Abstract
The interaction of coralyne with poly(A)•poly(U), poly(A)•2poly(U), poly(A) and poly(A)•poly(A) is analysed using spectrophotometric, spectrofluorometric, circular dichroism (CD), viscometric, stopped-flow and temperature-jump techniques. It is shown for the first time that coralyne induces disproportionation of poly(A)•poly(U) to triplex poly(A)•2poly(U) and single-stranded poly(A) under suitable values of the [dye]/[polymer] ratio (CD/CP). Kinetic, CD and spectrofluorometric experiments reveal that this process requires that coralyne (D) binds to duplex. The resulting complex (AUD) reacts with free duplex giving triplex (UAUD) and free poly(A); moreover, ligand exchange between duplex and triplex occurs. A reaction mechanism is proposed and the reaction parameters are evaluated. For CD/CP> 0.8 poly(A)•poly(U) does not disproportionate at 25°C and dye intercalation into AU to give AUD is the only observed process. Melting experiments as well show that coralyne induces the duplex disproportionation. Effects of temperature, ionic strength and ethanol content are investigated. One concludes that triplex formation requires coralyne be only partially intercalated into AUD. Under suitable concentration conditions, this feature favours the interaction of free AU with AUD to give the AUDAU intermediate which evolves into triplex UAUD and single-stranded poly(A). Duplex poly(A)•poly(A) undergoes aggregation as well, but only at much higher polymer concentrations compared to poly(A)•poly(U).
INTRODUCTION
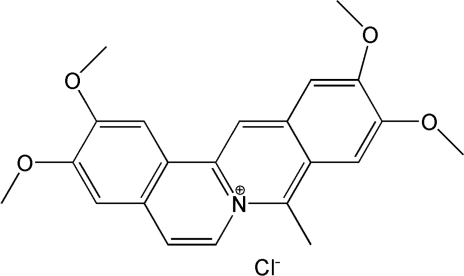
Coralyne, a synthetic crescent-shaped molecule (Figure 1), belongs to a class of compounds that play an important role in medicinal chemistry due to their extensive biological activity. Its antileukemic activity (1,2) and low toxicity (3) are the basis for the high interest aroused by this molecule in recent years (4,5). A number of studies on the mechanism of this antileukemic action have demonstrated that coralyne is able to interact with DNA via intercalation (6,7). Subsequent researches have shown that the binding to double helices is site-specific, and displays a preference for guanine–cytosine base-pairs (8,9). Moreover, coralyne can play an important role in the antigene and antisense strategies (10–12). Actually, coralyne has been found to stabilize poly(dA) single-strands (13–15) and DNA triple helices (16,17), showing a preference for T–A–T triplets (18,19). Although the binding mode is intercalation, for high dye concentrations external stacking on the DNA template has also found to occur (20). Finally, coralyne was shown to be able to induce G-quadruplex structures and therefore it has been the object of studies concerning the telomerase inhibition in cancer fight (21).
Figure 1.
Coralyne chloride (8-methyl-2,3,10,11-tetramethoxydibenzo[a,g]quinolizinium chloride) molecular formula.
Despite the number of articles published on the interaction of this molecule with DNA, detailed investigations on the binding mechanism accomplished by thermodynamic and kinetic analyses of the interaction features are still lacking. The interaction of coralyne-like molecules with RNA has been even far less investigated. It should be noticed that the data analysis is difficult because of the high tendency of coralyne to self-aggregation (6,22,23). Fluorescence techniques, which enable a precise observation of very low dye concentrations, provide a powerful tool to overcome difficulties due to aggregation. When the present manuscript was in progress, an article has appeared (24) in which the binding equilibria of some berberine analogues with poly(A)•poly(U) have been investigated; although the authors conclude that coralyne undergoes partial intercalation into the duplex, the profound structural changes we describe in this work were not reported. Actually, we have found that coralyne is able to induce the disproportionation of poly(A)•poly(U) to give triplex poly(A)•2poly(U) and a single poly(A) strand. Static methods including spectrofluorometric, spectrophotometric, viscometric and circular dichroism experiments, which provide information both on the binding processes and the conformational changes, have been combined in this study. Moreover, kinetic experiments by the stopped-flow and T-jump techniques enabled a deeper analysis of the binding mechanism.
MATERIALS AND METHODS
Materials
Coralyne chloride (Sigma-Aldrich, 99.9%) was used without further purification. Stock solutions of the dye (ca. 2 × 10−3 M) were prepared by dissolving weighed amounts of the solid in water and kept in a refrigerator (4°C). Polyadenylic acid (poly(A)), polyuridylic acid (poly(U) and poly(A)•poly(U) were all lyophilized sodium salts from Sigma-Aldrich (Germany). Other reagents were analytical grade. Stock solutions of the polynucleotides were prepared in [NaCl] = 0.1 M, brought to pH = 7.0 using sodium cacodylate [(CH3)2AsO2Na, 1.0 × 10−2 M] and then standardised spectrophotometrically using ε = 10 100 M−1 cm−1 (257 nm) for poly(A), ε = 8900 M−1 cm−1 (260 nm) for poly(U) and ε = 14 900 M−1 cm−1 (260 nm) for poly(A)•poly(U) (25). Doubly distilled water was used throughout.
Experiments with poly(A) were carried out at pH = 7.0, where the polynucleotide is in the single strand form, and at pH = 5.2, where poly(A) assumes a stable double-strand structure. Actually, the transition of poly(A) from the single- to the double-strand conformation depends on pH and ionic strength (I); at I = 0.10 M, 25°C and pH 5.2 the transition is fully accomplished (26) and the duplex of poly(A) is half protonated (27). Poly(A)•2poly(U) stock solutions were prepared by mixing equimolar amounts of poly(A)•poly(U) and poly(U) at pH = 7.0 and leaving the mixture to stay overnight. Note that in a previous study (28) on poly(A)•2poly(U) formation from poly(A)•poly(U) and poly(U) under the same experimental conditions employed in the present work it was found that strand union attains almost completion in a few seconds; the annealing process was revealed by a further modest change lasting for some minutes only. The polynucleotide concentration is denoted as CP, where CP is expressed in molarity of single bases for single-stranded poly(A), of base pairs for duplexes and in molarity of base triplets for poly(A)•2poly(U). The molar concentration of coralyne is denoted as CD. The absorbance of the dye and polynucleotide stock solutions, kept in the refrigerator, showed no noticeable changes over a time interval of a month, thus revealing that the reagents are stable over this period. Sodium chloride was used to adjust the ionic strength and sodium cacodylate [(CH3)2AsO2Na, 1.0 × 10−2 M] was employed to keep the solutions pH at the value of 7.0 [or 5.2 for poly(A)•poly(A)].
Methods
The pH measurements were taken with a Metrohm 713 (Herisau, Switzerland) pH-meter equipped with a combined glass electrode.
Spectrophotometric measurements
Spectrophotometric measurements were performed on a Perkin Elmer (Überlingen, Germany) Lambda 35 spectrophotometer and on a Hewlett-Packard 8453A (Agilent Technologies, Palo Alto, CA) photodiode array spectrophotometer. A typical titration was carried out by adding with a Mitutoyo (Kawasaki, Japan) syringe increasing micro-amounts of a polynucleotide solution 4 × 10−3 M directly into the cell containing 1.02 ml of the dye solution 5 × 10−5 M. The volumes added stepwise, initially small (0.33 µl), were progressively increased up to 83 µl in the final addition. After each addition the system was allowed to equilibrate for at least 5 min, since it was found that after such a time lapse the signal was stable. The sample was not illuminated during this period. The data have been corrected for dilution effect.
Spectrofluorometric measurements
Fluorescence titrations were performed on a Perkin Elmer (Überlingen, Germany) LS55 spectrofluorometer at λex = 420 nm and λem = 470 nm. The titrations were carried out using the same procedure described above for absorbance titrations. The only difference was that the typical polymer concentration in the syringe was 4 × 10−4 M and the typical dye concentration in the cell was 7 × 10−7 M.
