Abstract
IRG are a family of IFN-regulated proteins that are critical for resistance to infection. Mouse IRG proteins are divided into GMS and GKS subfamilies, based on a sequence within the G1 GTP-binding motif. The GMS proteins have a particularly profound impact on immunity, as typified by Irgm1, of which absence leads to a complete loss of resistance to a variety of intracellular bacteria and protozoa. The underlying molecular and cellular mechanisms are not clear. Here, we use time-lapse microscopy and cell-tracking analysis to demonstrate that Irgm1 is required for motility of IFN-γ-activated macrophages. The absence of Irgm1 led to decreased actin remodeling at the leading edge of migrating macrophages, as well as decreased Rac activation. Although Irgm1 did not localize to the leading edge of migrating macrophages, it was found to regulate the localization of a GKS IRG protein, Irgb6, which in turn, concentrated on the plasma membrane in the advancing lamellipodia, in close apposition to molecular components that regulate membrane remodeling, including Rac, paxillin, and actin. Thus, Irgm1 likely controls macrophage motility by regulating the positioning of specific GKS IRG proteins to the plasma membrane, which in turn, modulate cytoskeletal remodeling and membrane dynamics.
Keywords: innate immunity, trafficking, migration, interferon, macrophage
Introduction
IRG proteins (also known as p47 GTPases) are a family of proteins found in vertebrate species from fish to humans [1,2,3,4]. The mouse contains 23 IRG genes, the expression for most of which is stimulated by IFNs. Several mouse IRG proteins are critical mediators of IFN-γ-stimulated immunity to intracellular bacterial and protozoan pathogens; for instance, mice lacking expression of Irgm1 and/or Irgm3 display almost complete losses of host resistance to Salmonella typhimurium, Mycobacteria, and Toxoplasma gondii [1, 5]. Studies of a human protein from this family, IRGM, indicate that it also plays a key role in immune responses, particularly in resistance to Mycobacteria [6], and in the pathogenesis of Crohn’s disease [7,8,9,10,11,12,13,14,15,16].
It remains unclear what the molecular and cellular functions of IRG proteins are that underlie their key roles in host resistance in vivo. Several general functions have been assigned to the proteins in cellular systems; the ability to regulate pathogen survival in IFN-activated host cells such as macrophages is prominent. This function is manifested differently depending on the pathogen: For T. gondii, IRG proteins regulate the breakdown of the parasitophorous vacuole that precedes pathogen elimination [17,18,19]; for Mycobacteria, they regulate lysosomal fusion and acidification of the phagosome, as well as its entry into an autophagic pathway [6, 20, 21]. However, other functions have been attributed to IRG proteins that are likely important for their contributions to IFN-γ-regulated host resistance; these include regulation of macrophage adhesion [22], inflammatory cytokine secretion [23], and T lymphocyte production [24, 25]. The degree to which each of these activities is a truly separable function and the degree to which each represents different manifestations of central molecular/cellular function(s) are not known.
The mouse IRG proteins are characterized as 47–48 kDa polypeptides that have functional GTPase domains and are able to form dimers/oligomers in a GTP-dependent manner [1, 2]. They also have varying affinities for lipid membranes, including the ER, the Golgi, the plasma membrane, and phagosomal membranes, a property that is likely key for some of their functions [26,27,28]. There is diversity in structure and function among the mouse IRG proteins, with their separation into Irga, Irgb, Irgc, Irgd, and Irgm subfamilies based on homology across the GTPase domain [4]. The Irgm proteins possess a noncanonical GMS sequence in the G1 GTP-binding sequence, and the remaining subfamilies possess the canonical GKS sequence. The Irgm/GMS proteins appear to play a much more dominant role in host resistance at the physiological level than do the other subfamilies [22, 29].
In an effort to explore the function of Irgm1 (originally known as LRG-47), our group has previously generated and characterized Irgm1-deficient mice [29], finding them to be markedly susceptible to multiple intracellular bacterial and protozoan pathogens [1, 5, 30]. Underscoring this loss of host resistance is impaired functioning of IFN-γ-activated macrophages [31, 32], including marked changes in morphology and adhesion to certain substrata. In the current work, we investigate further the regulation of macrophage function by Irgm1 and the underlying mechanism. We find that Irgm1 is critically required for normal motility of activated macrophages, impacting molecular events central to cellular movement. Importantly, the protein seems to function indirectly through the regulation of other IRG proteins. These results implicate the IRG proteins as key modulators of macrophage motility following IFN-γ activation, a function that may well be pivotal in controlling the innate response to infection in vivo.
MATERIALS AND METHODS
Mice and cell culture
Irgm1 (LRG-47)-deficient mice were generated as described previously [29]. Primary murine BMM were isolated from the tibia and femurs of 2- to 4-month-old mice. BM was flushed from the bones using a 27G needle fitted to a syringe filled with DMEM (Life Technologies, Gaithersburg, MD, USA); the marrow was dispersed by drawing through the needle three to four times; red cells were lysed with ACK lysing buffer (Life Technologies). Adherent cells were cultured for 6 days in BMM medium [DMEM supplemented with 10% (v/v) FBS (Hyclone, Logan, UT, USA)] and 30% (v/v) L929 cell-conditioned medium. The cells were cultured on Petri dishes that were not cell culture-treated, which resulted in cultures that were loosely adherent and easily removed from the plates with cell dissociation buffer (#13150-016, Gibco/Invitrogen, Carlsbad, CA, USA). Twenty-four hours prior to all experiments, the cells were placed in medium lacking L929-conditioned media [DMEM supplemented with 10% (v/v) FBS] on polylysine-coated coverslips. After 24 h under these conditions, the cells were cultured an additional 24 h in the presence of 100 U/ml IFN-γ (#407320, Calbiochem, EMD Biosciences, San Diego, CA, USA). In some cases, the cells were exposed to 30 ng/mL CSF-1 (M-CSF; #234376, Calbiochem, EMD Biosciences).
