Abstract
The toxic gas H2S is produced by enzymes in the body. At moderate concentrations, H2S elicits physiological effects similar to hibernation. Herein, we describe experiments that imply that the phenomenon probably results from reversible inhibition of the enzyme cytochrome c oxidase (CcO), which reduces oxygen during respiration. A functional model of the oxygen-reducing site in CcO was used to explore the effects of H2S during respiration. Spectroscopic analyses showed that the model binds two molecules of H2S. The electro-catalytic reduction of oxygen is reversibly inhibited by H2S concentrations similar to those that induce hibernation. This phenomenon derives from a weak, reversible binding of H2S to the FeII porphyrin, which mimics heme a3 in CcO's active site. No inhibition of CcO is detected at lower H2S concentrations. Nevertheless, at lower concentrations, H2S could have other biological effects on CcO. For example, H2S rapidly reduces FeIII and CuII in both the oxidized form of this functional model and in CcO itself. H2S also reduces CcO's biological reductant, cytochrome c, which normally derives its reducing equivalents from food metabolism. Consequently, it is speculated that H2S might also serve as a source of electrons during periods of hibernation when food supplies are low.
Keywords: cytochrome c oxidase, reversible inhibition, synthetic functional model, H2S complex
There is an emerging paradigm concerning what can be learned biologically from functional synthetic models. Previous studies elucidate that the synthetic “picket-fence-porphyrins” are excellent structural and functional analogs of the active sites of hemoglobin and myoglobin (1, 2). They not only mimic the spectroscopic, magnetic, and structural characteristics of the two proteins but also reversibly bind O2 and have O2-binding affinities similar to the two proteins (3–5). The R and T states of oxyhemoglobin can be replicated demonstrating the effect on equilibrium binding of O2 as well as the role steric hindrance plays in that effect (6). The relative CO/O2 binding in model hemes can be varied by a factor of >4000 by manipulating the distal pocket of models (7, 8). Recently, a functional cytochrome c oxidase (CcO) model was created that reduces O2 to H2O under conditions of biomimetic rate limiting electron flux (9). This functional model allowed us to explore the interaction of NO with CcO and to demonstrate how NO could protect CcO (10). A subsequent publication on the enzyme CcO itself showed that the same reactions occur with “the real thing” (11). In the current account, we use this same functional model to examine the inhibition of CcO by hydrogen sulfide (H2S) and demonstrate a plausible inhibitory mechanism.
H2S, a ubiquitous gas that smells like rotten eggs, is found in natural gas, volcanic springs, petroleum, and decomposed organic matter (12). At concentrations >600 ppm, H2S is very toxic, even lethal, but at lower concentrations it elicits a variety of biological effects. Long-term exposure to H2S is reported to produce cytotoxic effects such as cerebral stroke, inflammatory diseases, mental retardation, and cell death (13, 14). In the context of its toxicity, it is surprising that H2S is produced in humans and other mammals by two enzymes, cystathione gamma-lyase and cystathione beta-synthase, acting on a simple amino acid, L-cysteine (15, 16). Although toxic in high concentrations, H2S has been shown to induce various cytoprotective effects in lower, micromolar concentrations. It stimulates ATP sensitive potassium channels, causing inhibition of insulin secretion in smooth-muscle cells, neurons, cardiomyocytes, and pancreatic beta-cells. H2S is also involved in myocardial contractility, neurotransmission, vascular tone, and blood pressure regulation (15, 17).
Recently, an amazing effect has been reported: when mammals are exposed to moderate (≈80 ppm) amounts of H2S, a state of hypothermia is induced. After 6 h of incubation, the core body temperature of a mouse decreased to a level as low as 15 °C, and its metabolic rate decreased by nearly 90% (18–20). When the mouse was returned to ambient conditions in fresh air, it was restored to normal and showed no apparent behavioral or functional changes. This phenomenon appears similar to hibernation and aestivation that have been observed in other mammals, reptiles, and amphibians (19, 21). The potential ability to chemically induce such physiological states might become useful as regulated induction of hypothermia has been beneficially applied to ischemia, pyrexia, reperfusion, transplant organ preservation, traumas, and during surgery (15, 20, 22).
It has been suggested that moderate concentrations of H2S could evoke these effects by reversibly and competitively inhibiting the mitochondrial enzyme cytotochrome c oxidase, (CcO), thus slowing respiration. But at lower concentrations, H2S has been claimed to be a “noncompetitive inhibitor” of CcO (23, 24). By using our recently developed functional models of the oxygen-reducing site in CcO (6), we have been able to explore some of the chemistry behind the diverse effects that H2S has on the mitochondria at different concentrations.
