Abstract
We have found repeatedly that medial septal (MS) infusions of glucose impair memory when co-infused with the γ-amino butyric acid (GABA) agonist muscimol. The present experiment sought to determine whether the memory-impairing effects of this concentration of glucose would generalize to another GABAA receptor agonist and to an agonist from another neurotransmitter system that is known to impair memory. Specifically, we determined whether the dose of glucose that produces memory deficits when combined with muscimol in the MS would also impair memory when co-infused with the GABAA receptor modulator chlordiazepoxide (CDP) or the opiate morphine. Male Sprague-Dawley rats were given MS co-infusions and then 15 min later tested for spontaneous alternation or given shock avoidance training (retention tested 48 hours later). The results showed that MS infusions of the higher dose of glucose with morphine did not produce memory deficits, whereas, the performance of rats given MS co-infusions of CDP with glucose was impaired. These findings suggest that the memory-impairing effects of brain glucose administration may involve an interaction with the GABAA receptor.
Keywords: Muscimol, GABA, Septum, Spontaneous Alternation, Inhibitory Avoidance, Morphine, Chlordiazepoxide, Benzodiazepine, Memory
Glucose typically has a positive effect on memory [1–3]; however, evidence is accumulating that under some conditions, elevations in glucose can be deleterious to memory in rodents and humans [2, 4–12]. The mechanisms underlying the memory-modulating effects of glucose, particularly the conditions that differentiate the memory-enhancing and -impairing effects of glucose, are poorly understood.
The memory-modulating effects of peripheral elevations in glucose are mediated, at least in part, via an effect on the brain [13–20]. One method that has been used extensively to understand the neural mechanisms underlying the mnemonic effects of glucose is to infuse small volumes of glucose into specific brain areas. The results of such studies indicate that glucose can have positive or negative effects on memory, that the effects of glucose vary by brain region, and that glucose does not interact with all memory-modulating substances in the same way [15, 16, 18, 19, 21–24]. For instance, in the medial septum (MS), infusions of glucose reverse the memory-impairing effects produced by co-infusions of morphine [21, 25–28], but exacerbate the memory deficits produced by GABA-A receptor agonist muscimol and interact with sub-effective doses of muscimol to impair memory [18, 19, 23, 24].
The dose of glucose (~ 18 nmol) that reverses memory deficits produced by morphine and galanin [16, 29] is smaller than the dose of glucose that produces memory deficits when combined with muscimol (33 nmol; [18, 30–32]). Therefore, the goal of Experiment 1 was to test whether the higher 33 nmol dose of glucose would produce memory deficits when combined with the opiate agonist morphine. It is not known whether the memory-impairing interaction between muscimol and glucose is specific to muscimol or can be generalized to other drugs that affect the GABAA receptor. If GABAA receptor activation is required for the memory-impairing effects of glucose, then co-infusions of glucose with other GABAA receptor agonists should also impair memory. Benzodiazepines have a binding site on the GABAA receptor and enhance the inhibitory effects of GABA [33, 34]. Enhancement of MS GABAA receptor activity by benzodiazepines, such as chlordiazepoxide (CDP), also impair memory [35, 36]. The goal of the Experiment 2 was to determine whether medial MS infusions of glucose would produce memory deficits when co-infused with sub-effective doses of CDP as they do with muscimol.
Experiment 1
Methods
The goal of Experiment 1 was to determine whether the dose of glucose that consistently impairs memory when co-infused with the GABA agonist muscimol [18, 30– 32, 37], would also produce memory deficits when co-infused with the opiate morphine.
Subjects
Sixty-three (n = 12–17 per group) male Sprague-Dawley-derived rats (Charles River, Wilmington, MA) were used for the SA task and 55 (n = 9–12 per group) were used for the continuous multiple trial inhibitory avoidance (CMIA) task. CMIA training was given at least 3 days after SA was assessed. The two behavioral tasks were used because they both depend on the integrity of the MS [18, 20, 38], and because they assess different types of memory that vary in motivational, temporal, and cognitive demands. This allowed us to determine whether the manipulations affected memory rather than some process that influences performance on a memory task.
The rats weighed 200–250 g upon arrival and were housed individually in polycarbonate cages (20×40×20 cm) with corncob bedding. They were located in a temperature-controlled colony room (70–74°F) and kept on a 12 hour light-dark cycle (lights on at 7:00 a.m.). The rats had free access to food and water and were acclimated to lab conditions for approximately 1 week prior to surgery. The Georgia State University Institutional Animal Care and Use Committee (IACUC) approved all procedures involving rats.
Surgery
Rats were placed in a clear, plastic gas induction chamber and anesthetized with 5% isoflurane gas (Baxter, Deerfield, IL) delivered in 1000 ml/minute medical grade oxygen. After the rat was no longer ambulatory, it was removed from the chamber and placed on a face-mask that delivered 3% isoflurane gas. Rats were then given injections of atropine sulfate (0.4 mg/kg, ip, Baxter, Deerfield, IL) and penicillin (1500 units, im, Hanfords US Vet, Syracuse, NY). The incision site was shaved with a #50 electric clipper blade (Oster) and betadine solution was applied to the surgical area. Anesthesia was maintained by delivering 1–3% isoflurane gas in 500 ml/minute oxygen to the face-mask. The percentage of isoflurane gas given to the rats was adjusted (from 1–3%) to maintain a surgical plane of anesthesia as determined by the toe pinch test. A stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) that was equipped with an anesthesia mask was used to implant one 22-gauge stainless-steel injection guide cannulae (Plastics One, Inc., Roanoke, VA) aimed at the MS (0.5 mm anterior to bregma, 4.9 mm ventral to dura, and 0 mm from the interaural line; [39]). The incision site was anesthetized with a 2% lidocaine/.001% epinephrine cocktail (0.5–2.0 cc, sc, Abbott Labs, Chicago, IL). After the incision, the 2% lidocaine/.001 % epinephrine cocktail solution (.05– 1.0 cc) was applied topically to the skull to facilitate viewing lambda and bregma. The cannulae were secured to the skull with three jeweler’s screws and a cranioplastic cement and acrylic mixture (Duralay, Worth, IL). A dummy cannula (Plastics One, Roanoke, VA) was inserted to keep the cannulae free of debris. Immediately after surgery, the rats were given an injection of 0.9 % sterile saline (3.0 cc, sc) and the non-steroidal anti�inflammatory analgesic flunixin meglumine (2.5 mg/kg, ip, Fort Dodge Animal Health, Fort Dodge, IA) and then wrapped with a paper towel and kept under a warm lamp (60 W) until recovery from anesthesia. Two days following surgery, the patency of each cannula was checked and betadine was applied to the surgical wound. If signs of infection were evident, the rats were anesthetized with isoflurane gas (5%) delivered in 1000 ml/minute of oxygen and given an additional injection of penicillin (1500 units im).
