Abstract
Aim
An association was recently reported between a low cesarean section delivery rate and a method of obstetrical care that involved the frequent use of risk-guided prostaglandin-assisted preventive labor induction. We sought to confirm this finding in a subsequent group of pregnant women.
Methods
A retrospective cohort study design was used to compare the outcomes of 100 consecutively delivered women, who were exposed to the alternative method of care, with the outcomes of 300 randomly chosen women who received standard management. The primary outcome was group cesarean delivery rate. Secondary outcomes were rates of neonatal intensive care unit admission, low 1-minute Apgar score, low 5-minute Apgar score, and major perineal trauma.
Results
Women exposed to the alternative method of obstetrical care had a higher induction rate (59% vs. 16.3%, p < 0.001), a more frequent use of prostaglandins for cervical ripening (32% vs. 13%, p < 0.001), and a lower cesarean delivery rate (7% vs. 20.3%, p = 0.002). Exposed women did not experience higher rates of other adverse birth outcomes.
Conclusions
Exposure to an alternative method of obstetrical care that used high levels of risk-driven prostaglandin-assisted labor was again associated with two findings: a lower group cesarean delivery rate and no increases in levels of other adverse birth outcomes. An adequately powered randomized controlled trial is needed to further explore this alternative method of care.
Introduction
United States rates of cesarean delivery have risen every year since 1997 and in 2006 reached an all time high of 31.1%.1 Possible reasons for these recent increases include changing patient demographics, medical-legal pressures, and an increasing number of indications for cesarean delivery.2–4 Despite calls from various sectors to slow or reverse these trends,5,6 there has been little success in developing methods of care that safely avoid cesarean delivery.6
Prevention of cesarean delivery is important because, as compared with simple vaginal delivery, cesarean delivery is associated with multiple adverse outcomes, including but not limited to higher rates of major postpartum infection,7 increased need for neonatal intensive care unit (NICU) admission,8 longer hospital length of stay for mother and baby,9 increased maternal mortality,10 and higher overall costs.9 Despite suggestions that elective primary cesarean delivery could improve maternal perineal health,11,12 the long-term benefits of this proposed intervention are unproven,13–15 and the long-term risks of elective cesarean delivery have probably been minimized.16,17 Consequently, a method of care that could safely prevent cesarean delivery is still being sought.
An association between a very low group cesarean delivery rate (4%) and exposure to an alternative method of care in an urban setting was recently described.18 This alternative method of care is called the Active Management of Risk in Pregnancy at Term (AMOR-IPAT). AMOR-IPAT begins with the use of each woman's constellation of prenatal risk factors to estimate an upper limit of her optimal of delivery (UL-OTD).19 Women who do not develop spontaneous labor by their UL-OTD are offered preventive labor induction to decrease the possibility of one of two events: (1) that their fetus will be too large to pass through the maternal pelvis or (2) that the placental will be too old to support the fetus during labor. Accordingly, AMOR-IPAT requires a high rate of preventive labor induction. In addition, because cervical ripening is required if labor induction is planned for a woman with an unfavorable cervix (i.e., modified Bishop's score ≤5), AMOR-IPAT also involves a high rate of vaginal prostaglandin medication use.18,20 The purpose of this investigation was to see if the previously reported association between AMOR-IPAT exposure and low cesarean delivery rates in an urban setting could be replicated within the context of a second fully detailed study.
Materials and Methods
As in the first AMOR-IPAT study,18 we employed a retrospective cohort study design to compare the outcomes of the 100 consecutively delivered women exposed to the AMOR-IPAT method of care with the outcomes of 300 randomly chosen women who received usual obstetrical care. All deliveries for this study occurred between April 2001 and October 2002, and no deliveries that were included in the original study were included in this study. AMOR-IPAT exposure revolved around estimating the UL-OTD for each exposed woman. For UL-OTD estimation, each woman's personal constellation of risk factors for cesarean delivery was used to estimate the number of days before 41 weeks 0 days gestation she should deliver in order to minimize her chances of developing either cephalopelvic disproportion or uteroplacental insufficiency.18–20 A scoring sheet for UL-OTD estimation is included as the Appendix. In order to minimize the risk of neonatal pulmonary problems related to preventive labor induction, confirmation of pregnancy dating with early ultrasound and the use of 38 weeks 0 days as the lower limit of the optimal time of delivery (LL-OTD) were integral parts of the AMOR-IPAT protocol. Once a woman's final UL-OTD was determined, the term period of her pregnancy was managed so as to increase the likelihood that she delivered before her ULOTD. If a woman developed spontaneous labor before her UL-OTD, her labor and delivery were managed in the usual manner. However, if spontaneous labor did not develop by a week or so before her UL-OTD, she was scheduled for preventive labor induction so that she delivered 1–3 days before her UL-OTD. In addition, if a woman was scheduled for a preventive induction but her cervix was not favorable (i.e., modified cervical Bishop's score ≤5,21 cervical ripening was provided. Prostaglandin E2 (PGE2) (dinoprostone [Cervidil, Forest Laboratories, Inc., New York, NY]), prostaglandin E1 (PGE1) (misoprostol [Cytotec, GD Searle LLC, Chicago, IL], or a Foley bulb catheter (30–60 mL) was used, as needed, for this purpose. All women receiving preventive labor induction were counseled with regard to the theoretical increased risk of cesarean delivery, and women offered preventive labor induction prior to 39 weeks 0 days gestation were counseled with regard to the theoretical increased risk of NICU admission because of possible fetal lung immaturity in the early term period of pregnancy.
In this study, women exposed to AMOR-IPAT received their prenatal care from three different family medicine offices. Women who received the standard of care, that is, the nonexposed group, received their care from four obstetrical clinic practices and three obstetrical faculty practices. Inclusion criteria included singleton pregnancy, at least one prenatal visit with a maternity care provider from our institution, and delivery after 37 weeks 3 days gestation. Exclusion criteria included >2 previous cesarean deliveries, any other transmural uterine surgery, HIV infection, history of major pelvic injury, major fetal anomaly, or any other factor precluding a trial of labor.
Our method of selecting nonexposed women was similar to the methods previously employed.18 The date of delivery of each exposed woman was determined. For each exposed subject, a random numbers chart was used to identify a number between 1 and 7. The number so selected was added to the delivery date of the exposed subject to obtain a random date. Next, a random numbers table was used to select three random numbers between 1 and 60. Each number so selected was used to randomly select control subjects by sequentially counting off women in the labor and delivery log book of the Hospital of the University of Pennsylvania. The first woman, on or after each of the three counts, who met the study's inclusion and exclusion criteria was included in the study. If the woman selected for any given random date and number had already been selected as a study subject, then the next eligible woman was selected.
We improved on our original study design in two ways. First, we matched for parity status. Exposed nulliparous woman (nulliparas) were matched with three nonexposed nulliparas. Exposed multiparous women (multiparas) were matched with three nonexposed multiparas. Exposed women with a previous cesarean delivery Vagina birth after cesarean (VBACs) were matched with three nonexposed VBACs. All VBAC women had to be eligible for a trial of labor (e.g., no history of >2 cesarean deliveries or classical incision). Second, we excluded women who received their prenatal care from maternal fetal medicine offices or high-risk clinics because these women are generally thought to have higher levels of prenatal risk than women who received their prenatal care from either general obstetricians or family physicians. This study was powered to test for a 60% reduction in cesarean delivery rate with a standard rate of 20%, alpha = 0.05, and beta = 0.80. The Institutional Review Board of the University of Pennsylvania approved this investigation.
