Abstract
Following the priming and contraction phases of the T-cell response, latent persistent herpesviruses lead to an accumulation of large pools of virus-specific CD8 T-cells, also known as memory inflation (MI). The mechanism of this inflation is incompletely understood, largely because molecular reactivation of these viruses in vivo and its impact upon T-cell biology have not been resolved in mice, and because the relevant observations in humans remain, by necessity, correlative. Understanding these processes is essential from the standpoint of the proposed critical role for latent herpesviruses in aging of the immune system. We studied the causes of memory CD8 T-cell accumulation following systemic HSV-1 administration, as a model of widespread latent viral infection in humans. Direct role of viral latency and Ag-specific restimulation in driving accumulation and maintenance of inflated CD8 T-cells, and strongly suggestive role of viral reactivation in that process, were shown by: (i) lack of MI in the absence of established latency; (ii) prevention or delay of MI with drugs that curtail viral replication; and (iii) abrogation of MI by transfer of inflated T-cells into virus-free environment. These results strongly suggest that periodic, subclinical reactivations of a latent persistent virus cause dysregulation of memory CD8 T-cell homeostasis similar to the one in humans. Moreover, results with antiviral drugs suggest that this approach could be considered as treatment modality to maintain T-cell diversity and/or function in the old age.
Keywords: CD8 T cells, HSV, memory inflation, virus reactivation, aging
INTRODUCTION
Herpesviruses are amongst the most prevalent latent human pathogens, infecting the vast majority (>90%) of the human population in the Western hemisphere. These viruses persist for the life of the host, and rarely cause manifest disease in the absence of profound immunosuppression. Because of that, there is considerable interest in understanding the impact of lifelong herpesvirus infections upon aging, long-term health, survival and immunity (1). How exactly the balance is maintained between the virus and the immune system over the long periods of time and into the old age remains incompletely understood. The latent virus should be kept at bay to prevent systemic and potentially devastating reactivation, however, the long-term immune response against the virus must also remain under control. This can be challenging, as is illustrated in the case of the Cytomegalovirus (CMV), a latent β-herpesvirus which elicits T-cell responses unusual in strength, breadth and complexity (2, 3). CMV seropositivity in humans has been correlated with an age-related increase in the fraction of CMV-specific memory T-cells (rev. in (1) and shorter lifespan in the octo- and nona-generians (4). This spurred the hypothesis that immune aging is precipitated by persistent infections, yet direct causality and the mechanistic connection between the two remain to be established. This is due to both the ethical constraints in the human model and because it is difficult to demonstrate subclinical reactivation of infectious CMV in blood of asymptomatic humans (5, 6) and mice (7, 8) late after the primary infection.
Herpes Simplex Virus type 1 (HSV-1) is a ubiquitous α-herpesvirus that establishes lifelong latent infection in the sensory ganglia and the central nervous system. In humans, this lifelong infection is associated with periodic viral reactivation and migration of the reactivated virus from the site of latency into the periphery. HSV readily infects mice, however, unlike in humans, spontaneous clinical HSV-1 reactivation does not occur in mice (9, 10). Much work has been done analyzing the relationship of the latent HSV virus with the sentinel CD8 T-cells present in infected sensory ganglia (11–13), particularly as it pertains to CD8 T-cells specific for the gB-8p, the immunodominant, glycoprotein B-derived HSV-1 epitope recognized by CD8 T-cells C57BL/6 (B6) mice (14–16).
By contrast, far less is known about the effect of the lifelong HSV-1 infection on the systemic memory CD8 T-cell pool. In that regard, our recent analysis (17) of circulating memory T-cells in ocularly infected B6 mice demonstrated that they were maintained at a stable frequency until late in life and exhibited a resting central memory phenotype. In addition, the size and phenotype of the memory CD8 T-cell pool were the same in control mice and in mice treated with antiviral drug famciclovir, suggesting that in the ocular infection model, HSV-1 does not massively reactivate from the trigeminal ganglia (TG) to the extent so as to palpably influence the systemic CD8 T-cell pool (17). Dysregulation of the systemic CD8 compartment in these mice occurred only late in life, appeared to be virus-independent and was indistinguishable from the spontaneously arising antigen-independent T-cell clonal expansions.(17), rev in (18). This stands in contrast to systemic murine and human CMV (mCMV) infection, where there is an accumulation of virus-specific T-cells several months to years after the priming phase (19–21), and these large cell populations are maintained at high numbers throughout lifespan. This leaves several unanswered questions: (i) Is memory accumulation driven by viral reactivation, or by persistence of viral antigen (Ag) regardless of viral reactivation?; (ii) Are the expanded CD8 T-cells programmed to expand, or are they able to contract upon antigenic withdrawal?; and (iii) What characteristics of latent herpesvirus infection (viral load, spread, number of latent sites, etc.) determine whether MI will occur?
To answer some of these questions and thereby elucidate the potential impact of latent herpesviruses on the adult and aging immune system, we established and characterized a model of systemic HSV infection, in which MI readily occurs. This model permits precise dissection of relative contributions of various virus-driven and virus-independent factors to the long-term (dys)regulation of the diversity and composition of the CD8 T-cell compartment. In the present work, we report that the size and phenotype of HSV-1-specific CD8 T-cell systemic memory is absolutely dependent on the establishment of HSV-1 latency, on the presence of viral Ag and can be modulated by drugs that block viral replication and reduce viral Ag expression. We discuss these results from the standpoint of maintenance of optimal T-cell diversity over lifetime.
MATERIALS AND METHODS
Mice
Male C57BL/6-NCr (B6) mice and B6-Ly5.2/Cr (Ly-5.2) were purchased from the National Cancer Institute colony (Frederick, MD). B6.gBT-I (gBT-I in the text) TCR transgenic mice (22), carrying rearranged tcr genes encoding TCR that recognizes the immunodominant HSV-1 epitope gB-8p + H-2Kb, were generously provided by Dr. F. R. Carbone (University of Melbourne, Melbourne, Australia). All animals were housed under the SPF conditions, and experiments conducted under IACUC approval and in accordance with the applicable federal, state and local regulations.