Drug Preparation and Intracranial Infusions
Two days prior to behavioral testing, the experimenter handled each rat for 2 minutes. Before and after all handling and behavioral testing, the rats were allowed a minimum of 30 minutes to habituate to the laboratory environment. Behavioral testing was conducted between 7:00 a.m. and 7:00 p.m. and drug treatments were counterbalanced over the course of the day. Fifteen minutes prior to behavioral testing, different groups of rats were given MS infusions of vehicle (0.5 µl, 0.5 µl/ minute; phosphate-buffered saline [PBS]; pH = 7.4), glucose (33 nmol), morphine (4 nmol: SA and CMIA or 8 nmol: CMIA; Sigma) or morphine combined with glucose in one solution. The drug solutions were prepared on the day of testing and the drugs that were to be combined in the same solution were prepared at double the desired concentration and then combined, which reduced the concentration of each by half. The drugs were infused through a 28-gauge injection needle that extended 1.0 mm beyond the guide cannulae. The needle was connected to a 25 µl Hamilton syringe by polyethylene tubing (PE-50), and the infusions were delivered using an infusion pump (Harvard Apparatus 11). Following the completion of the injections, the needle was left in place for 1 minute to facilitate drug diffusion. The 33 nmol dose of glucose was selected because it produces memory deficits when infused with the GABA receptor agonist muscimol in the MS [18, 30–32]. The dose of morphine was selected based on pilot data suggesting that 4 nmol dose of morphine is the maximum sub-effective dose (i.e., highest dose that does not cause a statistically significant deficit) for SA and CMIA, and the 8 nmol dose of morphine was chosen because it produces avoidance retention deficits.
Spontaneous Alternation (SA)
SA is assumed to be a hippocampal-dependent measure of spatial working memory [40–46]. The underlying assumption is that in order to alternate successfully between locations the rat must remember its visits to previous arms. This assumption is supported, in part, by data showing that SA is impaired by removing directional cues or by increasing the interval between arm choices [42–44]. Fifteen minutes after the drug injections, SA performance was assessed by placing each rat in a Y-maze composed of three equally spaced arms (60°; 60 cm long × 17.5 cm high). The floor of each arm was composed of stainless steel (3.5 cm wide) and the top (14 cm wide) was covered with a translucent plexiglass lid. All rats were placed in the same starting arm of the Y-maze and allowed to explore the maze freely for 8 minutes. The experimenter, who was blind to drug treatment, recorded the sequence and number of arms the rats entered during the 8 minute period. The maze was cleaned with 70% ethanol after each rat. The number of arms each rat entered was used as a measure of activity. A percent alternation score was computed for all rats that entered at least 10 arms. An alternation was defined as entering three different arms consecutively. The percent alternation score was computed by dividing the number of alternations each rat made by the number of arms entered minus two (i.e., the number of alternations possible) and then multiplying that resulting quotient by 100.
Continuous Multiple Trial Inhibitory Avoidance (CMIA)
CMIA training was given a minimum of 3 days after SA testing and the drug treatments were counterbalanced across the SA and CMIA task. The avoidance apparatus consisted of a trough-shaped alley (91 cm long, 15 cm high, 20 cm wide at the top, and 6.4 cm wide at the bottom) that was divided into a lighted (31 cm long) and a dark (60 cm long) compartment by a retractable guillotine door. The dark compartment had a metal floor through which shock could be delivered. A 15-watt lamp was placed over the lighted compartment and was the only source of illumination in the room. The table underneath the avoidance apparatus was lined with bench paper and the apparatus was cleaned with 70% ethanol after each rat was trained or tested.
For the training, the rat was placed in the lighted compartment with its head facing away from the door. Once the rat turned around to face the door or after 12 seconds (s) passed, the retractable door was opened and the rat was allowed to cross over to the dark (shock) compartment. After the rat crossed with all four paws, the rat was given a footshock (1.2 mA) until it returned to the lighted compartment (maximum 4 s). This sequence constituted one training trial. Training continued until the rat remained in the lighted compartment for 100 consecutive s or for a maximum of 5 trials. The rat was not removed from the avoidance apparatus between trials. The number of trials needed to reach the criterion was recorded and used as a measure of acquisition.
Retention of the training was tested 48 hours (+/− 2 hours) later. For the retention test, the rats were placed in the lighted compartment of the avoidance chamber with their heads facing away from the closed door. After each rat turned to face the door or 12 s passed, the door was opened and the latency (s) to cross over to the dark (shock) compartment was recorded and used as a measure of retention. Each rat was given a maximum of 600 s to enter the dark compartment during the retention test. Footshock was not delivered on the retention test.
Histology
After behavioral testing, the rats were euthanized with an overdose of sodium pentobarbital (Sleepaway; 400 mg/kg, ip, Fort Dodge, Fort Dodge, IA) and perfused intracardially with 0.9% saline followed by 10% formalin. Their brains were stored in a 10% formalin solution for at least 2 days before sectioning. All brains were sectioned on a cryostat (Leica CM 30510 S) and 45–60 µm sections were taken through the MS and hippocampal cannulae tracts. The brain sections were stained with thionin and an observer blind to experimental condition determined the cannulae placement using a light microscope (Olympus BX41). Acceptable MS cannulae placement was defined as injection sites located within the MS, but not within the lateral septum or the ventral diagonal band of Broca. Moreover, the cannula must not have penetrated the fimbria. Only rats with acceptable cannulae placements were included in the statistical analyses.
