Abstract
Expression of the calcium channels CaV2.1 and CaV2.2 is markedly suppressed by co-expression with truncated constructs containing Domain I. This is the basis for the phenomenon of dominant negative suppression observed for many of the episodic ataxia type 2 mutations in CaV2.1 that predict truncated channels. The process of dominant negative suppression has been shown previously to stem from interaction between the full-length and truncated channels and to result in downstream consequences of the unfolded protein response and endoplasmic reticulum-associated protein degradation. We have now identified the specific domain that triggers this effect. For both CaV2.1 and CaV2.2, the minimum construct producing suppression was the cytoplasmic N terminus. Suppression was enhanced by tethering the N terminus to the membrane with a CAAX motif. The 11-amino acid motif (including Arg52 and Arg54) within the N terminus, which we have previously shown to be required for G protein modulation, is also essential for dominant negative suppression. Suppression is prevented by addition of an N-terminal tag (XFP) to the full-length and truncated constructs. We further show that suppression of CaV2.2 currents by the N terminus-CAAX construct is accompanied by a reduction in CaV2.2 protein level, and this is also prevented by mutation of Arg52 and Arg54 to Ala in the truncated construct. Taken together, our evidence indicates that both the extreme N terminus and the Arg52, Arg54 motif are involved in the processes underlying dominant negative suppression.
Keywords: Biophysics, Calcium/Channels, Channels/Calcium, Diseases, Diseases/Neurological, Neurochemistry, Neurobiology/Neuroscience
Introduction
Voltage-gated calcium (CaV)3 channels are required for a number of essential physiological processes; in particular, they are essential for many neuronal functions, including neurotransmitter release (for review, see Ref. 1). They are heteromeric complexes consisting of the pore-forming CaVα1 subunit together (except in the case of the CaV3 channels) with an accessory β and α2δ subunit. The CaVα1 subunit consists of four homologous domains (I–IV), each consisting of six transmembrane (TM) segments (see Fig. 1A). The domains are linked by intracellular loops and have intracellular N and C termini. Ten mammalian α1 subunit genes have been cloned and divided into three subfamilies CaV1–3 (2).
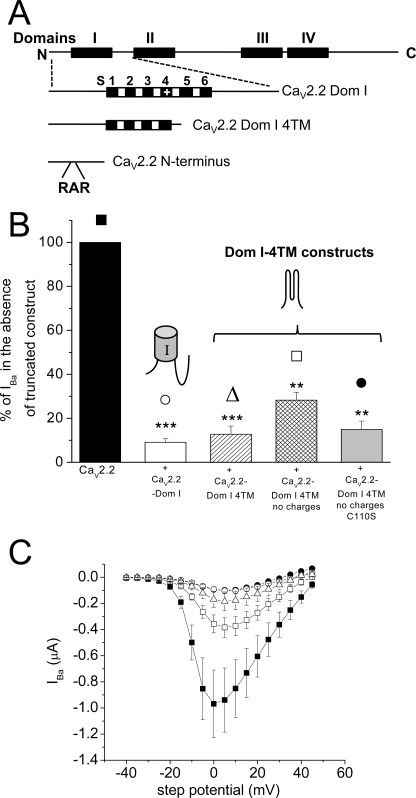
FIGURE 1.
Effect of CaV2.2-truncated domains on CaV2.2 IBa when expressed in Xenopus oocytes. A, diagram of the main CaV2.2 constructs used in this study, as described under “Experimental Procedures.” B, peak IBa for CaV2.2/α2δ-2/β1b expressed in Xenopus oocytes without any truncated domains (black bar, 100%) or with CaV2.2-Dom I (white bar, n = 12; ***, p < 0.001), CaV2.2-Dom I-4TMs (hatched bar, n = 17; ***, p < 0.001), CaV2.2-Dom I-4TMs no charges (cross-hatched bar, n = 14; **, p < 0.01), CaV2.2-Dom I-4TMs no charges C110S (light gray bar, n = 23; **, p < 0.01). Data are pooled from several experiments all recorded in 5 mm Ba2+ and normalized to the respective control in each experiment, and the statistical differences were determined compared with their respective control data, using one-way ANOVA and Bonferroni's post hoc test. Error bars indicate S.E. The symbols above the bars refer to the I-V relationship for the representative data in C. C, mean I-V relationship from two pooled experiments for CaV2.2/α2δ-2/β1b expressed in Xenopus oocytes without any truncated domains (■, n = 7) or with CaV2.2-Dom I (○, n = 8), CaV2.2-Dom I 4TMs (▵, n = 4), CaV2.2-Dom I-4TMs no charges (□, n = 11), CaV2.2-Dom I-4TMs no charges C110S (●, n = 7). The symbols are identified above the bars in B. All recordings are in 5 mm Ba2+.
Mutations of calcium channel α1 subunits can contribute to a number of pathological states (3). In particular, mutations in the CACAN1A gene encoding CaV2.1 result in familial hemiplegic migraine and episodic ataxia type 2 (4). Many of the episodic ataxia type 2 mutations in CaV2.1 predict truncated forms of this channel, although missense mutations are also found (4–7). This disease is dominant, and thus there is one wild-type (WT) allele and one mutant allele, both of which are likely to be expressed, although nonsense-mediated decay would reduce the expression of some mutant alleles (8). In many cases, the mutant channels, as well as either being nonfunctional or having reduced functionality, are dominant negative, in that they also suppress the function of the WT channel (9–11).
In our initial study on truncated CaVα1 subunits, we found that truncated constructs containing Domain I suppressed CaV2.2 currents and reduced the level of full-length CaV2.2 protein (12). We then showed that for both CaV2.2 and CaV2.1, this suppression required interaction between the full-length and the mutant construct (9). In this study, we also examined the effect of a two-domain construct predicted by an episodic ataxia type 2 mutation (9). We and others have also identified previously that the suppressive mechanism involves a reduction in protein synthesis resulting from the unfolded protein response (9) and an acceleration of proteasome-mediated decay (10).
Here, we have dissected the determinants required for suppression, which has increased our understanding of the mechanisms involved in the pathophysiology of episodic ataxia type 2. We find that the interaction between a truncated construct and a related full-length channel, identified previously (9), requires the presence of the N terminus on either or both of the full-length or the truncated channels. We also show that the N terminus of CaV2.2 or CaV2.1 alone is sufficient to suppress expression of the full-length channel. Suppression can be prevented by incorporation of a bulky tag on the N terminus or by removal of part of the N terminus. We further identify the motifs within the N terminus that are essential for suppression to occur and show that suppression can also be induced of endogenous channels in neurons.