Detailed data concerning prenatal variables, intrapartum events, and clinical outcomes were abstracted for each identified mother-baby pair using a validated data abstraction process. The resulting data were then entered into an Access database using a validated data entry process. At least 10% of abstractions and 10% of entries were repeated as a quality assurance procedure. Missing values were obtained by rechecking hospital records or by contacting a woman's primary maternity care provider. Data were analyzed using the STATA Statistical Program (version 8, College Station, TX).
The Student's t test and the Wilcoxon rank-sum test were used to compare distributions of demographic variables, past medical/surgical history details, and obstetrical risk factors present in the two study groups. Univariate chi-square tests were used to compare levels of various covariables and rates of common intrapartum variables. Statistical significance was determined by a p value ≤0.05. Time intervals for the various stages of spontaneous labor, labor induction, and delivery were determined and compared using Wilcoxon rank-sum testing. These analyses were performed on both the two main study groups and on the three parity groups previously described. Data relating to the timing of delivery, mode of labor onset, and method of delivery were collated according to gestational age, collapsed into half-week substrata, and encoded to enable graphic representation.
The strength of association between cesarean delivery and a variety of covariates, including AMOR-IPAT exposure, was initially determined using univariate chi-square analysis. These associations were further assessed using stepwise multiple logistic regression. Clinical problems developing between 38 weeks 0 days gestation and delivery (including preeclampsia, oligohydramnios, thick meconium passage at rupture of membranes, and intrapartum fever) were excluded from the final model because of concerns that they might lie in the causal chain between lack of delivery before the UL-OTD and cesarean section. In order to more fully evaluate the association between AMOR-IPAT exposure and cesarean delivery risk, however, additional models were developed that included these variables.
To evaluate the possibility that AMOR-IPAT exposure could have been associated with increased rates of various adverse outcomes, risk differences for salient adverse birth outcomes between the two groups were calculated using chi-square analysis. Rates of salient birth outcomes were also determined as a function of each of the three parity subgroups. Finally, a number needed to treat analysis was performed to estimate the number of women who would need to be exposed to the AMOR-IPAT method of care to prevent a single cesarean section.
Results
Table 1 shows levels of prenatal risk factors present in the study groups. Although distributions of age, race, ethnicity, marital status, and prepregnancy hypertension were present at statistically different levels, the two groups of this study appeared more similar than the two groups in our first urban study.18 Table 2 presents levels of intrapartum factors in the two study groups. As in the initial study, the overall labor induction rate was significantly higher (60% vs. 16%, p < 0.001), the percentage of women receiving cervical ripening was significantly higher (21% vs. 13%, p < 0.001), and the average gestational age of delivery was significantly lower (38 weeks 4 days vs. 39 weeks 6 days, p < 0.001) in the AMOR-IPAT-exposed group compared with the nonexposed group. The average cervical Bishop's score on admission, the rate of spontaneous rupture of membranes prior to admission, and the rate of thick meconium at rupture of membranes were all significantly lower in the exposed group.
Table 1.
Levels of Risk Factors by Study Group
| Variable | Exposed n = 100 | Nonexposed n = 300 | Risk ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median, years | 23 | 25.9 | <0.00 | ||
| Advanced age (≥35 years at delivery) | 4% | 10.3% | 0.39 | 0.14-1.07 | 0.06 |
| African American | 81% | 69.3% | 1.17 | 1.03-1.32 | 0.03 |
| Private medical insurance | 38% | 47.3% | 0.80 | 0.61-1.06 | 0.13 |
| Single marital status | 82% | 69.3% | 1.18 | 1.05-1.33 | 0.01 |
| Past medical history | |||||
| Chronic hypertension | 7% | 2.3% | 3.0 | 1.08-8.34 | 0.05 |
| Asthma | 20% | 11.7% | 1.71 | 1.04-2.83 | 0.04 |
| Type I diabetes | 0% | 0.3% | 0.00 | – | 1.00 |
| Sickle cell trait | 4% | 5.7% | 0.71 | 0.24-2.05 | 0.61 |
| Cigarette use | 22% | 19.7% | 1.12 | 0.72-1.73 | 0.67 |
| Alcohol use | 3% | 3% | 1.00 | 0.28-3.62 | 1.00 |
| Past obstetric/gynecological history | |||||
| Previous SABa | 25% | 25% | 1.00 | ||
| Previous TAB | 31% | 31.3% | 0.99 | 0.71-1.39 | 1.00 |
| Previous small infant (<6 lb 8 oz) | 19% | 12.7% | 1.42 | 0.85-2.37 | 0.19 |
| Previous large infant (>8 lb 8 oz) | 11% | 6% | 1.83 | 0.90-3.75 | 0.12 |
| Previous vacuum or forceps delivery | 7% | 4% | 1.75 | 0.71-4.32 | 0.28c |
| Laboratory | |||||
| 1-hour 50 gm Glucola ≥135 gm/dl | 7% | 11.3% | 0.62 | 0.29-1.35 | 0.26 |
| Hemoglobin (1st or 2nd trimester ≤11) | 26% | 21% | 1.24 | 0.83-1.84 | 0.33 |
| Group B Streptococcus culture positive | 32% | 28% | 1.14 | 0.81-1.60 | 0.45 |
| Maternal habitus | |||||
| Preconception weight, median | 152 lb | 149 lb | 0.20b | ||
| Preconception weight >180 lb | 30% | 22.3% | 1.34 | 0.93-1.94 | 0.14 |
| Height, median | 65 in | 64 in | 0.19b | ||
| Short stature (≤62 inches) | 22% | 22.3% | 0.99 | 0.64-1.51 | 1.00 |
| Preconception BMI, mean (kg/m2) | 26.6 | 25.0 | 0.26b | ||
| High BMI (≥30 m/kg2) | 26.3% | 25.2% | 1.04 | 0.71-1.53 | 0.83 |
| Index pregnancy | |||||
| Nulliparous status | 34% | 34% | 1.00 | 0.73-1.37 | 1.00 |
| Multiparous (all) | 66% | 66% | 1.00 | 0.85-1.18 | 1.00 |
| Multiparous without cesarean | 58% | 58% | 1.00 | 0.82-1.21 | 1.00 |
| Multiparous with previous cesarean | 8% | 8% | 1.00 | 0.46-2.15 | 1.00 |
| Previous cesarean, previous vaginal birth | 62.5% | 37.5% | 1.67 | 0.79-3.51 | 0.22 |
| Excess weight gain (≥30 lb) | 47% | 53.7% | 0.88 | 0.69-1.11 | 0.25 |
| Gestational diabetes | 3% | 2.3% | 1.29 | 0.34-4.88 | 0.72 |
| Elevated AFP | 0% | 2.7% | 0.00 | – | 0.21 |
| Weight gain during pregnancy, median | 29 lb | 30 lb | 0.00 | – | 0.19 |
| Excess weight gain (≥30 lb) | 47% | 53.7% | 0.88 | 0.69-1.11 | 0.25 |
SAB, spontaneous abortion; TAB, therapeutic abortion; BMI, body mass index; AFP, alpha-feto protein.