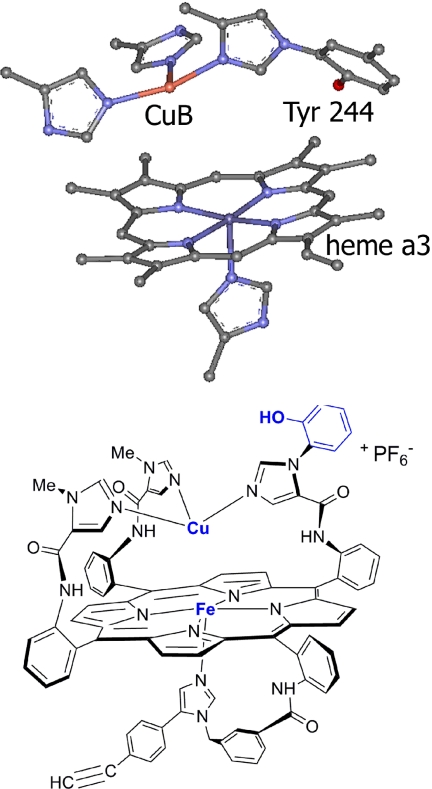
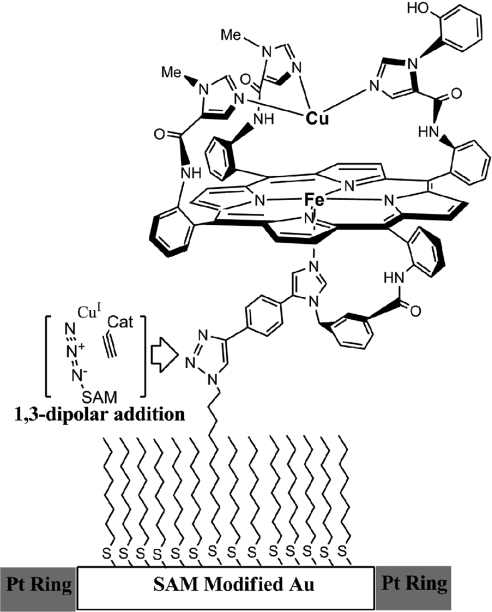
At its active site, CcO has a heme-Cu complex (Fig. 1A) that catalyzes the four-electron reduction of O2 to H2O during the terminal step of respiration (25–27). By translocating protons across the membrane, this process maintains a transmembrane proton gradient, which provides the energy required to phosphorylate ADP to ATP and generates heat in the mitochondria (28, 29). A molecule having all of the components of CcO's active site has been synthesized. This CcO model has a myoglobin-like heme fitted with a covalently attached proximal imidazole, and an array of three imidazoles coordinated to Cu rigidly attached to the distal face of the Fe porphyrin (6). A phenol is appended to one distal imidazole. These components replicate heme a3, CuB, and Tyrosine244, respectively (30). Using a copper catalyzed azide-alkyne coupling reaction, this synthetic enzyme mimic was covalently attached to a liquid-crystalline self-assembled-monolayer (SAM) film coating a gold-disc electrode (9). The CcO/SAM was used as a rotating ring-disc electrode assembly (Fig. 2). These CcO mimics selectively catalyze the four-electron reduction of O2 at physiological pH and potential, under conditions of both rate-limiting electron transfer through a “slow SAM” and fast-electron transfer through a “fast SAM” (9). In addition, it has recently been shown that in solution, the actual biological reductant, cytochrome c, reacts with the CcO model to selectively reduce O2 to water (31). The latter process reproduces the four-electron catalytic reduction of O2 without employing an electrode or a SAM film. Both functional CcO models are well suited to examine the effect of H2S on the catalytic reduction of O2.
Fig. 1.
Active site structures. CcO (A) and synthetic model (B) of CcO (Fe Cu and phenol analogue).
Fig. 2.
Schematic representation of the FeCuPhOH catalyst “clicked” onto an Au electrode (white) with Pt ring (gray) around it. “Cat” refers to the catalyst (Fig. 1B), excluding the terminal alkyne moiety.
Herein, we use this functional model to understand the reactions that may be involved in the reversible inhibition of CcO by H2S. These results provide insight into the potential role of H2S in decreasing metabolic activity and consequently lowering the core body temperature of animals by slowing the reduction of O2 by CcO.
Results and Analysis.
The electrochemical catalytic reduction of O2 can be used to mimic O2 reduction in the mitochondria. A linear sweep voltammogram of the CcO model on modified electrodes shows catalytic O2 reduction current under conditions of both fast and slow electron transfer (Fig. 3 A and C, black).
Fig. 3.