EXPERIMENTAL PROCEDURES
Full-length, Mutant, and Truncated CaV Constructs
The following cDNAs were used: CaV2.2 (GenBank Accession number D14157), α2δ-1 (GenBank Accession number M86621), α2δ-2 (13), β1b (14), Cav2.1 (GenBank Accession number M64373), Kir2.1-AAA (15), and GFP-mut3b (16) in pMT2. The CaV2.2-Dom I and YFP-CaV2.2 constructs (12) and the Δ1–55 CaV2.2 truncation (17) have been described previously. Other constructs were made by standard techniques and verified by automated sequencing. They were: Cav2.2 N terminus (residues 1–95), Cav2.1 N terminus (residues 1–100), CaV2.2-Dom I-4TMs (residues 1–225 plus a C-terminal myc-His6 tag), CaV2.2-Dom I-4TMs no charges (residues 1–225, with all charged residues in S1, S2, S3 and S4 replaced by the noncharged residues: valine, leucine, or isoleucine), CaV2.2-DomI-4TMs no charges-C110S, CaV2.2 Δ2–91 with an N-terminal SS motif to create a construct starting MSSTEW, CaV2.2-Dom I Δ2–91 and CaV2.2-Dom I Δ1–55. When a CAAX motif was added, this was the C-terminal 10 amino acids of H-Ras (GCMSCKCVLS). It was added to the C terminus of GFP-mut3b, CaV2.2 N terminus (residues 1–95), CaV2.2 N terminus containing the R52A/R54A mutation, CaV2.2 N terminus (Δ2–42), CaV2.1 N terminus (residues 1–100), and CaV2.1 N terminus R57A/R59A. These were all subcloned into an in-frame XhoI site of the CAAX pMT2 vector, which then creates an additional arginine residue between the construct and CAAX motif.
Cell Culture and Heterologous Expression
COS-7 cells were cultured as described previously (18). The tsA-201 cells were cultured in a medium consisting of Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum, 1% Glutamax, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were transfected using FuGENE 6 (Roche Diagnostics). The cDNAs (all at 1 μg/μl) for CaVα1 subunits, truncated domain constructs, α2δ-1 or α2δ-2, β1b, and GFP, when used as a reporter of transfected cells, were mixed in a ratio of 3:1.5:2:1:0.2, unless stated otherwise. When particular subunits were not used, the volume was made up with water or blank vector, or the volume of transfection reagent was reduced, all with equivalent results.
Dorsal root ganglion (DRG) neurons isolated from Sprague-Dawley rats (175–250 g) in ice-cold Hanks' balanced salt solution (Invitrogen) were transferred to DMEM nutrient mixture F-12 (DMEM/F12) containing 0.4 mg/ml trypsin, 0.6 mg/ ml collagenase type 1 (both from Worthington Biochemical Corp.), and 100 units/ml DNase (Invitrogen), saturated with 95% O2/5% CO2, and incubated for 1 h at 37 °C. The neurons were washed in DMEM/F12 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% Glutamax (10% DMEM). Neurons were dissociated by vigorous shaking, centrifuged twice for 9 min at 800 × g, and the pellet was resuspended in 200 μl of Amaxa rat neuron nucleofector solution (Lonza Cologne AG, Cologne, Germany). Suspended neurons were mixed with cDNA for the truncated domain constructs (40 ng/μl) and YFP (20 ng/μl) DNA and transfected with nucleofector program O-003 following the manufacturer's instructions. The effect of the constructs was compared with DRG neurons expressing only YFP cDNA. The transfection reagent was neutralized with 500 μl of 10% DMEM supplemented with 50 ng/ml nerve growth factor. Neurons from each group were plated on poly-l-lysine (0.5 mg/ml)-coated 22-mm coverslips (BDH), placed in 35-mm polystyrene tissue culture dishes for 2 h to settle, flooded with 10% DMEM supplemented with 50 ng/ml nerve growth factor, and cultured for 3–4 days at 37 °C. Prior to experiments, the numerous neurite processes were eliminated by replating to improve voltage-clamp recording. Culture medium was removed, and cells were incubated for 5 min at 37 °C in 1 ml of 10% DMEM containing 0.2% type 1 collagenase. The enzymatic reaction was stopped with 1 ml of 10% DMEM, and the neurons were triturated and spun for 9 min at 800 × g. The pellet was resuspended in 300 μl of 10% DMEM, and the cells from each group were plated on poly-l-lysine-coated coverslips and left to recover for at least 2 h at 37 °C before recording.
Xenopus oocytes were prepared, injected, and utilized for electrophysiology as described previously (17), with the following exceptions. Plasmid cDNAs for the different calcium channel subunits α1, α2δ, β1b, and truncated or mutated domains and other constructs were mixed in 2:1:2:2 ratios at 1 μg/μl, unless stated otherwise, and 9 nl was injected intranuclearly, after 2-fold dilution of the cDNA mixes. When the truncated domain was not included it was replaced by an equivalent volume of empty vector, water, or a cDNA for a nonfunctional transmembrane protein, Kir-AAA (15) with equivalent results.
Electrophysiology
For tsA-201 cells, the patch pipette solution contained 140 mm cesium aspartate, 5 mm EGTA, 2 mm MgCl2, 0.1 mm CaCl2, 2 mm K2ATP, 10 mm Hepes, pH 7.2, 310 mOsm with sucrose. The external solution contained 150 mm tetraethylammonium bromide, 3 mm KCl, 1.0 mm NaHCO3, 1.0 mm MgCl2, 10 mm Hepes, 4 mm glucose, 1 mm BaCl2, pH 7.4, 320 mOsM with sucrose. For DRGs, the patch pipette solution contained 140 mm cesium aspartate, 10 mm EGTA, 2 mm MgCl2, 5 mm K2ATP, 10 mm Hepes, pH 7.2, 310 mOsm with sucrose. The external solution was identical to that described above, except 10 mm BaCl2 was used, and 1 μm tetrodotoxin was included in the medium to suppress voltage-gated Na+ currents. IBa was recorded using an Axopatch 1D amplifier (Axon Instruments, Molecular Devices, Sunnyvale CA), and data were filtered at 2 kHz and digitized at 10 kHz. Analysis was performed using pClamp9 (Axon) and Origin 7 (Microcal Origin, Northampton, MA). Current records are shown following leak and residual capacitance current subtraction (P/4 protocol). Incompletely subtracted capacitative transients have been truncated in traces shown. Recordings in Xenopus oocytes were performed as described (19), and all recordings were performed 48–60 h after injection for CaV2.2 and 72–80 h after injection for CaV2.1. The Ba2+ concentration was 10 mm, unless stated otherwise. When stated, current-voltage (I-V) plots were fit with a modified Boltzmann equation as described, for determination of the voltage for 50% activation (19).