Calculated with Mann Whitney rank-sum test.
Calculated for patients with at least one previous birth (vaginal and/or previous cesarean).
Table 2.
Levels of Intrapartum Variables/Factors by Study Group
| Variable | Exposed n = 100 | Nonexposed n = 300 | Risk ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Status on admission | |||||
| Gestational age at delivery, all (median) | 38.6 weeks | 39.9 weeks | <0.001a | ||
| Gestational age on admission, nullipara (median) | 38.7 weeks | 40.0 weeks | <0.001a | ||
| Gestational age on admission, multipara (median) | 38.4 weeks | 39.9 weeks | <0.001a | ||
| Gestational age on admission previous cesarean (median) | 38.5 weeks | 39.6 weeks | 0.15a | ||
| Post dates delivery (>41 weeks) | 9% | 16% | 0.56 | 0.29-1.10 | 0.10 |
| Ruptured membranes on admission | 11% | 28.3% | 0.39 | 0.22-0.70 | 0.004 |
| Initial Bishop's score (mean) | 3.5 | 4.9 | <0.001a | ||
| Initial Bishop's score ≤5 | 88% | 51.3% | 1.71 | 1.50-1.96 | <0.001a |
| Mean arterial pressure (MAP, mean) | 87.2 mmHg | 89.5 mmHg | 0.02a | ||
| Mean arterial pressure (MAP) ≥105 mm Hg | 5% | 6.3% | 0.78 | 0.30-2.06 | 0.81a |
| Vertex presentation | 99% | 98% | 1.01 | 0.98-1.04 | 0.69 |
| Intrapartum interventions | |||||
| Induction of labor | 59% | 16.3% | 3.61 | 2.67-4.89 | <0.001 |
| Augmentation of labor | 26% | 50.7% | 0.51 | 0.37-0.73 | <0.001 |
| Prostaglandin use (any) | 32% | 13.0% | 2.46 | 1.64-3.71 | <0.001 |
| Misoprostol (PGE1) | 13% | 11% | 1.18 | 0.65-2.15 | 0.59 |
| Dinoprostone (PGE2) | 21% | 1.3% | 15.75 | 5.54-44.78 | <0.001 |
| Use of pitocin (any) | 74% | 51% | 1.45 | 1.24-1.70 | <0.001 |
| Artificial rupture of membranes | 50% | 28.3% | 1.76 | 1.35-2.30 | <0.001 |
| Epidural analgesia, overall | 67% | 80.3% | 0.83 | 0.72-0.97 | 0.009 |
| Epidural analgesia, nullipara | 76.5% (26/34) | 88.2% (90/102) | 0.91 | 0.76-1.10 | 0.27 |
| Epidural analgesia, multipara, no previous cesarean | 56.9% (33/58) | 73.0% (127/174) | 0.87 | 0.71-1.06 | 0.10 |
| Epidural analgesia, multipara, previous cesarean | 100% (8/8) | 100% (24/24) | – | – | – |
| Placement of internal electrode | 34% | 33.3% | 1.02 | 0.74-1.40 | 0.90 |
| Placement of IUPCb | 12% | 30% | 0.40 | 0.23-0.70 | <0.001 |
| Episiotomy (second degree) | 0% | 11% | 0.00 | – | <0.001 |
| Use of vacuum or forceps | 16% | 11.3% | 1.41 | 0.82-2.44 | 0.23 |
| Intrapartum findings | |||||
| Thick meconium at ROM | 4% | 16% | 0.25 | 0.92-0.68 | 0.001 |
| Fetal intolerance to laborc | 3% | 12% | 0.25 | 0.08-0.79 | 0.006 |
| Maternal Fever (Tmax > 100.5) | 6% | 5.3% | 1.12 | 0.45-2.80 | 0.80 |
Mann Whitney rank-sum test.
IUPC, intra-uterine pressure catheter; ROM, rupture of membranes; Tmax, maximum temperature.
Repetitive late decelerations.
Table 3 presents information about labor induction in the study groups. The preventive labor induction rate was significantly more frequent in the exposed group (52% vs. 2%, p < 0.001), and the reasons for both indicated and elective induction were different in the exposed group as compared with the nonexposed group. The most common reasons for labor induction in the AMOR-IPAT-exposed group were impending cephalopelvic disproportion and impending uteroplacental insufficiency.
Table 3.
Induction of Labor Information: Overall Rates and Indicationsa
| Induction of labor informationa | Exposed n = 100 | Nonexposed n = 300 | Risk ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Induction of labor rate, overalla | 59% | 16.3% | 3.75 | 2.77-5.09 | <0.001 |
| Indicated induction | 12% | 13.7% | 0.88 | 0.48-1.60 | 0.67 |
| Preventive induction | 43% | 1.7% | 17.6 | 8.6-36.0 | <0.001 |
| Elective inductionb | 4% | 1.0% | |||
| Reasons for indicated induction | |||||
| Severe preeclampsia | 1% | 3.3% | 0.3 | 0.04-2.31 | 0.22 |
| Oligohydramnios/IUGR | 6% | 3.7% | 1.64 | 0.62-4.31 | 0.32 |
| >41 weeks | 5% | 4.3% | 1.15 | 0.42-3.16 | 0.78 |
| >42 weeks | 0% | 1.0% | 0 | – | 0.32 |
| Gestational diabetes | 0% | 0.7% | 0 | – | 0.41 |
| Chronic hypertension | 0% | 0.3% | 0 | – | 0.85 |
| Acute cholestasis | 0% | 0.3% | 0 | – | 0.85 |
| Polyhydramnios | 0% | 0% | – | – | – |
| Nonreassuring antenatal testing | 0% | 0% | – | – | – |
| Reasons for preventive induction | |||||
| Impending preeclampsia | 5% | 0% | – | – | <0.001 |
| Impending cephalopelvic disproportion | 27% | 0.67% | 40.5 | 9.8-167 | <0.001 |
| Impending uteroplacental insufficiency | 8% | 0.33% | 24.0 | 3.04-189 | <0.001 |
| Gestational diabetes | 0% | 0.67% | 0 | – | 0.41 |
| History of precipitous labor | 2% | 0% | – | – | 0.05 |
| Unstable fetal liec | 1% | 0% | – | – | 0.08 |
All rates entire study group as denominator.
Elective reasons—convenience, patient request, frequent false labor visits.
Induction immediately following successful repeat external cephalic version.
The exposed group had a longer mean interval between admission and delivery (15.3 hours vs. 9.00 hours, p < 0.001). This was largely because of a longer mean interval between admission and the onset of labor (7.36 hours vs. 2.75 hours, p < 0.001). For women in the exposed nulliparous group, compared with nulliparous women in the nonexposed group, the median length of the first stage of labor was 522 minutes vs. 418 minutes (p = 0.18), and the median length of the second stage was 49 vs. 46 minutes (p = 0.80). For women in the exposed multiparous group, compared with multiparous women in the nonexposed group, the median length of the first stage of labor was 285 minutes vs. 260 minutes (p = 0.15), and the median length of the second stage was 15 minutes vs. 16 minutes (p = 0.95). The data concerning length of first-stage and second-stage duration were potentially impacted by right censoring in the nonexposed groups related to higher cesarean delivery use in these groups. Finally, because of the lower cesarean delivery rates, all exposed parity groups had a shorter median postdelivery hospital stay than the corresponding nonexposed group. Overall, women exposed to AMOR-IPAT and their babies had significantly shorter mean lengths of total hospital stay (63.1 hours vs. 66.8 hours, p = 0.38; 55.0 hours vs. 63.2 hours, p < 0.001, respectively).
