (1997) Biochemistry 62, 1196–1201 [PubMed] [Google Scholar]
- 3.McEachern M. J., Krauskopf A., Blackburn E. H. (2000) Annu. Rev. Genet. 34, 331–358 [DOI] [PubMed] [Google Scholar]
- 4.Bodnar A. G., Ouellette M., Frolkis M., Holt S. E., Chiu C. P., Morin G. B., Harley C. B., Shay J. W., Lichtsteiner S., Wright W. E. (1998) Science 279, 349–352 [DOI] [PubMed] [Google Scholar]
- 5.Greider C. W. (1999) Cell 97, 419–422 [DOI] [PubMed] [Google Scholar]
- 6.de Lange T. (2005) Genes Dev. 19, 2100–2110 [DOI] [PubMed] [Google Scholar]
- 7.Counter C. M., Meyerson M., Eaton E. N., Weinberg R. A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 9202–9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura T. M., Morin G. B., Chapman K. B., Weinrich S. L., Andrews W. H., Lingner J., Harley C. B., Cech T. R. (1997) Science 277, 955–959 [DOI] [PubMed] [Google Scholar]
- 9.Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. (1995) Science 269, 1236–1241 [DOI] [PubMed] [Google Scholar]
- 10.Hahn W. C., Stewart S. A., Brooks M. W., York S. G., Eaton E., Kurachi A., Beijersbergen R. L., Knoll J. H., Meyerson M., Weinberg R. A. (1999) Nat. Med. 5, 1164–1170 [DOI] [PubMed] [Google Scholar]
- 11.Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. (1994) Science 266, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 12.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. (1994) Science 266, 66–71 [DOI] [PubMed] [Google Scholar]
- 13.Monteiro A. N., August A., Hanafusa H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 13595–13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen E. M., Fan S., Pestell R. G., Goldberg I. D. (2003) J. Cell. Physiol. 196, 19–41 [DOI] [PubMed] [Google Scholar]
- 15.Rosen E. M., Fan S., Ma Y. (2006) Cancer. Lett. 236, 175–185 [DOI] [PubMed] [Google Scholar]
- 16.Xiong J., Fan S., Meng Q., Schramm L., Wang C., Bouzahza B., Zhou J., Zafonte B., Goldberg I. D., Haddad B. R., Pestell R. G., Rosen E. M. (2003) Mol. Cell. Biol. 23, 8668–8690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan S., Wang J. A., Yuan R. Q., Ma Y. X., Meng Q., Erdos M. R., Brody L. C., Goldberg I. D., Rosen E. M. (1998) Oncogene 16, 3069–3082 [DOI] [PubMed] [Google Scholar]
- 18.Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. (1988) Cancer. Res. 48, 589–601 [PubMed] [Google Scholar]
- 19.Loayza D., De Lange T. (2003) Nature 423, 1013–1018 [DOI] [PubMed] [Google Scholar]
- 20.Weisberg T. F., Cahill B. K., Vary C. P. (1996) Mol. Cell. Probes 10, 139–146 [DOI] [PubMed] [Google Scholar]
- 21.Ma Y., Katiyar P., Jones L. P., Fan S., Zhang Y., Furth P. A., Rosen E. M. (2006) Mol. Endocrinol. 20, 14–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L., Carter D. B., Xu J., Yang X., Prather R. S., Tian X. C. (2004) Biol. Reprod. 70, 1589–1593 [DOI] [PubMed] [Google Scholar]
- 23.Igbavboa U., Pidcock J. M., Johnson L. N., Malo T. M., Studniski A. E., Yu S., Sun G. Y., Wood W. G. (2003) J. Biol. Chem. 278, 17150–17157 [DOI] [PubMed] [Google Scholar]
- 24.Meeker A. K., Gage W. R., Hicks J. L., Simon I., Coffman J. R., Platz E. A., March G. E., De Marzo A. M. (2002) Am. J. Pathol. 160, 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahara H., Kusunoki M., Yamanaka Y., Matsumura S., Ide T. (2005) Nat. Methods 2, 829–831 [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Hoshiyama H., Shay J. W., Wright W. E. (2008) Nucleic Acids Res. 36, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Q., Chen C. F., Li S., Chen Y., Wang C. C., Xiao J., Chen P. L., Sharp Z. D., Lee W. H. (1999) Science 285, 747–750 [DOI] [PubMed] [Google Scholar]
- 28.Zhu X. D., Küster B., Mann M., Petrini J. H., de Lange T. (2000) Nat. Genet. 25, 347–352 [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Xiao S., Zhu X. D. (2007) Nat. Struct. Mol. Biol. 14, 832–840 [DOI] [PubMed] [Google Scholar]
- 30.Chai W., Sfeir A. J., Hoshiyama H., Shay J. W., Wright W. E. (2006) EMBO Rep. 7, 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan S., Yuan R., Ma Y. X., Xiong J., Meng Q., Erdos M., Zhao J. N., Goldberg I. D., Pestell R. G., Rosen E. M. (2001) Oncogene 20, 4827–4841 [DOI] [PubMed] [Google Scholar]
- 32.Bae I., Rih J. K., Kim H. J., Kang H. J., Haddad B., Kirilyuk A., Fan S., Avantaggiati M. L., Rosen E. M. (2005) Cell Cycle 4, 1641–1666 [DOI] [PubMed] [Google Scholar]
- 33.Al-Wahiby S., Slijepcevic P. (2005) Cytogenet. Genome Res. 109, 491–496 [DOI] [PubMed] [Google Scholar]
- 34.McPherson J. P., Hande M. P., Poonepalli A., Lemmers B., Zablocki E., Migon E., Shehabeldin A., Porras A., Karaskova J., Vukovic B., Squire J., Hakem R. (2006) Hum. Mol. Genet. 15, 831–838 [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Liu L., Montagna C., Ried T., Deng C. X. (2007) Cell Death Differ. 14, 924–931 [DOI] [PubMed] [Google Scholar]
- 36.French J. D., Dunn J., Smart C. E., Manning N., Brown M. A. (2006) Genes Chromosomes Cancer 45, 277–289 [DOI] [PubMed] [Google Scholar]
- 37.Wu G., Jiang X., Lee W. H., Chen P. L. (2003) Cancer Res. 63, 2589–2595 [PubMed] [Google Scholar]
- 38.Chen L., Nievera C. J., Lee A. Y., Wu X. (2008) J. Biol. Chem. 283, 7713–7720 [DOI] [PubMed] [Google Scholar]
- 39.Wilson C. A., Ramos L., Villaseñor M. R., Anders K. H., Press M. F., Clarke K., Karlan B., Chen J. J., Scully R., Livingston D., Zuch R. H., Kanter M. H., Cohen S., Calzone F. J., Slamon D. J. (1999) Nat. Genet. 21, 236–240 [DOI] [PubMed] [Google Scholar]
- 40.Staff S., Isola J., Tanner M. (2003) Cancer Res. 63, 4978–4983 [PubMed] [Google Scholar]