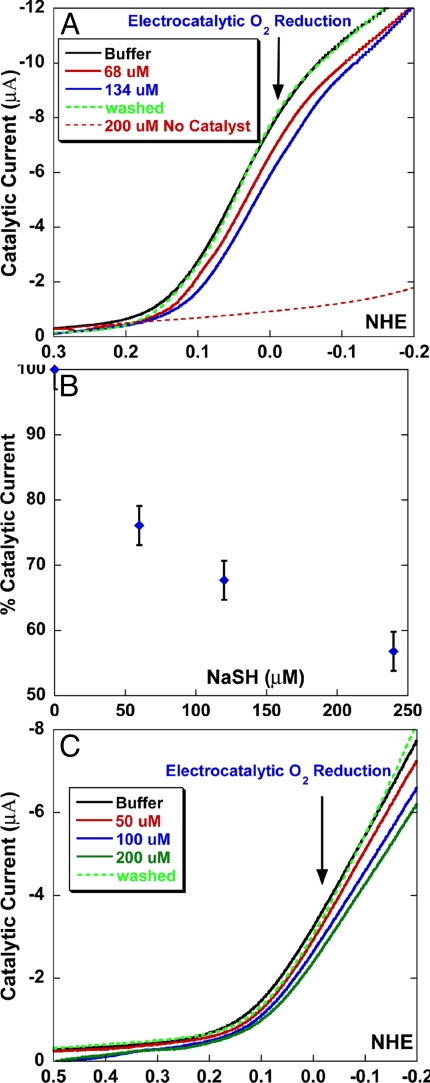
The electrocatalytic reduction of oxygen is reversibly inhibited by H2S. (A) A linear sweep voltammogram of the FeCuPhOH catalyst modified electrode showing catalytic O2 reduction current under fast electron flux in the absence of NaSH (black), in the presence of 68 μM NaSH (red), 134 μM NaSH (blue), after removal of NaSH (dotted green), and in 200 μM NaSH in absence of any catalyst (dotted red). (B) Plot of percent catalytic current vs. NaSH concentration. (C) Linear sweep voltammogram of the FeCuPhOH catalyst modified electrode showing catalytic O2 reduction current under slow (rate determining) electron flux in the absence of NaSH (black), in the presence of 50 μM NaSH (red), 100 μM NaSH (blue), 200 μM NaSH (green, and afterremoval of NaSH (dotted green) .
Introduction of H2S (pKa = 7) from a NaSH buffer solution sharply reduces the electrocatalytic current that represents catalytic O2 reduction (Fig. 3A, red and blue). The catalytic O2 reduction at 0 mV vs. NHE is almost fully restored (95–100%) when the NaSH buffer is replaced by an air-saturated buffer, thus removing H2S. This result demonstrates that H2S reversibly inhibits catalysis by this functional CcO model, both under fast and slow electron flux. The latter condition mimics steady state O2 reduction during respiration because the rate-limiting step during catalysis is the relatively slow stepwise arrival of electrons from cytochrome c (9). A plot of the catalytic current against the NaSH concentration shows that the current is reduced to ≈60% in presence of 240 μM NaSH in buffer (Fig. 3B).
We then explored the competitive binding of H2S to the reduced enzyme mimic. When 0.5 mM NaSH in pH 7 buffer solution was anaerobically added to 5 μM of the reduced FeIICuIPhOH catalyst (in a 50:50 aqueous buffer acetonitrile solvent) (i.e., 100 equivalents of SH−), the Soret band of the porphyrin became slightly red shifted with decreased intensity and the Q band intensity slightly increased (Fig. 4A, black to red), indicating that H2S binds to the reduced active site. Note that SH−, also present in the solution at this pH, should have a weaker binding affinity for the Fe(II) porphyrin site.
Fig. 4.
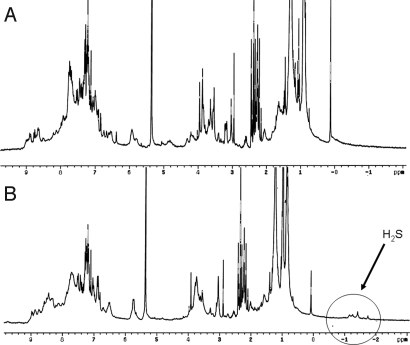
1H-NMR spectrum (recorded in CD2Cl2 at −50 °C, 300 MHz) of FeII complexes (14 mM) before A and after B exposure to H2S.
The H2S complex of the FeII-only and FeIICuI species (Fig. 1B, phenol is replaced by Me group) was further characterized by 1H-NMR, infrared, high-resolution nanospray, and UV/Vis spectroscopies (Figs. 4–8).
Fig. 5.
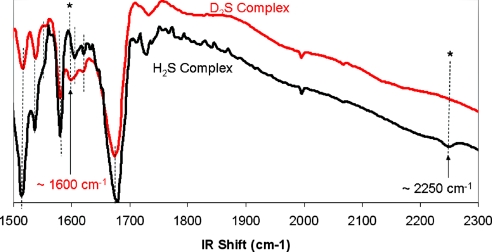
Infrared spectrum of the H2S- and D2S-complexes (in black and red, respectively) on KBr pellets or in solution in dichloromethane in a KBr cell (10 mM).
Fig. 6.
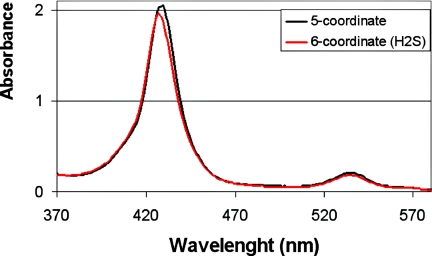
UV/Vis spectrum of a 18 μM Fe(II) complex before reaction with H2S (dry, 5-coordinate, 430 nm in black) and after reaction (6-coordinate, 427 nm in red).
Fig. 7.
