SUMMARY
In this issue of Structure, Frank and colleagues (Taylor et al., 2009) present the most complete model of a eukaryotic ribosome to date. This achievement represents a critical milestone along the path to structurally defining the unique aspects of the eukaryotic protein synthetic machinery.
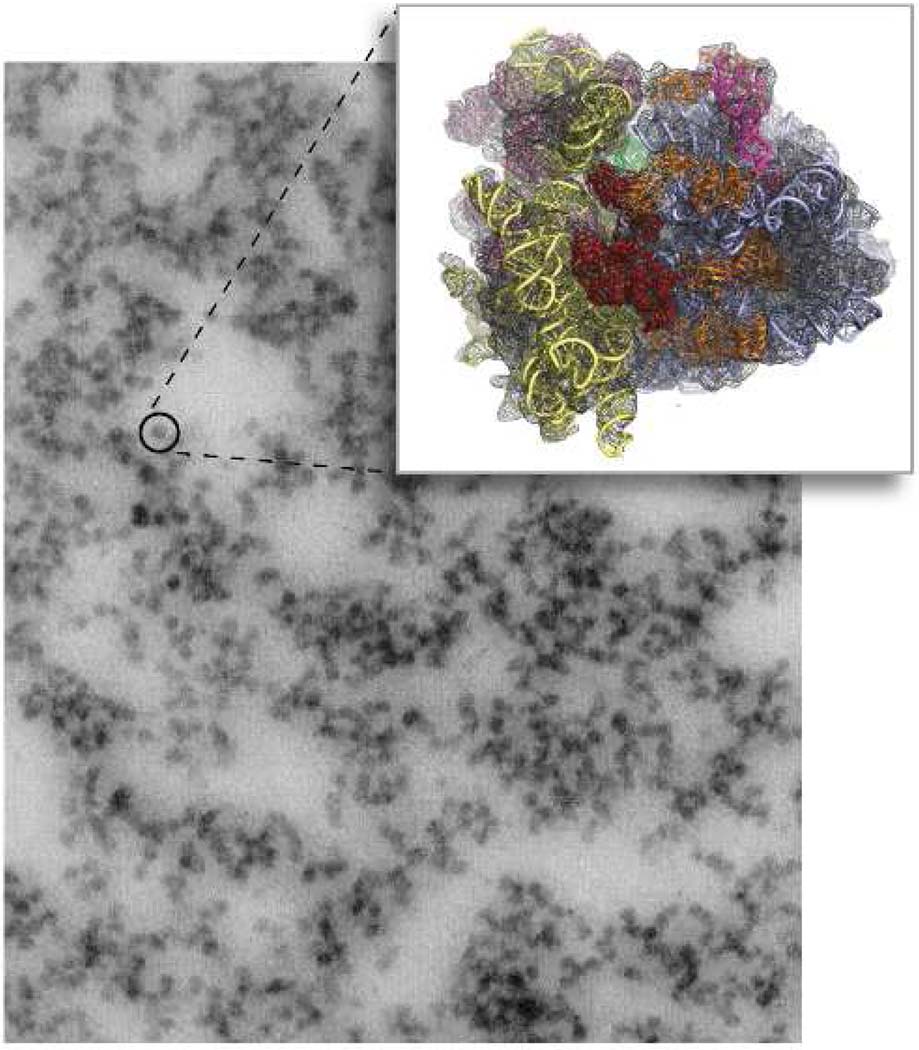
Since Palade and co-workers used transmission electron microscopy (EM) methods to visualize eukaryotic ribosomes (Fig. 1, Kirsch et al., 1960), our modern understanding of the relationship between molecular structure and function have time and again returned to analysis of this ancient molecule. Ribosomes serve a fundamental role in all kingdoms of life as the platform for the synthesis of proteins. While peptide bond formation occurs as an intrinsic activity of the ribosome, the efficiency and accuracy of the initiation, elongation and termination steps of translation require the interaction of the ribosome with soluble protein factors and aminoacylated transfer RNAs. Thus understanding the structures of the factors and the ribosome, as well as the structural and functional interactions between these essential components of the translational apparatus, is essential for understanding this key step in gene regulation.
Figure 1. The eukaryotic ribosome over 40 years.