Viruses and viral infections
Localized (intracorneal, i.c.) and intraperitoneal (i.p.) infections with 106 PFU HSV-1 (strain 17) per mouse were performed as described (23). Recombinant Vaccinia Virus expressing the SSIEFARL minigene (rVV-gB-8p) was a generous gift from Dr. Satvir Tevethia (Pennsylvania State University, Hershey, PA) and was grown on thymidine kinase deficient L-cells and titered on Vero cells. Mice were infected i.p. with 4×106 PFUs of rVV-gB-8p.
Determination of titers of replicating virus
The amount of replicating virus in the indicated organs (Table) was determined by plaque assay of organ homogenates as previously described (23).
Table 1. Wide viral spread after systemic HSV-1 infection.
Mice were infected as in Figure 1 and presence of actively replicating virus in the listed tissues at the time of harvest was determined by a plaque assay. The data shows number of mice with actively replicating virus in the given tissue over the total number of mice tested.
| infection route |
day post- infection |
spinal cord |
brain | trigeminal ganglia |
spleen | lungs | liver | eye | draining lymph nodes |
kidneys | peritoneal wash |
fat pads |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| localized (corneal) | day 1 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 5/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| day 3 | 0/13 | 4/13 | 9/9 | 0/13 | 0/5 | 0/5 | 13/13 | 0/5 | 0/5 | 0/5 | 0/5 | |
| day 5 | 0/9 | 7/9 | 9/9 | 0/9 | 0/5 | 0/5 | 9/9 | 0/5 | 0/5 | 0/5 | 0/5 | |
| systemic | day 1 | 5/5 | 0/5 | 0/5 | 1/5 | 0/5 | 1/5 | 0/5 | 2/5 | 4/5 | 5/5 | 5/5 |
| day 3 | 13/22 | 1/14 | 0/14 | 13/25 | 1/9 | 2/9 | 0/9 | 0/5 | 0/5 | 4/11 | 9/19 | |
| day 5 | 3/9 | 4/9 | 0/9 | 0/9 | 0/9 | 1/9 | 0/9 | 0/5 | 0/5 | 0/5 | 0/5 | |
Famciclovir treatment
Where indicated, Famciclovir (Famvir, Novartis) was administered to mice in their drinking water at a concentration of 2mg/ml. The famvir water was changed twice a week.
BrdU labeling
Mice were given BrdU in drinking water at 0.8 mg/ml as described (24, 25) for 3 weeks. BrdU incorporation was measured by FCM using a kit from BD Pharmingen as per the manufacturer’s recommendation.
Adoptive transfers and CFSE labeling
Splenocytes from naïve gBT-I transgenic mice or from HSV-immune mice were enriched for CD8 T-cells and labeled with CFSE as previously described (23). Splenocytes containing 2 ×106 Tet+ naïve CD8 T-cells (naïve gBT-I cell transfers) were adoptively transferred into each congenic B6-Ly5.2/Cr female recipient by i.v. injection 24 hours prior to infection, as described previously (23). Alternatively, splenocytes containing 3×105 Tet+ memory CD8 T-cells (memory cell transfers) were transferred into congenic naïve or HSV-1 infected B6-Ly5.2/Cr mice. The transferred and recipient T-cells were distinguished by FCM detection of the CD45.2 (Ly-5.1) molecule (mAb clone 104, BD Pharmingen).
Reagents, antibodies and flow cytofluorometric (FCM) analysis
The gB-8p peptide (SSIEFARL) was purchased from SynPep Corporation (Dublin, CA), and the gB-8p :Kb tetramer was obtained from the NIH Tetramer Core Facility (Emory University, Atlanta, GA). Monoclonal antibodies were purchased from commercial sources.
FCM analysis was performed as previously described (23). Anti-Ki-67 mAb was purchased from BDPharmingen and the intranuclear staining was performed per manufacturer's instructions. FCM data was acquired on the FACS LSRII instrument using the Diva software (Becton Dickinson, Mountain View, CA), and analysis performed using FlowJo software (Tree Star).
Statistics
Student’s t-tests were performed with Excel (Microsoft), using a 2-tailed analysis with equal variance.
RESULTS
Systemic HSV-1 Infection Causes Robust MI
In the present study, we analyzed the impact of HSV-1 infection on the memory CD8 T-cell maintenance in systemically infected B6 mice. Previously, we found that after intracorneal (i.c., ocular) infection, there was little to no evidence for the continuous interaction between the latent virus (residing in the trigeminal ganglion and the brain) and the systemic CD8 T-cell pool (17). We speculated that the localized nature of ocular infection, where the virus spread is limited to the corneal epithelium, the TG and the brain (23), may contribute to this. A different situation could occur in the course of intraperitoneal (i.p.) infection, where there is potential for broad initial viral dissemination and spread, conducive to wider establishment of latency, and consequently a wider base for viral reactivation. This could result in increased access to viral Ag, which could influence CD8 T-cell priming and/or maintenance.
To test this prediction, we first compared the total viral titer in the nervous system of mice infected via localized (i.c.) and systemic (i.p.) route, by comparing total titers obtained from the brain, spinal cord and sensory ganglia. We reasoned that the total amount of virus present in the nervous system would correlate with the viral load during latency, as has been suggested in the literature (26–28). Unexpectedly, the total viral titers in the nervous system (sum of viral titer in brain, the TG, spinal cord and associated ganglia) at the peak of viral replication were identical on day 3, and somewhat higher, but not significantly so in systemic infection on day 5 (day 3 – 1.3×103± 3×102 pfu for both routes; day 5 p.i., - 8 × 102± 4×102 pfu for i.c. and 4 × 103±1.1×103 pfu for i.p.). However, we found that in many mice, systemic infection resulted in variable, but greater systemic viral spread than localized infection (Table 1), with the virus reaching the fat pads, spinal cord, brain, spleen, lung, liver, kidney and lymph nodes.