Fluorescence quenching
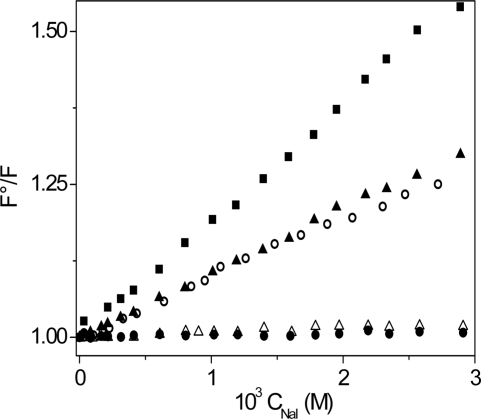
The experiments on fluorescence quenching were done at λex = 420 nm and λem = 470 nm by adding increasing amounts of the quencher solution (0.1 M NaI) directly into the spectrofluorometric cell containing the polynucleotide/dye system. The experimental conditions were such that complex formation was (almost) quantitative and the concentrations of free coralyne could be neglected. The details of the experiments are given in the legend of Figure 4.
Figure 4.
Stern–Volmer plots for the NaI quenching of the light emitted by different polynucleotide/coralyne systems. CD = 6.2 × 10 − 7 M, λex = 420 nm, λem = 470 nm, T = 25°C. (filled square) coralyne alone; (filled triangle) poly(A)•poly(U)/coralyne: CP = 6.90 × 10−7 M, CD/CP = 0.9, %complex = 86, pH = 7.0, [NaCl] = 0.01 M; (open triangle) poly(A)•poly(U)/coralyne: CP = 1.15 × 10−5 M, CD/CP = 0.05, %complex = 98, pH = 7.0, [NaCl] = 0.01 M; (filled circle) poly(A)•2poly(U)/coralyne: CP = 1.15 × 10−5 M, CD/CP = 0.05, %complex = 99.6, pH = 7.0, [NaCl] = 0.1 M; (open circle) poly(A)•poly(A)/coralyne: CP = 5.34 × 10−5 M, CD/CP = 0.01, %complex = 97, pH = 5.2, [NaCl] = 0.01 M. The maximum quenching effect is exerted on free coralyne in solution, whereas no quenching is exhibited by the poly(A)•2poly(U)/coralyne and poly(A)•poly(U)/coralyne at CD/CP = 0.05 systems because, owing to intercalation, coralyne is widely protected from the iodide action. The poly(A)poly(A) and poly(A)poly(U)/coralyne at CD/CP = 0.9 systems exhibit an intermediate behaviour, which suggests that in these systems coralyne is partially intercalated.
Salt effect and ethanol addition measurements
The spectrofluorometric titrations of the poly(A)•poly(U)/coralyne system have been done also under low added salt conditions following the same procedure described above. Both the dye and the polynucleotide solutions contained 0.01 M NaCl and 2.5 × 10−3 M sodium cacodylate (pH = 7.0). Titrations of the poly(A)•poly(U)/coralyne system in the presence of ethanol were made using the same procedure above described. The analysed range goes from 0 to 30% EtOH (v:v).
Melting experiments
The thermal denaturation studies were performed on a Hewlett-Packard 8453A (Agilent Technologies, Palo Alto, CA) spectrophotometer outfitted with diode array detection and computer-assisted temperature control systems. The raise in temperature was in all experiments 0.2°C/min. The coralyne concentration is kept constant at 1.7 × 10−5 M whereas the polynucleotide concentration is varied in order to obtain the desired CD/CP ratio. Before starting the temperature increase, the polynucleotide/dye mixture was let to stay some hours.
Circular dichroism (CD)
CD measurements were made with a thermostatted MOS-450 Biologic spectrometer (Bio-Logic SAS, Claix, France) fit out with a 1.0 cm path length cell. CD titrations were carried out at 25°C by adding increasing calibrated micro-amounts of the dye to a known volume of the polymer solution (∼2 ml), following the same procedure used for spectrophotometric and spectrofluorometric titrations. The typical dye concentration in the syringe was 1 × 10−3 M and the typical polymer concentration in the cell was 6 × 10−5 M poly(A)•poly(U) and poly(A)•2poly(U) or 2 × 10−4 M poly(A)•poly(A) and ss-poly(A).
Viscometric measurements
Viscometric measurements were performed by means of a Micro-Ubbelohde viscometer (Schott Geräte Gmbh) whose temperature was controlled by an external thermostat (±0.1°C). Titrations were accomplished at 25°C by adding increasing calibrated micro-amounts of a dye solution (1 × 10−3 M) to a known volume of the polymer solution (2.0 ml), following the same procedure described above for other titrations. The typical polymer concentration in the viscometer was 1.6 × 10−4 M.
Kinetics
The kinetic measurements were made using a stopped-flow equipment described elsewhere (29) and a home-made T-jump apparatus based on the Riegler et al. (30) prototype with the photomultipliers replaced by suitable silica photodiodes (Hamamatsu, S1336, Japan). A tungsten lamp–monochromator system was used as the light source. The relaxation curves were collected by an Agilent 54622A (Palo Alto, CA) storage oscilloscope, transferred to a PC and evaluated with the fitting package by Jandel (AISN software, Mapleton, OR). Each shot was repeated at least ten times and the resulting relaxation curves were averaged via an accumulation procedure.
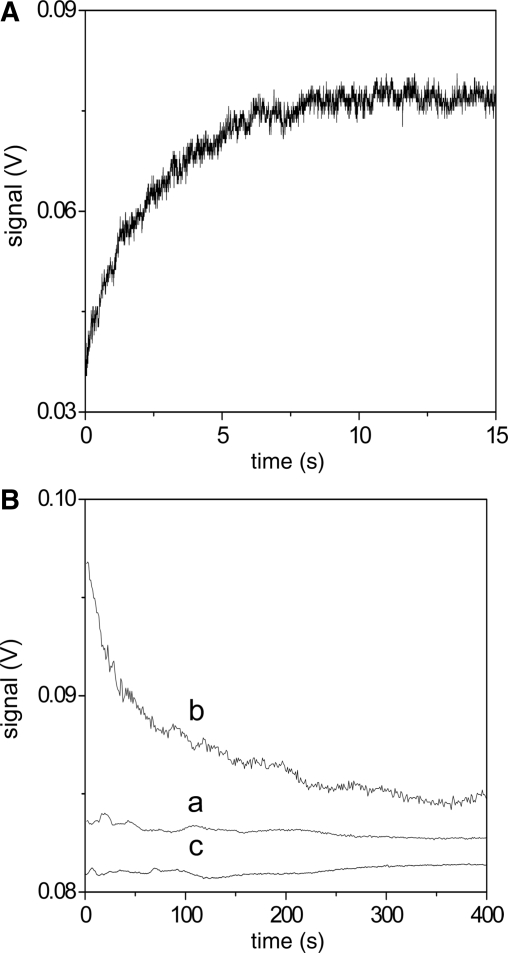
As regards the stopped-flow experiments, the apparatus is able to measure absorbance and/or fluorescence changes and in the present study both detection modes were employed. The details of the experiments are given in the legend of Figure 8, where the concentrations given are the final values after 1:1 mixing of reagents (mixing time 5 ms).
Figure 8.
Kinetic experiments showing triplex formation at [NaCl] = 0.10 M, pH = 7.0, T = 25°C, mixing time 5 ms. (A) Formation of the poly(A)•2poly(U)/coralyne complex is observed on mixing poly(A)•poly(U)/coralyne with poly(U); Cpoly(A)·poly(U)/coralyne = 4.7 × 10−6 M, CpolyU = 4.7 × 10−6 M (the signal corresponds to the fluorescence change using λex = 405 nm); (B) The experiment performed mixing poly(A)•poly(U) and coralyne at CD/CP = 3.0 (curve a) does not display any kinetic effect, thus indicating that triplex does not form under these conditions. In contrast, triplex formation is revealed by the absorbance decrease at 280 nm at CD/CP = 0.3 (curve b). Curve (c) shows that mixing the triplex poly(A)•2poly(U) with coralyne at CD/CP = 0.3 does not exhibit any signal change. (a) Ccoralyne = 1.70 × 10−5 M; Cpoly(A)•poly(U) = 5.67 × 10−6 M; (b) Ccoralyne = 1.70 × 10−5 M; Cpoly(A)•poly(U) = 5.67 × 10−5 M; (c) coralyne: Ccoralyne = 1.70 × 10−5 M; Cpoly(A)•2poly(U) = 5.67 × 10−5 M.