The pioneering work of Palade and co workers demonstrated ribonucleic particles from eukaryotes (Kirsch et al., 1960), rats), which has evolved (insert) into molecular level understanding of the ribosome (Taylor et al., 2009) showing the 80S density map (mesh) and fitted atomic structure, reproduced from (Frank, 2009). Structure in front, in red, is eEF2. Courtesy Lila Rubenstein and Joachim Frank.
Enhanced computational resources and technical prowess have dramatically expanded our ability to solve extremely complex molecular structures. Currently, X-ray crystallography and cryo-EM provide complementary approaches for meshing atomic resolution structure with conformational dynamics. As evidenced by the Nobel Prizes recently awarded for the atomic scale resolution of archael and eubacterial ribosome structures (Wittmann et al., 1982; Ban et al., 2000; Brodersen et al., 2001), the ribosome has re-emerged as the “molecule of the century”. The past thirty years have seen tremendous advances in single particle reconstruction applied elegantly to the ribosome (Frank, 2009). Looking forward, determining the structure of the eukaryotic ribosome represents the next major achievement. The work by Frank and coworkers (Taylor et al., 2009) represents a critical milestone toward this goal, identifying structural elements specific to eukaryotic ribosomes. In addition, it highlights the key advances that occur when the structure is considered in the presence of trans acting factors. This includes eukaryotic Elongation Factors for which all the X-ray structures are known and for which a battery of mutants alleles in yeast are available (Taylor et al., 2006), using a method that can shed light on the dynamics of the steps in protein synthesis.
The current study employed a combination of cryo-EM and structural homology modeling to create the most comprehensive model of the eukaryotic ribosome to date. Based on a structure initially determined by Taylor et al. (Taylor et al., 2007), this work partnered several teams with key tools and insights into modeling the eukaryotic ribosome and continuing the task of fitting eukaryote-specific ribosomal proteins (rps) into the overall scheme. It provides a critical update of the eukaryotic ribosome structure and pays careful attention to those parts that have remained mysterious even in the context of recent structural studies. A critical breakthrough in this work was the fitting of Saccharomyces cerevisiae rRNA and rp sequence into the Thermomyces lanuginosus structure by capitalizing on their being over 85% identical. As such, it is a terrific boon to the eukaryotic ribosome community in general, and provides a well defined roadmap for yeast geneticists to test the hypotheses developed from these models,
One of the major differences between archael/eubacterial ribosomes and their eukaryotic counterparts is size: eukaryotic ribosomes are significantly larger, primarily due to the presence of and additional 12 small and 41 large ribosomal subunit rRNA-based structures known as expansion segments (ES). The structural elucidation of these is of tremendous value as they give form to elements that are thought to play important roles in both the enormously complex eukaryotic ribosome biogenesis program, and as receptors for eukaryote-specific ribosomal protein and trans-acting regulatory factors. The new resolution allows not only for enhanced visualization of major ES, such as the 18S ES6, but also for ES-ES interactions to be proposed such as between 18S ES3 and ES6. The location of 18S ES7 supports a role in recruiting the more complicated eukaryotic initiation factors. Previously merged S. cerevisiae 25S ES7 and ES39 regions are now found to be not only distinct from, but in different conformations, as compared to the thermophilic and more stable T. lanuginosus ribosomes.
The identification of additional (but not all) rps is tremendous useful for researchers employing the power of yeast molecular genetics. The clustering of the ES structures with eukaryotic specific rps indicates their likely link in ribosome function and assembly. In addition, Supplemental Table 1 in Taylor et al., 2009 represents a “Rosetta Stone” for translating eubacterial, archael, and eukaryotic ribosomal protein equivalents, and already adorns numerous surfaces of our laboratories as a quick reference guide. The docking of available atomic resolution apo-structures of unique eukaryotic rps onto the ribosome greatly enhances our understanding of these elements. For example, this analysis clearly reveals details of the interaction between the rp components of the rpL1 stalk base, rpP0 and eEF2. Similarly, docking of the RACK complex onto the small subunit reveals unique functional interactions between these two elements that could not be discerned in previous structures. The localization of rpS19, utilizing the Pyrococcus abyssi RPS19 structure (Gregory et al., 2007), allows for localization of a protein linked to Diamond-Blackfan anemia (DBA, see Taylor et al., 2006). This allows for a structural interpretation and genetic dissection of the interface between this clearly important rp and the 18S rRNA. This analysis also demonstrates the value of individual structures of the “missing” rps and the importance of ribosome structures determined in the context of associated factors like eEF2. Hypotheses are developed when modeling allows for 2 potential locations e.g. for rpS24e, integrating biochemical and immunolocalization data. While some limitations still exist to mapping the apo-rp structures onto the model, this represents an important advancement in the field and highlights the need for more structures to augment our understanding.