Live cell imaging
BMM were plated subconfluently onto 35 mm dishes containing a polylysine-coated glass coverslip (Matek, Ashland, MA, USA). These cells were activated with 100 U/ml IFN-γ for 24 h and then stimulated with CSF-1—at which point, time-lapse imaging was started. The images were collected on an Olympus IX70 microscope equipped with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI, USA) and a 37°C incubation chamber (Solent Scientific, Segensworth, UK). Single cell tracking was carried out using NIH Image J software and the Manual Tracking plugin.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (w/v) in PBS for 15 min and permeabilized with 0.2% (w/v) saponin in PBS for 10 min. As indicated in the text, the cells were then stained with phalloidin-conjugated AlexaFluor-594 to stain actin (Molecular Probes/Invitrogen, Carlsbad, CA, USA) or with various primary antibodies followed by AlexaFluor-conjugated secondary antibodies (Molecular Probes/Invitrogen). The primary antibodies included anti-Irga6 rabbit polyclonal antisera (gift of Jonathan Howard, University of Cologne, Köln, Germany); anti-Irgb6 rabbit polyclonal antisera [22], anti-Irgm1 monoclonal antisera [32], and anti-Irgm3 mouse monoclonal antisera (#610880, BD Transduction Labs, San Jose, CA, USA); anti-Rac1 mouse monoclonal antisera (#05-389, UpState/Millipore, Temecula, CA, USA); anti-vinculin mouse monoclonal antisera (#V4505, Sigma Chemical Co., St. Louis, MO, USA); and anti-paxillin mouse monoclonal antisera (#610051, BD Transduction Labs). Cell imaging was performed using an Olympus IX70 microscope and a SPOT RT camera. Wide-field z-stack fluorescence images were collected using Metamorph 6.2.3.5 and deconvolved using Auto Quant 9.3 software. The cells were originally magnified 1000×.
Analysis of membrane ruffling
Cells cultured as described above were maintained under control conditions or were exposed to IFN-γ for 24 h, followed by stimulation with CSF-1 for an additional 5 min. The cells were then fixed and stained with phalloidin-AlexaFuor-594 and imaged at the ventral/basal and dorsal surfaces. The amount of actin membrane ruffling was quantified in a blinded manner on a 4-point scale with 1 representing no ruffling, 2 representing little to no ruffling on the ventral surface and small amounts on the dorsal surface, 3 representing moderate ruffling on portions of the ventral and dorsal surfaces, and 4 representing elaborate ruffling along the lamellipodia at the ventral surface and at the dorsal surface.
Rac activation assay
BMM were maintained under control conditions or were exposed to IFN-γ for 24 h and were stimulated with CSF-1 for an additional 5 min. They were then lysed and used for quantification of active Rac using an ELISA method (#BK125, Cytoskeleton, Denver, CO, USA), according to the direction of the manufacturer. (Rac1, 2, and 3 are detected using this kit.)
Statistical analysis
Student’s paired t-test was used to assess statistical significance.
RESULTS
Altered motility in Irgm1 KO macrophages
Previously, we have shown that IFN-γ-activated macrophages that lack Irgm1 expression (Irgm1 KO macrophages) exhibited an adherence defect, which varied with the particular substrate and was accompanied by a marked change in cellular morphology [31]. Additionally, in the same studies, we found that Irgm1 KO macrophages exhibited a reduced capacity to transit through a transwell apparatus and bind to the underlying membrane. Although the transwell phenotype may reflect a change in motility in Irgm1 KO macrophages, this technique is not a direct assessment of cellular motility, and the observed response of Irgm1 KO macrophages in this assay could also be explained by the demonstrated change in adhesive properties of the cells. We, therefore, began the current studies with a direct analysis of the motility of Irgm1 KO macrophages.
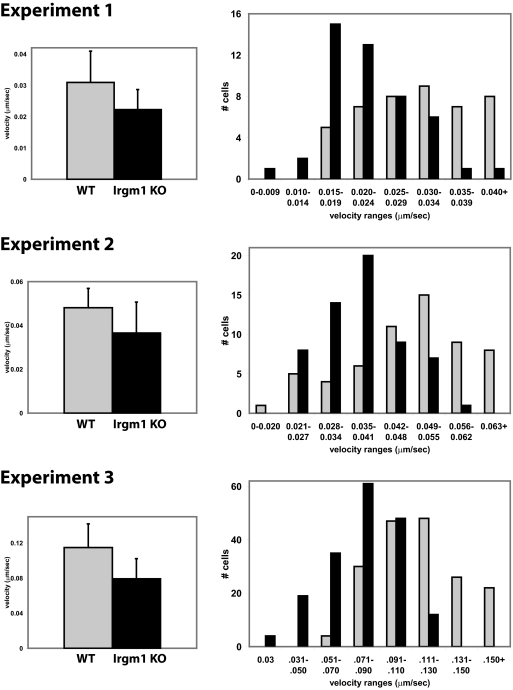
The motility of WT and Irgm1 KO BMM was compared by time-lapse video microscopy and cell-tracking analysis. The cells were activated by exposure to IFN-γ for 24 h, which markedly increases the expression of Irgm1 and most other IRG proteins [1, 2, 33]. To stimulate their motility, the cells were also exposed to CSF-1 for 5 min, a well-characterized activator of macrophage motility pathways [34]. Although both populations of cells displayed variability in morphology and rates of movement, Irgm1 KO BMM demonstrated less CSF-1-stimulated movement and less membrane ruffling than did WT BMM, which was easily observable in the videos (see Supplemental Movies 1 and 2). Tracking of individual cells showed that Irgm1 KO macrophages exhibited, on average, slower random migration than did WT cells (Fig. 1). When the velocities of individual cells , were stratified in histograms (Fig. 1), it was apparent that although some Irgm1 KO cells were able to obtain the same velocities as those of the most rapidly moving WT cells, many fewer Irgm1 KO cells were in the higher velocity ranges. The data were analyzed further to determine whether there was any change in persistence in the Irgm1 KO BMM, i.e., the ability to maintain directional motility. In this analysis, persistence was defined as the net distance moved from the origin divided by the total distance moved. The Irgm1 KO BMM displayed a slightly higher average persistence (0.416±0.271) than did the WT BMM (0.331±0.271); however, the difference was not statistically significant (P=0.13). Based on these data, it was concluded that Irgm1 is required for IFN-γ-activated macrophages to achieve normal motility rates, and the protein is not required to maintain persistence once the cells are in motion.