Statistical Analysis
The data were expressed as means and standard errors of the mean (S.E.M.) and analyzed using a 1-way analysis of variance (ANOVA) and Tukey post hoc tests where appropriate. The acquisition and retention latency data were not normally distributed due to the fact that several of the rats reached the maximum trials to criterion and 600 s retention latency cut-off. Consequently, these data were expressed as medians and inter�quartile ranges (I.Q.) and the non-parametric Kruskal-Wallis and Mann-Whitney U tests were used to detect differences between treatment groups. An alpha level of 0.05 was used as the criterion for statistical significance. Bonferonni corrections were used for the Mann-Whitney U tests based on the number of planned comparisons.
Results
Two rats showed signs of infection 2 days after surgery and thus required reanesthetization to accommodate the intramuscular injection of penicillin. Behavioral testing occurred at least 5 days later. The two rats were in different treatment groups and their behavioral scores were consistent with those of others in their treatment condition.
Figure 1 shows the approximate location of drug infusions in Experiment 1. There were no apparent systematic differences in cannula placement for the different treatment conditions. As Figure 2 shows, the drug infusions into the MS did not significantly affect SA performance [F (3, 62) = 0.36; p > .05] or the number of arms that the rats entered in the maze [F (3,62) = 1.54; p > .05].
Figure 1.
Schematic illustration of coronal sections of the rat brain showing the approximate location of MS infusion sites in Experiment 1. Atlas plates were adapted from Paxinos and Watson (1998).
Figure 2.
Figure 2A. MS co-infusions of glucose with morphine did not significantly decrease mean (+/− S.E.M.) percent alternation scores (p > .05 vs. PBS).
Figure 2B. There were no significant effects of any of the manipulations on the mean (+/− S.E.M.) number of arm entries (p > .05 vs. PBS).
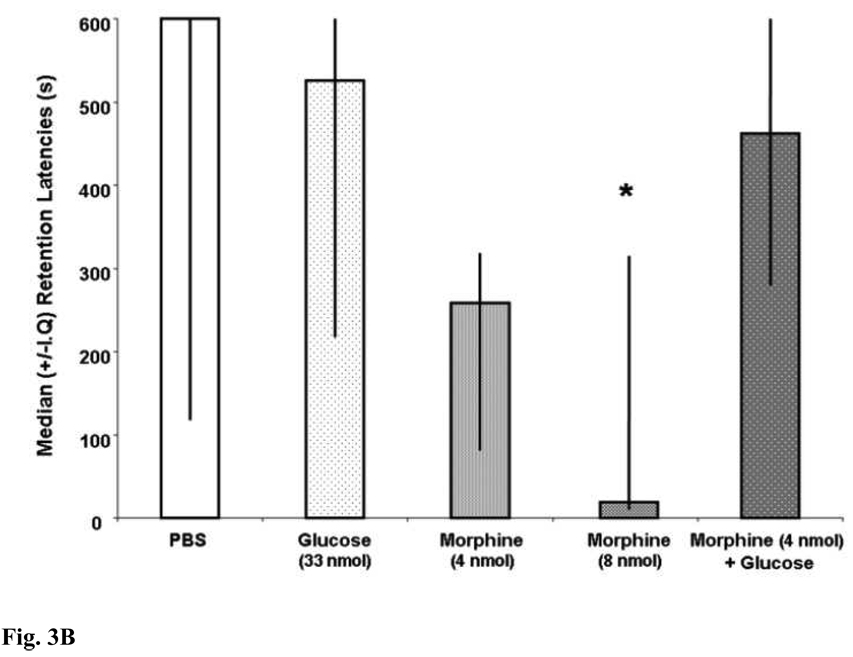
Figure 3A demonstrates that MS drug infusions did significantly affect trials to criterion during CMIA training [H (4,55) = 10.77; p < .05]. After Bonferonni correction (p <.007), the post-hoc tests revealed no significant differences between any of the groups on the number of trial to criterion during CMIA training. Figure 3B illustrates that the pre-training drug infusions into the MS significantly affected subsequent CMIA retention [H (4,55) = 11.47; p < .05]. MS infusions of morphine dose-dependently impaired memory. Specifically, rats that were given MS infusions of 8 nmol of morphine had significantly shorter retention latencies than did those rats that that were given MS infusions of vehicle [U (1,21) = 28; p < .007]. MS infusions of morphine (4 nmol) or glucose alone did not significantly impair CMIA retention. The retention latencies of rats that were given MS infusions of 4 nmol of morphine [U (1,22) = 40, p > .007] or glucose [U (1,19) = 48; p > .007] were not statistically different from those of rats given vehicle. MS infusions of glucose did not produce deficits when combined with morphine. The retention latencies of rats that were given MS infusions of morphine (4 nmol) with glucose did not significantly differ from those of rats that were given MS infusions of vehicle [U (1,22) = 65; p > .007].
Figure 3.
Figure 3A. There were no significant effects of any of the manipulations on the mean (+/− S.E.M.) number of trials to criterion (p > .05 vs. PBS).
Figure 3B. MS infusions of morphine (8 nmol) decreased median (+/− I.Q.) retention latencies (*p < .05 vs. PBS). MS infusions of glucose did not produce deficits when combined with subeffective doses of morphine (p > .05 vs. PBS).
Experiment 2
The goal of Experiment 2 was to determine whether MS infusions of glucose would produce memory deficits when combined with sub-effective doses of CDP as they do with muscimol.