The supplemental information is of great value, and should not be dismissed. The discussion of the rps19e mutations associated with DBA is highly insightful, and is the first example of human mutations whose functional consequences may benefit from this analysis. The extended discussions of yeast large subunit ribosomal proteins, the ES structures, and the changes in the B1b/B1c bridge during intersubunit ratcheting serve as a timely complement to the functional, biochemical and structural analyses of rpl11p mutants in this region currently being analyzed in the Dinman laboratory.
It is clear that the eukaryotic ribosome represents the next great challenge in the structural analysis of translation. The foundation for this effort is strong: the high resolution structures of bacterial ribosomes illuminate many of the conserved elements of the structure and function, while the continuing evolution of the cryo EM structures and modeling techniques reveal the structurally dynamic nature of this complex molecular machine. The ability to identify and clarify expansion of both the rRNA and protein repertoire in the eukaryotic ribosome promises to illuminate critical new information, e.g. locations of mutations linked to human diseases and structural elements critical for eukaryotic regulatory processes. The use of fungal systems also establishes a linkage to the wealth of genetic and biochemical information generated from many laboratories exploring rRNA processing and ribosome assembly to the structural biology and the functional biochemistry of these unique rRNA and rp expansions (Dinman, 2009). In summary, Frank and co-workers clearly establish the unanswered questions in the eukaryotic ribosome structure research, and demonstrate a pathway to their solution. There are even more challenges ahead, including how the eukaryotic specific rps and rRNA ES may affect other ribosome-associated processes such as IRES mediated translation and miRNA directed regulation of gene expression. This work represents both a rich source of new information and a challenge in the quest to understand the eukaryotic ribosome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- Brodersen DE, Carter AP, Clemons WM, Jr, Morgan-Warren RJ, Murphy FV, Ogle JM, Tarry MJ, Wimberly BT, Ramakrishnan V. Atomic structures of the 30S subunit and its complexes with ligands and antibiotics. Cold Spring Harb.Symp.Quant.Biol. 2001;66:17–32. doi: 10.1101/sqb.2001.66.17. [DOI] [PubMed] [Google Scholar]
- Dinman JD. The eukaryotic ribosome: current status and challenges. J.Biol.Chem. 2009;284:11761–11765. doi: 10.1074/jbc.R800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J. Single-particle reconstrution of biological macromolecules in electron microscopy - 30 years. Quarterly Reviews of Biophysics. 2009 doi: 10.1017/S0033583509990059. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory LA, Aguissa-Toure AH, Pinaud N, Legrand P, Gleizes PE, Fribourg S. Molecular basis of Diamond-Blackfan anemia: structure and function analysis of RPS19. Nucleic Acids Res. 2007;35:5913–5921. doi: 10.1093/nar/gkm626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch JF, Siekevitz P, Palade GE. Amino acid incorporation in vitro by ribonucleoprotein particles detached from guinea pig liver microsomes. J.Biol.Chem. 1960;235:1419–1424. [PubMed] [Google Scholar]
- Taylor DJ, Devkota B, Huang AD, Topf M, Narayanan E, Sali A, Harvey SC, Frank J. Comprehensive molecular structure of the eukaryotic ribosome. Structure. 2009 doi: 10.1016/j.str.2009.09.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, Frank J, Kinzy TG. Eukaryotic Ribosomes and the Elongation Pathway. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational control of gene expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 59–85. [Google Scholar]
- Taylor DJ, Nilsson J, Merrill AR, Andersen GR, Nissen P, Frank J. Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation. EMBO J. 2007;26:2421–2431. doi: 10.1038/sj.emboj.7601677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann HG, Mussig J, Piefke J, Gewitz HS, Rheinberger HJ, Yonath A. Crystallization of Escherichia coli ribosomes. FEBS Lett. 1982;146:217–220. doi: 10.1016/0014-5793(82)80739-4. [DOI] [PubMed] [Google Scholar]