We next compared the dynamics of the CD8 T-cell activation during localized and systemic infection. Cohorts of B6 mice were infected i.c. or i.p. and the expansion, contraction and maintenance of Ag-specific cells were followed longitudinally in blood (Fig. 1A). While i.c. infection produced slightly lower percentage of tetramer+ (Tet+) cells, there was no statistically significant difference in the extent of expansion during the effector phase. At the later timepoints, however, the HSV-specific CD8 T-cells have exhibited significant differences, with the systemically infected mice having significantly higher percentages of Tet+ cells at all points starting from day 45 (Fig. 1a). While Fig. 1A shows a particularly dramatic increase in HSV-specific memory T-cells, such memory inflation was consistently observed in all experiments, and was typically ranging between 12–20% of total CD8 repertoire. This inflation was not only due to differences in the set-point of memory (day 45); whereas after ocular infection the frequency of Tet+ cells remained stable throughout the experiment, in systemic infection the frequency of memory Tet+ cells continued to rise from the levels observed at set-point and was significantly higher than in localized infection at all time points tested (p < 0.00001, Fig. 1A). Of note, inflation did not continue indefinitely, in concert with the fact that expanded cells were not malignantly transformed, and suggesting strongly that some level of homeostatic control remained to keep these cells from continuous accumulation. Examination of cell numbers at 12 months pi showed that this expansion was absolute, and not only relative (Fig. 1B). Therefore, HSV-1, like CMV, was capable of producing early MI, if administered systemically. The onset of MI was not likely a result of the particular conditions granted by the systemic infection route, because systemic infection with an acute virus, recombinant Vaccinia Virus expressing the gB-8p epitope (rVV-gB) was not associated with MI early after infection (Fig. 1C& D). This is consistent with our previous results (17) as well as those of others (29), showing that late-life T-cell clonal accumulations are only rarely specific for viral antigens present during acute viral infections; if such T-cell clonal accumulations occur, they then share the characteristics of spontaneous T-cell clonal expansions (TCE) (30, 31), in that they appear antigen-independent and instead are maintained by cytokines.
Figure 1.
Early onset of memory inflation following systemic HSV-1 infection A–B. Two cohorts of young mice were infected with 106 PFU HSV-1 via localized infection (triangles, n = 10) or systemic (circles, n = 8) infection route. Blood samples were taken at indicated timepoints and stained with anti-CD8 and gB-8p:Kb tetramer. The values (A) represent the average percent of tetramer+ cells within CD8 T-cells over time (± SD), which was significantly different between groups from day 45pi onwards (at least at p < 0.05). The difference in numbers (B) of tetramer+ cells was determined at 12 m.p.i. and was also statistically significant. C – D. Infection with acute virus, rVV-gB, does not results in memory inflation. Two cohorts of young mice were infected with 106 PFU HSV-1 (C, n=11) or rVV-gB (D, n=11) via systemic infection route. Blood samples were taken at indicated timepoints and analyzed as in (A). Each data point represents the percent of tetramer+ cells within CD8 T-cells of individual mice. Memory inflation was observed only in the HSV-1 infected group.
Kinetics Of CD8 T-Cell Activation Following Systemic And Local Infection And In The Course Of MI
While the above data did not provide a formal proof, they could be interpreted to suggest that the difference in memory CD8 T-cell maintenance is linked to differences in viral spread. However, it was also possible that infection via different routes resulted in generation of different CTLs, with different responsiveness to homeostatic stimuli, or different ability to survive beyond the effector phase. The onset of MI could be either a result of early CTL programming unique to the systemic infection route, or a response to different cell extrinsic stimuli, such as ongoing exposure to viral antigen, experienced by the memory cells during the latent phase of infection. To discriminate between these possibilities, we compared the detailed kinetics of CD8 T-cell activation, as determined by the expression/downmodulation of markers associated with activation and also by expansion and contraction in the course of i.p. and i.c. infection (Fig. 2). The analysis of proliferation kinetics of transferred, indicator CD8 cells (gBT-1, specific for the immunodominant HSV gB-8p epitope, (22) (Fig. 2A), and of frequencies of endogenous gB-8p Tet+ CD8 T-cells (Fig. 2B, C), as well as of their activation phenotype measured by the downregulation of CD62L and CD127 (Fig. 2D, E) all revealed faster activation and proliferation in systemic infection, but these differences were greatly reduced or disappeared by day 10 (Fig. 2, B–E), when the replicating virus became undetectable [(23) and not shown]. By that time CD8 T-cells exhibited super-imposable frequencies and absolute numbers (Fig. 2,B & C), as well as the activation phenotype (Fig.2 D,E). Of importance, activation phenotype became again significantly different between the groups during the MI phase (Fig. 3), and these cells did not appear to exhibit any notable functional defects (Fig. 3C, D). This is similar to MI described in the MCMV model, where the memory CD8 T cells specific for the inflationary MCMV epitopes retain their function(32). In contrast, accumulation of functionally defective memory CD8 T cells has been documented in CMV-infected people, particularly the elderly, by the Pawelec/Wikby group (33–35). It is at the present unclear what accounts for these differences, although it is tempting to speculate that the impact of MI on accumulation of dysfunctional memory cells varies with the longevity of the host. We are currently investigating the combined effects of MI and aging on the functionality of memory CD8 T-cells in the HSV infection model (in preparation).
Figure 2.