Methods
The same procedures were used as in Experiment 1 with the following exceptions: Fifteen minutes prior to behavioral testing, different groups of rats were given MS infusions of vehicle (0.5 µl, 0.5 µl/ minute; saline, pH = 3.0), phosphate-buffered saline (PBS; pH = 7.4), glucose (33 nmol), CDP (15 nmol: SA or 30 nmol: SA and CMIA, Sigma) or CDP combined with glucose in one solution. The doses of CDP were selected based on pilot data suggesting that 15 nmol of CDP is the maximum sub-effective dose (i.e., highest dose that does not cause a statistically significant deficit) for SA, and the 30 nmol dose of CDP was chosen to produce SA deficits. Although our pilot data indicated that a large range of doses of CDP (15–200 nmol) did not produce shock avoidance retention deficits, the 30 nmol dose of CDP was chosen for the CMIA task because it was the dose that had a tendency to produce a mild deficit. CDP could only go into solution at low pH levels (pH = 3.0); therefore, infusions of PBS (pH = 7.4) served as a pH control in comparison to the saline vehicle (pH=3.0). Eighty-two (n = 11–17 per group) rats were used for the SA task and 56 (n = 9–15 per group) were used for the CMIA task.
Results
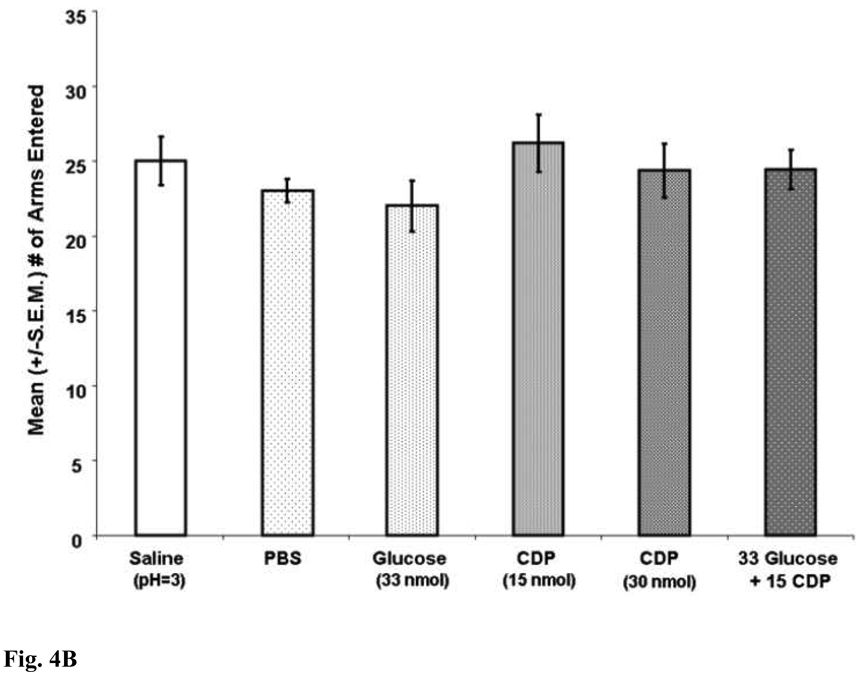
Drug infusions into the MS [F (5, 81) = 3.91; p < .05] significantly affected SA performance (see Figure 4A). Consistent with previous research, MS infusions of CDP dose-dependently impaired spatial working memory performance [35, 36, 47–50]. Specifically, the percent alternation scores of rats given CDP (30 nmol) were significantly lower than of those rats given MS infusions of vehicle (p < .05). MS infusions of glucose or CDP (15 nmol) alone did not significantly affect SA performance. The percent alternation scores of rats given glucose or CDP (15 nmol) or glucose alone did not differ significantly from those of rats given MS infusions of vehicle (p > .05). Low pH did not likely contribute to the memory-impairing effects of CDP because the percent alternation scores of rats given low pH saline (pH = 3.0) were not significantly different from those of rats given MS infusions of PBS (p > .05). Interestingly, the findings showed that MS co-infusions of glucose with CDP (15 nmol), at doses that produced no affect alone, significantly impaired SA performance. Specifically, the percent alternation scores of rats given glucose with CDP (15 nmol) in the same solution were significantly lower than those of rats given MS infusions of vehicle (p < .05). The same drug infusions into the MS did not significantly affect the number of arms that the rats entered in the maze ([F (5,81) = .98; p > .05]; see Figure 4B).
Figure 4.
Figure 4A. MS infusions of CDP (30 nmol) significantly decreased mean (+/− SEM) percent alternation scores (*p < .05 vs. saline). More importantly, MS co-infusions of CDP (15 nmol) with glucose, at doses that had no affect on their own (p > .05 vs. saline), significantly decreased mean percent alternation scores (*p < .05 vs. saline).
Figure 4B. MS infusions of CDP did not significantly impair mean (+/−S.E.M.) number of arm entries (p > .05 vs. saline).
Drug infusions into the MS significantly affected the number of trials to criterion in CMIA training ([H (4,55) = 11.750; p < .05]; see Figure 5A). After Bonferonni correction (p <.017), the post-hoc tests revealed no significant differences between any of the groups on the number of trial to criterion during CMIA training. Figure 5B shows that drug infusions into the MS also did not significantly affect avoidance retention latencies [H (4, 55) = 3.421; p > .05].
Figure 5.
Figure 5A. MS infusions of CDP did not significantly impair median (+/−I.Q.) trials to criterion (p > .05 vs. saline).
Figure 5B. MS infusions of CDP did not significantly impair median (+/−I.Q.) retention latencies (p > .05 vs. saline).
Discussion
The present findings show that the same dose of glucose that produces memory deficits when combined with the GABA agonist muscimol [18, 23] does not produce deficits when combined with the opiate morphine (present findings). Importantly, the present findings show that MS co-infusions of glucose do impair memory when combined with the benzodiazepine CDP. The memory-impairing interaction between glucose and CDP was observed in the spatial working memory task, but not in the emotional, long-term memory task. These findings add to the growing body of evidence indicating that medial MS co-infusions of glucose produce memory deficits when combined with drugs that influence medial MS GABA receptors [18, 19, 24, 51]. These data suggest that the memory-impairing effects of glucose may involve activation of the GABA neurotransmitter system.
The present findings demonstrated that MS infusions of the GABAA modulator CDP impair spontaneous alternation in a dose-dependent manner. These results are consistent with previous research showing that systemic and medial MS infusions of CDP impair spatial working memory [35, 36, 47, 50, 52]. More importantly, the present data show that co-infusions of glucose with CDP, at doses that individually have no effect, impair spontaneous alternation performance. The deficits appear to reflect a spatial working memory deficit, rather than a performance deficit, because the manipulations affected the sequence of arms entered, but not the number of arms entered.