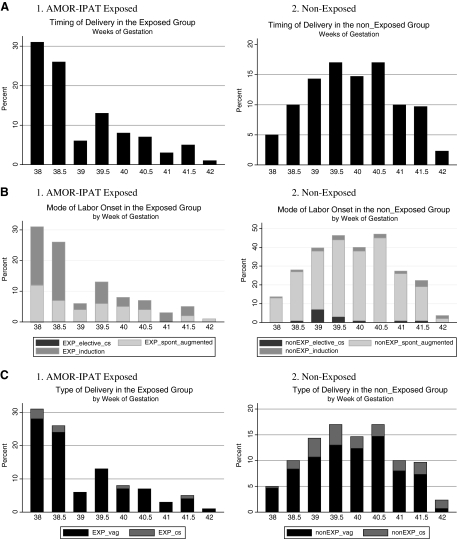
Figure 1A demonstrates graphically that as a function of gestational age at the time of delivery, exposed women delivered earlier in the term period than did nonexposed women. Not all exposed women agreed to preventive induction on or before 41 weeks 0 days of gestation, and 8 exposed women delivered after 41 weeks 0 days of gestation. Figure 1B demonstrates that exposed women experienced induction of labor more frequently and at earlier gestational ages than did nonexposed women.
FIG. 1.
(A) Distribution of timing of delivery as a function of gestational age, by study groups. (B) Spontaneous vs. induced labor as a function of gestational age, by study group. (C) Vaginal vs. cesarean delivery as a function of gestational age, by study group.
Table 4 presents information related to cesarean delivery in the study groups. The exposed group had a significantly lower overall cesarean delivery rate (7% vs. 20.3%, p = 0.002) and a significantly lower cesarean delivery in the multiparous group without history of previous cesarean delivery (0% vs. 10.9%, p = 0.005). Exposed cesarean delivery rates trended lower in the nulliparous group and in the VBAC group. When all women with previous cesarean delivery were excluded, there was a significantly lower cesarean delivery rate in the AMOR-IPAT-exposed group (5.4% vs. 14.3%, OR 0.34, 95% CI 0.10–0.91, p = 0.02). Despite 11 elective cesarean deliveries in the nonexposed group (1 elective cesarean delivery for presumed fetal macrosomia and 10 elective repeat cesarean deliveries), the nonexposed group had a significantly greater number of cesarean deliveries for failure to progress (8.7% vs. 2%, p = 0.02). The cesarean delivery rate remained significantly lower in the exposed group even if the elective cesarean deliveries were excluded from the nonexposed group: 7% (7/100) vs. 17.3% (50/289), RR 0.40, p = 0.012. Figure 1C demonstrates the mode of delivery in the two study groups as a function of gestational age at delivery.
Table 4.
Cesarean Delivery Overall Rates and Indicationsa
| Cesarean delivery informationa | Exposed n = 100 | Nonexposed n = 300 | Risk ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Overall cesarean delivery rate | 7% | 20.3% | 0.34 | 0.16-0.73 | 0.002 |
| Cesarean rate by different subgroups | |||||
| Nulliparousb | 14.7% (5/34) | 26.5% (27/102) | 0.56 | 0.23-1.33 | 0.24 |
| Multiparous, no previous cesareanb | 0% (0/58) | 10.9% (19/174) | 0 | – | 0.005 |
| Multiparous with history of previous cesareanb | 25% (2/8) | 62.5% (15/24) | 0.4 | 0.12-1.38 | 0.11 |
| Multiparous, history of cesarean, no prior vaginal birth | 33.3% (1/3) | 80% (12/15) | 0.42 | 0.08-2.11 | 0.10 |
| Multiparous, history of cesarean, prior vaginal birth | 20% (1/5) | 33.3% (3/9) | 0.60 | 0.08-4.35 | 0.60 |
| Multiparous, history of previous cesarean and with trial of laborb | 25% (2/8) | 35.7% (5/14) | 0.71 | 0.19-2.69 | 0.60 |
| All women without history of previous cesarean | 5.4% (5/92) | 16.7% (46/276) | 0.33 | 0.13-0.80 | 0.007 |
| Indications for cesarean delivery | |||||
| Fetal intolerance of labor | 4% | 6.3% | 0.5 | 0.15-1.66 | 0.31 |
| Failure to progress | 3% | 8.0-% | 0.23 | 0.56-0.96 | 0.02 |
| Malpresentation (breech) | 0% | 1.7% | 0 | – | 0.34 |
| Active herpes infection | 0% | 0.7% | 0 | – | 1.00 |
| Elective repeat cesarean | 0% | 3.3% | 0 | – | 0.07 |
| Elective cesarean for macrosomia | 0% | 0.33% | – | – | – |
| Cesarean delivery by mode of labor onset | |||||
| Noninduced onset (all)b | 2.4% (1/41) | 18.3% (32/238) | 0.18 | 0.03-1.29 | 0.04 |
| Spontaneous labor, no ROM on admission, no augmentationb | 10% (1/10) | 3.6% (2/56) | 2.8 | 0.28-28.0 | 0.37 |
| Spontaneous labor, no ROM on admission, with augmentationb | 0% (0/20) | 16.0% (16/100) | 0 | – | 0.05 |
| ROM on admission, spontaneous labor, no augmentationb | 0% (0/5) | 13.8% (4/29) | 0 | – | 0.38 |
| ROM on admission, with augmentationb | 0% (0/6) | 18.9% (10/53) | 0 | – | 0.24 |
| Induced onset (all)b | 10.2% (6/59) | 30.6% (15/49) | 0.33 | 0.14-0.79 | 0.008 |
| Indicated induction of laborb | 16.7% (2/12) | 36.7% (15/41) | 0.46 | 0.12-1.72 | 0.19 |
| Elective induction of laborb | 8.5% (4/47) | 0% (0/8) | 0 | – | 0.39 |
| Elective repeat cesareanb | 0% (0/0) | 100% (13/13) | 0 | – | – |
When not specified, rates are based on entire study group.
Rates calculated based on substrata.
Table 5 provides information from the final logistic regression modeling of the association between exposure and cesarean delivery. The final model included seven risk factors: AMOR-IPAT status (exposed vs. nonexposed), parity group (nullipara, multipara, or VBAC), cocaine abuse, alcohol abuse, insurance status (Medicaid vs. private insurance), advanced maternal age (<35 years of age or ≥35 years of age at delivery), and epidural analgesia use. Adjustment for these important covariates did not alter the magnitude of association between AMOR-IPAT exposure and lower cesarean delivery rate. There was also no significant impact on this association when clinical problems that developed between 38 weeks 0 days gestation and delivery (e.g., maternal fever, preeclampsia, oligohydramnios, and thick meconium at rupture of membranes) were added to the model (data not shown).