Western Blotting and Calcium Channel Subunit Quantification
COS-7 cells were processed for SDS-PAGE as described (12). Samples (50 μg of cell lysate protein/lane) were separated using Novex 4–12% Tris-glycine or 4–12% BisTris NuPAGE gels (Invitrogen) and transferred electrophoretically to polyvinylidene fluoride membranes. The membranes were blocked with 3% bovine serum albumin and 0.02% Tween 20 and then incubated overnight at room temperature with the relevant primary antibody: 1:1000 dilution of anti-Cav2.2 (12). Detection was performed either with a 1:1000 dilution of goat anti-rabbit (or anti-mouse) IgG-horseradish peroxidase conjugate (Bio-Rad) and ECL Plus (Amersham Biosciences), or with a 1:1000 dilution of goat anti-rabbit IgG-Cy5 conjugate (Amersham Pharmacia Biotech), all in conjunction with a Typhoon 9410 Variable Mode Imager (Amersham Pharmacia Biotech), set in chemiluminescence or fluorescence mode, respectively. Protein bands were quantified using ImageQuant 5.2. The same amount of total protein was loaded for all samples on a gel for accurate comparison between lanes.
RESULTS
To extend our studies on the suppression of CaV2.x channels by truncated domains containing Domain I (Fig. 1A) (9, 12), in terms of the specific truncated domain involved, we first mutated various structural motifs in Domain I and expressed the resultant constructs to narrow down the element(s) responsible for the suppression. We have used a number of different expression systems and methods to ensure that our results are able to generalize beyond a single system. Key experiments have been reproduced by more than one method.
Which Structural Elements within Domain I of CaV2 Channels Are Required for Suppression?
CaV2.2-Dom I alone produced ∼90% suppression of CaV2.2 currents in Xenopus oocytes (Fig. 1, B and C). CaV2.2-Dom I was previously found to be more effective than CaV2.2-Dom I-II to inhibit CaV2.2 currents (12). We then found that a construct consisting of the N terminus and the first four TM segments (S1–S4) of CaV2.2 (CaV2.2-Dom I-4TMs) was as effective as CaV2.2-Dom I, CaV2.2 IBa being reduced to 13% of control (Fig. 1, B and C). A conserved set of structural motifs in this region of CaV2.2-Dom I is the charged amino acids in TM segments S1–S4, which might mediate inappropriate interaction between the full-length and truncated channel. However, mutation of all charged amino acids in the TM segments S1, S2, S3, and S4 to hydrophobic residues, within the truncated CaV2.2-Dom I-4TMs construct, did not significantly affect the ability of this construct to suppress CaV2.2 currents (Fig. 1, B and C). A second potential source of interaction is the conserved cysteines (in S1 and S2) which might form disulfide bonds with the full-length WT channel. However, when the cysteine in S1 (Cys110) was also mutated to serine to form CaV2.2-Dom I-4TMs (no charges, C110S), suppression of IBa was again undiminished (Fig. 1, B and C).
Role of the N Terminus of CaV2.2 in Dominant Negative Suppression
We then surmised that the cytoplasmic N terminus might contain structural elements involved in suppression. To examine the role of the N terminus, we utilized truncated and full-length constructs of CaV2.2, in which either or both were engineered to contain N-terminal deletions. We have previously shown that Δ1–55 CaV2.2 produced functional channels (17).
We first utilized expression in tsA-201 cells and compared the ability of two N-terminally deleted, truncated constructs of CaV2.2-Dom I (Δ1–55 CaV2.2-Dom I and Δ2–91 CaV2.2-Dom I) for their ability to suppress expression of CaV2.2 and Δ1–55 CaV2.2 IBa (Fig. 2A). We found that the two N-terminally truncated Domain I constructs showed consistently less suppression of Δ1–55 CaV2.2 than of CaV2.2 itself (Fig. 2), and there was no suppression of Δ1–55 CaV2.2 by the construct with the longer N-terminal deletion, Δ2–91 CaV2.2-Dom I (Fig. 2).
FIGURE 2.
Effect of N-terminally truncated CaV2.2 domains on CaV2.2 IBa when expressed in tsA-201 cells. A, peak IBa was determined from I-V relationships in 1 mm Ba2+ following expression in tsA-201 cells. The currents in the presence of the stated truncated domain are expressed as a percentage of control currents in its absence for CaV2.2/α2δ-2/β1b (filled bars) or Δ1–55 CaV2.2/α2δ-2/β1b (open bars). Data were pooled from several experiments, each examining the effect of one truncated construct, and normalized to the respective control in each experiment. The statistical significances of the differences compared with control were determined by Student's t test; *, p < 0.05. The numbers of determinations are given above each bar. Error bars indicate S.E. B, representative current traces (from −30 to +15 mV at Δ5 mV, from a holding potential of −90 mV), for CaV2.2/α2δ-2/β1b (upper panel) and Δ1–55 CaV2.2/α2δ-2/β1b (lower panel) in the absence or presence of CaV2.2-Dom I, Δ1–55 CaV2.2-Dom I, or Δ2–91 CaV2.2-Dom I. The scale bars refer to all traces.
We then examined whether Δ2–91 CaV2.2 was functional and found that, unlike truncations up to residue 55, its expression did not result in any discernible calcium channel currents (Fig. 3A). We next examined whether this N-terminally truncated channel would suppress expression of the full-length WT CaV2.2 IBa and found substantial inhibition of CaV2.2 currents in Xenopus oocytes (Fig. 3A). The peak CaV2.2 IBa at +5 mV was reduced to 41.4 ± 10.1% of control (p = 0.016) in the additional presence of Δ2–91 CaV2.2. However, there was no effect on any other properties of the currents, including steady-state inactivation (Fig. 3B). A similar result was observed when the same constructs were expressed in tsA-201 cells (Fig. 3C). Taken together, these data suggest that at least one intact N terminus, on either the full-length or the truncated channel construct, is required for suppression; and in agreement with this hypothesis, we found no suppression of Δ1–55 CaV2.2 currents by the Δ2–91 CaV2.2 construct in the same experiment (Fig. 3C).