To our knowledge, there are not any studies showing that MS infusions of CDP impair shock avoidance (although systemic infusions do impair shock avoidance; [53]). This finding is consistent with a recent finding that MS infusions of the hyperpolarization-activated cyclic nucleotide-gated channel blocker ZD7288 impair spontaneous alternation, but not inhibitory avoidance [54]. It is not clear why CDP and ZD7288 have these task-dependent effects on memory. One possibility is that shock avoidance memory can be based on the formation of different types of associations mediated by different brain regions. That is, when MS function is impaired by CDP or ZD7288, other brain areas may mediate the spared retention that is observed. For instance, avoidance retention can reflect stimulus-stimulus associations mediated by the hippocampus [55–62], stimulus-affect associations mediated by the amygdala [63–67], or stimulus-response associations mediated by the striatum [67–74]. It is not clear, though, why CDP and ZD7288 do not produce deficits when MS infusions of morphine or muscimol do. Another possibility is that HCN channels and GABAA receptor modulation are involved in working memory, but not long term memory.
There are multiple potential mechanisms that may contribute to the memory-modulating effects of glucose. The negative effects of the higher glucose concentration are not likely due to extracellular hyperosmolarity, because previous research has shown that equiosmolar concentrations of other sugars do not produce memory deficits when combined with muscimol [31, 32]. This interpretation is supported by the present finding showing that infusions of the same concentration of glucose into the MS did not produce memory deficits when combined with morphine.
The fact that the interaction between elevated glucose and GABA receptor activation in the MS is synergistic suggests that both are acting on a common mechanism to impair memory [75]. One candidate is glucose metabolism, because GABA agonists inhibit brain glucose metabolism [76, 77]. This possibility seems unlikely, though, because both morphine and muscimol inhibit glucose metabolism [76, 78], yet glucose potentiates deficits produced by muscimol, but prevents deficits produced by morphine. A second possibility is that the synergistic interaction is somehow due to decreases in the membrane potential produced by the various GABA agonists. This is also not likely because glucose reverses deficits produced by galanin and morphine [16, 29, 79], two peptides that also hyperpolarize neurons [80, 81]. Glucose could perhaps impair memory by directly influencing the binding properties of the GABA receptor, although there is no evidence to date showing that glucose can do so. There is evidence, though, showing that glucose does reduce the binding of morphine to opioid receptors [82], which could account for the ability of glucose to reverse morphine-induced memory deficits. Another possibility is that adenosine tri-phosphate (ATP)-dependent potassium (K+) channels (K+-ATP) are involved in the negative effects of glucose on memory. Glucose closes K-ATP channels [83–85] and affects neurotransmitter release [86, 87]. It is not clear how K-ATP channels could participate in the memory–impairing effects of glucose, however, because the opening of the MS K-ATP channels impairs memory [16, 22, 88, 89] and blocking MS K-ATP channels with glucose enhances memory and reverses memory deficits [16, 22, 88, 89].
The present finding adds to the growing evidence indicating that glucose, despite its traditional role as an energy source, does not have global effects on memory but rather influences memory in a specific manner. Collectively, the evidence indicates that the effects of glucose on memory depend on the chemical systems that are activated, the brain region in which glucose is elevated, and the type of memory process that is involved. For instance, elevating glucose in the amygdala reverses deficits produced by MS infusions of morphine in spontaneous alternation, but not in inhibitory avoidance [90]. Similarly, elevating glucose in the striatum impairs place learning, but has no effect on response learning [91]. In the amygdala, glucose reverses memory deficits produced by the opioid morphine [92], but not the beta-noradrenergic antagonist propranolol [93]. Likewise, when infused into the MS, glucose reverses deficits produced by morphine [21, 25], galanin [16], but not those produced by the GABA agonist muscimol [18, 19, 23, 24].
In summary, MS co-infusions of glucose with the GABAA modulator CDP, at doses that individually have no effect on memory, produce spatial working memory deficits. In contrast, MS co-infusions of the same concentration of glucose with the opiate morphine do not produce spatial working memory deficits or emotional, long-term memory deficits. Collectively, these findings suggest that the memory-impairing effects of glucose in the MS may be specific to GABA receptor activation.