Figure 1.
Reduced motility of macrophages lacking Irgm1. WT and Irgm1 KO BMM were plated onto polylysine-coated glass coverslips, activated with 100 U/mL IFN-γ for 24 h, and exposed to CSF-1 to stimulate motility. The cells were then used for time-lapse video microscopy isolating images every 5 min. A 150-min time period was used for cell-tracking analysis, as detailed in the text. Shown are the average velocities, with error bars indicating sd, and a histogram stratifying the variation in velocities for each of three separate experiments. WT cells are represented by gray bars and Irgm1 KO cells by black bars. The differences in average velocities were statistically significant in each study, with P values <0.000005. In Experiment 1, 47 WT and 44 Irgm1 KO cells were tracked; in Experiment 2, 59 WT and 59 Irgm1 KO cells; and in Experiment 3, 177 WT and 179 Irgm1 KO.
Altered actin dynamics in Irgm1 KO macrophages
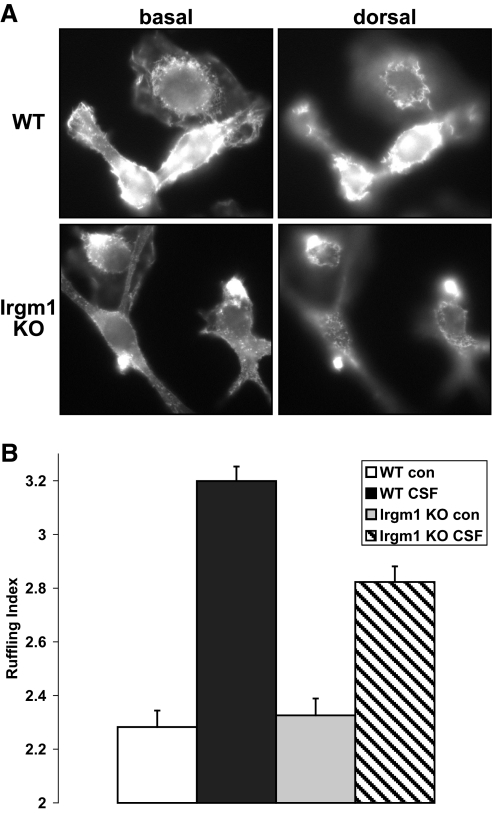
Movement of cells is driven by remodeling of the actin cytoskeleton. CSF-1R activation is known to stimulate a signaling cascade that leads to Rac activation, recruitment of WASP and WASP family verprolin-homologous protein, and modulation of actin polymerization through the Arp2/3 complex [34]. Accompanying CSF-1-stimulated motility is a marked increase in membrane ruffling [35], which can serve as a marker of actin remodeling. In the current studies, membrane ruffling was assessed at the ventral and dorsal surface of cells following CSF-1 stimulation. There was markedly less CSF-1-induced ruffling in the Irgm1 KO BMM (Fig. 2, A and B), indicating that less actin remodeling was occurring in those cells. The decreased actin remodeling was also reflected by a decrease in F-actin staining, which was noted consistently in phalloidin-stained Irgm1 KO BMM (e.g., see Fig. 7). Thus, the absence of Irgm1 in macrophages leads to a decrease in the ability of the cells to respond to CSF-1 and induce actin remodeling.
Figure 2.
Decreased membrane ruffling in macrophages lacking Irgm1. IFN-γ-activated WT and Irgm1 KO BMM were exposed to CSF-1 or control conditions for 5 min and then used for staining with AlexaFluor-594-phalloidin to stain F-actin. The cells were analyzed in a blinded manner to score their degree of membrane ruffling using a 4-point index, with 4 representing the highest degree of ruffling. (A) Representative images of CSF-1-stimulated WT and Irgm1 KO BMM. The cells were originally magnified ×1000. Note the higher degree of ruffling at the ventral and dorsal surfaces of the WT cells. (B) The average ruffling indices for WT and Irgm1 KO BMM, with and without [control (con)] activation by CSF-1. The average indices were derived from three separate experiments with at least 60 cells analyzed per group in each experiment. The error bars represent sd based on the average results from the three experiments. Note that the difference between WT/CSF-1 and Irgm1 KO/CSF-1 was statistically significant (P=0.037).
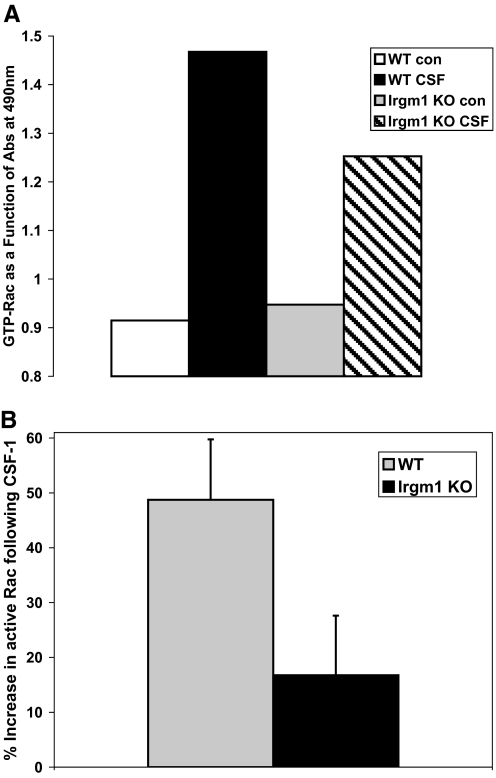
Figure 3.
Decreased CSF-1-induced Rac activation in Irgm1 KO macrophages. BMM were maintained under control conditions or were exposed to IFN-γ for 24 h and then stimulated with CSF-1 for an additional 5 min. The cells were lysed and used for quantification of active Rac using an ELISA method described in the text that specifically measures GTP-bound Rac. Shown are (A) a representative study and (B) the CSF-1-stimulated increase in Rac activity averaged across four experiments. The error bars represent sd. Note that there is nearly equal basal Rac in activated WT and Irgm1 KO BMM under control conditions, and the CSF-1-stimulated increase in active Rac is reduced substantially in Irgm1 KO cells (P=0.0014). Abs, Absorbance.