FIGURE 3.
Effect of Δ2–91 CaV2.2 and YFP-tagged CaV2.2 on CaV2.2 IBa. A, mean I-V relationship for CaV2.2 (■, n = 12) or Δ2–91 CaV2.2 (▵, n = 3) co-expressed with α2δ-2/β1b in Xenopus oocytes, either alone or together (○, n = 13). All recordings are in 10 mm Ba2+. The I-V curves are fit with a modified Boltzmann relationship, as described under “Experimental Procedures.” Inset, bar chart of peak IBa determined from these I-V relationships. The currents in the presence of Δ2–91 CaV2.2 (open bar, n = 13) are expressed as a percentage of control IBa in its absence (black bar), for CaV2.2/α2δ-2/β1b. B, lack of effect of co-expression of CaV2.2 with Δ2–91 CaV2.2 (○, n = 6) on the voltage-dependence of steady-state inactivation of CaV2.2/α2δ-2/β1b IBa (■, n = 8) from the same experiments as in A. Data are fit with a Boltzmann function, as described under “Experimental Procedures.” C, peak IBa was determined from I-V relationships in 1 mm Ba2+ following expression of constructs in tsA-201 cells. The currents in the presence of Δ2–91 CaV2.2 are expressed as a percentage of control IBa in its absence (black bar), for CaV2.2/α2δ-2/β1b (open bar, n = 20), or Δ1–55 CaV2.2/α2δ-2/β1b (gray bar, n = 16). Data were pooled from several experiments and normalized to the respective control in each experiment. The statistical significances of the differences compared with control were determined by Student's t test, p < 0.05. Error bars indicate S.E. D, lack of effect of YFP-CaV2.2-Dom I on YFP-CaV2.2 IBa in Xenopus oocytes. Peak currents at +5 mV are shown for YFP-CaV2.2/α2δ-1/β1b alone (black bar, n = 24) or plus YFP-CaV2.2-Dom I (open bar, n = 20). Data were obtained in three different experiments, all with similar results. No significant differences were observed between the conditions, p > 0.05, Student's t test.
Dominant Negative Suppression Requires a Free N Terminus
To examine further the role of the N terminus, we then investigated whether its effect could be prevented by addition of a bulky tag attached to the extreme N terminus of CaV2.2. We found that there was no significant inhibition of YFP-CaV2.2 currents by YFP-CaV2.2-Dom I (14.8 ± 11.4% inhibition, Fig. 3D). This result indicates that the presence of an intact free N terminus, unencumbered by the bulky YFP tag, is required for dominant negative suppression.
Construct Consisting of the N Terminus of CaV2.2 Suppresses CaV2.2 Channel Expression
We had previously shown that an N-terminal construct of CaV2.2 did not inhibit GFP-CaV2.2 currents (12). This result was confirmed in the present study, the peak IBa resulting from expression of GFP-CaV2.2 was nonsignificantly reduced in the presence of the N terminus of CaV2.2 (residues 1–95), by 8.9 ± 19.5% (n = 13; Fig. 4A).
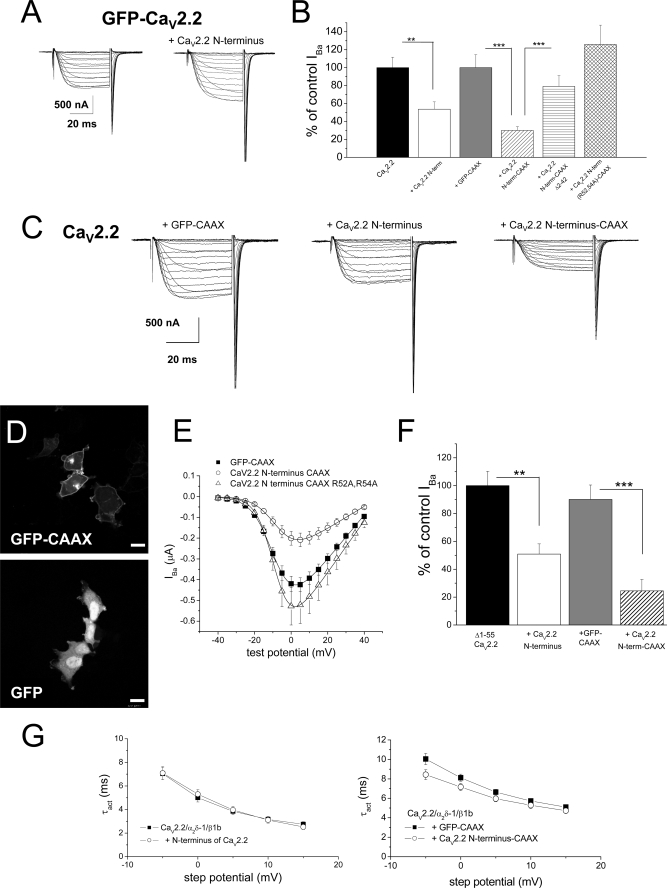
FIGURE 4.