Acknowledgements
This research was supported in part by grants from NINDS-HIDDK-JDF (RO1NS41173-02), the STC Program of the National Science Foundation under Agreement No. IBN-9876754, and the Georgia State University Brains and Behavior Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korol DL. Enhancing cognitive function across the life span. Annals of the New York Academy of Sciences. 2002;959:167–179. doi: 10.1111/j.1749-6632.2002.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 2.Messier C. Glucose improvement of memory: a review. Eur J Pharmacol. 2004;490(1–3):33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 3.Korol DL, Gold PE. Epinephrine converts long-term potentiation from transient to durable form in awake rats. Hippocampus. 2008;18(1):81–91. doi: 10.1002/hipo.20372. [DOI] [PubMed] [Google Scholar]
- 4.Craft S, Murphy C, Wemstrom J. Glucose effects and glucose regulation in memory and nonmemory tasks: The influence of age, sex, regulation, and glucoregulatory response. Psychobiology. 1994;22:95–105. [Google Scholar]
- 5.Gold PE, Vogt J, Hall JL. Glucose effects on memory: behavioral and pharmacological characteristics. Behav Neural Biol. 1986;46(2):145–155. doi: 10.1016/s0163-1047(86)90626-6. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez WA, et al. Comparable dose-response functions for the effects of glucose and fructose on memory. Behav Neural Biol. 1994;61(2):162–169. doi: 10.1016/s0163-1047(05)80070-6. [DOI] [PubMed] [Google Scholar]
- 7.Craft S, et al. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci. 1993;107(6):926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- 8.Awad N, et al. Impact of peripheral glucoregulation on memory. Behav Neurosci. 2002;116(4):691–702. doi: 10.1037/0735-7044.116.4.691. [DOI] [PubMed] [Google Scholar]
- 9.Vanhanen M, et al. Risk for non-insulin-dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. Neuroreport. 1997;8(6):1527–1530. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- 10.Gradman TJ, et al. Verbal learning and/or memory improves with glycemic control in older subjects with non-insulin-dependent diabetes mellitus. J Am Geriatr Soc. 1993;41(12):1305–1312. doi: 10.1111/j.1532-5415.1993.tb06480.x. [DOI] [PubMed] [Google Scholar]
- 11.Meneilly GS, et al. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol. 1993;48(4):M117–M121. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- 12.Naor M, et al. Cognitive function in elderly non-insulin-dependent diabetic patients before and after inpatient treatment for metabolic control. J Diabetes Complications. 1997;11(1):40–46. doi: 10.1016/1056-8727(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 13.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proceedings of the National Academy of Sciences U S A. 2000;97(6):2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MK, Graham SN, Gold PE. Memory enhancement with posttraining intraventricular glucose injections in rats. Behavioral Neuroscience. 1988;102(4):591–595. doi: 10.1037//0735-7044.102.4.591. [DOI] [PubMed] [Google Scholar]
- 15.Ragozzino ME, et al. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. The Journal of Neuroscience. 1998;18(4):1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefani MR, Gold PE. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Research. 1998;813(1):50–56. doi: 10.1016/s0006-8993(98)00876-2. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur J Neurosci. 2003;17(7):1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- 18.Parent M, et al. Intraseptal infusions of muscimol impair spontaneous alternation performance: Infusions of glucose into the hippocampus, but not the medial septum, reverse the deficit. Neurobiology of Learning and Memory. 1997;68(1):75–85. doi: 10.1006/nlme.1997.3769. [DOI] [PubMed] [Google Scholar]
- 19.Shah AA, Parent MB. Septal infusions of glucose or pyruvate, but not fructose, produce avoidance deficits when co-infused with the GABA agonist muscimol. Neurobiology of Learning and Memory. 2003;79(3):243–251. doi: 10.1016/s1074-7427(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 20.Krebs DL, Parent MB. The enhancing effects of hippocampal infusions of glucose are not restricted to spatial working memory. Neurobiol Learn Mem. 2005;83(2):168–172. doi: 10.1016/j.nlm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68(4):981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- 22.Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: a potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93(2):557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 23.Parent MB, Gold PE. Intra-septal infusions of glucose potentiate inhibitory avoidance deficits when co-infused with the GABA agonist muscimol. Brain Research. 1997;745(1–2):317–320. doi: 10.1016/s0006-8993(96)01206-1. [DOI] [PubMed] [Google Scholar]
- 24.Shah AA, Parent MB. Septal infusions of glucose or pyruvate with muscimol impair spontaneous alternation. Brain Research. 2004;996(2):246–250. doi: 10.1016/j.brainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Ragozzino ME, Parker ME, Gold PE. Spontaneous alternation and inhibitory avoidance impairments with morphine injections into the medial septum. Attenuation by glucose administration. Brain Research. 1992;597(2):241–249. doi: 10.1016/0006-8993(92)91480-3. [DOI] [PubMed] [Google Scholar]
- 26.Ragozzino ME, Gold PE. Task-dependent effects of intra-amygdala morphine injections: attenuation by intra-amygdala glucose injections. J Neurosci. 1994;14(12):7478–7485. doi: 10.1523/JNEUROSCI.14-12-07478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ragozzino ME, et al. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci. 1995;109(6):1074–1080. doi: 10.1037//0735-7044.109.6.1074. [DOI] [PubMed] [Google Scholar]
- 28.McNay EC, et al. Modulation of memory with septal injections of morphine and glucose: effects on extracellular glucose levels in the hippocampus. Physiology and Behavior. 2006;87(2):298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Ragozzino ME, Parker ME, Gold PE. Spontaneous alternation and inhibitory avoidance impairments with morphine injections into the medial septum. Attenuation by glucose administration. Brain Res. 1992;597(2):241–249. doi: 10.1016/0006-8993(92)91480-3. [DOI] [PubMed] [Google Scholar]
- 30.Parent MB, Gold PE. Intra-septal infusions of glucose potentiate inhibitory avoidance deficits when co-infused with the GABA agonist muscimol. Brain Res. 1997;745(1–2):317–320. doi: 10.1016/s0006-8993(96)01206-1. [DOI] [PubMed] [Google Scholar]