Despite early differences, the size and phenotype of HSV-specific CD8 T-cell response after systemic and localized HSV-1 infection are the same by day 14. A. Splenocytes from naïve gBT-I TCR transgenic mice were labeled with CFSE and splenocytes containing 2×106 TCR transgenic CD8 T-cells were transferred into each congenic Ly5.2+ recipient. 24 hours post-transfer the recipient mice were infected with HSV-1 via the localized (n = 5) or the systemic (n = 5) infection route. Blood samples were taken at day 3, 5 and 10 p.i.. The graphs are gated on HSV-specific CD8 T-cells (CD8+ gB-8p:Kb tetramer+ Ly5.1+) from representative mice and show dilution of CFSE in HSV-specific CD8 T-cells at a given time point. The numbers above each gate marker represent percent of undivided cells within the HSV-specific CD8 T-cells. The data is representative of 2 independent experiments. B. Cohorts of B6 mice were infected with 106 PFUs HSV-1 via localized (n = 5, triangles) or systemic (n= 5, circles) infection route. Blood samples were taken at indicated time p.i.. and stained with anti-CD8 and gB-8p:Kb tetramer. The values show the average percent of gB-8p:Kb tetramer+ cells within CD8 T-cells (± SD) The data is representative of two independent experiments. C. The mice were infected as in B. On day 14 p.i. the absolute number of HSV-specific CD8 T-cells in spleens of infected mice was determined by staining splenocytes with gB-8p:Kb tetramer and CD8. The values show the average number of tetramer+ CD8 T-cells (± SD). D –E. Expression of CD62L (D) and CD127 (E) by gB-8p:Kb tetramer+ CD8 T-cells from part B was determined by FCM. The values show the average percent of CD62Lhi and CD127+ cells within gB-8p:Kb tetramer+ CD8 T-cells (± SD). The data is representative of two independent experiments. MI = memory inflation.
Figure 3.
Cells undergoing MI are characterized by an effector/effector memory phenotype and retain their effector function. A. Examples of phenotypes displayed by inflating and non-inflating memory CD8 T-cells. At 12 m.p.i., blood samples from mice infected with HSV-1 via localized or systemic route were stained with CD8, tetramer, CD62L, CD127, and CD27. The graphs are gated on tetramer+ cells (infected mice) or on total CD8 T-cells from naïve age-matched mouse. The numbers represent the percent of gated cells expressing the given marker, and are representative of values obtained from the entire experimental cohort. B. Summary of expression of CD62L, CD127 and CD27 on mice infected with HSV-1 via localized (n = 10) or systemic (n = 8) route. The values show the average (± SD) percentage of CD62Lhi, CD127+ and CD27+ within the tetramer+ CD8 T-cells at 12 m.p.i. The data is representative of 3 independent experiments. C–D. Splenocytes from mice at 12 m.p.i. (n=10) were stimulated for 6 hours with 10−5 M gB-8p peptide in presence of brefeldin A, and then stained using gB-8p:Kb tetramer and CD8 (panel C, top plots) or CD8 and intracellular IFNγ (panel C, bottom plots). Representative data from three individual mice is shown in C. The data from all tested mice is summarized in D. The X values show the percent of tetramer+ cells, and the Y values shows the percent of IFNγ+ cells within total CD8+ T-cell pool of individual mice. The values for IFNγ show the net IFNγ (background IFNγ staining from unstimulated wells was subtracted; background was < 0.2%). Excellent correlation of the tetramer and IFNγ staining was observed (R2 = 0.948), indicating that the expanded Tet+ cells are functional.
MI in systemic infection was accompanied by an overwhelmingly effector/effector memory phenotype, with most Tet+ T-cells being CD62Llo,CD127lo,CD27−. Since the virus-specific CD8 T-cells regain expression of CD127 early during the effector-to-memory transition phase (day 14 p.i., Fig. 2E), lack of CD127 expression by memory CD8 T-cells 12 months p.i. (Fig. 3) suggested that this molecule may have been downregulated again by an activation event beyond the acute phase of infection. Moreover, downregulation of CD27 has been linked with presence of repeated Ag stimulation (36, 37). The above described effector memory phenotype suggests that CD8 T-cells from systemically infected mice have received additional stimulation in vivo following resolution of acute infection. We sought to test the possibility that this stimulation resulted from presentation of the gB-8p to the memory T-cells as a result of viral reactivation.
Ag-Dependence Of Development And Maintenance Of MI
To decisively test whether onset of MI is dependent on Ag, we performed adoptive transfer studies in which memory CD8 cells isolated from systemically infected mice were transferred into congenic naïve or infected mice (Fig. 4). We first addressed the issue of MI development, using splenocytes from mice at 14 days p.i., which is an early phase of effector-to-memory CD8 T-cell transition that precedes the onset of MI. The recipients were either naïve, or infected via the localized or systemic route, and they were in the same stage of infection as the donor mice (day 14). The frequencies of donor-derived memory CD8 T-cells were monitored in blood (Fig. 4A). The results were unequivocal. The frequency of donor-derived memory CD8 T-cells transferred into naïve or locally infected recipients declined over time. By contrast, in systemically infected recipients their frequencies remained initially stable and may have slightly increased over time. In a related pilot experiment (data not shown) we have transferred cells from locally infected mice on d14 pi into locally or systemically infected recipients and again saw MI only in systemically infected hosts. We next performed an adoptive transfer of CD8 T-cells that underwent MI for 4 months (systemic infection) to test whether maintenance of already expanded memory CD8 T-cell populations requires presence of Ag (Fig. 4B). The frequency of Tet+ cells within the donor CD8 T-cells remained high early after transfer (day 2), but declined over time in naïve recipients (Fig. 4B). By contrast, the same cell population continued to be maintained in infected mice (Fig. 4B). While our results suggest that these cells may be even increasing in percentages and numbers over time, future experiments will be needed to decisively answer whether there is a net expansion of the transferred cells over long periods of time, particularly in aging. Therefore, maintenance of an elevated frequency of memory CD8 T-cells was absolutely dependent on presence of the virus, and, by inference, of viral Ag.