Examination of the effect of the N terminus of CaV2.2 on functional expression of CaV2.2. A, example of current traces for voltage steps from −40 mV to +40 mV from a holding potential of −100 mV for GFP-CaV2.2/α2δ-1/β1b alone (left), and together with CaV2.2 N terminus (right). Recordings were made with 10 mm Ba2+ in Xenopus oocytes. B, peak IBa for CaV2.2/α2δ-1/β1b alone (black bar, n = 22) or together with CaV2.2 N terminus 1–95 (open bar, n = 36), GFP-CAAX (gray bar, n = 16), CaV2.2 N terminus 1–95-CAAX (hatched bar, n = 37), CaV2.2 N terminus Δ2–42-CAAX (horizontal striped bar, n = 25), and R52A/R54A CaV2.2 N terminus-CAAX (cross-hatched bar, n = 19). The statistical significances of the differences indicated were determined by one-way ANOVA and Bonferroni's post hoc test. **, p = 0.0016; ***, p < 0.001. Error bars indicate S.E. C, example of current traces for voltage steps from −40 mV to +40 mV for CaV2.2/α2δ-1/β1b with GFP-CAAX (left), with CaV2.2 N terminus (center), and with CaV2.2 N terminus-CAAX (right). Recordings were made with 10 mm Ba2+. D, representative images showing the distribution of GFP-CAAX (upper panel) and free GFP (lower panel) expression in tsA-201 cells. Scale bars, 20 μm. E, mean I-V relationship for CaV2.2/α2δ-1/β1b expressed in Xenopus oocytes, co-expressed with GFP-CAAX (■, n = 18), CaV2.2 N terminus-CAAX (○, n = 18), or R52A/R54A CaV2.2 N terminus-CAAX (▵, n = 19). All recordings were performed in parallel using 10 mm Ba2+. The I-V curves are fit with a modified Boltzmann relationship, as described under “Experimental Procedures.” The V50, act was −8.6 mV for GFP-CAAX, −7.1 mV for CaV2.2 N terminus-CAAX, and −8.4 mV for R52A/R54A CaV2.2 N terminus-CAAX. F, peak IBa (at 0 mV) for Δ1–55 CaV2.2/α2δ-1/β1b alone (black bar, n = 34) or together with CaV2.2 N terminus (open bar, n = 36), GFP-CAAX (gray bar, n = 10), and CaV2.2 N terminus-CAAX (hatched bar, n = 9). The statistical significances of the differences indicated were determined by Student's two-tailed t test. **, p = 0.002; ***, p < 0.0001. Recordings were made with 10 mm Ba2+. G, voltage dependence of time constant of activation (τact): left, for CaV2.2/α2δ-1/β1b without (■, n = 12) or with (○, n = 7) the free CaV2.2 N terminus; and right, for CaV2.2/α2δ-1/β1b with GFP-CAAX (■, n = 10) or with the free CaV2.2 N terminus-CAAX (○, n = 13).
In the light of the results described above, we then examined whether a construct consisting of the CaV2.2 cytoplasmic N terminus alone would be capable of inhibiting WT CaV2.2 IBa. We found a 40% reduction in WT CaV2.2 currents when the CaV2.2 N terminus was co-expressed (Fig. 4, B and C).
To examine how the CaV2.2 N terminus was inhibiting CaV2.2 currents, we attached an extended CAAX motif to its C terminus, consisting of the last 10 amino acids of H-Ras. This would promote both prenylation and palmitoylation of the polypeptide and thus enhance the concentration associated with both plasma and internal membranes (20). To confirm the localization of constructs to which a CAAX motif was attached, we examined the distribution of GFP-CAAX, which was found to be associated both at the plasma membrane and also with cytoplasmic organelles (Fig. 4D), in contrast to free GFP, which was observed uniformly throughout the cytoplasm and also in the nucleus (Fig. 4D). The membrane-tethered CaV2.2 N terminus-CAAX produced a very strong inhibition of CaV2.2 currents, by 70% at 0 mV, whereas a control prenylated protein (GFP-CAAX) produced no significant inhibition (Fig. 4, B, C, and E).
We then examined whether truncation of the CaV2.2 N terminus would prevent this inhibition and found that no significant inhibition was produced by CaV2.2 N terminus (Δ2–42)-CAAX (Fig. 4B), indicating that the extreme N terminus is involved in the process of suppression of CaV2.2 currents. We previously identified an 11-amino acid motif (residues 45–55) in the N terminus of CaV2.x channels, YKQSxAQRART, which was essential for G protein-mediated inhibition of these channels (17, 21). Two key amino acids involved in this process were found to be the two arginine residues in this motif. We therefore examined whether the same motif was involved in suppression of CaV2.2 current expression by mutating these two amino acids to alanine in the CaV2.2 N terminus-CAAX construct. We found that these mutations prevented the effect of the N terminus on CaV2.2 currents, even producing a small increase compared with CaV2.2 alone (Fig. 4, B and E). Together with the previous result, this suggests that docking of the free N terminus via a motif including Arg52 and Arg54 is involved in its ability to suppress CaV2.2 currents but that the extreme N terminus of CaV2.2 must also play a role.
Further studies showed that co-expression of the CaV2.2 N-terminal construct also produced a significant inhibition (∼50%) of Δ1–55 CaV2.2 IBa (Fig. 4F), and co-expression of CaV2.2 N terminus-CAAX strongly inhibited (by 75%) Δ1–55 CaV2.2 IBa (Fig. 4F), indicating that the suppressive effect of the CaV2.2 N terminus does not require the presence of the same motif on the full-length channel. This provides evidence that the suppressive effect is probably not via dimerization of the N termini.
One potential explanation for the reduction of CaV2.2 IBa by the CaV2.2 N-terminal construct, given the involvement of the amino acids 45–55 (17, 21), was that there could be tonic modulation of the calcium channel currents (22). However, co-expression of the CaV2.2 N-terminal constructs was not associated with slowed activation of the CaV2.2 currents (Fig. 4G). This was therefore discarded as a possibility.
Inhibition of CaV2.1 by the N Terminus of CaV2.1
We next examined the effect of the N terminus of CaV2.1 on the expression of the P/Q-type channel CaV2.1. Co-expression of the CaV2.1 N terminus with CaV2.1/α2δ-2/β4 produced 37.9% inhibition of the peak IBa currents (Fig. 5, A and B). The relevance of this combination of channel subunits is that it is likely to be one of the main channel complexes in cerebellar Purkinje neurons (13), which mediates many of the effects of the mutations in episodic ataxia type 2. The effect of co-expression of the N terminus of CaV2.1 attached to a CAAX motif was a 43.8% reduction in peak IBa, and this was also reversed by the corresponding mutations in the key arginine residues, Arg57 and Arg59 (Fig. 5B).
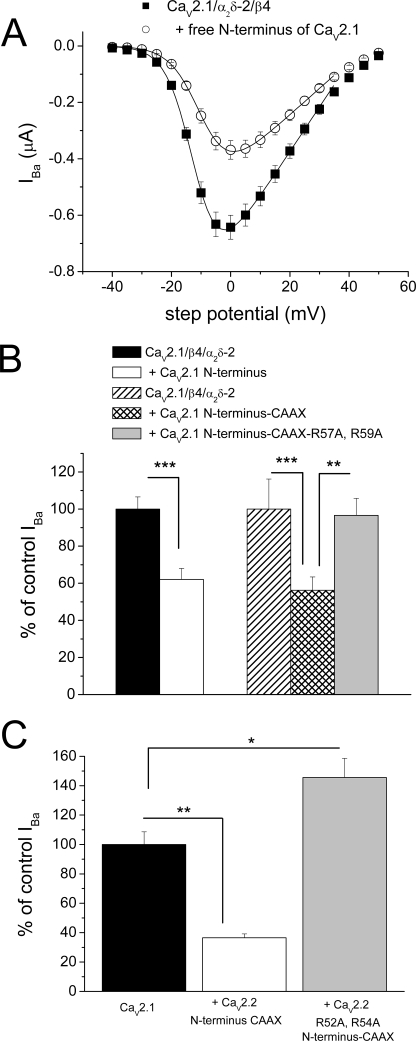
FIGURE 5.