Table 5.
Logistic Regression Modeling of AMOR-IPAT Exposure on Cesarean Delivery
| Variablesa | Unadjusted OR | Adjusted OR | Adjusted 95% CI |
|---|---|---|---|
| AMOR-IPAT exposure | 0.29* | 0.29* | (0.12-0.73) |
| Nulliparity | 1.00* | 1.00* | – |
| Multiparity | 0.29* | 0.25* | (0.12-0.50) |
| Previous cesarean section | 3.68* | 3.31* | (1.39-7.91) |
| Epidural analgesia | 12.27* | 8.41* | (1.84-38.41) |
| Alcohol use | 2.53 | 6.62* | (1.50-29.2) |
| Medicaid insurance | 1.37 | 1.70 | (0.91-3.16) |
| Cocaine use | 6.85* | 5.23 | (0.72-38.17) |
| Age ≥ 35 | 2.48* | 2.09 | (0.91-3.16) |
Listed according to decreasing significance within the final logistic model.
Statistically significant (p < 0.05).
Table 6 lists important clinical outcomes other than cesarean section that occurred in the two study groups. Rates of major perineal trauma, thick meconium at rupture of membranes, and repetitive late decelerations were low enough in the AMOR-IPAT-exposed group to indicate that it is unlikely that AMOR-IPAT exposure could have been associated with increased rates of these outcomes. Rates of assisted vaginal delivery were higher in the exposed group but did not reach statistical significance (16% vs. 11.3%, p = 0.23). The only outcome that was present at significantly higher rates following AMOR-IPAT exposure was birth weight <6 lb 8 oz (32% vs. 18.3%, p = 0.005). Rates of very small infants (birth weight <5 lb 8 oz) trended higher after exposure (6% vs. 2.3%, p = 0.10), but none of the AMOR-IPAT-exposed infants who weighed <6 lb 8 oz at birth or were delivered following labor induction required NICU admission. In addition, none of the 13 infants in the exposed group delivered after labor induction prior to 39 weeks 0 days of gestation developed transcient tachypnea of the newborn, required therapy for hyperbilirubinemia, or were admitted to the NICU. In all parity groups, women in the exposed study group were significantly more likely to undergo preventive labor induction (nulliparous women: 50% vs. 0%, p < 0.001; multiparous women without history of cesarean delivery: 50% vs. 2.9%, p < 0.001; multiparous women with history of cesarean delivery: 75% vs. 4.2%, p < 0.001). Except for a nonstatistically significant upward trend in third-degree or fourth-degree perineal injury in exposed nulliparous group (14.7% [5/34] vs. 7.8% [8/102]), every other major outcome in every parity group was similiar or was lower in the exposed group compared with the nonexposed group (data not shown) (Table 7). Finally, the number of women who needed to be exposed to AMOR-IPAT in order to prevent one cesarean delivery was calculated to be 7.5.
Table 6.
Major Outcomes and Risk Differences for Outcomes Based on AMOR-IPAT Exposure vs. Nonexposure Statusa
| Variable | Exposed n = 100 | Nonexposed n = 300 | Risk difference | 95% CI | p value |
|---|---|---|---|---|---|
| Maternal—Delivery | |||||
| 3rd or 4th degree perineal injury, all | 5% | 5.3% | −0.003 | (−0.05)-(0.05) | 1.0 |
| 3rd or 4th degree tear in women with vaginal delivery only | 5.4% (5/93) | 6.7% (16/239) | 0.013 | (−0.07)-(0.04) | 0.66 |
| 3rd or 4th degree tear in women without previous cesarean delivery | 5.4% (5/92) | 5.8% (17/276) | −0.004 | (−0.06)-(2.49) | 0.90 |
| Assisted vaginal delivery, all | 16% | 11.3% | 0.05 | (−0.03)-(0.13) | 0.22 |
| Assisted vaginal delivery, vaginal delivery only | 17.2% (16/93) | 13.0% (31/239) | 0.04 | (−0.04)-(0.13) | 0.38 |
| Assisted vaginal delivery in women without previous cesarean delivery | 16.3% (15/92) | 12.0% (33/276) | 0.04 | (−0.04)-(0.13) | 0.28 |
| Estimated blood loss, all | 356 mL ± 245 | 445 mL ± (260) | – | – | <0.001 |
| Estimated blood loss, vaginal delivery women only | 312 mL ± 188 | 344 mL ± 124 | – | – | 0.001 |
| Excess blood loss (>500 mL), all | 19% | 36% | −0.17 | (−0.26)-(−0.08) | 0.002 |
| Excess blood loss (>500 mL), vaginal delivery women only | 12.9% | 20.1% | −0.07 | (−0.16)-(0.01) | 0.15 |
| Maternal–Postpartum | |||||
| Maternal fever (>100.4°F) | 3% | 6% | −0.03 | (−0.07)-(0.01) | 0.31 |
| Postpartum anemia (Hgb<8.0 g/dL) | 10% | 8.3% | 0.02 | (−0.05)-(0.08) | 0.68 |
| Flux in hemoglobin with delivery | 1.24 g ± 0.94 | 1.48 g ± 1.03 | – | – | 0.06 |
| Neonatal | |||||
| NICU admission, all | 7% | 9% | −0.02 | (−0.08)-(0.04) | 0.68 |
| NICU Admission in women without previous cesarean delivery | 6.5% | 8.7% | −0.02 | (−0.08)-(0.04) | 0.51 |
| Sepsis, suspected or actual | 5% | 5.7% | −0.01 | (−0.01)-(0.04) | 1.00 |
| Meconium aspiration | 1% | 3.7% | −0.03 | (−0.06)-(0.002) | 0.31 |
| Cephalohematoma | 2% | 4% | −0.02 | (−0.06)-(0.02) | 0.53 |
| Birth weight | 3167 g ± 447 | 3394 g ± 476 | – | – | <0.001 |
| Large (>8 lb 7 oz) | 7% | 19.3% | −0.13 | (−0.19)-(−0.06) | 0.003 |
| Small (<6 lb 8 oz) | 32%b | 18.3% | 0.14 | (0.04)-(0.24) | 0.004 |
| Very Small (<5 lb 8 oz) | 6%c | 2.3% | 0.04 | (−0.01)-(0.09) | 0.10 |
| Head circumference | 33.6 cm ± 1.6 | 34.0 cm ± 1.8 | 0.02 | ||
| Apgar 1 < 7 | 10% | 13.7% | −0.04 | (−0.11)-(0.03) | 0.39 |
| Apgar 1 < 4 | 4% | 3.3% | 0.01 | (−0.04)-(0.05) | 0.76 |
| Apgar 5 < 7 | 1% | 0.7% | 0.003 | (−0.02)-(0.02) | 1.00 |
| Apgar 5 < 4 | 1% | 0.3% | 0.01 | (−0.01)-(0.03) | 0.44 |
Calculated with the Mann Whitney rank-sum test.
Of the 17 of these infants delivered following induction, none required NICU admission.
Of the 5 of these infants delivered following induction, none required NICU admission.
Table 7.