Figure 4.
Dependence of memory inflation development and maintenance on presence of antigen. A. CD8-enriched splenocytes (see Materials and Methods) pooled from mice infected i.p. with HSV-1 14 days earlier (n = 12, average % of tetramer+ CD8 T cells in donors was 3.5% at the time of transfer) were transferred into congenic Ly5.2+ recipients that were either naïve (n = 3), or infected with HSV-1 via localized (n = 3) or systemic (n = 3) route. Each recipient received 3×105 tetramer+ CD8 T-cells. The percentage of tetramer+ cells within the donor CD8 T-cell population was monitored in blood on days 2, 30 and 60 post-transfer. Average values (±SD) are shown. B. Splenocytes from mice infected i.p. with HSV-1 4 months earlier (n = 3, average % of tetramer+ CD8 T cells in donors was 12% at the time of transfer) were transferred into naïve (n = 3) or systemically infected (n = 3) congenic Ly5.2+ recipients (the infected recipients were infected i.p. with HSV-1 4 months prior to transfer). Each recipient received 5×105 tetramer+ CD8 T-cells. The percentage of tetramer+ cells within the donor CD8 T-cell population was monitored in blood on days 2, 7, and 30 post-transfer. Average values (±SD) are shown.
MI Depends On Establishment Of Latency And Likely Involves Viral Reactivation
Next we sought to specifically address the role of latency and viral reactivation in MI. Famciclovir, also known as famvir, is an inhibitor of HSV-1 replication(38). Famvir is a nucleoside analog with strong affinity for the viral, but not cellular, DNA polymerase, and the drug is ingested in its inactive form and becomes activated by viral thymidine kinase, selectively targeting cells with actively replicating virus (38–40). We and others have previously demonstrated that famvir treatment during acute ocular HSV-1 infection successfully blocks viral replication in vivo (17, 40), and significantly reduces, although does not completely block, the gB transcription during viral reactivation (17). We therefore used this drug to test whether establishment of latency and viral reactivation were necessary for MI.
We first administered famvir to mice in a manner so as to prevent latency (17). Upon such treatment, the virus exhibited greatly curtailed replication at the site of infection (Fig. 5A) and showed no spread to the spleen (Fig. 5B), in contrast to the control mice. That, however, did not prevent CD8 priming in famvir-treated animals (Fig. 5C, D), which in absolute numbers was not significantly different from that in untreated animals on day 7 p.i. (Fig. 5D), most likely due to the substantial amounts of pre-formed gB protein (41). Consequently, the control mice developed robust MI in relative (Fig. 5C) and absolute (Fig. 5D) terms, whereas famvir-treated animals showed no MI. Analysis of the phenotypes of these cells further corroborated the Ag and latency-dependent nature of MI, because while the control cells exhibited effector memory phenotype, famvir-treated cells exhibited clear central-memory phenotype (Fig. 5E).
Figure 5.
Decreasing initial viral load and interfering with viral reactivation can prevent memory inflation after systemic HSV-1 infection. Two groups of mice (n = 4 per group) were infected with HSV-1 via systemic route and their CD8 T-cell responses were followed longitudinally by staining blood samples with CD8 and tetramer. One cohort (HSV+FVR d(−7)) was given antiviral drug famciclovir (FVR) starting on day (−7), and continuing thereafter. The second cohort (HSV) was left untreated. On day 3 p.i., mice from FVR-treated and control group (n = 4/group) were sacrificed and the amount of actively replicating virus was determined in fat pads (A) and in spleen (B). In the FVR treated group, only 2 out of 4 mice had any detectable virus in their fat pads, and no virus was detected in their spleens (n.d.). The average percentage (C) and number (D) of tetramer+ cells within CD8 T-cells was determined. The asterisks in panels C and D indicate a statistically significant (p < 0.05, Student's t-test) difference between the FVR-treated and control groups. (E) Significant differences in expression of CD62L, CD127 and CD27 were observed between the FVR-treated and control groups at 180 d.p.i. The values show the average percentage (±SD) of cells with given phenotype within the tetramer+ CD8 T-cells.
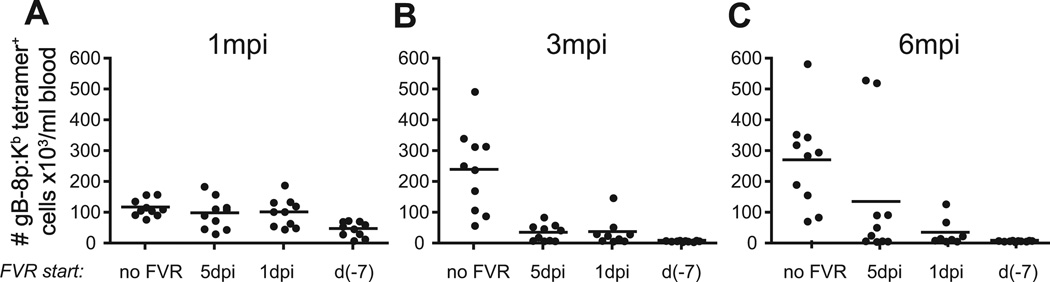
Next, we sought to address the issue of reactivation from latency. Time of onset of famvir treatment directly correlated with MI, so that pretreatment with famvir completely inhibited MI, whereas later onset of famvir administration correlated with increased magnitude and accelerated kinetics of MI (Fig. 6A). Even when famvir treatment has been initiated as late as day 14, by which time acute viral replication was controlled by the immune system, there was an initial prevention, and subsequently a delay, in MI (not shown and Figs. 6 and 7A). That delayed drug administration, however, was not enough to prevent MI over long time periods, and was therefore very different compared to the sharp, and nearly absolute, differences between animals pre-treated with Famvir (Fig. 5) and controls, or between systemic and ocular infection (Figs 1–3).