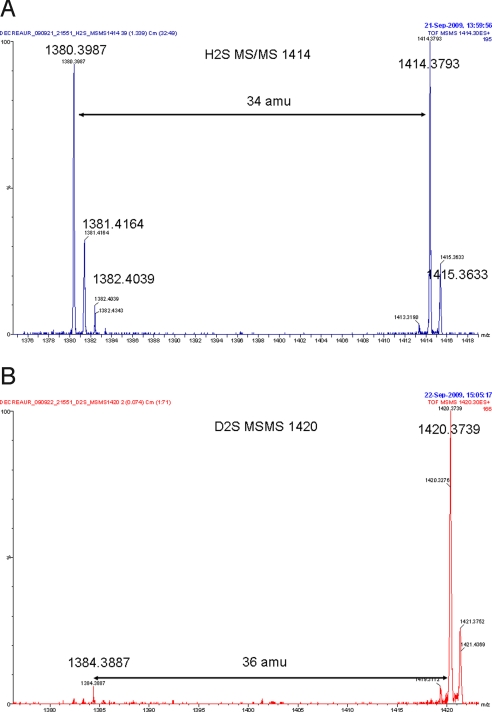
MSMS analysis on the most aboundant isotopic peak in the high-resolution nano-spray mass spectrum of the Fe(II) H2S (A, Calcd: 1414.3771, Found: 1414.3793) and D2S complexes (B, Calcd: 1420.4148, Found: 1420.3739), respectively. For this peak
Fig. 8.
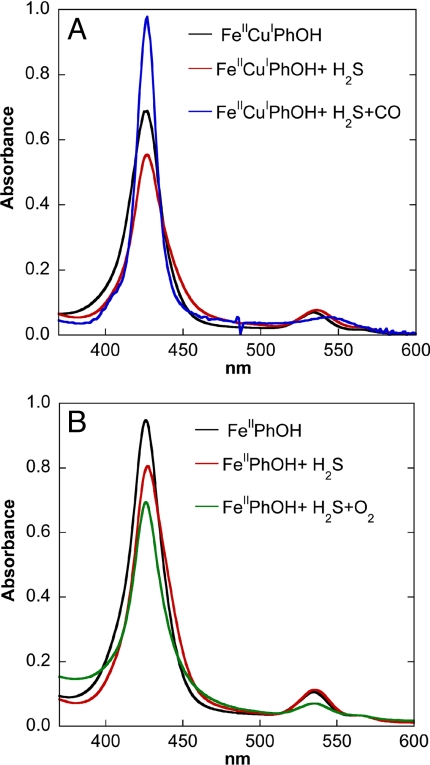
The reactivity of Fe(II) H2S complexes with CO and O2 monitored by UV-Vis spectroscopy. (A) UV–Vis spectra of reduced 5 μM FeIICuIPhOH (black), reduced FeIICuIPhOH + 0.5 mM NaSH solution (red), and reduced FeIIPhOH + 0.5 mM NaSH solution + excess CO gas (blue). (B) Reduced 8 μM FeIIPhOH (black), reduced FeIIPhOH + 80 μM NaSH solution (red), and reduced FeIIPhOH + 80 μM NaSH solution + excess O2 gas (green).
The 1H-NMR analysis of the low-spin ferrous-H2S complex was carried out at low temperature (−50 °C) (Fig. 4 A and B). To avoid possible signal exchange between H2O and H2S in the distal pocket, we used water-free Fe-only and FeCu complexes. Upon careful drying of the Fe-only CcO model, either in refluxing toluene over dry sodium sulfate or by azeotropic distillation in toluene, the NMR of the resulting sample does not display upfield chemical shifts. This spectrum shows that no molecules of water are in the distal pocket. This dry five-coordinate FeII complex reacts with H2S. The NMR spectrum shows the presence of four individual upfield peaks, at −1.16 ppm, −1.26ppm, −1.42ppm, and −1.75 ppm, which are shifted from that of free H2S (≈1 ppm) (32). These upfield peaks result from the porphyrin ring current. The S-H chemical shifts are significantly broader in a wet complex; one of them is further shifted upfield. Integration shows that these signals have equal intensity and that this intensity is comparable with that of the alkyne proton (≈2.9 ppm). This result suggests that two molecules of H2S are bound to iron and may be in a locked conformation. The NMR of the FeCu H2S complex shows the same features than an Fe-only complex but with one peak shifted downfield (−0.6 ppm).
The infrared spectrum of both wet ferrous H2S Fe-only and FeCu complexes exhibit weak S-H stretch at 2250 cm−1 (Fig. 5). This vibration shifts to 1600 cm−1 using D2S. This isotopic shift is consistent with those reported for free H2S and D2S (2600 and 1800 cm−1, respectively) (33). This S-H vibration is much lower than that reported for free H2S but is similar to that of a structurally characterized Ru(II) complex, which is claimed to be hydrogen bonded (34). It is notable that the first H2S complex of a transiton metal, Ru(II), was prepared by Taube (35) who reported that this labile complex had a weak S-H stretching frequency at 2547 cm−1. The difference between the stretching frequencies reported by Taube (35) and Sellman (32) were ascribed by Sellman to hydrogen binding, which is consistent with the lower value that we have observed. When CO was added to either of our Fe(II) H2S complexes, CO replaced H2S as evidenced by immediate changes in both the NMR or IR spectra.