Labor Onset and Outcome Information According to Parity Groupa
| Variable | Exposed | Nonexposed | Risk ratio | 95% CI | p value |
|---|---|---|---|---|---|
| Labor onset | |||||
| Indicated induction | |||||
| Nullipara | 11.8% (4/34) | 18.6% (19/102) | 0.63 | 0.23-1.73 | 0.36 |
| Multipara without previous cesarean | 8.6% (5/58) | 10.9% (19/174) | 0.82 | 0.36-1.84 | 0.80 |
| Multipara with previous cesarean | 37.5% (3/8) | 12.5% (3/24) | 3.0 | 0.75-12.0 | 0.12 |
| Preventive induction | |||||
| Nullipara | 41.2% (14/34) | 2.0% (2/102) | 21 | 5.0-87.7 | <0.001 |
| Multipara without previous cesarean | 50% (29/58) | 3.4% (6/174) | 14.5 | 6.3-33.2 | <0.001 |
| Multipara with previous cesarean | 50% (4/8) | 0% (0/24) | – | – | <0.001 |
| Elective cesarean delivery | |||||
| Nullipara | 0.0% (0/34) | 2.0% (2/102) | 0.0 | – | 1.00 |
| Multipara without previous cesarean | 0.0% (0/58) | 1.2% (2/174) | 0.0 | – | 1.00 |
| Multipara with previous cesarean | 0.0% (0/8) | 41.7% (10/24) | 0.0 | – | 0.04 |
| Prostaglandin E2 use | |||||
| Nullipara | 47.1% (16/34) | 1.0% (1/102) | 48 | 6.61-348.57 | <0.001 |
| Multipara without previous cesarean | 3.5% (2/58) | 1.2% (2/174) | 3.0 | 0.43-20.82 | 0.26 |
| Multipara with previous cesarean | 37.5% (3/8) | 4.2% (1/24) | 9.0 | 1.08-74.76 | 0.04 |
| Major outcomes | |||||
| 3rd or 4th degree perineal injury | |||||
| Nullipara | 14.7% (5/34) | 7.8% (8/102) | 1.88 | 0.66-5.35 | 0.31 |
| Multipara without previous cesarean | 0.0% (0/58) | 4.6% (8/174) | 0.0 | – | 0.21 |
| Multipara with previous cesarean | 0.0% (0/8) | 0.0% (0/24) | – | – | – |
| NICU admission (overall) | |||||
| Nullipara | 8.8% (3/34) | 12.8% (13/102) | 0.69 | 0.21-2.28 | 0.76 |
| Multipara without previous cesarean | 5.2% (3/58) | 6.3% (11/174) | 0.82 | 0.24-2.83 | 1.00 |
| Multipara with previous cesarean | 12.5% (1/8) | 12.5% (3/24) | 1.0 | 0.12-8.31 | 1.00 |
| Thick meconium passage | |||||
| Nullipara | 2.9% (1/34) | 17.7% (18/102) | 0.17 | 0.23-1.20 | 0.04 |
| Multipara without previous cesarean | 5.2% (3/58) | 16.1% (28/174) | 0.32 | 0.10-1.02 | 0.04 |
| Multipara with previous cesarean | 0.0% (0/8) | 8.3% (2/24) | 0.00 | – | 0.40 |
| Apgar 1 < 7 | |||||
| Nullipara | 8.8% (3/34) | 17.7% (18/102) | 0.50 | 0.16-1.59 | 0.28 |
| Multipara without previous cesarean | 12.1% (7/58) | 12.1% (21/174) | 1.00 | 0.45-2.23 | 1.00 |
| Multipara with previous cesarean | 0.0% (0/8) | 8.3% (2/24) | 0 | – | 0.40 |
| Apgar 5 < 4 | |||||
| Nullipara | 2.9% (1/34)b | 0% (0/102) | – | – | 0.25 |
| Multipara without previous cesarean | 0% (0/58) | 1% (1/174)b | 0 | – | 1.00 |
| Multipara with previous cesarean | 0% (0/8) | 0% (0/24) | – | – | – |
Calculated with the Mann Whitney rank-sum test.
Associated with spontaneous labor onset rather than induction of labor.
Discussion
This article corroborates the association recently reported between exposure to AMOR-IPAT, with its high group labor induction rate and high group prostaglandin cervical ripening rate, and a lower group cesarean delivery rate. As in the first study,18 this association was statistically significant after adjustment for possible confounding variables. Rates of other adverse birth outcomes were either lower or unchanged in the AMOR-IPAT exposed study group compared with the nonexposed group.
Most previous investigations have reported an association between indicated labor induction and increased use of cesarean delivery.22–26 However, AMOR-IPAT uses labor induction primarily in a preventive mode rather than in response to standard indications. In addition, published studies of AMOR-IPAT evaluate the impact of labor induction from a practice-based perspective rather than a labor onset-based perspective.18–20 To support this analytical perspective, a practice-based statistical approach is routinely used to measure U.S. national and state cesarean delivery rates.1 In contrast to the the 2006 national cesarean delivery rate of 31.1%, however, all published retrospective studies involving AMOR-IPAT have reported cesarean delivery rates of <11% in the exposed groups.18,20,26–30
On the surface, these findings are at odds with multiple studies that have reported an association between labor induction and an increased risk of cesarean delivery.22–26 However, the first AMOR-IPAT article18 described a variety of theoretical problems with previous studies, including both confounding by indication and problems with study design. Important questions have not been asked or answered: (1) In retrospective studies, are the higher rates of cesarean delivery in women who are induced using standard obstetrical protocols due to the induction event per se, or are they due to the reasons that the inductions were initiated? (2) Should retrospective study group designation be determined by mode of labor onset (spontaneous labor vs. induction) or by practice-based criteria (group A with a lower rate of labor induction vs. group B with a higher rate of labor induction)? A recent article suggested that the association between induction and cesarean delivery vanished after adjustment for potential confounding variables.27 In addition, a recent meta-analysis of randomized prospective studies28 determined that the use of labor induction to encourage delivery at 41 weeks of gestation, compared with expectant management until 42 weeks gestation, modestly decreased cesarean delivery risk. Both the first AMOR-IPAT article and this investigation suggest that the careful use of preventive labor induction prior to 41 weeks of gestation might further reduce rates of adverse birth outcomes.
Several recent studies have suggested a continuous increase in the risk of adverse birth outcomes as a function of increasing gestational age during the term period of pregnancy.29–33 The higher induction of labor rate in the exposed group of this study necessarily lowered its average gestational age. Lower gestational age may favorably impact the mode of delivery in two important ways. First, because fetal weight increases continuously during the term period,34 earlier delivery through AMOR-IPAT may have reduced cesarean delivery for failure to progress (FTP) by encouraging labor when the fetus was mature but relatively small. A lower median birth weight and a lower rate of macrosomia (birth weight >4000 g) were noted in the exposed group compared with the nonexposed group. Second, because placental function decreases continuously during the term period,34 earlier delivery through AMOR-IPAT may have reduced cesarean delivery for uteroplacental insufficiency by encouraging labor at the time of optimal placental functioning. Lower rates of both thick meconium at rupture of membranes and fetal intolerance of labor (severe variable and repetitive late decelerations) were noted in the exposed group compared with the nonexposed group. AMOR-IPAT employed the concept that certain risk factors accelerate fetal growth and certain risk factors accelerate placental senescence.19 Hence, AMOR-IPAT-exposed women with risk factors were encouraged to deliver earlier in the term period than exposed women without risk factors, and the timing of induction was directly related to the quantity of any given exposed patient's cumulative risk.