- 31.Shah AA, Parent MB. Septal infusions of glucose or pyruvate with muscimol impair spontaneous alternation. Brain Res. 2004;996(2):246–250. doi: 10.1016/j.brainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Shah AA, Parent MB. Septal infusions of glucose or pyruvate, but not fructose, produce avoidance deficits when co-infused with the GABA agonist muscimol. Neurobiol Learn Mem. 2003;79(3):243–251. doi: 10.1016/s1074-7427(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 33.Choi DW, Farb DH, Fischbach GD. Chlordiazepoxide selectively potentiates GABA conductance of spinal cord and sensory neurons in cell culture. J Neurophysiol. 1981;45(4):621–631. doi: 10.1152/jn.1981.45.4.621. [DOI] [PubMed] [Google Scholar]
- 34.Duman RS, et al. Molecular biology of inhibitory amino acid receptors. Mol Neurobiol. 1987;1(1–2):155–189. doi: 10.1007/BF02935267. [DOI] [PubMed] [Google Scholar]
- 35.Stackman RW, Walsh TJ. Anatomical specificity and time-dependence of chlordiazepoxide-induced spatial memory impairments. Behav Neurosci. 1995;109(3):436–445. doi: 10.1037//0735-7044.109.3.436. [DOI] [PubMed] [Google Scholar]
- 36.Stackman RW, Walsh TJ. Distinct profile of working memory errors following acute or chronic disruption of the cholinergic septohippocampal pathway. Neurobiol Learn Mem. 1995;64(3):226–236. doi: 10.1006/nlme.1995.0005. [DOI] [PubMed] [Google Scholar]
- 37.Parent M, Krebs D. The memory-enhancing effects of hippocampal glucose infusions override the memory-impairing effects of septal glucose infusions. XXVIII Winter Conference on the Neurobiology of Learning and Memory; Park City, UT. 2004. [Google Scholar]
- 38.Krebs DL, Parent MB. Hippocampal infusions of pyruvate reverse the memory-impairing effects of septal muscimol infusions. Eur J Pharmacol. 2005;520(1–3):91–99. doi: 10.1016/j.ejphar.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth Edition ed. San Diego: Academic Press; 1998. [Google Scholar]
- 40.Stevens R, Cowey A. Effects of dorsal and ventral hippocampal lesions on spontaneous alternation, learned alternation and probability learning in rats. Brain Res. 1973;52:203–224. doi: 10.1016/0006-8993(73)90659-8. [DOI] [PubMed] [Google Scholar]
- 41.Will B, Deluzarche F, Kelche C. Does post-operative environment attenuate or exacerbate symptoms which follow hippocampal lesions in rats? Behav Brain Res. 1983;7(1):125–132. doi: 10.1016/0166-4328(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 42.Richman C, Dember W, Kim P. Spontaneous alternation behavior: A review. Current Psychological Research & Review. 1987;5:358–391. [Google Scholar]
- 43.Dember W. Spontaneous alternation behavior. New York: Springer-Verlag; 1989. [Google Scholar]
- 44.Lalonde R. The neurological basis of spontaneous alternation. Neuroscience and Biobehavioral Reviews. 2002;26(1):91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 45.Deacon RM, et al. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133(1):57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 46.Pacteau C, Einon D, Sinden J. Early rearing environment and dorsal hippocampal ibotenic acid lesions: long-term influences on spatial learning and alternation in the rat. Behav Brain Res. 1989;34(1–2):79–96. doi: 10.1016/s0166-4328(89)80092-0. [DOI] [PubMed] [Google Scholar]
- 47.Stackman RW, Walsh TJ. Chlordiazepoxide-induced working memory impairments: site specificity and reversal by flumazenil (RO15–1788) Behav Neural Biol. 1992;57(3):233–243. doi: 10.1016/0163-1047(92)90206-j. [DOI] [PubMed] [Google Scholar]
- 48.Herzog C, et al. Effects of intraseptal zolpidem and chlordiazepoxide on spatial working memory and high-affinity choline uptake in the hippocampus. Neurobiology of Learning and Memory. 2000;73:168–179. doi: 10.1006/nlme.1999.3928. [DOI] [PubMed] [Google Scholar]
- 49.Tonkiss J, et al. Chlordiazepoxide-induced spatial learning deficits: dose-dependent differences following prenatal malnutrition. Pharmacol Biochem Behav. 2000;65(1):105–116. doi: 10.1016/s0091-3057(99)00182-3. [DOI] [PubMed] [Google Scholar]
- 50.Tonkiss J, et al. Prenatally protein-malnourished rats are less sensitive to the amnestic effects of medial septal infusions of chlordiazepoxide. Behav Pharmacol. 2000;11(6):437–446. doi: 10.1097/00008877-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Erickson EJ, Watts KD, Parent MB. Septal co-infusions of glucose with a GABAB agonist impair memory. Neurobiol Learn Mem. 2006;85(1):66–70. doi: 10.1016/j.nlm.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farber HT. Chlordiazepoxide improves the performance of septal lesioned but not hippocampal lesioned animals in a Morris maze. Brain Res. 1996;725(2):257–262. doi: 10.1016/s0006-8993(96)00346-0. [DOI] [PubMed] [Google Scholar]
- 53.Tohyama K, et al. Involvement of GABAergic systems in benzodiazepine-induced impairment of passive avoidance learning in mice. Psychopharmacology (Berl) 1991;105(1):22–26. doi: 10.1007/BF02316859. [DOI] [PubMed] [Google Scholar]
- 54.Cisse RS, Krebs-Kraft DL, Parent MB. Septal infusions of the hyperpolarization-activated cyclic nucleotide-gated channel (HCN-channel) blocker ZD7288 impair spontaneous alternation but not inhibitory avoidance. Behav Neurosci. 2008;122(3):549–556. doi: 10.1037/0735-7044.122.3.549. [DOI] [PubMed] [Google Scholar]
- 55.Compton DM. Encoding of a nonmonotonic serial pattern: role of the dorsal hippocampus and amygdala. Physiol Behav. 1993;53(4):657–665. doi: 10.1016/0031-9384(93)90170-k. [DOI] [PubMed] [Google Scholar]
- 56.Compton DM, et al. The flexible use of multiple cue relationships in spatial navigation: a comparison of water maze performance following hippocampal, medial septal, prefrontal cortex, or posterior parietal cortex lesions. Neurobiol Learn Mem. 1997;68(2):117–132. doi: 10.1006/nlme.1997.3793. [DOI] [PubMed] [Google Scholar]
- 57.Eichenbaum H. The hippocampal system and declarative memory in animals. Journal of Comparative Nerology. 1992;4:217–231. doi: 10.1162/jocn.1992.4.3.217. [DOI] [PubMed] [Google Scholar]
- 58.Eichenbaum H, et al. Hippocampal system dysfunction and odor discrimination learning in rats: impairment of facilitation depending on representational demands. Behavioral Neuroscience. 1988;102:331–339. doi: 10.1037//0735-7044.102.3.331. [DOI] [PubMed] [Google Scholar]
- 59.Eichenbaum H, Matthews P, Cohen NJ. Further studies of hippocampal representation during odor discrimination learning. Behavioral Neuroscience. 1989;103:1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- 60.Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in place learning. Journal of Neuroscience. 1991;10:3531–3142. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behav Biol. 1974;12:421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- 62.Sutherland RJ, Rudy JW. Configural association theory: the role of the hippocampal formation in learning, memory, and amnesia. Psychobiology. 1989;17:129–144. [Google Scholar]