UV/Vis studies show that the dry five-coordinate iron (II) complex with a characteristic Soret band at 430 nm reacts with H2S resulting in a rapid blue shift of the Soret band to 427 nm, characteristic of a six-coordinate species (Fig. 6). By comparison, the reactions with either our Fe-only or FeCu wet models are much slower and the shifts in the Soret are modest (≈1 nm). In the presence of CO gas, H2S is replaced by CO, thus leading to a CO complex with a characteristic Soret band at 428 nm having increased intensity.
The H2S and D2S complexes of Fe-only and FeCu were also characterized by high-resolution nanospray (Fig. 7 A and B and Fig. S1 A–F). Nanospray is the only technique that employs sufficiently mild ionization to allow detection of a fragile H2S complex, but it seems difficult to prevent exposure of these samples to air. As a result, contamination with the oxygen complex was clearly observed in several cases with Fe-only, and the resulting peak shows an isotopic distribution that corresponds to overlap between an H2S (+34 amu) and an O2 (+32 amu) complex (Fig. S1A). To disentangle the two signals, MS–MS analyses were carried out on each isotopic peak. The peaks at m/z = 1414, 1415, and 1416 showed loss of 34 amu, leading to peaks at m/z = 1380 and 1381, which corresponds to H2S (Fig. 7A and Fig. S1 A and B). The same experiment was carried out with the Fe-only complex using D2S, but because fast exchange with the amide protons occurs, an analogous iron porphyrin species with four deuterated amides was prepared by exposure to D2O for 10 min (m/z = 1384 amu) before the reaction with D2S. After the sample was reacted with D2S, fragmentation of the peaks at m/z = 1419, 1420, and 1421 showed loss of 36 amu, which corresponds to loss of one D2S molecule (Fig. 7B and Fig. S1C). Similarly, the MS analyses of FeCu complex that was treated with H2S showed peaks at m/z = 1511 and 1478, which were subjected to MS–MS and showed loss of 2 × 34 amu and 34 amu, respectively (Fig. S1 D and E). This corresponds to two and one H2S, respectively. These results seem to concur with an earlier report that CcO itself binds two molecules of H2S, one at heme a3 and the other at CuB.
However, to examine binding to Cu, we examined the Zn/Cu complex (Zn in the porphyrin) and found no evidence for H2S binding. We conclude that the second H2S must be hydrogen bound to the one attached to Fe(II).
To obtain the binding affinity of H2S to the metal centers, sequential addition of H2S to the FeIICuI (Fig. 1B, phenol is replaced by Me group) analogue was monitored by following its UV–Vis spectra (Fig. S2). The data indicate that two molecules of H2S bind the reduced catalyst with approximate binding constants of 0.8 and 0.1 (or 0.0125 and 0.01 mM). These binding constants indicate that H2S is a weak ligand for the reduced CcO functional model; this assumption is further supported by the facile replacement of bound H2S by other ligands.
When the H2S-bound FeIICuIPhOH complex was exposed to CO, an increase in the soret band intensity and a red shift of the Q-band (Fig. 8A, blue), characteristic of the formation of a FeII-CO complex, were observed (Fig. S3). This again shows that H2S is readily replaced by the stronger ligand, CO. Furthermore, in these complexes, CO can be replaced by O2 (the physiological substrate of CcO), which implies that O2 should also be able to replace the weaker ligand H2S. Replacing H2S by O2 in the FeIICuIPhOH complex leads to O–O bond cleavage and hence is hard to probe. To determine whether O2 can replace the H2S bound to the reduced catalyst, the FeIIPhOH complex (Fig. 1B, in the absence of Cu) was used. This complex was chosen because it lacks all of the reducing equivalents (one additional reducing equivalent from Cu) necessary for O–O bond cleavage and therefore would not reduce O2. The UV–Vis spectrum of the reduced FeIIPhOH complex (Fig. 8B, black) shows a similar decrease in intensity and a red shift of the soret band upon addition of H2S (Fig. 8B, black to red). When the H2S bound FeIIPhOH complex was exposed to O2, the soret and q band decreased in intensity, characteristic of the formation of an FeIII-O2− complex (36, 37)(Fig. 8B, green), implying that the bound H2S is readily replaced by O2. These results clearly show that H2S binds to the reduced FeIICuIPhOH site, but it is a weak ligand, and can be readily replaced by either CO or O2.