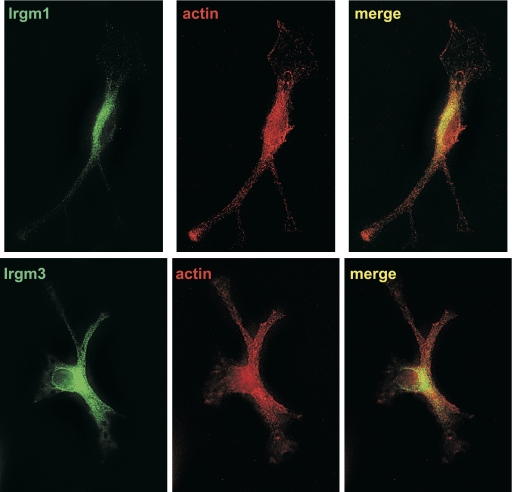
Figure 4.
Lack of Irgm1 localization at the leading edge of motile macrophages. WT BMM were activated with IFN-γ for 24 h and then exposed to CSF-1 for 5 min to stimulate motility. The cells were then used for immunostaining with anti-Irgm1 antibodies, anti-Irgm3 antibodies, or AlexaFluor-594-phalloidin, as indicated. The cells were originally magnified ×1000. These images are representative of those from three independent experiments. Note that the cells lack discernable Irgm1 or Irgm3 staining along the plasma membrane in the advancing lamellipodia.
Figure 5.
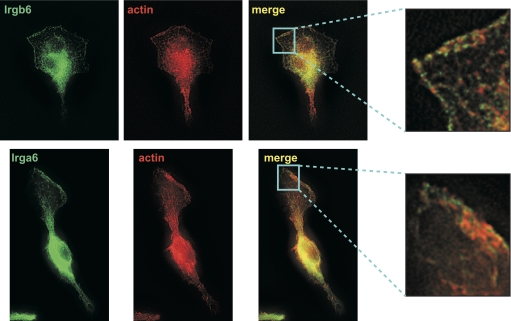
Irgb6 and Irga6 localization at the leading edge of motile macrophages. WT BMM were activated with IFN-γ for 24 h and then exposed to CSF-1 for 5 min to stimulate motility. The cells were then used for immunostaining with anti-Irgb6 antibodies, anti-Irga6 antibodies, or AlexaFluor-594-phalloidin, as indicated. The cells were originally magnified ×1000. These images are representative of those from three independent experiments. Note that the cells display strong Irgb6 and Irga6 staining on the plasma membrane in the advancing lamellipodia, which is closely apposed to actin-stained areas.
Figure 6.
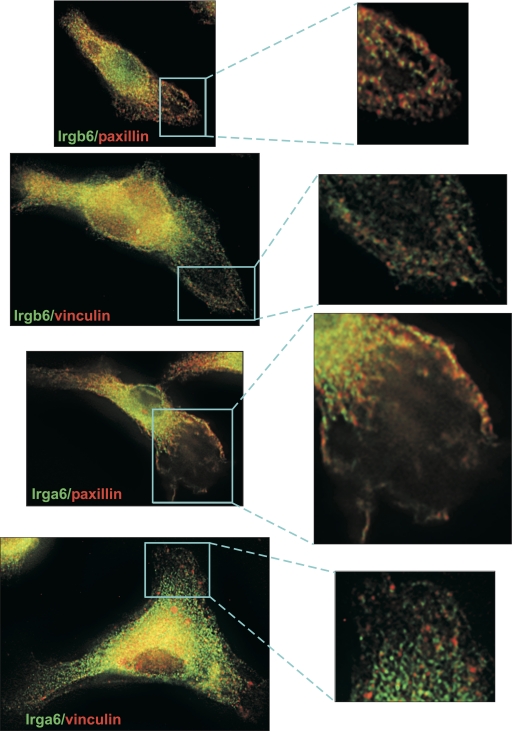
Irgb6 and Irga6 costaining with paxillin in motile macrophages. WT BMM were activated with IFN-γ for 24 h and then exposed to CSF-1 for 5 min to stimulate motility. The cells were then used for immunostaining with anti-Irgb6, Irga6, paxillin, and/or vinculin antibodies, as indicated. The cells were originally magnified ×1000. These images are representative of those from three independent experiments. Note that Irgb6- and Irga6-stained areas in the advancing lamellipodia are closely apposed or coincident with paxillin-stained areas.
Figure 7.
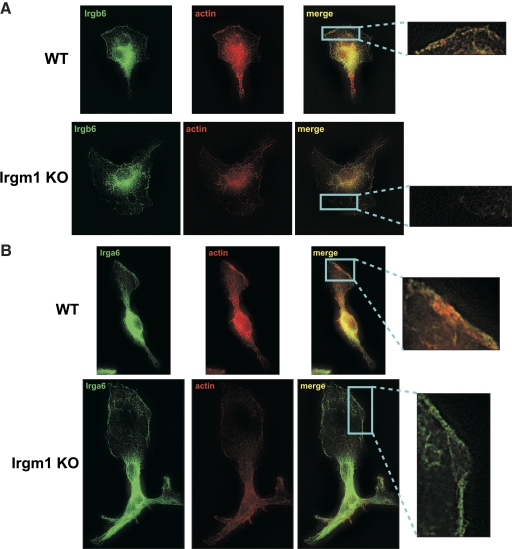
Loss of Irgb6, but not Irga6, at the leading edge of motile macrophages that lack Irgm1 expression. WT or Irgm1 KO BMM were activated with IFN-γ for 24 h and then exposed to CSF-1 for 5 min to stimulate motility. The cells were then used for immunostaining with (A) anti-Irgb6 antibodies, (B) anti-Irga6 antibodies, and/or AlexaFluor-594-phalloidin, as indicated. Exposure times were equal for all cells. The cells were originally magnified ×1000. These images are representative of those from three independent experiments. Note that in Irgm1 KO cells, there is a decrease in the intensity of actin staining and a substantial loss of Irgb6 staining on the plasma membrane in the advancing lamellipodia. In contrast, strong Irga6 staining is maintained in Irgm1 KO cells.