The T-jump apparatus is also able to measure absorbance and/or fluorescence changes; in the present study the fluorescence detection mode at λex = 420 nm was employed to monitor the binding of coralyne to the RNAs. The rate dependence on the reactants concentration has been investigated by varying both coralyne and polynucleotide concentrations (legends of Figure 9 and Supplementary Figure S11) in a way appropriate to obtain the desired values of CD/CP.
Figure 9.
T-jump experiments: dependence of the reciprocal relaxation time, 1/τ, on varying concentrations for the poly(A)•poly(U)/coralyne system at [NaCl] = 0.10 M, pH = 7.0, T = 25°C, λex = 420 nm. (A) CD from 3.4 × 10−7 M to 1.7 × 10 − 6 M, CP from 3.2 × 10−7 M to 4.9 × 10−6 M, CD/CP > 0.8, the binding of coralyne to duplex is observed [Step (1) of the reaction scheme], fit to Equation (8); (B) CD from 1.1 × 10−6 M to 3.4 × 10−6 M, CP from 2.6 × 10−5 M to 6.9 × 10−5 M, 0 < CD/CP < 0.8, dye exchange between duplex and triplex is observed [Step (3) of the reaction scheme], fit to Equation (9).
RESULTS
Equilibria
Absorption spectra
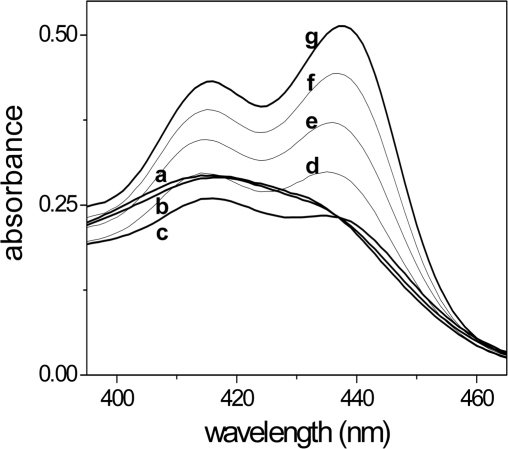
The visible spectra of the poly(A)•poly(U)/coralyne system, recorded at different values of the [dye]/[polymer] ratio (CD/CP), show a rather complex behaviour (Figure 2). As the polynucleotide was added to the spectrophotometric cell containing the dye solution, the intensity of the spectral band of coralyne (420 nm), initially present mainly as an aggregate (23), is progressively reduced as the polymer level increases, while the monomer band (438 nm) raises and becomes more intense than the band of the aggregate. The inversion of the band intensity ratio reveals that, if free coralyne is present as an aggregate, the bound coralyne is present mainly as a monomer. This finding suggests that the dye binds to poly(A)•poly(U) by intercalation. Figure 2 also shows that in the course of the titration the absorbance of the system first decreased and then increased. This behaviour reveals the occurrence of profound changes of the poly(A)•poly(U) structure induced by coralyne, which will be confirmed and analysed below.
Figure 2.
Absorption spectra of the poly(A)•poly(U)/coralyne system. CD = 4.9 × 10−5 M, CP from 0 (a) to 1.3 × 10−3 M [CD/CP = 7.4 (b), 1.9 (c), 0.49 (d), 0.15 (e), 0.049 (f), 0.038 (g)], pH = 7.0, [NaCl] = 0.1 M, T = 25°C. As the RNA content is raised, the absorption first decreases and then increases, revealing that the reaction of dye binding to RNA is coupled to other processes.
Spectrofluorometric titrations
Poly(A)•poly(U)/coralyne
The spectrofluorometric method allows use of dye concentrations much lower (10−7 M) than those needed for absorbance titrations and has enabled us to overcome the difficulties occasioned by the coralyne self-aggregation processes (23) coupled with the dye binding to the polynucleotides.
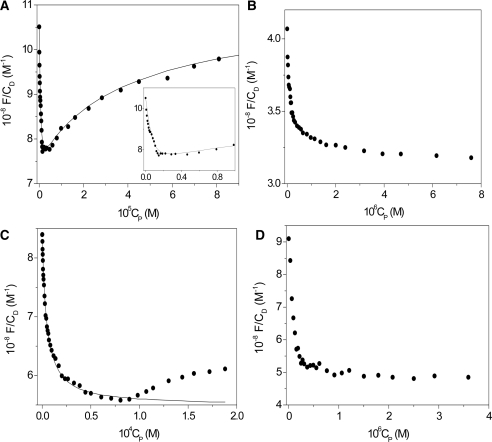
The binding of coralyne to the poly(A)•poly(U) duplex displays, surprisingly, a biphasic behaviour in which the fluorescence first decreases and then, for higher values of CP such that the CD/CP ratio is lower than 0.8, increases (Figure 3A). The biphasic features of the binding isotherms remain upon changing temperature and/or ionic strength. The observed behaviour can be explained according to Scheme (1–3), where the interaction between base-pairs (AU) and coralyne (D) leads first to formation of the AUD complex (Step 1). This process is associated to the first branch of the binding isotherm. When a sufficient dye amount is bound to the double strand, further addition of AU favours the formation of the UAUD triplex (Step 2). Moreover, the T-jump experiments, described below, suggest the occurrence of a third process involving fast ligand exchange between duplex and triplex (Step 3). Steps 2 and 3 give rise to the second branch of the binding isotherm.
| 1 |
| 2 |
| 3 |
The equilibria involved in the proposed model are described by Equation (4)
| 4 |
where φD, is the optical parameter (31) of D, Δφ1 =(φAUD – φD) and Δφ2 = (φUAUD − φD). The iteration procedure presented in the Supplementary Data was employed to analyze the data. The continuous line plotted in Figure 3A shows the goodness of the fit. The values of the parameters obtained are collected in Tables 1 and 2.
Figure 3.
Fluorescence binding isotherms for different polynucleotide/coralyne systems. [NaCl] = 0.1 M, λex = 420 nm, λem = 470 nm, T = 25°C. (A) poly(A)•poly(U), CD = 7.6 × 10−7 M, pH = 7.0 (fit to Equation (4), the insert is an enlargement of the first part of the curve); (B) poly(A)•2poly(U), CD = 7.2 × 10−7 M, pH = 7.0; (C) poly(A)•poly(A), CD = 6.6 × 10 − 7 M, pH = 5.2 (fit to Equation (6) of the first branch); (D) poly(A), CD = 4.8 × 10−7 M, pH = 7.0. The biphasic behaviour displayed by poly(A)•poly(U) is also exhibited by the poly(A)•poly(A)/coralyne system, although to a limited extent. The usual form of the binding isotherm of poly(A)•2poly(U)/coralyne system reveals that in this case only dye binding to triplex is operative.
Table 1.
Thermodynamic and kinetic parameters for the interaction of coralyne with different polynucleotides
| pH | 10−5K1 (M−1) | 10−4K0 (M−1) | k1 (s−1) | k−1 (s−1) | ΔH1 (kcal mol−1) | ΔS1 (cal mol−1 K−1) | |
|---|---|---|---|---|---|---|---|
| poly(A)•poly(U) | 7.0 | 18 ± 7a 39 ± 18b ∼300c | 29 ± 13 | 419 ± 107 | 80 ± 10 | − 17 ± 5 | − 27 ± 10 |
| poly(A)•2poly(U) | 7.0 | >100b 230d | |||||
| poly(A) | 7.0 | 54 ± 7b | |||||
| poly(A)•poly(A) | 5.2 | 1.2 ± 0.5a 2.4 ± 0.1b | 4.5 ± 0.8 | 10.0 ± 0.8 | 6 ± 1 | − 7 ± 2 | 2 ± 5 |
[NaCl] = 0.1 M, 25°C.
aKinetics K1 = K0(1 + k1/k−1).
bFluorescence titrations.
c[NaCl] = 0.01 M.
dK1(triplex) = K1(duplex) × (k−3/k3).
Table 2.
Thermodynamic and kinetic parameters for the binding of coralyne with poly(A)•poly(U) under 0 < CD/CP < 0.8 conditions [Reactions (2) and (3)]
| 104 K2 | 102 K3 | 10−6 k3 (M−1s−1) | 10−7 k−3 (M−1s−1) |
|---|---|---|---|
| 8 ± 20 a | 0.6 ± 2a 8 ± 3b | 2.1 ± 0.4 | 2.7 ± 0.7 |
[NaCl] = 0.1 M, pH = 7.0, 25°C.
aFluorescence titrations.
bKinetics: K3 = k3/k−3.