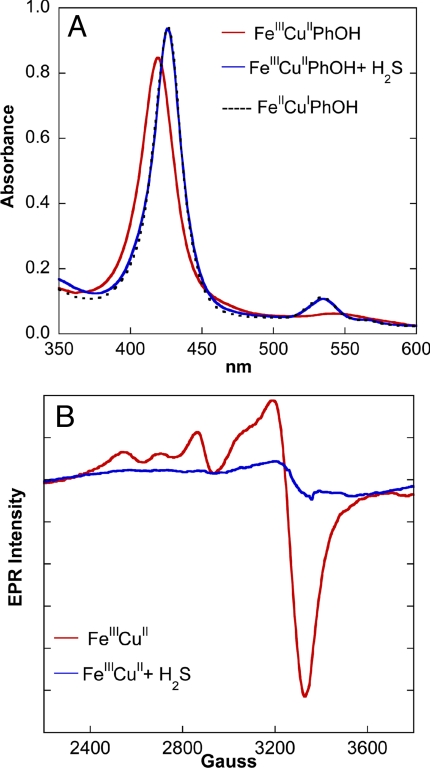
At this juncture, where the reversible binding of H2S to the reduced catalyst had been established, the possible effects of H2S at lower concentrations were examined. H2S is known to be a two-electron reducing agent (HS− → So + H+ + 2e−; Eo = 0.17 V at pH = 7) and has been reported to reduce metal centers of CcO (38, 39). When a NaSH solution in a pH 7 buffer was added to our oxidized CcO model, quantitative reduction of both the FeIII heme and distal CuII centers was observed. When 1 equivalent of NaSH was added, the UV–Vis spectrum of the oxidized catalyst (Fig. 9A, red) was red shifted, generating the spectrum of the FeIICuIPhOH catalyst (Fig. 9A, black). This reaction was also followed using EPR spectroscopy. The EPR spectrum of the FeIIICuII complex (in frozen tetrahydrofuran) (Fig. 9B, red) shows the low-spin FeIII signal overlapping with the CuII signal between 2200 and 3800 G. Addition of 1 equivalent of (t-Bu4N)SH results in the loss of this signal. These results show that H2S can completely reduce the oxidized complex even at very low H2S concentrations.
Fig. 9.
The reactivity of fully oxidized complexes with H2S monitored by UV-Vis and EPR spectroscopies. (A) UV–Vis spectra (in a 50:50 volume ratio of pH 7 aqueous buffer and acetonitrile) of 10 μM oxidized FeIIICuIIPhOH (red), oxidized FeIIICuIIPhOH + 70 μM NaSH solution (blue) overlaid with a reduced FeIICuIPhOH spectrum (dotted black). (B) EPR spectra of 1 mM oxidized FeIIICuII (red) in THF and oxidized FeIIICuII + 1 mM (t-Bu4N)SH solution (blue) at 77 K.
Significantly, H2S also reduces oxidized cytochrome c, the protein that is the electron source for CcO. When 10 μM of oxidized cytochrome c, from horse heart, was exposed to 10 μM NaSH solution, the FeIII form is completely reduced to the FeII form (Fig. S4).
Discussion
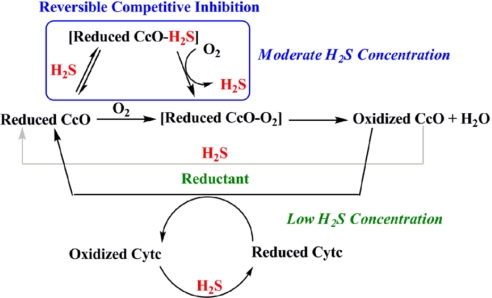
Scheme 1 summarizes the reactions we have observed between H2S and the functional CcO model. These reactions suggest the possible effect that H2S might have on CcO at moderate and low concentrations.
Scheme 1.
Schematic representation of the possible roles of H2S affecting CcO activity. The blue box indicates that H2S binds to the reduced CcO active site, but can be subsequently replaced by O2. This inhibition should occur at moderate H2S concentrations. At lower concentrations, H2S does not compete with the substrate O2 but can still reduce CcO's active site and/or cytochrome c (Cytc) during catalytic O2 reduction.
At higher concentrations, H2S binds to FeII in the reduced active site (Figs. 4–8) and can act as a competitive inhibitor of CcO, competing with its substrate, O2, for binding to the reduced FeIICuI active site. However, as the ligand binding data clearly indicate, this inhibition should be reversible as O2 can easily replace H2S bound to a reduced FeII site. This hypothesis is supported by electrochemical experiments where catalytic inhibition by H2S is readily reversed by flushing H2S from the medium with air.
At lower H2S concentrations, the affinity of H2S for the FeII state is too low to result in measurable inhibition. But at low concentrations H2S can exert other effects by virtue of its ability to act as a reducing agent. For example, H2S quantitatively reduces the oxidized CcO site. Thus, H2S could provide reducing equivalents to the active site during O2 reduction. Moreover, H2S can also reduce cytochrome c, and therefore, could provide electrons to cytochrome c substituting for NADH during physiological O2 reduction.
The experiments reported herein provide a basis for understanding the origin of the effect that H2S has on CcO and therefore on respiration. Electrocatalytic experiments demonstrate that at low H2S concentrations, O2 reduction is not measurably inhibited, apparently because of its rather low affinity for the FeII heme. But these electrocatalytic results demonstrate that at higher H2S concentrations, the catalytic reduction of O2 is reversibly inhibited. These results are reminiscent of the effects that H2S has on respiration in mammals – no discernable effect at low concentration but reversible inhibition at higher concentrations. The low affinity that H2S is shown to have for hemes might also explain why H2S may not be tied up in the body by the huge reservoir of myoglobin and hemoglobin. However, at low concentrations, H2S does carry out relevant chemistry: it rapidly and irreversibly reduces oxidized forms, both of CcO and of oxidized cytochrome c, the natural reductant. We speculate that this rapid reduction by H2S could have biological ramifications, for example, under conditions of low food supply during periods of hibernation and we note that this redox process should produce elemental sulfur. An in vivo experiment, demonstrating the presence of increased elemental sulfur within the cells of a hibernating animal would be confirmatory. As demonstrated herein, functional synthetic models can be useful in understanding biological phenomena.