Examination of the role of the N terminus of CaV2.1 on functional expression of CaV2.1. A, mean I-V relationship for CaV2.1/α2δ-2/β4 in Xenopus oocytes, either alone (■, n = 15) or co-expressed with CaV2.1 N terminus (○, n = 15). All recordings were performed in parallel, using 10 mm Ba2+. The I-V curves were fit with a modified Boltzmann relationship, up to +35 mV. The V50,act was −11.9 mV for control and −9.3 mV in the presence of the CaV2.1 N terminus. B, left panel, peak IBa (at 0 mV) for CaV2.1/α2δ-2/β4 alone (black bar, n = 28) or together with CaV2.1 N terminus (open bar, n = 26), from two independent experiments, including that depicted in A. Right panel, peak IBa for CaV2.1/α2δ-2/β4 alone (hatched bar, n = 9) or together with CaV2.1 N terminus-CAAX (cross-hatched bar, n = 20) or CaV2.1 N terminus R57A/R59A-CAAX (gray bar, n = 20). The statistical significances of the differences indicated were determined by Student's two-tailed t test. **, p = 0.0046; ***, p < 0.001. Error bars indicate S.E. C, peak IBa (at 0 mV) for CaV2.1/α2δ-2/β4 alone (black bar, n = 23) or together with CaV2.2 N terminus-CAAX (open bar, n = 22) or R52A/R54A CaV2.2 N terminus-CAAX (gray bar, n = 22). The statistical significances of the differences indicated were determined by ANOVA and Bonferroni's post hoc test. *, p < 0.01; **, p < 0.001.
Furthermore, the CAAX motif-linked N terminus of CaV2.2, which has a strong homology to CaV2.1, also inhibited CaV2.1 currents by 63.5%, whereas the mutant R52A/R54A CaV2.2 N terminus-CAAX construct produced no inhibition, rather resulting in an increase in the peak IBa compared with CaV2.1/α2δ-2/β4 alone (Fig. 5C).
Biochemical Basis for Suppression by the CaV2.2 N Terminus of CaV2.2 Channel Expression
We previously established that dominant negative suppression by truncated constructs involves a reduction of CaVα1 subunit protein expression (9, 12). In the present study, we found that co-expression of the CaV2.2 N terminus-CAAX with CaV2.2/β1b/α2δ-1 in tsA-201 cells resulted in a consistent decrease in the level of CaV2.2 protein (Fig. 6A). This was normalized to the total amount of protein in each sample and quantified as a 53% reduction, from six separate transfections (Fig. 6B). We also confirmed that expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was not altered by these manipulations (supplemental Fig. 1).
FIGURE 6.
Examination of the effect of the N terminus of CaV2.2 on CaV2.2 protein expression. A, expression of CaV2.2 (upper panel) and α2δ-1 (lower panel) protein in untransfected tsA-201 cells (first lane), when CaV2.2/α2δ-1/β1b were expressed, alone (second lane) and together with CaV2.2 N terminus-CAAX (third lane), or R52A/R54A CaV2.2 N terminus-CAAX (fourth lane). The same amount of total protein was loaded for all samples on a gel, for accurate comparison among lanes. B, bar chart from quantification of results, including those in A, showing the effect of CaV2.2 N terminus-CAAX (open bars, n = 6) or R52A/R54A CaV2.2 N terminus-CAAX (gray bars, n = 4) relative to control levels (black bars), for CaV2.2 (left) and α2δ-1 (right) protein levels. The statistical significance of the differences indicated were determined by Student's t test. *, p = 0.0162; **, p = 0.0041. Error bars indicate S.E.
In our previous study implicating the unfolded protein response in this process, we also examined the level of co-expressed α2δ protein and found it also to be reduced (9). The level of α2δ-1 was also reduced by co-expression of the CaV2.2 N terminus in the present study, by 51% (n = 6; Fig. 6, A and B). To confirm whether the RAR motif in the N terminus was involved in this response, we examined the effect of the R52A/R54A CaV2.2 N terminus-CAAX construct. In agreement with our electrophysiological results, the R52A/R54A CaV2.2 N terminus-CAAX construct produced no suppression of expression of CaV2.2 and α2δ-1 protein (Fig. 6, A and B).
The inclusion of GFP as a marker of transfection and cell survival showed that the effect of CaV2.2 N terminus-CAAX was not due to a reduction in transfected cell number at the time of harvesting the cells. The proportion of GFP-positive cells present 48 h after transfection was 4.6 ± 0.5%, 5.8 ± 0.5%, and 4.9 ± 0.5% in tsA-201 cells transfected with CaV2.2/β1b/α2δ-1/pMT2, CaV2.2/β1b/α2δ-1/CaV2.2 N terminus-CAAX, and CaV2.2/β1b/α2δ-1/R52A/R54A CaV2.2 N terminus-CAAX, respectively. These were not significantly different (n = 3, one-way ANOVA and Tukey's post hoc test).
Is There an Interaction between the N Terminus of CaV2.2 and Other Domains of CaV2.2?
We were unable to demonstrate any positive interactions between the N terminus of CaV2.2 and the CaV2.2 I-II loop, CaV2.2 Dom I or a number of other CaV2.2 sequences, using the yeast two-hybrid assay (supplemental data), in contrast to a previous study (22). Therefore we cannot identify a high affinity interaction of the CaV2.2 N terminus with a particular peptide domain of CaV2.2 using this system, indicating that it is perhaps more likely to interact in a complex binding pocket, made up of multiple elements.
Effect of Truncated Calcium Channels on Endogenous Calcium Channel Currents in DRG Neurons
We wished to examine whether the truncated constructs would also affect endogenous calcium channel currents, and we therefore expressed these constructs in DRG neurons using Amaxa transfection. We performed experiments 4 days after transfection, to allow synthesis of endogenous channels to occur, and in the presence of 10 μm nifedipine, to isolate native N-type calcium channel currents (Fig. 7,A–C). Expression of CaV2.2 N terminus-CAAX produced a statistically significant reduction in DRG IBa (Fig. 7B), whereas R52A/R54A CaV2.2 N terminus-CAAX produced no reduction in DRG IBa (Fig. 7D).