The reversibility of the reaction Schemes (1–3) with respect concentrations has been tested by addition of increasing amounts of poly(A) to a poly(A)•poly(U)/coralyne mixture brought to the plateau (Figure 3A) with a suitable excess of polymer. The back titration curve displayed a minimum as the forward titration (Figure S1A of the Supplementary Data).
Poly(A)•2poly(U)/coralyne
In this case the binding isotherm shows a simple monophasic decrease in fluorescence as the extent of binding increases (Figure 3B). The experimental data were thus analysed on the basis of reaction (5) by means of Equation (6) (32).
| 5 |
| 6 |
where ΔF = F − φDCD and Δφ = (φUAUD − φD). An iteration procedure, already described (31), was employed in order to obtain a linear representation of Equation (6) as in Figure S2 of the Supplementary Data. The intercept of the plot shown in the figure is too close to zero to allow a reliable evaluation of K1. This result is due to the very high extent of binding. This is the reason why the calculated fit has not been given in Figure 3B. One could guess that K1 > 107 M−1 (Table 1), suggesting that the affinity of coralyne for triplex is much stronger than for duplex. This hypothesis has been confirmed by T-jump experiments which allowed an estimation of K1.
Poly(A)•poly(A)/coralyne and poly(A)/coralyne
The binding isotherm of poly(A)•poly(A)/coralyne is somewhat similar to that of poly(A)•poly(U)/coralyne (Figure 3C) but the fluorescence rise is much weaker and, to be observed, requires much higher polymer amounts. Hence, it was disregarded and only the descending stretch of the binding isotherms was analysed (Figure S3 of the Supplementary Data) using Equation (6). The binding constant value obtained for duplex-coralyne complex formation (first stretch) is given in Table 1.
The binding isotherm of the poly(A)/coralyne system is shown in Figure 3D. The data were analysed by means of Equation (6) (Figure S4 of the Supplementary Data) and the binding constant value is given in Table 1.
Fluorescence quenching analysis
The light emission by the poly(A)•poly(U)/coralyne, poly(A)•2poly(U)/coralyne and poly(A)•poly(A)/coralyne systems in the presence of increasing amounts of sodium iodide [an efficient fluorescence quencher (33)], was measured; for comparison, the effect of iodide ion addition on coralyne alone has been measured as well. Concerning poly(A)•poly(U), the experiments were performed under two different experimental conditions corresponding to the two different branches of the binding isotherm shown in Figure 3A. The total dye concentration used is 6.2 × 10−7 M in all experiments. Using appropriate CP values the fraction of complex was 99.6% for poly(A)•2poly(U)/coralyne, 97% for poly(A)•poly(A)/coralyne, 98% for poly(A)•poly(U)/coralyne (CD/CP = 0.05) and 86% for poly(A)•poly(U)/coralyne (CD/CP = 0.9); the percents were evaluated using the equilibrium constant values of Tables 1 and 2, corrected when necessary for the ionic strength effect. The resulting Stern–Volmer plots are shown in Figure 4.
Salt effect on equilibria
Fluorescence titrations of the poly(A)•poly(U)/coralyne system have been conducted also at 0.01 M NaCl content. The binding isotherm is still biphasic (Figure S5 of the Supplementary Data). The analysis of the data yields K1 = 3 × 107 M−1. It can be observed that, by decreasing the ionic strength from 0.1 to 0.01 M, K1 increases by a factor of ∼10, as expected for the interaction of a double-stranded nucleic acid with an intercalator bearing a monopositive charge (31).
Ethanol effect on equilibria
The effect of ethanol on the binding equilibria of the poly(A)•poly(U)/coralyne system has also been investigated (Figure S6 of the Supplementary Data). The K1 values decreased by a factor higher than 103 when the ethanol content was raised from 0 to 30% (v:v).
Temperature dependence of equilibria
The equilibria of coralyne binding to poly(A)•poly(U) and poly(A)•poly(A) have been measured at different temperatures between 10 and 32°C. The vant Hoff plots (Figure S7 of the Supplementary Data) yielded the ΔH1 and ΔS1 values for the two systems collected in Table 1.
Melting experiments
Figure 5 shows the melting curves for the poly(A)•poly(U)/coralyne system at different values of the CD/CP ratio (columns A and B). For comparison the melting curves of the poly(A)•2poly(U)/coralyne system, recorded under same experimental conditions, are reported as well (column C).
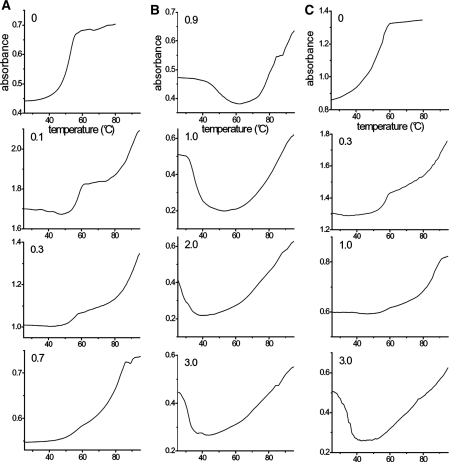
Figure 5.
Absorption melting profiles of the poly(A)•poly(U)/coralyne (columns A and B) and of the poly(A)•2poly(U)/coralyne systems (column C) at different CD/CP ratios. [NaCl] = 0.10 M, pH = 7.0, λ = 280 nm; (A) poly(A)•poly(U)/coralyne at CD/CP < 0.8, from top to bottom CD/CP = 0 (CD = 0 M, CP = 2.5 × 10−5 M), 0.1 (CD = 1.7 × 10−5 M, CP = 1.7 × 10 − 4 M), 0.3 (CD = 1.7 × 10−5 M, CP = 5.7 × 10−5 M), 0.7 (CD = 1.7 × 10−5 M, CP = 2.7 × 10−5 M); (B) poly(A)•poly(U)/coralyne at CD/CP > 0.8, from top to bottom CD/CP = 0.9 (CD = 1.7 × 10−5 M, CP = 1.9 × 10−5 M), 1.0 (CD = 1.7 × 10−5 M, CP = 1.7 × 10−5 M), 2.0 (CD = 1.7 × 10−5 M, CP = 8.5 × 10−6 M), 3.0 (CD = 1.7 × 10−5 M, CP = 5.7 × 10−6 M); (C) poly(A)•2poly(U)/coralyne, from top to bottom CD/CP = 0 (CD = 0 M, CP = 5.0 × 10−4 M), 0.3 (CD = 1.7 × 10−5 M, CP = 5.7 × 10−5 M), 1.0 (CD = 1.7 × 10−5 M, CP = 1.7 × 10−5 M), 3.0 (CD = 1.7 × 10−5 M, CP = 5.7 × 10−6 M). For the poly(A)•poly(U)/coralyne system at 0 < CD/CP < 0.8 the prevailing form at low temperatures is the triplex; two transitions can be observed (triplex → duplex and then duplex → single-strands) that tend to merge when CD/CP approaches the value of 0.8. For CD/CP > 0.8 the absorbance decrease is related to the transition duplex → triplex, whereas the absorbance increase corresponds to the UAUD → U + A + U + D process.
Concerning poly(A)•poly(U)/coralyne, for 0< CD/CP< 0.8 (Figure 5A) the form prevailing up to 50°C is the triplex, whose formation has been promoted by addition of coralyne to duplex some hours prior to starting the melting experiments. Increasing the temperature, a transition (Tm1 = 55°C) do occur which is ascribed to conversion of triplex to duplex with release of a poly(U) strand. Note that this transition becomes less and less evident as the CD/CP value is raised but the value of Tm1 stays constant. Further heating results in the dissociation of duplex into single strands of poly(A) and poly(U). This melting transition is broad, ending at a temperature close to 90°C.
The melting profile experiences a drastic change for CD/CP > 0.8 (Figure 5B). Here, the initially prevailing structure is that of the duplex. As the temperature increases the duplex disproportionates to triplex. Note that the temperature of this transition shifts to lower and lower values as the ratio CD/CP is raised from 0.9 to 3.0. The first transition is quite sharp, in agreement with a single cooperative melting process, whereas the second transition (Tm2) is broader, in particular for high CD/CP values. This behaviour reveals that the second melting effect is quite complex and possibly includes several melting reactions such as UAUD → U + A + U + D, AUD → A + U + D and AD → A + D. The helix–coil transition of poly(A) should contribute as well to make the melting profile broader.