Figure 6.
Timing of famciclovir (FVR) treatment initiation determines the kinetics and extent of MI. Cohorts of mice (8–10/group) were infected systemically with HSV-1 and their gB-8p-specific CD8 T-cell responses were monitored in blood over time. Four groups were analyzed: control (no famciclovir, no FVR in the legend), and three groups of mice in which FVR treatment was started on day 5 p.i., day 1 p.i. or 7 days prior to infection. Once started, FVR was administered continuosly throughout the duration of the experiment. The number of tetramer+ cells/ml blood is shown for individual mice per group at 1 (A), 3 (B) and 6 m.p.i. (C). The data is representative of two independent experiments.
Figure 7.
Dependence of in vivo proliferation of memory CD8 T-cells on presence of antigen. Cohorts of B6 mice were systemically infected with HSV-1 and their gB-8p-specific CD8 T-cell response was monitored in blood over time. (A). One group was left untreated (HSV only, n = 8), whereas the second group was continuously treated with FVR starting on day 14 p.i. (HSV + FVR, n = 8). At 7 m.p.i. mice from both groups were given BrdU in their drinking water for 3 weeks to monitor in vivo T cell proliferation. The loss of BrdU label (BrdU chase) was monitored over the course of 5 weeks following termination of BrdU treatment. Mice in HSV+FVR group continued to receive FVR during BrdU treatment. B–C. Percent of BrdU+ cells within tetramer+ or tetramer− CD8 T-cell populations in FVR treated (B) or untreated control (C) group. Average values (±SD) are shown. D. Percent of BrdU+ cells within tetramer+ CD8 T-cell populations from FVR treated or untreated control group. Average values (±SD) are shown. E. The change in frequency of Tet+ cells in both cohorts (famvir-treated - filled bars; control - open bars), respectively was monitored during the chase period. The frequency measured at the start of chase was taken as 100% and the frequencies detected at 0 and 5 week timepoints were calculated accordingly. Overall, this corresponded to absolute values of 3±1.6 and 6.3±1.1 × 105 of gB-specific CD8 cells in famvir-treated and control mice, respectively.
We used this delayed drug administration protocol to examine proliferation and accumulation of CD8 T-cells in the course of MI. Young animals were infected and the experimental group maintained on famvir as of d. 14 p.i. Seven months later, there was significant MI in the untreated, but significantly lower MI in the famvir-treated group (significant difference in the average percent of Tet+ CD8 cells, p=0.005,Figure 7A), at which time the mice were treated with BrdU in drinking water over 3 weeks. This long pulse allowed labeling of up to a third of total CD8 T-cells that had proliferated at least once over this time period. We next determined the kinetics of label loss, as a measure of long-term turnover amongst CD8 T-cells. In the famvir-treated group, HSV-specific cells did not show faster turnover than CD8 T-cells of irrelevant specificity (Fig. 7B). By contrast, Tet+ cells from control mice showed significantly greater loss of label (and therefore higher turnover) compared to Tet− cells (Fig. 7C). Finally, direct comparison of Tet+ cells from control and famvir-treated mice revealed significant increase in CD8 T-cell turnover in the absence of famvir (Fig. 7D). This was not a consequence of the direct action of the drug upon CD8 T-cells, because we saw no difference between CD8 T-cell turnover between naïve, uninfected mice in the presence and the absence of long-term administration of famvir (not shown). While the above differences in labeling rates were not dramatic, they could be expected to amplify over time and produce substantial differences in accumulation and maintenance of elevated numbers of memory CD8 T-cells in older animals, as seen in Fig. 1. Alternatively, gain due to proliferation in these cells could be offset by simultaneous loss due to death of some of these cells, and recruitment of new naïve cells into the response could also play a role in the overall population dynamics of this memory pool (32). Regardless of the precise source, an analysis of gB-specific CD8 T-cell representation over the same period revealed substantial increase in percentages (Fig. 7E) and absolute numbers (3±1.6 and 6.3±1.1 × 105 of gB-specific CD8 cells in famvir-treated and control mice, respectively) of gB-specific cells in the blood, which was not seen in famvir treated mice, consistent with the above hypothesis. However, one should bear in mind that viral reactivation would be expected to occur asynchroneously and this linear sharp increase in this period is unlikely to represent the whole memory inflation period, as judged from the plateau effect we have seen in absolute accumulation of inflated cells.
To better understand the mechanism of MI we next analyzed the maintenance of memory CD8 T cells by correlating their numbers and turnover over a longer period of time in blood (Fig. 8 A,C,E,G) and spleen (Fig. 8 B,D,F,H). Mice were infected via the i.p. route and either left untreated (HSV only group), or were treated with famvir from day 5 p.i. onwards (HSV + FVR group). The timing of initiation of famvir treatment was based on results presented in Fig. 6, which showed day 5 p.i. to be optimal for interfering with MI, but not preventing it alltogether. After resolution of the acute infection and establishment of gB-specific memory cell pools mice were given BrdU for 3 weeks (day 77–98 p.i.), and then the turnover of Tet+ CD8 T-cells was followed for 16 weeks by measuring the loss of BrdU (day 98–210 p.i.). Similarly to the experiment form Fig. 7, up to 30% of Tet+ cells found in blood got labeled with BrdU during the 3 week-long pulse, both in control and famvir treated mice (Fig. 8E). However, over the course of 16 weeks we found that the Tet+ cells from control mice (HSV only group) had a significantly greater turnover than Tet+ cells form famvir-treated mice, as shown by greater loss of BrdU (Fig. 8E). This increased turnover in control mice correlated with development of MI and maintenance of elevated numbers of Tet+ cells in blood (Fig. 8 A,C). Again, given that no major increase in percentages was seen over long periods of time, we conclude that there must be additional factors that result in loss of Tet+ cells to balance the increased proliferation, so that the entire population is either maintained or increases only slightly. These factors remain to be elucidated. In addition, significantly greater proportion of Tet+ cells from control than from famvir-treated mice had phenotype indicative of recent stimulation (fewer CD62Lhi, CD127+, CD27+ cells, Fig. 8G), suggesting that there is ongoing (likely periodic) Ag presentation in HSV infected mice, and that its extent can be decreased by treatment of latently infected animals with antiviral drugs.