Decreased Rac activation in Irgm1 KO macrophages
Rac is a small GTP-binding protein that assumes an active state when GTP-bound and functions as a key player in the CSF-1 signaling cascade that leads to actin polymerization. Rac activity is required for lamellipodial extension, membrane ruffling, and motility in macrophages [36, 37]. Rac also regulates the persistence of cell movement, with decreased Rac activity leading to an increase in the persistence [38]. Thus, the motility phenotype seen in cells with reduced active Rac (decreased motility and increased persistence) was similar to that observed in macrophages lacking Irgm1. Consequently, we next examined the impact of Irgm1 deficiency on Rac activation in IFN-γ-activated macrophages.
Rac activity in IFN-γ-activated WT and Irgm1 KO BMM was assessed with an ELISA technique that recognizes Rac in the GTP-bound/active form (Fig. 3). In these assays, CSF-1 induction was used to trigger cell motility and a coincident increase in the amount of Rac-GTP that was detected. Although the amounts of basal Rac-GTP that were present in WT and Irgm1 KO BMM were not significantly different, the increase in Rac-GTP observed in WT cells in response to CSF-1 stimulation was decreased markedly in the Irgm1 KO cells and was reduced by approximately two-thirds. Thus, Irgm1 is required for CSF-1-induced Rac activation that leads to motility in activated macrophages.
Differential localization of IRG proteins to the leading edge of migrating macrophages
As mentioned above, IRG proteins have the propensity to concentrate on various lipid membranes within the cell, including the plasma membrane. The ultimate function of the IRG proteins is likely exerted in the context of these lipid membranes. For instance, Irga6 and Irgb6 (originally called IIGP1 and TGTP/Mg21, respectively) are known to concentrate on the parasitophorous vacuole membranes, following entry of T. gondii into host cells, an event that precedes breakdown of the vacuole, presumably as a consequence of the activity of these IRG proteins [18, 19, 39]. Therefore, in the context of cellular motility, we tested the prediction that Irgm1 could concentrate on the plasma membrane in the lamellipodium at the leading edge of the migrating macrophage, where it might interact with Rac, actin, and/or the other molecular players that drive cellular movement. We examined CSF-1-stimulated BMM for the presence of Irgm1 at these sites. Although Irgm1 was easily detected in the Golgi of the cells, as reported previously [27, 32], there was no apparent Irgm1 immunostaining on the membranes at the leading edge of the macrophages (Fig. 4). Another protein in the GMS IRG subfamily, Irgm3, was similarly found to be absent from the advancing lamellipodium and from the plasma membrane in general (Fig. 4). In contrast to these results, however, we found strong localization of two GKS IRG proteins—Irgb6 and Irga6—along the leading edge of migrating macrophages and in dorsal membrane ruffles (Fig. 5). Both proteins colocalized with actin in some regions along the membrane, but more often, the concentrations of the two IRG proteins were found immediately adjacent to, but not coincident with, strong concentrations of actin along the membrane. Other studies were performed to determine whether Irgb6 and/or Irga6 complexed directly with actin; these included standard actin/phalloidin precipitation assays to assess coprecipitation of the GKS proteins, as well as GST-Irgb6 pull-down assays. None of these studies demonstrated a direct, high-affinity interaction between actin and either GKS IRG protein (data not shown).
The presence of Irgb6 and Irga6 at the leading edge of migrating macrophages was characterized further by examining potential costaining with two other proteins that are involved in cell adhesion and motility: paxillin and vinculin. The results were similar for the two GKS IRG proteins; both were concentrated immediately adjacent to areas of strong paxillin staining with some overlap, and neither displayed appreciable colocalization with vinculin (Fig. 6). Collectively, these and the above data suggest that Irgb6 and Irga6 are located on the advancing lamellipodium in areas where they might engage the molecular machinery of the cell that influences actin remodeling and cell motility.
Effect of Irgm1 deficiency on Irgb6 and Irga6 localization
We and others [22, 40] have shown previously that in general, GMS proteins regulate the expression and localization of GKS proteins. More specifically, in the absence of GMS proteins, GKS proteins display lower expression levels and take on a punctate staining pattern. The absence of any one GMS protein produces a relatively mild effect on GKS protein expression and localization, and the absence of two or more GMS proteins leads to much more marked effects. The mechanistic basis for these phenomena is not clear, although it has been suggested that GMS proteins may complex with GKS proteins in a manner that controls their activity and ability to oligomerize [40].
In the current studies, we addressed whether the absence of Irgm1 in macrophages affected the localization of Irgb6 and/or Irga6 to the advancing lamellipodium of macrophages. We found, in fact, that the absence of Irgm1 had a major impact on Irgb6 localization to the extent that there was little detectable Irgb6 staining on the advancing lamellipodium in Irgm1-deficient cells (Fig. 7A), although Irgb6 staining did remain high in the ER of those cells. In contrast, Irga6 staining on the lamellipodial membranes remained strong despite the absence of Irgm1 (Fig. 7B). Thus, Irgm1 specifically affected the localization of Irgb6. When considered with the above results, these data suggest that Irgm1 may control cell motility indirectly by controlling the positioning of particular GKS IRG proteins, such as Irgb6, on the plasma membrane at the advancing edge of the migrating cell, where the GKS proteins would be in position to engage the cytoskeleton and signaling molecules and modulate actin remodeling.