Materials and Methods
All reagents were of the highest grade commercially available and were used without further purification. All UV–Vis and kinetic measurements using NaSH as a source of H2S were performed in 1:1 mixture of an aqueous phosphate buffer (pH 7) acetonitrile solvent system. UV–Vis measurements using H2S gas from the tank (99.9% pur) were performed in dichloromethane or in toluene in a Young-Flask fitted with a UV cuvette (tonometer). All solutions were prepared anaerobically in a glove box. Absorption data were collected by an UV–Vis diode array spectrophotometer (Agilent 8453). The catalyst was synthesized as reported (30). The distal Cu metal was introduced before experiments. Horse heart cytochrome c was used for studying its reduction with H2S. H2S (pKa = 7) and was generated by dissolving NaSH in a pH 7 phosphate buffer. In aqueous solution at pH 7, H2S exists as a 50:50 mixture of H2S and SH−. In organic solvents, (t-Bu4N)SH was used as the source of SH−.
EPR spectra were obtained using a Bruker EMX spectrometer, ER 041 XG microwave bridge, and ER 4102ST cavity. X band EPR sample was run at 77 K in a liquid nitrogen finger dewar.
IR spectra were obtained on a Mattson Galaxy 4030 FT-IR spectrometer. Solid samples were prepared by dissolving a sample in solution in a glovebox, spotting on a KBr palate, allowing the solvent to evaporate, then covering it by another palate and sealing the sides with electrical tape. Liquid samples were prepared at ≈10 mM concentration of porphyrin in a septum-capped vial sitting in a septum-capped scintillation vial sealed with electrical tape. Approximately 200 μl of solution was transferred with a gas-tight Hamilton syringe into a sealed double-walled KBr cell, sealed with two septa.
Low-temperature NMR were run on a 300 MHz Bruker machine, in PTFE-capped NMR tubes, using CDCl3 or CD2Cl2 as the solvent and using the following parameters bs = 4, nt = 100, sw = 8000, with the temperature set at −50 °C. Nanospray experiments were carried out on a Q-Tof Ultima API.
The self-assembled monolayers (SAMs) were formed by depositing an ethanolic solution (total thiol concentration 0.4 mM) of 11-azido-undecane-thiol using octanethiol as diluent (fast SAM) or 18-azido-octadecane-thiol with octadecanethiol as diluent (slow SAM). The formation of mixed thiol SAM on Au coated Ti discs and clicking the catalysts on the SAM surface was done as described in ref. 9. The click reaction was performed in a N2 glove box to avoid catalyst decay during the process. All electrochemical measurements were performed at room temperature (≈21 °C) at pH = 7 buffer with 100 mM KPF6 electrolyte on a Pine AFCBP1 bipotentiostat (Pine Instruments). Rotating ring-disk experiments were performed after inserting the Au-coated Ti disks into ring-disk electrode mounts purchased from Pine (E6 series platinum ring with changeable disk) and were carried out on a Pine AFCBP1 bipotentiostat (Pine Instruments). The concentration of electroactive catalyst was estimated by integrating the current under the reversible cyclic voltammogram (CV) obtained at pH 7 with 100 mM imidazole (the latter inhibits O2 reduction). Turnover numbers for the electrocatalytic reduction of O2 were estimated by integrating the decay of the catalytic current over a period of 20 min. This was then normalized for catalyst decay that was estimated from a CV done before and after electrolysis in presence of 100 mM imidazole.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant GM-17880–38. We thank Dr. Todd A. Eberspacher for helpful discussions and Dr. Lei Fu and Dr. Ying Yang for additional assitance. R.A.D is supported by a Lavoisier fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904082106/DCSupplemental.