Figure 5C shows the melting curves of the poly(A)•2poly(U)/coralyne system investigated under the same conditions of duplex/coralyne, which have been included for comparison. Increasing the coralyne content induces profound changes in the triplex melting as well.
The reversibility of the poly(A)•poly(U)/coralyne system with respect temperature has been tested by performing two successive heatings of the system, separated by a time lapse of 24 h, at CD/CP = 0.4. The two melting curves, shown in Figure S1B of the Supplementary Data, should be considered in agreement if the alterations of the system secondary structure, caused by the denaturation–renaturation process, are left out.
CD measurements
Addition of coralyne to poly(A)•poly(U) brings about a fall in the intensity of the CD band centred at 260 nm, while a positive two-peak band arises between 315 and 400 nm (Figure S8A of the Supplementary Data). Moreover, new visible bands are generated in the 400–500 nm range, demonstrating dye interaction with helical strands. Figure 6 shows the profile recorded at 340 nm using the CD technique. Two inflections could be revealed at CD/CP = 0.05 and 0.9 respectively. These values limit the range of CD/CP values within which poly(A)poly(U) can undergo disproportionation at 25°C.
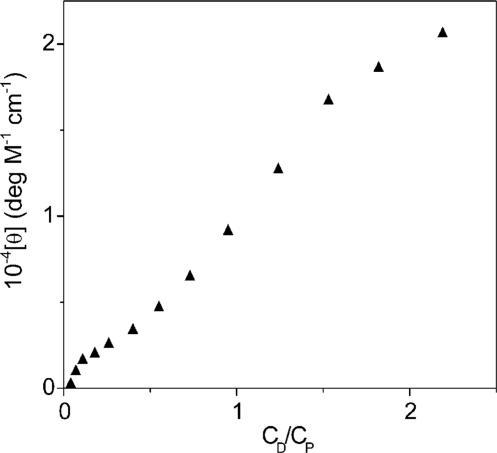
Figure 6.
CD profile for the poly(A)•poly(U)/coralyne system. CP = 6.2 × 10−5 M, CD from 0 to 2.0 × 10 − 4 M, [NaCl] = 0.10 M, pH = 7.0, T = 25°C, λ = 340 nm. The two inflexions can be observed in the CD profile. The first occurs at a value of CD/CP < 0.05 and the second at ∼0.9. The interval between the two inflections limits the range of CD/CP within which, at 25°C, poly(A)•poly(U) disproportionation do occur.
The CD spectra of poly(A)•2poly(U) (Figure S8B of the Supplementary Data) and poly(A)•poly(A) (Figure S8C of the Supplementary Data) show a general behaviour similar to that of poly(A)•poly(U), but plots as that of Figure 6 do not show any inflection.
CD spectra have been recorded also for the ss-poly(A)/coralyne system (pH = 7.0) for comparison purposes (Figure S8D of the Supplementary Data).
Viscometric measurements
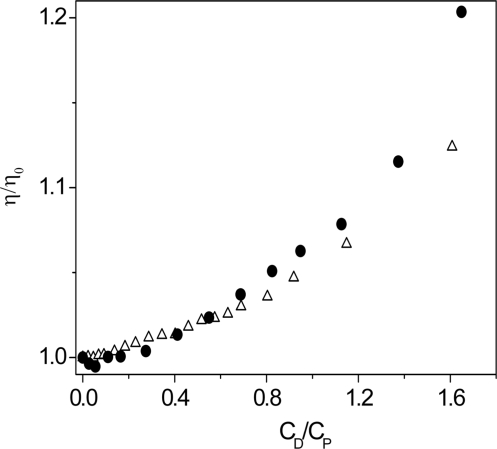
Viscometric experiments were performed by adding increasing dye amounts to a polymer solution directly into the viscometer. The viscosity ratio η/η0, where η0 is the viscosity of the polymer alone, was plotted as a function of the CD/CP ratio (Figure 7). The viscosity variation of poly(A)•poly(U) and of poly(A)•2poly(U) display trends that almost overlap for 0 < CD/CP < 0.8, thus confirming that under these conditions the AUD duplex converts to triplex (UAUD). For CD/CP > 0.8 the two trends diverge, revealing that poly(A)•poly(U) is now present as a duplex.
Figure 7.
Relative viscosity (η/η0) dependence of poly(A)•poly(U)/coralyne (open triangle) and poly(A)•2poly(U)/coralyne (closed circle) systems on the CD/CP ratio. CP = 1.6 × 10−4 M, CD from 0 to 2.6 × 10 − 4 M, [NaCl] = 0.10 M, pH = 7.0, T = 25°C. As expected, the two trends tend to overlap at low values of CD/CP because, under these circumstances, the polymer is present as a triplex in both systems. The trends become distinct at the highest values of CD/CP since the polymer is now present as a duplex in the poly(A)•poly(U)/coralyne system.
Kinetics
Kinetic evidence for triplex formation
Table 1 shows the highest affinity of the dye for poly(A)•2poly(U) compared to poly(A)•poly(U). Evidence of the highest affinity of coralyne for the triplex is also provided by the stopped-flow experiments shown in Figure 8. In the first experiment (Figure 8A) the preformed poly(A)•poly(U)/coralyne complex is mixed with single-stranded poly(U). The large fluorescence increase recorded during the reaction reveals that the conversion of the AUD duplex to the UAUD triplex not only occurs to a large extent but also that the stacking interaction between dye and polymer is strengthened. Further evidence for the ability of coralyne to induce triplex formation is provided by the second experiment shown in Figure 8B. Here, poly(A)•poly(U) is added to the dye contained in the spectrophotometric cell. Curve (a) refers to CD/CP = 3.0, where only the fast Step (1) of the reaction Scheme (1–3) can occur. No kinetic effects are displayed in the long time-scale since, under these conditions, the AUD complex, formed within the mixing time, does not react further. By contrast, run (b), carried out at CD/CP = 0.3 where triplex formation should occur, displays a kinetic effect in the long time scale caused by the AUD conversion to triplex UAUD.
A kinetic experiment, where coralyne is mixed with triplex poly(A)•2poly(U) at CD/CP = 0.3 has been performed for control (c). The binding of D to UAU to form UAUD is completed within the mixing time and no further reaction can occur in the long time range, as demonstrated by the horizontal trace.
Binding of coralyne to poly(A)•poly(U)
The analysis of the fast binding of coralyne to poly(A)•poly(U) has been performed using the T-jump technique in the fluorescence detection mode (λex = 420 nm). As the static experiments showed a biphasic behaviour, two sets of relaxation experiments were carried out, each under the conditions where either of the two phases shown in Figure 3A is prevailing. Simple mono-exponential relaxation curves were always observed (Figure S9 of the Supplementary Data).
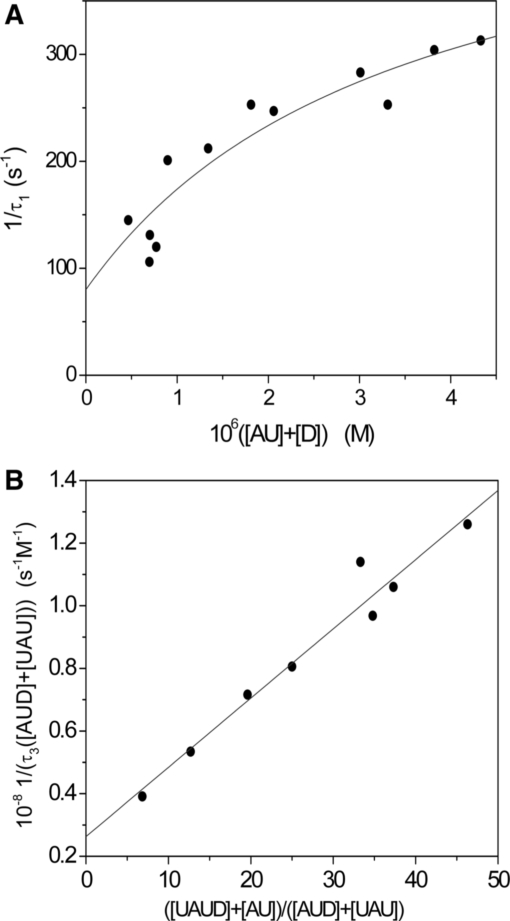
T-jump experiments at CD/CP > 0.8
The species distribution diagram (Figure S10 of the Supplementary Data), built on the basis of the reaction Scheme (1–3), shows that for CD/CP > 0.8 at 25°C the species UAUD can be neglected and reaction (1) is prevailing. A plot of the reciprocal relaxation time, 1/τ1 versus [AU] + [D] (Figure 9A) tends to a plateau, thus revealing that an intermediate species is rapidly formed prior to the rate-determining step, as in Scheme (7).
| 7 |
According to this scheme, the concentration dependence of 1/τ1 is derived (31) in the form of Equation (8), which has been used to analyze the data.
| 8 |
The obtained reaction parameters are collected in Table 1.