Association of Irgb6 with Rac
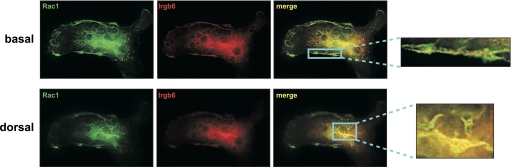
We next examined whether Irgb6 might engage Rac directly, which could lead to the Irgm1-dependent effects on Rac activity that were observed above (Fig. 3). This was first addressed with immunostaining experiments that showed strong and closely apposed Irgb6 and Rac staining on membranes at the advancing edge of WT macrophages, although there was no obvious overlap at the ventral surface (Fig. 8). When the dorsal surface of the cells was examined, there appeared to be substantial colocalization in membrane ruffles. In other studies, we similarly found closely apposed and coincident Irgb6-Rac staining on the surface of nascent latex bead phagosomes in WT BMM (Supplemental Fig. 1). The Irgb6-Rac interaction was also addressed with GST-Irgb6 pull-down assays and with coimmunoprecipitation studies (data not shown). By neither approach was any interaction detected between the two proteins. These data argue that any direct interactions between Irgb6 and Rac are weak and/or occur transiently, perhaps on membranes undergoing remodeling.
Figure 8.
Irgb6 and Rac localization in motile macrophages. WT BMM were activated with IFN-γ for 24 h and then exposed to CSF-1 for 5 min to stimulate motility. The cells were then used for immunostaining with anti-Irgb6 and Rac antibodies, as indicated. The cells were originally magnified ×1000. Note the closely apposed Irgb6 and Rac staining on the plasma membrane in the advancing lamellipodia, as well as coincident Irgb6 and Rac costaining in dorsal membrane ruffles.
DISCUSSION
Our data demonstrate that Irgm1 is required for normal cellular motility in IFN-γ-activated mouse macrophages (note model in Fig. 9). Absence of the protein leads to decreases in actin remodeling, as manifested by decreased overall actin staining in the cells and decreased CSF-1-stimulated membrane ruffling. Activation of the CSF-1R is known to trigger a signaling cascade that leads to Rac activation, followed by activation of its effector WASP, which in turn, interacts with the Arp2/3 complex to enhance actin polymerization [34, 41]. Our results indicate that Irgm1 deficiency leads to decreased Rac activation following CSF-1 stimulation; thus, the effect of Irgm1 on actin remodeling occurs by affecting Rac directly or by affecting an upstream signaling molecule. Although our current data do not distinguish definitively between these possibilities, close apposition between Irgb6 and Rac in imaging studies suggests a direct effect of IRG proteins on Rac. The fact that high-affinity Irgb6-Rac complexes were not identified in coimmunoprecipitation and GST pull-down assays suggests that any interaction between the two molecules is weak and/or transient, perhaps only occurring at the advancing lamellipodium during cell movement. Alternatively, Irgb6 and Rac may be present in a complex but may not interact directly. Regarding the signaling cascades upstream of Rac, a major effector of CSF-1 that leads to Rac activation is PI3K. A common approach to measure activation of class IA PI3K is the measurement of AKT phosphorylation, which has been shown previously to be up-regulated by CSF-1 stimulation in macrophages [42]. Along these lines, however, we found that Irgm1 deficiency did not affect AKT phosphorylation in primary macrophages (data not presented).
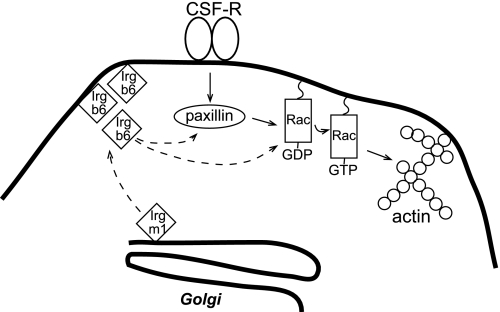
Figure 9.
Hypothetical model for Irgm1 regulation of macrophage motility. Binding of CSF-1 to its receptor is known to initiate a signaling cascade that includes activation of paxillin and Rac and ultimately, increases actin remodeling at the leading edge of motile macrophages. In the current work, Irgm1 has been found to control the localization of Irgb6 and likely other GKS IRG proteins to the plasma membrane at the leading edge of migrating IFN-γ-activated macrophages. On the membrane, Irgb6 is in position to interact with Rac, paxillin, and other molecular components of the actin machinery. The presence of Irgb6 at these sites leads to increased Rac activation, actin remodeling, and cellular movement.
A change in actin polymerization at the leading edge of the migrating macrophage would suggest that Irgm1 might be localized in the advancing lamellipodium, where it could interact with Rac and/or the other molecules that affect actin remodeling. It was therefore surprising that we did not find Irgm1 localized to this area of the cell; rather, we found GKS IRG proteins, Irgb6 and Irga6, to be concentrated at the membrane in these areas. Further, we found that in the absence of Irgm1, Irgb6 was decreased markedly in membranes at the leading edge of the macrophage. Thus, Irgm1 appears to act distally by controlling Irgb6 localization, with Irgb6 thereby being in a position where it may modulate actin remodeling and membrane dynamics. Irgb6 probably does not act as a lone IRG protein in modulating cell motility in this context, as there are thought to be ∼18 functional GKS IRG proteins in mouse cells [4], among which there is likely a substantial subset that is regulated by Irgm1 and that acts in concert to regulate motility. In support of this notion, we found that a small interfering RNA knock-down of Irgb6 alone in BMM did not affect motility (data not shown). It is important to point out that Irgm1 controls GKS IRG protein positioning and functioning in at least one other context. We have shown previously that although Irgm1 controls survival of T. gondii in macrophages, the protein does not localize to T. gondii vacuolar membrane [32]; in contrast, it has been shown by others that several GKS proteins do localize to the T. gondii vacuole when Irgm1 is expressed properly in the cells [27], and these GKS proteins affect parasite survival [17,18,19]. Thus, in general, Irgm1 appears to function distally by regulating localization of other IRG proteins to membranes, where they likely regulate membrane dynamics.
It is interesting that the effect of Irgm1 on GKS localization was selective; that is, there was an effect on Irgb6 but not Irga6. Although previous data have suggested that GMS proteins affect GKS protein localization in mouse cells [2, 40], they have not demonstrated any specificity in vivo. If GMS proteins do function by controlling GKS protein function, then GMS function almost certainly involves specificity, as mice lacking different GMS proteins (e.g., Irgm1 vs. Irgm3) have markedly different phenotypes with regard to host resistance [1]. The data presented here suggest that this might occur through differential regulation of particular GKS proteins that ostensibly have distinct functions in themselves.