Figure 8.
Comparison of in vivo proliferation and maintenance of memory CD8 T cells in blood and spleen. Cohorts of B6 mice were infected with HSV-1 as in Fig. 7. One group (HSV only, n=8)) was left untreated, and the second was continuously treated with FVR starting on day 5 p.i. (HSV + FVR, n=8). Both groups were given BrdU in their drinking water for 3 weeks (day 77–98 p.i.). The percentage (A, B) and number (C,D) of Tet+ cells, as well the loss of BrdU label by Tet+ CD8 T-cells (E, F) was analyzed in blood (A,C,E) and spleen (B,D,F) during 16 weeks following the end of BrdU labeling (day 98–210 p.i.). G–H. The expression of CD62L, CD127 and CD27 was evaluated on Tet+ CD8 T-cells in blood (G) and spleen (H) on day 210 p.i. The results are presented as percentage of CD62Lhi, CD127+ and CD27+ cells within Tet+ CD8+ T-cells (±SD).
Interestingly, we found that both development of MI and the differences in the turnover of Tet+ cells in control and famvir treated mice were less dramatic in spleen than in blood (compare Fig. 8A,C,E and Fig. 8B,D,F). During the latent phase (sampled on day 98 and 210 p.i.) the HSV-infected mice had both higher percentage (Fig. 8B) and higher absolute numbers (Fig. 8D) of Tet+ memory CD8 T cells in their spleens than did famvir treated mice. This correlated with increased percentage of memory cells in the spleens of control mice expressing effector memory phenotype (fewer CD62Lhi, CD127+, CD27+ cells, Fig. 8H). However, these differences were much smaller in spleen than in blood. Similarly, the Tet+ cells isolated from spleens of control mice showed a significantly greater loss of BrdU over the course of the experiment than did famvir treated mice, however that difference was not dramatic (Fig. 8F) and smaller than seen among circulating lymphocytes (Fig. 8E). We interpret these results to suggest that T-cells in the spleen, being at the site that does not contain latent virus (Table 1 and not shown), are less exposed to the viral Ag upon reactivation, compared to the cells that recirculate in the blood and presumably visit the sites of neuronal activation. l
Overall, together with the prior results on famvir efficacy and action (17, 40), these results strongly suggest that increased proliferation rate of Tet+ cells is driven by viral reactivation, and are consistent with the idea that this process is the key mechanisms leading to development and maintenance of MI in mice where viral reactivation is not controlled.
DISCUSSION
In this study, we show in a murine model of latent herpesvirus infection that dysregulation of CD8 T-cells responding to the virus depends upon establishment of latency and the presence of viral Ag, and present results that strongly suggest that viral reactivation drives the extent of MI. These results have direct bearing upon the understanding of human T-cell responses to latent herpesviruses, including the dysregulation of these responses with age. Namely, human memory T-cell pool gets progressively obsessed with latent herpesviruses (35, 42), chiefly with CMV, so that up to one-half of the entire pool is specific for this virus (2). It remains unclear whether these responses are the “last man standing” while the rest of the immune system crumbles around them, or whether they may actively contribute to the phenomena associated with immune senescence. Our experiments show that systemic infection with a herpesvirus which is normally localized to the epithelial and neuronal tissues, can produce CMV-like MI in mice, akin to the one described for CMV in mice (20, 21, 43) and humans (1, 42). We show that, similar to previously published results (21, 44), only latent, but not acute, viruses, can produce intense MI. More importantly, we show that the establishment of latency must occur for the onset of MI, that viral antigens are necessary for maintenance of inflated cells, and our data with inhibitors of viral replication strongly suggest that viral reactivation is necessary for this process. This all suggests that in mice, and likely in humans too, constant subclinical reactivation of herpesviruses contributes heavily towards MI. It should be noted that the experiments discussed in this paper focus solely on viral reactivation-induced MI, which is distinct from reactivation-independent, but age-dependent phenomenon of age-related T-cell clonal expansions previously documented to take place in old mice and to sometimes be specific for acute viral ag, but not dependent on them for maintenance(17, 45).
The results presented here are consistent with the recent work in the herpesvirus field suggesting that molecular reactivation of HSV-1 may occur in mice in the absence of clinical reactivation. Several studies demonstrated ongoing presence of inflammatory cell infiltrates at the site of latency, associated with an elevated level of proinflammatory cytokines(9, 10, 46–48), both of which could be curtailed by administration of acyclovir (49). With regard to HSV-1-specific CD8 T-cells, effector memory phenotype cells were shown to be continually present in the TG of ocularly infected mice (11, 13) and to proliferate at a greater rate than memory CD8 T-cells present in an uninfected lung (12). In addition, only virus-specific cells were retained in the latently infected ganglia and the maintenance of their activated phenotype depended on the ability of the host to present HSV Ag (50). This suggested ongoing presentation of viral Ag(s) within the tissue harboring the latent virus. However, in these situations there is no inflation of the virus-specific CD8 T-cells, and it was not clear how would this latent virus interact with the immune system if present systemically. We here show that systemic infection with HSV produces strong MI of gB-specific CD8 T-cells, similar to the one described for mCMV (19, 21, 43) and akin to the situation encountered in humans (51).