T-jump experiments at 0 < CD/CP < 0.8
This set of experiments has been carried out under conditions of polymer excess. Interestingly, in spite of the much higher amount of poly(A)•poly(U) employed, the values of the reciprocal relaxation time, 1/τ3, are somewhat lower than those provided by the experiments performed at CD/C P> 0.8. Moreover, the concentration dependence of the rate does not tend to level off. These findings indicate that, under conditions of RNA excess, the process observed in the T-jump scale relaxes independently of Step (1). On the other hand, this process, being of the magnitude of hundredths of a second, cannot be ascribed to reaction (2); actually, reaction (2) involves unwinding of AU and winding of U to AUD in order to form UAUD; this process is slow, as shown by the experiments of Figure 8 and by the literature (34). Instead, the relaxation effect observed reveals the occurrence of an interaction between dye and polymer, which manifests itself in the range of 10−2 s. Since for 0< CD/CP< 0.8, the concentration of free coralyne is largely minority (Figure S10 of the Supplementary Data), fast dye exchange between strands can occur according to Step (3) of the reaction Scheme (1–3). Actually, under these circumstances Step (1) cannot be operative due to the small concentration of D, whereas Step (2) is out of the observation window of the T-jump method owing to its slowness; hence, the observed relaxation effect should be attributed only to Step (3). The corresponding expression for 1/τ3 is given by Equation (9).
| 9 |
Note that Step (3) is thermodynamically unfavourable (K3 = 8 × 10−2); however, in this case the AU excess added is able to drive the equilibrium to the right-hand side to such an extent that the amplitude factor (35) experiences an increase suitable to produce relaxation effects of measurable amplitude. The plot of Figure 9B provides the values of the rate constants that are collected in Table 2.
Interaction of coralyne with poly(A)•poly(A)
A T-jump study of the interaction of coralyne with poly(A)•poly(A) was also performed. Simple mono-phasic relaxation curves were observed (Figure S9C of the Supplementary Data) and analysed by means of mono-exponential functions. The concentration dependence of 1/τ tends to a plateau, (Figure S11 of the Supplementary Data), thus indicating that the reaction mechanism likely to occur is the one described by a reaction scheme similar to Scheme (7) where AA is written instead of AU. Data were analysed according to Equation (8) (with AA written instead of AU) and the obtained reaction parameters are collected in Table 1.
DISCUSSION
The principal result stemming from the analysis of the data provided by the different experimental techniques employed in the present investigation is that coralyne induces the disproportionation of poly(A)•poly(U) into the triplex poly(A)•2poly(U) and a single-strand of poly(A). This result is particularly interesting since, although the ability of coralyne to induce dismutation of poly(dA)•poly(dT) into a mixture of triple- and single-strand structures was already recognised (17,36), such an ability concerning RNA so far was not observed.
The extent of the disproportionation process, at constant temperature, depends on the CD/CP ratio. A plot of the dependence of [UAUD]/[AUD] versus CD/CP, made using the equilibrium constants of Tables 1 and 2 (Figure S10 of the Supplementary Data), shows a sharp increase of the coralyne preference for triplex at low dye loadings while on increasing CD/CP the preference of the dye changes in favour of the duplex to which it binds by intercalation. Quite similar behaviour is displayed by ethidium (37) although the dye does not induce disproportionation of duplex.
The ΔH1 value related to the interaction of coralyne with the duplex AU is negative (−17 Kcal mol−1) in agreement with the intercalative nature of Step (1). Chaires (38) correlated the enthalpy with the entropy of a large number of reactions of dye binding to DNA and observed that a plot of ΔH versus −TΔS presents two linear segments, both with a negative slope. The points lying on the segment with the most negative slope belong to intercalation processes whereas the points fitting to the segment with less negative slope correspond to non-intercalative binding reactions. The ΔH1 and ΔS1 values for Step (1) lie on the first segment of the Chaires plot, thus supporting the intercalative nature of Step (1), under the assumption that DNA and dsRNA behave similarly with respect to intercalation.
The interaction of coralyne with the triplex UAU is characterised by a very high affinity, so high that only a lower limit could be provided for the binding constant (K1 > 107 M−1) even by the fluorescence method in which very small concentrations of reactants are used. However, we have been able to estimate K1 for the poly(A)•2poly(U)/coralyne system from the kinetic analysis of Step (3) (Figure 9B). Actually, Step (3) is a combination of the binding reactions of coralyne with AU and UAU, so it turns out that K1(triplex) = K1(duplex) × k−3/k3 = 2.3 × 107 M−1 (Table 1).
The affinity of coralyne for the double-stranded poly(A)•poly(A) is lower than that for the other investigated polynucleotides. The reduction of K1 could be ascribed to the fact the base-pair charge in poly(A)•poly(A) is (−1) compared to (−2) in poly(A)•poly(U) and (−3) in base triplets of poly(A)•2poly(U); moreover, in poly(A)•poly(A) the N1 nitrogen site is protonated (39) and one of the two phosphate groups is involved in a hydrogen bond. This feature can play a role in reducing the non-electrostatic interactions between polymer and dye that have been found so important to stabilize the bound forms of the dye.
The fluorescence quenching experiments (Figure 4) confirm the coralyne ability to convert poly(A)•poly(U) to the triplex structure; actually the Stern–Volmer plot yields horizontal lines (no quenching) both for poly(A)•poly(U)/coralyne at 0< CD/CP< 0.8 (white triangles) and poly(A)•2poly(U)/coralyne (black circles); the absence of quenching reveals that coralyne is fully intercalated into the triplex. On the other hand, the behaviours of poly(A)•poly(U)/coralyne at CD/CP> 0.8 (black triangles) and poly(A)•poly(A)/coralyne (white circles) are intermediate between that of free coralyne (black squares, maximum quenching) and that of the systems discussed above (absence of quenching). The reduced, but still remarkable, quenching effect suggests that in AUD and AAD the intercalation is only partial and part of the coralyne molecule stays outside of the polymer cavities, being exposed to the quenching action of the iodide ions. Hence, poly(A)•poly(A) as well can be converted by coralyne to aggregated forms, according to a process similar to Scheme (11) (see below) although with lower efficiency compared to poly(A)•poly(U). In fact, even if biphasic fluorescence binding isotherms are observed as with poly(A)•poly(U), the signal increase is on a smaller scale and occurs only in the presence of a very large excess of polynucleotide. It should be noted that formation of aggregates in solutions containing only poly(A) has been recognised for long time (40), and, more recently, structured AAD forms have been proposed (17,41).
The melting experiments shown in Figure 5 have revealed that for 0 < CD/CP ≤ 0.8 the prevailing form at low temperature is the triplex UAUD, which is formed from the duplex. This form, in turn, converts to duplex by increasing the temperature, the transition temperature (55°C) being independent of the CD/CP value. A comparison of the melting profiles of Figure 5A and C both recorded at CD/CP = 0.3 confirms that the transition at Tm1 = 55°C shown in Figure 5A indeed corresponds to the process triplex → duplex. Moreover, the amplitude of the transition of Figure 5A is half that shown in Figure 5C despite the fact that the initial concentrations of the two RNAs are the same. This means that the first transition shown in Figure 5A corresponds to the melting of an amount of triplex (UAUD) equal to half that present in the experiment 5C. This situation depends on the fact that the duplex/coralyne system has disproportionated, before starting the heating ramp, according to the reaction AUD → ½UAUD + ½AD. Comparison with the first melting temperature of poly(A)•2poly(U) alone (52°C) (28) shows that coralyne does not exert any important effect on the triplex stability, thus suggesting that coralyne is scarcely involved in the release of the third U strand. On the other hand, coralyne stabilizes to a large extent the duplex with respect to dissociation into A + U single strands (Tm2 > 80°C).