References
- 1.Collman JP, Gagne RR, Reed CA. A paramagnetic dioxygen complex of iron II derived from a picket fence porphyrin further models for hemoproteins. J Am Chem Soc. 1974;96:2629–2631. doi: 10.1021/ja00815a060. [DOI] [PubMed] [Google Scholar]
- 2.Collman JP, Gagne RR, Halbert TR, Marchon JC, Reed CA. Reversible oxygen adduct formation in ferrous complexes derived from a picket fence porphyrin model for oxy myoglobin. J Am Chem Soc. 1973;95:7868–7870. doi: 10.1021/ja00804a054. [DOI] [PubMed] [Google Scholar]
- 3.Collman JP, Brauman JI, Rose E, Suslick KS. Cooperativity in O2 binding to iron porphyrins. Proc Natl Acad Sci USA. 1978;75:1052–1055. doi: 10.1073/pnas.75.3.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collman JP, et al. Picket-Fence Porphyrins. Synthetic Models for Oxygen Binding Hemoproteins. J Am Chem Soc. 1975;97:1427–1439. doi: 10.1021/ja00839a026. [DOI] [PubMed] [Google Scholar]
- 5.Collman JP, Brauman JI, Suslick KS. Oxygen binding to iron porphyrins. J Am Chem Soc. 1975;97:7185–7186. doi: 10.1021/ja00857a050. [DOI] [PubMed] [Google Scholar]
- 6.Collman JP, Boulatov R, Sunderland CJ, Fu L. Functional analogues of cytochrome c oxidase, myoglobin, and hemoglobin. Chem Rev. 2004;104:561–588. doi: 10.1021/cr0206059. [DOI] [PubMed] [Google Scholar]
- 7.Collman JP, Fu L. Synthetic models for hemoglobin and myoglobin. Acc Chem Res. 1999;32:455–463. [Google Scholar]
- 8.Collman JP, Fu L, Zingg A, Diederich F. Dioxygen and carbon monoxide binding in dendritic iron(II)porphyrins. Chem Comm. 1997;2:193–194. [Google Scholar]
- 9.Collman JP, et al. A cytochrome c oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collman JP, et al. Interaction of nitric oxide with a functional model of cytochrome c oxidase. Proc Natl Acad Sci USA. 2008;105:9892–9896. doi: 10.1073/pnas.0804257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce LL, Manzano EL, Martinez-Bosch S, Peterson J. Antagonism of Nitric Oxide Toward the Inhibition of Cytochrome c Oxidase by Carbon Monoxide and Cyanide. Chem Res Toxicol. 2008;21:2073–2081. doi: 10.1021/tx800140y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beauchamp ROJ, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen-sulfide toxicity. Crit Rev Toxicol. 1984;65:18–25. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 13.Dorman DC, et al. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: Correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci. 2002;65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, et al. Effects of hydrogen-sulfide exposure on lung mitochondrial respiratory-chain enzymes in rats. Toxicol Appl Pharmacol. 1990;103:482–490. doi: 10.1016/0041-008x(90)90321-k. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discovery. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 16.Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 17.Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas for interest for pharmacologists. Pharmalogical Reports. 2007;59:4–24. [PubMed] [Google Scholar]
- 18.Volpato GP, et al. Inhaled hydrogen sufide - A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anestaesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CC. Is human hibernation possible? Annu Rev Med. 2008;59:177–186. doi: 10.1146/annurev.med.59.061506.110403. [DOI] [PubMed] [Google Scholar]
- 20.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 21.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 23.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J Bionenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 24.Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim Biophys Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa S, et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 26.FergusonMiller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2907. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 27.Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig B, et al. Cytochrome c oxidase and the regulation of oxidative phosphorylation. ChemBioChem. 2001;2:392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AID-CBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Babcock GT, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 30.Collman JP, Decreau RA, Zhang C. Synthesis of cytochrome c oxidase models bearing a Tyr(244) mimic. J Org Chem. 2004;69:3546–3549. doi: 10.1021/jo0499625. [DOI] [PubMed] [Google Scholar]
- 31.Collman JP, Gosh S, Dey A, Decreau RA, Yang Y. Catalytic Reduction of O2 by Cytochrome c Using a Synthetic Model of Cytochrome c Oxidase. J Am Chem Soc. 2009;131:5034–5035. doi: 10.1021/ja9001579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellman D, Lechner P, Knoch F, Moll M. Proof of Strong S-H * - S Bridges in [Ru(SH2)(PPh,)(‘S4′)] * THF, the First H2S Complex Characterized by X-Ray Crystallography. Angew Chem, Int Ed. 1991:552–553. [Google Scholar]
- 33.Anderson A, Binbrekt S, Tang HC. Raman and infrared study of the low temperature phase of solid H2S and D2S. J Raman Spectrosc. 1977;6:213–220. [Google Scholar]
- 34.Muladige DC. Vancouver, Canada: Univ of British Colombia; 1994. Binding and Activation of small molecules by Ruthenium complexes containing a chelated aminophosphine ligand. Ph. D. Thesis. [Google Scholar]
- 35.Kuehn CG, Taube H. Ammineruthenium Complexes of Hydrogen Sulfide and Related Sulfur Ligands. J Am Chem Soc. 1976;98:689–702. [Google Scholar]
- 36.Collman JP, Decreau RA, Yan Y, Yoon J, Solomon EI. Intramolecular single-turnover reaction in a cytochrome c oxidase model bearing a Tyr244 mimic. J Am Chem Soc. 2007;129:5794–5795. doi: 10.1021/ja0690969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collman JP, Sunderland CJ, Berg KE, Vance MA, Solomon EI. Spectroscopic evidence for a heme-superoxide/Cu(I) intermediate in a functional model of cytochrome c oxidase. J Am Chem Soc. 2003;125:6648–6649. doi: 10.1021/ja034382v. [DOI] [PubMed] [Google Scholar]
- 38.Hill BC, et al. Low-spin ferric forms of cytochrome-a3 in mixed-ligand and partially reduced cyanide-bound derivatives of cytochrome c oxidase. Biochem J. 1984;224:591–600. doi: 10.1042/bj2150057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls P, Kim JK. Sulfide as an inhibitor and electron-donor for the cytochrome c oxidase system. Can J Biochem. 1982;60:613–623. doi: 10.1139/o82-076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.