We also found that Irga6 and Irgb6 were not localized evenly across the membranes at the advancing edge of the macrophage, but they were often found in discrete concentrations along the membranes. Some of these Irgb6/a6 concentrations localized with filamentous actin on membrane ruffles, but there were also many cases in which the GKS IRG proteins were found in punctate concentrations that were immediately adjacent to concentrations of actin (and paxillin). This pattern in which the GTPase appears to interdigitate among punctate actin areas (some of which likely demarcate podosomes) is reminiscent of the manner in which dynamin concentrates along membranes adjacent to podosomes [43]. The IRG proteins have been shown previously to have biochemical and cellular properties similar to the dynamins: Both groups are GTPases; they are able to multimerize in a GTP-dependent manner; they have a propensity to bind membranes; and they regulate mechanical modulation of the membranes [2, 44]. Dynamins are known to regulate cell motility through multiple mechanisms [44] that include direct effects on actin polymerization [45] as well as indirect effects on Rac activity [46] and other signaling molecules in the CSF-1 cascade that are upstream of Rac, such as Cbl [43]. The data presented here suggest that the IRG proteins have functions that overlap those of the classical dynamins in regulating cell motility.
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health grant (AI57831; G. A. T.) and a VA Merit Review grant (G. A. T.). We are grateful to Dorothy Schafer for technical advice and her review of this manuscript.
Supplementary Material
Footnotes
Abbreviations: Arp2/3=actin-related protein 2/3, BMM=bone marrow macrophage(s), ER=endoplasmic reticulum, IRG=immunity-related GTPase(s), Irgm1=immunity-related GTPase family M member 1, KO=knockout, WASP=Wiskott-Aldrich syndrome protein, WT=wild-type
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
References
- Taylor G A. IRG proteins: key mediators of interferon-regulated host resistance to intracellular pathogens. Cell Microbiol. 2007;9:1099–1107. doi: 10.1111/j.1462-5822.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Martens S, Howard J. The interferon-inducible GTPases. Annu Rev Cell Dev Biol. 2006;22:559–589. doi: 10.1146/annurev.cellbio.22.010305.104619. [DOI] [PubMed] [Google Scholar]
- Shenoy A R, Kim B H, Choi H P, Matsuzawa T, Tiwari S, MacMicking J D. Emerging themes in IFN-γ-induced macrophage immunity by the p47 and p65 GTPase families. Immunobiology. 2007;212:771–784. doi: 10.1016/j.imbio.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekpen C, Hunn J P, Rohde C, Parvanova I, Guethlein L, Dunn D M, Glowalla E, Leptin M, Howard J C. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G A, Feng C G, Sher A. Control of IFN-γ-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Singh S B, Davis A S, Taylor G A, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313:1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Parkes M, Barrett J C, Prescott N J, Tremelling M, Anderson C A, Fisher S A, Roberts R G, Nimmo E R, Cummings F R, Soars D, Drummond H, Lees C W, Khawaja S A, Bagnall R, Burke D A, Todhunter C E, Ahmad T, Onnie C M, McArdle W, Strachan D, Bethel G, Bryan C, Lewis C M, Deloukas P, Forbes A, Sanderson J, Jewell D P, Satsangi J, Mansfield J C, Cardon L, Mathew C G. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Takahashi A, Takazoe M, Kubo M, Onouchi Y, Fujino A, Kamatani N, Nakamura Y, Hata A. Positive association of genetic variants in the upstream region of NKX2–3 with Crohn’s disease in Japanese patients. Gut. 2009;58:228–232. doi: 10.1136/gut.2007.140764. [DOI] [PubMed] [Google Scholar]
- Latiano A, Palmieri O, Cucchiara S, Castro M, D'Inca R, Guariso G, Dallapiccola B, Valvano M R, Latiano T, Andriulli A, Annese V. Polymorphism of the IRGM gene might predispose to fistulizing behavior in Crohn’s disease. Am J Gastroenterol. 2009;104:110–116. doi: 10.1038/ajg.2008.3. [DOI] [PubMed] [Google Scholar]
- Huett A, McCarroll S A, Daly M J, Xavier R J. On the level: IRGM gene function is all about expression. Autophagy. 2009;5:96–99. doi: 10.4161/auto.5.1.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R J, Huett A, Rioux J D. Autophagy as an important process in gut homeostasis and Crohn’s disease pathogenesis. Gut. 2008;57:717–720. doi: 10.1136/gut.2007.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R L, Hollis-Moffatt J E, Gearry R B, Kennedy M A, Barclay M L, Merriman T R. Confirmation of association of IRGM and NCF4 with ileal Crohn’s disease in a population-based cohort. Genes Immun. 2008;9:561–565. doi: 10.1038/gene.2008.49. [DOI] [PubMed] [Google Scholar]
- McCarroll S A, Huett A, Kuballa P, Chilewski S D, Landry A, Goyette P, Zody M C, Hall J L, Brant S R, Cho J H, Duerr R H, Silverberg M S, Taylor K D, Rioux J D, Altshuler D, Daly M J, Xavier R J. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser A L, Darfeuille-Michaud A. Abnormalities in the handling of intracellular bacteria in Crohn’s disease: a link between infectious etiology and host genetic susceptibility. Arch Immunol Ther Exp (Warsz) 2008;56:237–244. doi: 10.1007/s00005-008-0026-1. [DOI] [PubMed] [Google Scholar]