For CD/CP > 0.8 the prevailing form of poly(A)•poly(U) at the lowest temperatures is the AUD duplex, which is formed according to reaction (1) prior to start heating. By increasing the temperature, the duplex converts to triplex (absorbance decrease, Figure 5B) because of the disproportionation reaction 2AUD → UAUD + A + D which can replace reaction (2) when CD ≥ CP. The duplex → triplex transition temperature was found to decrease upon increasing CD/CP. Since the variation of CD/CP has been attained by changing CP and keeping CD constant, the transition temperature decreases by decreasing CP. This behaviour has been rationalised according to Equation (10) (42). A plot of the data according to Equation (10) is linear with negative slope and yields for the disproportionation reaction ΔH° = −18 Kcal mol−1 (the error on intercept is too large for a reliable value of ΔS° be provided).
| 10 |
Further increase of temperature causes a large increase of absorbance (Figure 5B), to be ascribed to triplex melting. This process is the same as that shown in Figure 5A but, whereas for 0 < CD/CP < 0.8 the two transitions triplex → duplex and duplex → single strands can be distinguished, now they tend to occur in one step.
It is useful to continue the discussion comparing the melting behaviour of poly(A)•poly(U)/coralyne with that of poly(dA)•poly(dT)/coralyne (17). In the latter system, the process AT → ½ TAT + ½ A is revealed by a decrease of ellipticity and is completed at 55°C for CD/CP = 0.25. In case of poly(A)•poly(U), under similar CD/CP conditions, the disproportionation process of AU occurs at remarkably lower temperatures. For CD/CP > 0.8, the transition duplex → triplex occurs between 50°C and 30°C, for CD/CP increasing from 0.9 to 3.0 (Figure 5B).
In the melting experiments of the triplex poly(dA)•2poly(dT), formed by duplex disproportionation, a transition was observed at 47°C and ascribed to the disruption of the coralyne-promoted structure of poly(dA). We were not able to observe, as a distinct process, the analogous transition of the poly(A) molecules released by the disproportionation reaction, in spite of the highest affinity of coralyne for poly(A) (9). However, an indication of the presence of structured poly(A)/coralyne complex could be obtained comparing the melting profiles of Figure 5B. While for CD/CP = 1.0 the melting curve shows that at high temperatures only the process UAU → U + A + U is operative, at CD/CP = 2.0 and 3.0 two phases could be distinguished just after the minimum. The first phase, ranging between 40 and 60°C falls in the temperature range of the melting temperature of the poly(A)/coralyne system (41,43).
Finally, the second transition (Figure 5B) is ascribed to reaction UAU → U + A + U similar to the behaviour of the poly(dA)•poly(dT) system where direct dissociation of triplex into three single-strands occurs at 82°C (17).
Our results are compared as well with those obtained by Islam et al. (24) which also refer to the poly(A)•poly(U)/coralyne system. At low CD/CP ratios these authors do observe, by differential scanning calorimetry, a single transition at ∼ 50°C, which corresponds to our first transition (Figure 5A). The second transition escaped their attention probably because they stopped the scanning at 70°C. Also, our experiments on triplex melting have been compared with the results of Sinha and Kumar (44) who have investigated the same system. In contrast with our melting curves which for 0 ≤ CD/CP ≤ 0.3 clearly show the triplex → duplex and duplex → single-strands sequence (Figure 5C), their melting profile at CD/CP = 0.1 shows a single step (Tm = 50°C) attributed to the triplex → single-strands process. Again, the transition at 80°C could not be detected because heating was stopped at 60°C.
The melting profile shown in Figure 5C for CD/CP = 3.0 closely resembles that of Figure 5B indicating that at the starting temperature the stable form is the duplex AUD. This behaviour is rather puzzling unless one could advance the idea that in excess of coralyne formation of a stable UD complex could drive the reaction UAUD + D → AUD + UD towards duplex formation. However, further investigations are needed on this issue.
The rise in the EtOH content is reflected in a reduction of the equilibrium constant of reaction (1). Based on electrostatics, an increase of K1 should be expected because of the reduction of the dielectric constant since the reactants are oppositely charged. Hence, the decrease of K1 indicates that forces opposed to the electrostatic ones, such as the hydrophobic interactions coupled with solvation, are operative. A possible explanation of the ethanol effect lies in the preferential solvation of coralyne by EtOH with respect to water (23). In other words, coralyne becomes more stable when water is replaced by ethanol at the dye surface. Since the penetration of coralyne between base-pairs requires extensive desolvation, the energy penalty to remove ethanol from the dye will be higher as the EtOH content is raised; in consequence, K1 becomes smaller. The effect of ethanol on the viscosity of the poly(A)•poly(U)/coralyne system has been as well investigated (Figure S12 of the Supplementary Data); the figure shows that the viscosity trend is only little affected by the ethanol addition. This finding suggests that the site size of the system do not change noticeably by the addition of ethanol.
CD experiments confirm the features of the poly(A)•poly(U) coralyne interaction. Comparing the CD spectra of poly(A)•2poly(U)/coralyne with those of poly(A)•poly(U)/coralyne it can be observed that the CD characteristics of the two systems for CD/CP < 0.8 are similar. Moreover, the spectrum of poly(A)•poly(U)/coralyne recorded after the first addition of the dye (CD/CP = 0.04) agrees with the characteristics of the triplex/coralyne spectrum. This means the lower limit of CD/CP duplex → triplex for conversion is <0.04. Actually, Figure 6 shows two inflections: one at CD/CP ca. 0.9 and the second at CD/CP < 0.05. The disproportionation process occurs within these limits. The viscosity trend of the poly(A)•poly(U)/coralyne system as a function of the CD/CP values is also in agreement with the above conclusions.
Concerning the discussion of the kinetic results, note that coralyne intercalation into duplex, although presenting the same mechanism of other dyes [Scheme (7)] leads to the formation of a much higher population of the precursor AU,D [K0 = 2.9 × 105 M−1 for poly(A)•poly(U)/coralyne versus, for instance, 2.3 × 103 M−1 for poly(A)•poly(U)/proflavine (45)]. Such high K0 value reflects in a high overall affinity of coralyne for poly(A)•poly(U) (K1 = 1.7 × 106 M−1), remarkably higher than that for DNA [K1 = 1 × 105 M−1 (9)] and cannot be ascribed only to electrostatics. The information from kinetics not only confirms the conclusions drawn from the ethanol effects on K1, but indicates that the non-electrostatic forces stabilize the intermediate complex AU,D (46). The population of this precursor is rather high, constituting ∼16% of the total bound population, as inferred from the value of k1/k−1 (5.2). Being coralyne a quite long molecule, a remarkable portion of the intercalated drug stays outside the cavity constituting the intercalation site, as suggested by the quenching experiments discussed above. Under these circumstances, when further polymer is added to the reaction mixture, i.e. for 0 < CD/CP < 0.8, a second molecule of duplex can bind to the outside protruding portion of AUD to form an intermediate species UADUA where coralyne acts as a bridge between two duplex molecules. This species, being unstable, evolves towards a triplex molecule and a single strand of poly(A). According to this view, Step (2) can be rewritten in a more detailed way according to the sequence (11)
| 11 |
It has to be noted that in the poly(dA)•poly(dT)/coralyne system the poly(dA) strand released by the duplex reacts with coralyne and the high affinity of this process provides the driving force of the disproportionation process (17). If poly(A)•poly(U)/coralyne would behave similarly to poly(dA)•poly(dT)/coralyne, Step (2) would be replaced by the alternative Step (12)
| 12 |
where the released A strand is bound to D.
In order to clarify this issue, we have measured the equilibrium constant for AD formation [K1(AD)] under the experimental conditions of the disproportionation process. We believe that this information is important also in view of the differences in the published binding constant values. Actually, the literature values for K1(AD) are 1.6 × 106 M−1 [T = 20°C, ionic strength (I) 0.04 M (14)], 5.4 × 103 M − 1 and 7.4 × 105 M−1 [T = 20°C, I = 0.128 M (41)], >1 × 107 M−1 [T = 22°C, I = 0.04 M (43)]. Our value, 5.4 × 106 M−1 (T = 25°C, I = 0.1 M), once reduced to 22°C using ΔH = −8.3 kcal mol−1 (14) and to I = 0.04M by means of the Record equation with zΨ′ = 1 (47), becomes 5.1 × 107 M−1, in agreement with the Cetinkol and Hud guess (43).