- 63.Compton DM. An investigation of the role of the hippocampus and the amygdale in the encoding of a serial pattern: effects of a long interelement interval. Behav Proc. 1995;34:113–128. doi: 10.1016/0376-6357(94)00058-o. [DOI] [PubMed] [Google Scholar]
- 64.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68(3):285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 65.McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behavioral and Neural Biology. 1995;61:260–270. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- 66.McGaughy J, et al. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a five-choice serial reaction time task. The Journal of Neuroscience. 2002;22(5):1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salinas JA, White NM. Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behavioral Neuroscience. 1998;112:812–826. doi: 10.1037//0735-7044.112.4.812. [DOI] [PubMed] [Google Scholar]
- 68.Kirkby RJ, Polgar S, Coyle IR. Caudate nucleus lesions impair the ability of rats to learn a simple straight-alley task. Percept Motor Skills. 1981;52:499–502. doi: 10.2466/pms.1981.52.2.499. [DOI] [PubMed] [Google Scholar]
- 69.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9(5):1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Packard MG, White NM. Lesions of the caudate nucleus selectively impair “reference memory” acquisition in the radial maze. Behav Neural Biol. 1990;53(1):39–50. doi: 10.1016/0163-1047(90)90780-a. [DOI] [PubMed] [Google Scholar]
- 71.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65(1):65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 72.Petri HL, Mishkin M. Behaviorism, cognitivism, and the neuropsychology of memory. Am Sci. 1993;82:3–37. [Google Scholar]
- 73.Phillips AG, Carr GD. Cognition and the basal ganglia: a possible substrate for procedural knowledge. Can J Neurol Sci. 1987;14:381–385. doi: 10.1017/s031716710003777x. [DOI] [PubMed] [Google Scholar]
- 74.Squire LR, Butters N, editors. Memories and habits: some implication for the analysis of learning and retention. Neuropsychology of memory. Guilford Press; New York: 1984. pp. 287–296. [Google Scholar]
- 75.Seeley RJ, Moran TH. Principles for interpreting interactions among the multiple systems that influence food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R46–R53. doi: 10.1152/ajpregu.00021.2002. [DOI] [PubMed] [Google Scholar]
- 76.Ito K, et al. Linear relationship between GABAA receptor occupancy of muscimol and glucose metabolic response in the conscious mouse brain. Clinical implication based on comparison with benzodiazepine receptor agonist. Drug Metab Dispos. 1994;22(1):50–54. [PubMed] [Google Scholar]
- 77.Ishizuka H, et al. Nonlinear relationship between benzodiazepine receptor occupancy and glucose metabolic response in the conscious mouse brain in vivo. J Pharmacol Exp Ther. 1989;251(1):362–367. [PubMed] [Google Scholar]
- 78.Cohen R, Kimes A, London E. Morphine decreases cerebral glucose utillization in limbic and forebrain regions while pain has no effect. Neuropharmacology. 1991;30(2):125–134. doi: 10.1016/0028-3908(91)90195-h. [DOI] [PubMed] [Google Scholar]
- 79.Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68(4):981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- 80.Hokfelt T, et al. Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm Metab Res. 1999;31(5):330–334. doi: 10.1055/s-2007-978748. [DOI] [PubMed] [Google Scholar]
- 81.Inturrisi C. Clinical pharmacology of opiods for pain. Clinical Journal of Pain. 2002;18(4 suppl):S3–S13. doi: 10.1097/00002508-200207001-00002. [DOI] [PubMed] [Google Scholar]
- 82.Brase DA, Han YH, Dewey WL. Effects of glucose and diabetes on binding of naloxone and dihydromorphine to opiate receptors in mouse brain. Diabetes. 1987;36(10):1173–1177. doi: 10.2337/diab.36.10.1173. [DOI] [PubMed] [Google Scholar]
- 83.Larsson O, et al. [Mechanisms of action of peroral antidiabetics Sulfonylurea preparations block the ATP-dependent potassium channels] Lakartidningen. 1997;94(48):4473–4477. [PubMed] [Google Scholar]
- 84.Straub SG, et al. Glucose activates both K(ATP) channel-dependent and K(ATP) channel-independent signaling pathways in human islets. Diabetes. 1998;47(5):758–763. doi: 10.2337/diabetes.47.5.758. [DOI] [PubMed] [Google Scholar]
- 85.Valdeolmillos M, et al. The relationship between glucose-induced K+ATP channel closure and the rise in [Ca2+]i in single mouse pancreatic beta-cells. J Physiol. 1992;455:173–186. doi: 10.1113/jphysiol.1992.sp019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.During MJ, et al. Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. J Clin Invest. 1995;95(5):2403–2408. doi: 10.1172/JCI117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amoroso S, et al. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- 88.Rashidy-Pour A. ATP-sensitive potassium channels mediate the effects of a peripheral injection of glucose on memory storage in an inhibitory avoidance task. Behavioral Brain Research. 2001;126(1–2):43–48. doi: 10.1016/s0166-4328(01)00242-x. [DOI] [PubMed] [Google Scholar]
- 89.Stefani MR, Gold PE. Intrahippocampal infusions of K-ATP channel modulators influence spontaneous alternation performance: Relationship to acetylcholine release in the hippocapus. The Journal of Neuroscience. 2001;21(2):609–614. doi: 10.1523/JNEUROSCI.21-02-00609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNay EC, Gold PE. Memory modulation across neural systems: intra-amygdala glucose reverses deficits caused by intraseptal morphine on a spatial task but not on an aversive task. Journal of Neuroscience. 1998;18(10):3853–3858. doi: 10.1523/JNEUROSCI.18-10-03853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pych JC, Kim M, Gold PE. Effects of injections of glucose into the dorsal striatum on learning of place and response mazes. Behavioral Brain Research. 2006;167(2):373–378. doi: 10.1016/j.bbr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Ragozzino ME, Gold PE. Task-dependent effects of intra-amygdala morphine injections: attenuation by intra-amygdala glucose injections. Journal of Neuroscience. 1994;14(12):7478–7485. doi: 10.1523/JNEUROSCI.14-12-07478.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lennartz RC, et al. Inhibitory avoidance impairments induced by intra-amygdala propranolol are reversed by glutamate but not glucose. Behavioral Neuroscience. 1996;110(5):1033–1039. doi: 10.1037//0735-7044.110.5.1033. [DOI] [PubMed] [Google Scholar]