We acknowledge that this study has a number of limitations. First, it was retrospective and therefore potentially influenced by unknown confounders. Although logistic regression demonstrated that adjustment for known confounding variables did not alter the strength of association between exposure and cesarean delivery rate reduction, it is possible that hidden confounders were present. Second, this study occurred at a single quaternary care teaching institution with primarily African American women, and it is unclear if our results are generalizable to other types of institutions, patient populations, or geographic areas. However, a large retrospective study (n = 1869) describing the association between AMOR-IPAT exposure and a very low cesarean delivery rate (5.3%) was published recently,20 and that study involved a rural nonteaching secondary hospital and a primarily Caucasian population. Third, the difference in the specialty of providers in the exposed and nonexposed groups of this study raises the possibility of selection bias, information bias, and differences in practice style, including threshold for cesarean delivery. It is unlikely, however, that the major findings of this study could be entirely explained by these factors. The decision to perform cesarean delivery in this study's population was always made by obstetrician specialists and not by family physicians. In addition, cesarean delivery rates as low as reported in this article have not been reported recently from urban family practice settings. Finally, the study was not powered to evaluate the association between AMOR-IPAT exposure and infrequent adverse outcomes, such as neonatal hyaline membrane disease, meconium aspiration syndrome, neonatal mortality, or maternal mortality. There were no apparent trends or near misses in the outcome data suggesting that maternal or neonatal outcomes would have been less favorable had the study been larger.
The benefit of the AMOR-IPAT approach may lie in the finding that in both this study and the previous urban study, the cesarean delivery rates declined with exposure in both the induced labor subgroup and the spontaneous labor subgroup. AMOR-IPAT-exposed women, especially those with increased risk, were usually induced prior to their due date. This led to labor that occurred at a time when there was a reduced likelihood that patient risk factors had transformed into an indication for cesarean delivery. Additionally, because many exposed women with risk were induced, the exposed women who did develop spontaneous labor carried, on average, lower levels of risk into labor than did the nonexposed women who developed spontaneous labor. Despite the finding that cesarean delivery occurred at higher rates in the exposed/induced subgroup compared with the exposed/spontaneous labor subgroup, the greater use of induction, in a preventive mode, was associated with a lower cesarean delivery in both exposed subgroups compared with the corresponding nonexposed subgroup. This may have led to the significant overall reduction in group cesarean delivery rate. As noted in our previous study, the full benefit of higher use of preventive induction in retrospective studies can be seen only if a treatment group perspective, rather than a mode of labor onset perspective, is taken.
AMOR-IPAT has been widely challenged for not routinely performing amniocentesis prior to preventive labor induction in the 38th week of gestation. However, the use of AMOR-IPAT in the setting of a randomized controlled trial was reported recently, and with AMOR-IPAT exposure, the rate of NICU admission was significantly lower than in the group that was not exposed.30 In addition, all other previous retrospective studies of AMOR-IPAT have found either a lower or an unchanged rate of NICU admission following AMOR-IPAT exposure.18,20 In this study and in the randomized clinical trial,35 the rate of NICU admission among infants delivered following preventive labor induction within the 38th week of gestation has been unusually low. From the standpoint of safely, however, it is important to note that the use of preventive labor induction in the 38th week of gestation probably requires both the use of AMOR-IPAT risk scoring to justify early term labor induction and solid ultrasound-based dating.
This study confirms our previous finding that exposure to AMOR-IPAT, with its high rates of cervical ripening and labor induction rate, was associated with a significantly lower group cesarean delivery rate. As in our previous study, rates of other major adverse birth outcomes were not increased following exposure to AMOR-IPAT. Hence, AMOR-IPAT may represent a strategy that provides for the safe reduction of group cesarean delivery rates. Adequately powered multisite prospective randomized trials are needed to study the impact of AMOR-IPAT on major pregnancy outcomes.
Appendix: UL-OTD Scoring Sheet
| A. Uteroplacental factors: | Odds ratio | Time units | |
|---|---|---|---|
| History of chronic hypertension | 1.8 | 6 days | __________ |
| Gestational diabetes | 1.8 | 6 days | __________ |
| Insulin-dependent diabetes | 2.4 | 10 days | __________ |
| Sickle cell trait | 1.5 | 3 days | __________ |
| Elevated AFP | 1.4 | 3 days | __________ |
| Cigarette use | 1.3 | 2 days | __________ |
| Size < dates (≤3 cm) | 1.6 | 4 days | __________ |
| Advanced age (≥35 years at delivery) | 1.8 | 6 days | __________ |
| Anemia (1st trimester ≤10.0) | 1.6 | 4 days | __________ |
| Total UPI time units | |||
| UL-OTD-upi (utero-placental insufficiency) = (41 weeks − total UPI time units) = | |||
| B. Cephalopelvic factors: | Odds ratio | Time units | |
|---|---|---|---|
| Elevated BMI (≥30) | 1.3 | 2 days | __________ |
| Short stature (≤62 inches) | 1.8 | 6 days | __________ |
| Excess weight gain (≥30 lbs) | 1.8 | 6 days | __________ |
| Size > dates (≥3 cm) | 1.7 | 4 days | __________ |
| Gestational diabetes | 1.8 | 6 days | __________ |
| Type 1 diabetes | 2.4 | 10 days | __________ |
| History of vacuum/forceps | 2.2 | 9 days | __________ |
| Previous macrosomia (≥4000 g) | 2.0 | 7 days | __________ |
| Total CPD time units | |||
Disclosure statement
The authors have no conflicts of interest to report.