Overall, the above results shed light on the molecular basis of this systemic memory accumulation, and support the scenario whereby reactivation of a latent virus over lifetime, followed by Ag presentation to virus-specific CD8 T-cells, lead to MI. We show, to the best of our knowledge for the first time, that transferring inflated cells into the virus-free environment leads to their contraction and/or loss of most of the activated cells. We therefore conclude that accumulating cells are not dysregulated in an absolute sense. This is consistent with the observation that while MI cells accumulate to impressive levels, they rarely occupy more than half of the total CD8 compartment and there is no absolute increase of the CD8 compartment in animals undergoing MI (21)(A. L. and J.N-Z., in preparation). In addition, we found no evidence that the inflated memory cell population lost its ability to function in response to antigenic stimulation. We found that a minor population of memory CD8 T-cells was still detectable 120 days post-infection upon these transfers. It will be of interest to determine, using sorted CD8 T-cell subsets, whether these cells originated from effector memory cells converting into central memory, or whether they were perhaps the central memory cells present in the original inoculum. Moreover, limits of expansion of cells already undergoing memory inflation need to be established experimentally. Finally, it will be important to further understand the precise role of various virus and host mechanisms involved in this phenomenon, as well as the impact of this form of clonally expanded T-cells upon the residual T-cell diversity and immune defense of the host. This is of particular importance in aging organisms, where the size of the expanded T-cell populations can reach levels that have the potential to severely constrict the remaining T-cells in the body. Such constriction could potentially impinge upon productive immune defense, as was shown in the case of spontaneously arising T-cell clonal expansions (TCE) in uninfected mice (52). If so, our results would open the possibility of intervention with antiviral treatments to attenuate viral reactivation, and to consequently dampen expansion of inflated virus-specific T-cells. Experiments are in progress to address these possibilities.
ACKNOWLEDGEMENTS
Authors wish to thank Dr. Ilhem Messaoudi for the help with experimental troubleshooting and helpful discussions and the members of Nikolich lab for input and help.
Supported by USPHS awards AG20719 (to J.N-Z.) and RR0163 (to ONPRC) from the National Institute on Aging and National Institute of Research Resources, NIH.
ABBREVIATIONS
- CMV
cytomegalovirus
- FCM
flow cytofluorometry
- HSV-1
herpes simplex virus type 1
- MI
memory inflation
- TCE
T-cell clonal expansions
- Tet+
T-cells staining with the cognate peptide:MHC tetramer
- TG
trigeminal ganglion
REFERENCES
- 1.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 4.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 5.Mocarski ES, C CT. Cytomegaloviruses and their replication. In: Knipe DM, Howley PM, editors. Fields - Virology. Philadelphia: Lippincot Williams & Wilkins; 2001. pp. 2629–2674. [Google Scholar]
- 6.Jarvis MA, Nelson JA. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. 2002;5:403–407. doi: 10.1016/s1369-5274(02)00334-x. [DOI] [PubMed] [Google Scholar]
- 7.Pollock JL, Virgin HWt. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddehase MJ, Podlech J, Grzimek NK. Mouse models of cytomegalovirus latency: overview. J Clin Virol. 2002;25(Suppl 2):S23–S36. doi: 10.1016/s1386-6532(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 9.Shimeld C, Hill TJ, Blyth WA, Easty DL. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J Gen Virol. 1990;71(Pt 2):397–404. doi: 10.1099/0022-1317-71-2-397. [DOI] [PubMed] [Google Scholar]
- 10.Willey DE, Trousdale MD, Nesburn AB. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984;25:945–950. [PubMed] [Google Scholar]
- 11.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. Immunity. 2003;18:593–608. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 14.Hanke T, Graham FL, Rosenthal KL, Johnson DC. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilakos JP, Michael JG. Herpes simplex virus class I-restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+ T cells. Journal of Immunology. 1993;150:2346–2355. [PubMed] [Google Scholar]
- 16.Bonneau RH, Salvucci LA, Johnson DC, Tevethia SS. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 17.Lang A, Brien JD, Messaoudi I, Nikolich-Zugich J. Age-related dysregulation of CD8+ T cell memory specific for a persistent virus is independent of viral replication. J Immunol. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol. 2000;74:11495–11503. doi: 10.1128/jvi.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podlech J, Holtappels R, Pahl-Seibert MF, Steffens HP, Reddehase MJ. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J Virol. 2000;74:7496–7507. doi: 10.1128/jvi.74.16.7496-7507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 22.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 23.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 24.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. Journal of Experimental Medicine. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 26.Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–6892. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawtell NM, Poon DK, Tansky CS, Thompson RL. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino Y, Qin J, Follmann D, Cohen JI, Straus SE. The number of herpes simplex virus-infected neurons and the number of viral genome copies per neuron correlate with the latent viral load in ganglia. Virology. 2007 doi: 10.1016/j.virol.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, Woodland DL. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- 30.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 31.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 32.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 37.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur J Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 38.Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66:2397–2416. doi: 10.2165/00003495-200666180-00016. [DOI] [PubMed] [Google Scholar]
- 39.Darby G, Field HJ, Salisbury SA. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981;289:81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- 40.LeBlanc RA, Pesnicak L, Godleski M, Straus SE. The comparative effects of famciclovir and valacyclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice. J Infect Dis. 1999;180:594–599. doi: 10.1086/314962. [DOI] [PubMed] [Google Scholar]
- 41.Brehm MA, Bonneau RN, Knipe DM, Tevethia SS. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J. Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term Cytomegalovirus infection leads to significant changes in the composition of the CD8 T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holtappels R, Podlech J, Geginat G, Steffens HP, Thomas D, Reddehase MJ. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8(+) T cell immunity to respiratory virus infections. Exp Gerontol. 2006 doi: 10.1016/j.exger.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 49.Halford WP, Gebhardt BM, Carr DJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 50.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. Journal of Immunology. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 51.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 52.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]