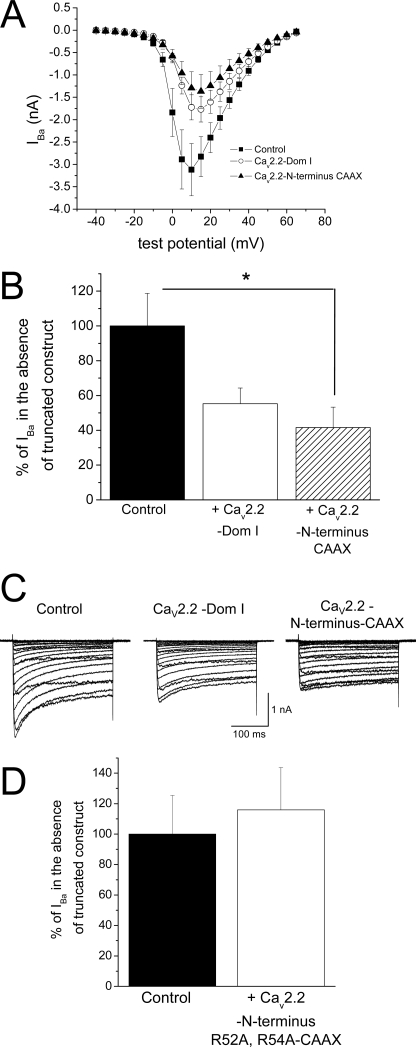
FIGURE 7.
Effect of truncated constructs containing CaV2.2 on expression of endogenous calcium channel currents in DRG neurons. A, I-V relationship recorded in the presence of 10 μm nifedipine for DRG neurons expressing YFP (control, ■, n = 14), CaV2.2 Dom I (○, n = 12), and CaV2.2 N terminus-CAAX) (▴, n = 8). All recordings were performed 4 days after transfection. The mean ± S.E. cell capacitances were 26.4 ± 3.8, 28.4 ± 6.0, and 27.3 ± 5.5 picofarads, respectively, for the three different conditions. B, peak IBa (recorded in the presence of 10 μm nifedipine, at +10 mV) for DRG neurons expressing CaV2.2 Dom I (open bar, n = 12) and CaV2.2 N terminus-CAAX) (hatched bar, n = 8), normalized as a percentage of control (black bar, n = 14). The statistical significances of the differences were determined by one-way ANOVA followed by post-hoc Dunnett's test. *, p < 0.05. Error bars indicate S.E. C, example of current traces for voltage steps between −40 mV and +65 mV for neurons expressing YFP only (control) or with CaV2.2 Dom I or CaV2.2 N terminus-CAAX (left to right). Recordings were made with 10 mm Ba2+ in the presence of 10 μm nifedipine. D, IBa (recorded in the presence of 10 μm nifedipine at +10 mV) for DRG neurons expressing R52A/R54A CaV2.2 N terminus-CAAX (white bar, n = 10), normalized as a percentage of control (black bar, n = 9).
DISCUSSION
Here, we have examined the process of dominant negative suppression of CaV2.1 and CaV2.2 currents by truncated CaVα1 constructs, which was identified by Raghib et al. (12). Our previous conclusion was that if CaV2.2 is co-expressed with truncated constructs of CaV2.2 containing Domain I, expression of the full-length CaV2.2 channel protein is almost completely prevented. We subsequently identified that there was a requirement for interaction between the full-length and truncated constructs (9), which has been confirmed and extended by others (10).
We previously observed cross-suppression between the different subclasses of CaV2 channels (CaV2.1, 2.2, 2.3), where conservation both within the TM segments and in the cytoplasmic N and C termini and loops is fairly high. However, there was no significant cross-suppression between full-length CaV3.1 and truncated constructs of CaV2.2, and vice versa (9). This suggests that the response is induced by the association of part of the truncated domain with segments of a cognate full-length channel with which it shows an affinity. Our present results identify specific motifs involved in the interaction.
We have found that one of the main regions involved in interaction is the N terminus of these channels. We observed that the optimum requirements for this to occur are that the N terminus should be both full-length and have a free N terminus (i.e. not tagged with XFP). When these conditions are met, in either or both of the truncated and the full-length CaV2.2 channels, suppression occurs, but when the extreme N terminus is missing from both constructs, or both are tagged with XFP, suppression is markedly reduced or absent.
Our finding that an N-terminal XFP tag hinders the dominant negative suppression process may also explain some anomalies in the literature, where such tagged constructs have been used in the study of this phenomenon (7, 23).
Amino acids 1–55 of CaV2.2 contain the N-terminal motif MVRFGDEL attached to a highly flexible region GGRYGGTGGGERARGGGAGGAGGPGQGGLPPG, representing amino acids 9–40 and identified as being glycine-rich (56%) and hence of low complexity. This region is followed by YKQSIAQRART, which is the 11-amino acid motif that we have previously identified to be essential for G protein modulation in CaV2.x channels. This motif is highly conserved in the CaV2 family (17, 21) and predicted to form an α-helix (PSIPRED 2.6). An additional motif residing within amino acids 56–95 (MALYNPIPVKQNCFTVNRSLFVFSEDNVVRKYAKRITEWPPFE) was also identified as being involved in the role of the N terminus of CaV2.2 to mediate G protein modulation by Gβγ (22). We have now found that this region is essential for functional expression of the channel.
A possible scenario is that the high mobility of the low complexity region in the N terminus will allow the N terminus to interact with a distant binding pocket on the channel and that the motif containing the key amino acids Arg52 and Arg54 is involved in this process. This intramolecular interaction might be required as a quality check point for correct folding during channel synthesis. RXR motifs have previously been identified to regulate endoplasmic reticulum retention and retrieval in other channels (24) and may be acting by a similar mechanism here.
In agreement with this hypothesis, our results show that the CaV2.x N terminus, particularly when attached C-terminally to a CAAX motif, results in strong suppression of CaV2.2 expression, both in terms of functional currents and at the level of CaV2.2 protein. This suppression by the N terminus is prevented when the N terminus is truncated and when the amino acids Arg52 and Arg54 are mutated to Ala. We hypothesize that once the N terminus, either as a free domain or attached to a truncated channel, has interacted intermolecularly with the full-length channel, the misfolded aggregate may both be directed to the proteasomal pathway as suggested by others (10, 25) and may also trigger the unfolded protein response to suppress further translation (9, 26).