References
- 1.Hamilton BE. Martin JA. Ventura SJ. National vital statistics reports. 7 Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Births: Preliminary data for 2006. [Google Scholar]
- 2.Ecker JL. Frigoletto FD., Jr Cesarean delivery and the risk-benefit calculus. N Engl J Med. 2007;356:885–888. doi: 10.1056/NEJMp068290. [DOI] [PubMed] [Google Scholar]
- 3.Bailit JL. Love TE. Mercer B. Rising cesarean rates: Are patients sicker? Am J Obstet Gynecol. 2004;191:800–803. doi: 10.1016/j.ajog.2004.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Declercq E. Menacker F. Macdorman M. Maternal risk profiles and the primary cesarean rate in the United States, 1991–2002. Am J Public Health. 2006;96:867–872. doi: 10.2105/AJPH.2004.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healthy people 2000. National health promotion and disease prevention objectives: Full report, with commentary. Government Printing Office. Vol. 378. Washington, DC: 1990. pp. 91–50212. DHHS publication No. (PHS). [Google Scholar]
- 6.Flamm B. Kabcenell A. Berwick DM. Roessner J. Reducing cesarean rates while maintaining maternal and infant outcomes. Boston: Institute for Healthcare Improvement; 1997. [Google Scholar]
- 7.Liu S. Liston RM. Joseph KS. Heaman M. Sauve R. Kramer MS. Maternal Health Study Group of the Canadian Perinatal Surveillance System. Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. Can Med Assoc J. 2007;176:455–460. doi: 10.1503/cmaj.060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogelson NS. Menard MK. Hulsey T. Ebeling M. Neonatal impact of elective repeat cesarean delivery at term: A comment on patient choice cesarean delivery. Am J Obstet Gynecol. 2005;192:1433–1436. doi: 10.1016/j.ajog.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Druzin ML. El-Sayed YY. Cesarean delivery on maternal request: Wise use of finite recources? A view from the trenches. Semin Perinatol. 2006;30:305–308. doi: 10.1053/j.semperi.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Deneux-Tharaux C. Carmona E. Bouvier-Colle MH. Breart G. Postpartum maternal mortality and cesarean delivery. Obstet & Gynecol. 2006;108:541–548. doi: 10.1097/01.AOG.0000233154.62729.24. [DOI] [PubMed] [Google Scholar]
- 11.Minkoff H. Chervenak FA. Elective primary cesarean delivery. N Engl J Med. 2003;348:946–950. doi: 10.1056/NEJMsb022734. [DOI] [PubMed] [Google Scholar]
- 12.Lal M. Prevention of urinary and anal incontinence: Role of elective cesarean delivery. Cur Opin in Obstet Gynecol. 2003;15:439–448. doi: 10.1097/00001703-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Lukacz E. Lawrence J. Contreras R. Nager CW. Luber K. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107:1253–1260. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 14.Dietz HP. Pelvic floor trauma following vaginal delivery. Curr Opin Obstet Gynecol. 2006;18:528–537. doi: 10.1097/01.gco.0000242956.40491.1e. [DOI] [PubMed] [Google Scholar]
- 15.Hannah ME. Whyte H. Hannah WJ, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: The Internation Randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191:917–927. doi: 10.1016/j.ajog.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Kennare R. Tucker G. Heard A. Chan A. Risks of adverse outcomes in the next birth after a first cesarean delivery. Obstet Gynecol. 2007;109:270–276. doi: 10.1097/01.AOG.0000250469.23047.73. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JF. Roberts CL. Currie M. Ellwood DA. Prevalence and persistence of health problems after childbirth: Associations with parity and method of birth. Birth. 2002;29:83–94. doi: 10.1046/j.1523-536x.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JA. Kellar LC. Cronholm PF. Macones GA. Active management of risk in pregnancy at term: An association between a higher induction of labor rate and a lower cesarean delivery rate. Am J Obstet Gynecol. 2004;191:1516–1528. doi: 10.1016/j.ajog.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson JM. Holt M. Will active management of obstetric risk lower C/S rates? Contemp Obstet Gynecol. 2005;50:38–53. [Google Scholar]
- 20.Nicholson JM. Yeager D. Macones G. A preventive approach to obstetric care in a rural hospital: Association between higher rates of preventive induction of labor and lower rates of cesarean delivery. Ann Fam Med. 2007;5:310–319. doi: 10.1370/afm.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bujold E. Blackwell SC. Hendler I. Berman S. Sorokin Y. Gautheir RJ. Modified Bishop's score and induction of labor in patient with a previous cesarean delivery. Am J Obstet Gynecol. 2004;191:1644–1648. doi: 10.1016/j.ajog.2004.03.075. [DOI] [PubMed] [Google Scholar]
- 22.Cammu H. Martens G. Ruyssinck G. Amy JJ. Outcome after elective induction in nulliparous women: A matched cohort study. Am J Obstet Gynecol. 2002;186:240–244. doi: 10.1067/mob.2002.119643. [DOI] [PubMed] [Google Scholar]
- 23.Yeast JD. Jones A. Poskin M. Induction of labor and the relationship to cesarean delivery: A review of 7001 consecutive inductions. Am J Obstet Gynecol. 1999;180:628–633. doi: 10.1016/s0002-9378(99)70265-6. [DOI] [PubMed] [Google Scholar]
- 24.Coonrod DV. Bay RC. Kishi GY. The epidemiololgy of labor induction: Arizona, 1997. Am J Obstet Gynecol. 2000;182:1355–1362. doi: 10.1067/mob.2000.106248. [DOI] [PubMed] [Google Scholar]
- 25.Seyb ST. Berka RJ. Socol ML. Dooley SL. Risk of cesarean delivery with elective induction of labor at term in nulliparous women. Obstet Gynecol. 1999;94:600–607. doi: 10.1016/s0029-7844(99)00377-4. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Haroush A. Yogev Y. Bar J. Glickman H. Kaplan B. Hod M. Indicated labor induction with vaginal prostaglandin E2 increases the risk of cesarean section even in multiparous women with no previous cesarean section. J Perinatal Med. 2004;32:31–36. doi: 10.1515/JPM.2004.005. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JM. MCIntire DD. Leveno KJ. Prolonged pregnancy: Induction of labor and cesarean births. Obstet Gynecol. 2001;97:911–915. doi: 10.1016/s0029-7844(01)01354-0. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Ramos L. Olivier F. Delke I. Kaunitz AM. Labor induction versus expectant management for postterm pregnancies: A systematic review with meta-analysis. Obstet Gynecol. 2003;101:1312–1318. doi: 10.1016/s0029-7844(03)00342-9. [DOI] [PubMed] [Google Scholar]
- 29.Caughey AB. Musci TJ. Complications of term pregnancies beyond 37 weeks of gestation. Obstet Gynecol. 2004;103:57–62. doi: 10.1097/01.AOG.0000109216.24211.D4. [DOI] [PubMed] [Google Scholar]
- 30.Heimstad R. Romundstad PR. Eik-Nes SH. Salvesen KA. Outcomes of pregnancy beyond 37 weeks of gestation. Obstet Gynecol. 2006;108:500–508. doi: 10.1097/01.AOG.0000227783.65800.0f. [DOI] [PubMed] [Google Scholar]
- 31.Caughey AB. Washington AE. Laros RK. Neonatal complications of term pregnancy: Rates by gestational age increase in a continuous, not threshold, fashion. Am J Obstet Gynecol. 2005;192:185–190. doi: 10.1016/j.ajog.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson JM. Kellar LC. Kellar GM. The impact of the interaction between increasing gestational age and obstetrical risk on birth outcomes: Evidence of a varying optimal time of delivery. J Perinatol. 2006;26:392–402. doi: 10.1038/sj.jp.7211528. [DOI] [PubMed] [Google Scholar]
- 33.Caughey AB. Bishop JT. Maternal complications of pregnancy increase beyond 40 weeks of gestation in low-risk women. J Perinatol. 2006;26:540–545. doi: 10.1038/sj.jp.7211560. [DOI] [PubMed] [Google Scholar]
- 34.Ramsey MM. James DK. Steer PJ. Weiner CP. Gonik B. Normal values in pregnancy. London: WB Saunders; 1996. [Google Scholar]
- 35.Nicholson JM. Parry S. Caughey AB. Rosen S. Keen A. Macones GA. The impact of the active management of risk in pregnancy at term on birth outcomes: A randomized clinical trial. Am J Obstet Gynecol. 2008;198:511.e1–511.e15. doi: 10.1016/j.ajog.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]