- Fisher S A, Tremelling M, Anderson C A, Gwilliam R, Bumpstead S, Prescott N J, Nimmo E R, Massey D, Berzuini C, Johnson C, Barrett J C, Cummings F R, Drummond H, Lees C W, Onnie C M, Hanson C E, Blaszczyk K, Inouye M, Ewels P, Ravindrarajah R, Keniry A, Hunt S, Carter M, Watkins N, Ouwehand W, Lewis C M, Cardon L, Lobo A, Forbes A, Sanderson J, Jewell D P, Mansfield J C, Deloukas P, Mathew C G, Parkes M, Satsangi J. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40:710–712. doi: 10.1038/ng.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Master S, Singh S. Autophagy gives a nod and a wink to the inflammasome and Paneth cells in Crohn’s disease. Dev Cell. 2008;15:641–642. doi: 10.1016/j.devcel.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y O, Khaminets A, Hunn J P, Howard J C. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNγ-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y M, Shaw M H, Ayala C, Coppens I, Taylor G A, Ferguson D J, Yap G S. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard J C. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J D, Taylor G A, McKinney J D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Gutierrez M G, Master S S, Singh S B, Taylor G A, Colombo M I, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Henry S C, Daniell X G, Burroughs A R, Indaram M, Howell D N, Coers J, Starnbach M N, Hunn J P, Howard J C, Feng C G, Sher A, Taylor G A. Balance of Irgm protein activities determines IFN-{γ}-induced host defense. J Leukoc Biol. 2009;85:877–885. doi: 10.1189/jlb.1008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafica A, Feng C G, Santiago H C, Aliberti J, Cheever A, Thomas K E, Taylor G A, Vogel S N, Sher A. The IFN-inducible GTPase LRG47 (Irgm1) negatively regulates TLR4-triggered proinflammatory cytokine production and prevents endotoxemia. J Immunol. 2007;179:5514–5522. doi: 10.4049/jimmunol.179.8.5514. [DOI] [PubMed] [Google Scholar]
- Feng C G, Collazo-Custodio C M, Eckhaus M, Hieny S, Belkaid Y, Elkins K, Jankovic D, Taylor G A, Sher A. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004;172:1163–1168. doi: 10.4049/jimmunol.172.2.1163. [DOI] [PubMed] [Google Scholar]
- Feng C G, Weksberg D C, Taylor G A, Sher A, Goodell M A. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell. 2008;2:83–89. doi: 10.1016/j.stem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G A, Stauber R, Rulong S, Hudson E, Pei V, Pavlakis G N, Resau J H, Vande Woude G F. The inducibly expressed GTPase localizes to the endoplasmic reticulum, independently of GTP binding. J Biol Chem. 1997;272:10639–10645. doi: 10.1074/jbc.272.16.10639. [DOI] [PubMed] [Google Scholar]
- Martens S, Sabel K, Lange R, Uthaiah R, Wolf E, Howard J C. Mechanisms regulating the positioning of mouse p47 resistance GTPases LRG-47 and IIGP1 on cellular membranes: retargeting to plasma membrane induced by phagocytosis. J Immunol. 2004;173:2594–2606. doi: 10.4049/jimmunol.173.4.2594. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Choi H P, Matsuzawa T, Pypaert M, MacMicking J D. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol. 2009;10:907–917. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo C M, Yap G S, Sempowski G D, Lusby K C, Tessarollo L, Woude G F, Sher A, Taylor G A. Inactivation of LRG-47 and IRG-47 reveals a family of interferon γ-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med. 2001;194:181–188. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G A, Feng C G, Sher A. p47 GTPases: regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4:100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- Henry S C, Daniell X, Indaram M, Whitesides J F, Sempowski G D, Howell D, Oliver T, Taylor G A. Impaired macrophage function underscores susceptibility to Salmonella in mice lacking Irgm1 (LRG-47) J Immunol. 2007;179:6963–6972. doi: 10.4049/jimmunol.179.10.6963. [DOI] [PubMed] [Google Scholar]
- Butcher B A, Greene R I, Henry S C, Annecharico K L, Weinberg J B, Denkers E Y, Sher A, Taylor G A. p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect Immun. 2005;73:3278–3286. doi: 10.1128/IAI.73.6.3278-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorace J M, Johnson R J, Howard D L, Drysdale B E. Identification of an endotoxin and IFN-inducible cDNA: possible identification of a novel protein family. J Leukoc Biol. 1995;58:477–484. doi: 10.1002/jlb.58.4.477. [DOI] [PubMed] [Google Scholar]
- Pixley F J, Stanley E R. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ampel N M, Wing E J, Waheed A, Shadduck R K. Stimulatory effects of purified macrophage colony-stimulating factor on murine resident peritoneal macrophages. Cell Immunol. 1986;97:344–356. doi: 10.1016/0008-8749(86)90405-3. [DOI] [PubMed] [Google Scholar]
- Heasman S J, Ridley A J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behavior. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada K M. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer T, Duffy A, Weiss L M, Halonen S K. IGTP is necessary for Toxoplasma vacuolar disruption and induces parasite egression in IFN{γ} stimulated astrocytes. Infect Immun. 2008;76:4883–4894. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunn J P, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Kaiser F, Zerrahn J, Martens S, Howard J C. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J. 2008;27:2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Weiss-Haljiti C, Pasquali C, Ji H, Gillieron C, Chabert C, Curchod M L, Hirsch E, Ridley A J, Hooft van Huijsduijnen R, Camps M, Rommel C. Involvement of phosphoinositide 3-kinase γ, Rac, and PAK signaling in chemokine-induced macrophage migration. J Biol Chem. 2004;279:43273–43284. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- Bruzzaniti A, Neff L, Sanjay A, Horne W C, De Camilli P, Baron R. Dynamin forms a Src kinase-sensitive complex with Cbl and regulates podosomes and osteoclast activity. Mol Biol Cell. 2005;16:3301–3313. doi: 10.1091/mbc.E04-12-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D A. Regulating actin dynamics at membranes: a focus on dynamin. Traffic. 2004;5:463–469. doi: 10.1111/j.1600-0854.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Schafer D A, Weed S A, Binns D, Karginov A V, Parsons J T, Cooper J A. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol. 2002;12:1852–1857. doi: 10.1016/s0960-9822(02)01228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunck G, Damke H, Kiosses W B, Rusk N, Symons M H, Waterman-Storer C M, Schmid S L, Schwartz M A. Modulation of Rac localization and function by dynamin. Mol Biol Cell. 2004;15:256–267. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.