The relevance to episodic ataxia type 2 is that many of the mutations found in this dominant disease result in premature protein truncation, but in all the mutations to date, the N terminus is intact, the first known mutation being in Domain I (6). A number of studies have shown previously that the truncated channels predicted by episodic ataxia type 2 mutations interact with the full-length channel (9–11, 23). Here, we have identified that an intact N terminus is essential for interaction between the truncated domain and the full-length channel. Future work to discover the site of interaction may now allow the development of therapeutic agents that hinder this process.
Supplementary Material
This work was supported by Wellcome Trust Grant 077883 and Medical Research Coucil Grant G0700368.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods,” “Results,” Table 1, Fig. 1, and additional references.
- CaV channel
- voltage-gated calcium channel
- ANOVA
- analysis of variance
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- DMEM
- Dulbecco's modified Eagle's medium
- Dom
- Domain
- DRG
- dorsal root ganglion
- GFP
- green fluorescent protein
- S1–S4
- segments S1 through S4
- TM
- transmembrane
- WT
- wild type
- YFP
- yellow fluorescent protein
- XFP
- any of the members of the GFP family.
REFERENCES
- 1.Catterall W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 2.Ertel E. A., Campbell K. P., Harpold M. M., Hofmann F., Mori Y., Perez-Reyes E., Schwartz A., Snutch T. P., Tanabe T., Birnbaumer L., Tsien R. W., Catterall W. A. (2000) Neuron 25, 533–535 [DOI] [PubMed] [Google Scholar]
- 3.Pietrobon D. (2002) Mol. Neurobiol. 25, 31–50 [DOI] [PubMed] [Google Scholar]
- 4.Ophoff R. A., Terwindt G. M., Vergouwe M. N., van Eijk R., Oefner P. J., Hoffman S. M., Lamerdin J. E., Mohrenweiser H. W., Bulman D. E., Ferrari M., Haan J., Lindhout D., van Ommen G. J., Hofker M. H., Ferrari M. D., Frants R. R. (1996) Cell 87, 543–552 [DOI] [PubMed] [Google Scholar]
- 5.Denier C., Ducros A., Vahedi K., Joutel A., Thierry P., Ritz A., Castelnovo G., Deonna T., Gérard P., Devoize J. L., Gayou A., Perrouty B., Soisson T., Autret A., Warter J. M., Vighetto A., Van Bogaert P., Alamowitch S., Roullet E., Tournier-Lasserve E. (1999) Neurology 52, 1816–1821 [DOI] [PubMed] [Google Scholar]
- 6.Jen J. C., Graves T. D., Hess E. J., Hanna M. G., Griggs R. C., Baloh R. W. (2007) Brain 130, 2484–2493 [DOI] [PubMed] [Google Scholar]
- 7.Wappl E., Koschak A., Poteser M., Sinnegger M. J., Walter D., Eberhart A., Groschner K., Glossmann H., Kraus R. L., Grabner M., Striessnig J. (2002) J. Biol. Chem. 277, 6960–6966 [DOI] [PubMed] [Google Scholar]
- 8.Maquat L. E. (2002) Curr. Biol. 12, R196–R197 [DOI] [PubMed] [Google Scholar]
- 9.Page K. M., Heblich F., Davies A., Butcher A. J., Leroy J., Bertaso F., Pratt W. S., Dolphin A. C. (2004) J. Neurosci. 24, 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezghrani A., Monteil A., Watschinger K., Sinnegger-Brauns M. J., Barrère C., Bourinet E., Nargeot J., Striessnig J., Lory P. (2008) J. Neurosci. 28, 4501–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeng C. J., Chen Y. T., Chen Y. W., Tang C. Y. (2006) Am. J. Physiol. Cell Physiol. 290, C1209–C1220 [DOI] [PubMed] [Google Scholar]
- 12.Raghib A., Bertaso F., Davies A., Page K. M., Meir A., Bogdanov Y., Dolphin A. C. (2001) J. Neurosci. 21, 8495–8504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barclay J., Balaguero N., Mione M., Ackerman S. L., Letts V. A., Brodbeck J., Cantí C., Meir A., Page K. M., Kusumi K., Perez-Reyes E., Lander E. S., Frankel W. N., Gardiner R. M., Dolphin A. C., Rees M. (2001) J. Neurosci. 21, 6095–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson W. J., Stea A., Bourinet E., Charnet P., Nargeot J., Snutch T. P. (1993) Neuropharmacology 32, 1117–1126 [DOI] [PubMed] [Google Scholar]
- 15.Tinker A., Jan Y. N., Jan L. Y. (1996) Cell 87, 857–868 [DOI] [PubMed] [Google Scholar]
- 16.Cormack B. P., Valdivia R. H., Falkow S. (1996) Gene 173, 33–38 [DOI] [PubMed] [Google Scholar]
- 17.Cantí C., Page K. M., Stephens G. J., Dolphin A. C. (1999) J. Neurosci. 19, 6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell V., Berrow N., Brickley K., Page K., Wade R., Dolphin A. C. (1995) FEBS Lett. 370, 135–140 [DOI] [PubMed] [Google Scholar]
- 19.Cantí C., Davies A., Berrow N. S., Butcher A. J., Page K. M., Dolphin A. C. (2001) Biophys. J. 81, 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choy E., Chiu V. K., Silletti J., Feoktistov M., Morimoto T., Michaelson D., Ivanov I. E., Philips M. R. (1999) Cell 98, 69–80 [DOI] [PubMed] [Google Scholar]
- 21.Page K. M., Cantí C., Stephens G. J., Berrow N. S., Dolphin A. C. (1998) J. Neurosci. 18, 4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agler H. L., Evans J., Tay L. H., Anderson M. J., Colecraft H. M., Yue D. T. (2005) Neuron 46, 891–904 [DOI] [PubMed] [Google Scholar]
- 23.Jeng C. J., Sun M. C., Chen Y. W., Tang C. Y. (2008) J. Cell. Physiol. 214, 422–433 [DOI] [PubMed] [Google Scholar]
- 24.Schwappach B., Zerangue N., Jan Y. N., Jan L. Y. (2000) Neuron 26, 155–167 [DOI] [PubMed] [Google Scholar]
- 25.Chevet E., Cameron P. H., Pelletier M. F., Thomas D. Y., Bergeron J. J. M. (2001) Curr. Opin. Struct. Biol. 11, 120–124 [DOI] [PubMed] [Google Scholar]
- 26.Ron D. (2002) J. Clin. Invest. 110, 1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.