A comparison between the binding constant of poly(A)/coralyne (Table 1) and poly(dA)/coralyne K1(dAD) = 5.7 × 104 M−1 [obtained by reduction at 25°C and I = 0.1 M of the value measured by Ren and Chaires (9)] confirms the assertion (14) that poly(A) binds coralyne more tightly than poly(dA).
Having measured K1(AD) under our experimental conditions we are able to evaluate the equilibrium constant of reaction (12), K2′. Actually, being K2′/K2 = K1(AD) × K3/K1(AUD), the data of Table 1 yield K2′ = 8.3 × 10−3K2. Based on these results we have excluded step (12) from the reaction scheme.
The kinetic analysis can be of help in the interpretation the melting experiments. According to Scheme (11), the disproportionation process involves the formation of the intermediate species UADAU, where coralyne makes a bridge between the two double strands. This structure could favour or disfavour, depending of the extent of site filling, the intramolecular occupation of the major groove of one of the two AU chains by the U strand of the other. At low coralyne loading, the cavities are only sporadically occupied by the dye, so there is room for groove occupation by U from the second AU chain. By contrast, at high dye loading the cavities are occupied by the dye molecules to a large extent; so, there is no room for groove occupation by U and the duplex is prevailing. By increasing the temperature, site saturation by coralyne is reduced and the conditions for major groove occupation by U to form the triplex with release of A are restored.
CONCLUSIONS
Different aspects of the interaction of coralyne with poly(A)•poly(U), poly(A)•2poly(U), poly(A) and poly(A)•poly(A) have been analysed using a variety of physico-chemical methods. Dye intercalation is the prevailing binding mode observed for the RNAs investigated. Moreover, concerning poly(A)•poly(U) one comes to the important conclusion that, under suitable values of dye to polymer concentration ratios, coralyne is able to promote the disproportionation of the duplex poly(A)•poly(U) into the triplex poly(A)•2poly(U) and a single-strand of poly(A). Double-stranded poly(A)•poly(A) as well tends to aggregate in the presence of coralyne, although to a much lower extent compared to poly(A)•poly(U). The effect of ethanol on the thermodynamic parameters indicates that considerable dye–dye and dye–base hydrophobic forces are involved in the binding processes. The ability of coralyne to induce the formation of triple-stranded structures, here observed for the first time with double-stranded-RNA, is also displayed in the case of poly(dA)•poly(dT). Hence, it is recommended to exert great care when studying nucleic acid/coralyne-like systems.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Ministerio de Educación y Ciencia, Projects CTQ2006-14734/BQU and CTQ2009-13051/BQU, supported by FEDER and Junta de Castilla y León, Projects BU013A09 and GR257, Spain. University of Pisa and Cassa di Risparmio di Pisa Lucca e Livorno (CIVR).
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Gatto B, Sanders MM, Yu C, Wu HY, Mahkey D, LaVoie EJ, Liu LF. Identification of Topoisomerase I as the cytotoxic target of the protoberberine alkaloid coralyne. Cancer Res. 1996;56:2795–2800. [PubMed] [Google Scholar]
- 2.Zee-Cheng K, Paull KD, Cheng CC. Experimental antileukemic agents. Coralyne, analogs, and related compounds. J. Med. Chem. 1974;17:347–351. doi: 10.1021/jm00249a020. [DOI] [PubMed] [Google Scholar]
- 3.Whang L, Rogers BD, Hecht SM. Inhibition of Topoisomerase I function by coralyne and 5,6-dihydrocoralyne. Chem. Res. Toxicol. 1996;9:75–83. doi: 10.1021/tx950080y. [DOI] [PubMed] [Google Scholar]
- 4.Maiti M, Kumar GS. Protoberberine alkaloids. Physicochemical and nucleic acid binding properties. Top. Heterocyclic Chem. 2007;10:155–209. [Google Scholar]
- 5.Maiti M, Kumar GS. Molecular aspects on the interaction of protoberberine, benzophenanthridine, and aristolochia group of alkaloids with nucleic acid structures and biological perspectives. Med. Res. Rev. 2007;27:649–695. doi: 10.1002/med.20087. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WD, Gough AN, Doyle JJ, Davidson MW. Coralyne. Intercalation with DNA as a possible mechanism of antileukemic action. J. Med. Chem. 1976;19:1261–1263. doi: 10.1021/jm00232a020. [DOI] [PubMed] [Google Scholar]
- 7.Ihmels H, Faulhaber K, Vedaldi D, dall’Acqua F, Viola G. Intercalation of organic dye molecules into double-stranded DNA. Part 2: the annelated quinolizinium ion as a structural motif in DNA intercalators. Photochem. Photobiol. 2005;81:1107–1115. doi: 10.1562/2005-01-25-IR-427. [DOI] [PubMed] [Google Scholar]
- 8.Pal S, Das S, Suresh Kumar G, Maiti M. Antitumor agent coralyne: a guanine-cytosine specific DNA-binding alkaloid. Curr. Sci. 1998;75:496–500. [Google Scholar]
- 9.Ren J, Chaires JB. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry. 1999;38:16067–16075. doi: 10.1021/bi992070s. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WD, Tanious FA, Mizan S, Yao S, Kiselyov AS, Zon G, Srekowski L. DNA triple-helix specific intercalators as antigene enhancers: unfused aromatic cations. Biochemistry. 1993;32:10614–10621. doi: 10.1021/bi00091a011. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WD, Mizan S, Tanious FA, Yao S, Zon G. The interaction of intercalators and groove-binding agents with DNA triple-helical structures: The influence of ligand structure, DNA backbone modifications and sequence. J. Mol. Recognit. 1994;7:89–98. doi: 10.1002/jmr.300070206. [DOI] [PubMed] [Google Scholar]
- 12.Murayama K, Yamauchi T, Tsuchiya K, Komiya K, Kito M, Isono Y, Inomata N, Suwa Y, Wakamatsu A, Yamamoto J, et al. Combinations of known drugs and target proteins and application to the screening of the novel drugs that can bind and/or regulate the functions and expression levels of the target proteins. PCT Int. Appl. 2008:532. patent number: W02008102912. [Google Scholar]
- 13.Persil O, Santai CT, Jain SS, Hud NV. Assembly of antiparallel homo-adenine DNA duplex by small molecule binding. J. Am. Chem. Soc. 2004;126:8644–8645. doi: 10.1021/ja0492891. [DOI] [PubMed] [Google Scholar]
- 14.Xing F, Song G, Ren J, Chaires JB, Qu X. Molecular recognition of nucleic acids: Coralyne binds strongly to poly(A) FEBS Lett. 2005;579:5035–5039. doi: 10.1016/j.febslet.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 15.Islam MM, Pandya P, Kumar S, Kumar GS. RNA targeting through binding of small molecules: Studies on t-RNA binding by the cytotoxic protoberberine alkaloid coralyne. Mol. BioSyst. 2009;5:244–254. doi: 10.1039/b816480k. [DOI] [PubMed] [Google Scholar]
- 16.Keppler MD, Neidle S, Fox KR. Stabilization of TG- and AG-containing antiparallel DNA triplexes by triplex-binding ligands. Nucleic Acids Res. 2001;29:1935–1942. doi: 10.1093/nar/29.9.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak M, Hud NV. Complete disproportionation of duplex poly(dT)•poly(dA) into triplex poly(dT)•poly(dA)•poly(dT) and poly(dA) by coralyne. Nucleic Acids Res. 2002;30:983–992. doi: 10.1093/nar/30.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moraru-Allen AA, Cassidy S, Alvarez JA, Fox KR, Brown T, Lane AN. Coralyne has a preference for intercalation between TA•T triples in intramolecular DNA triple helices. Nucleic Acids Res. 1997;25:1890–1896. doi: 10.1093/nar/25.10.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JS, Latimer LJP, Hampel KJ. Coralyne binds tightly to both TAT and CGC+ containing DNA triplexes. Biochemistry. 1993;32:5591–5597. doi: 10.1021/bi00072a014. [DOI] [PubMed] [Google Scholar]
- 20.Wilson WD, Gough AN, Doyle JJ, Davidson MW. Coralyne. Intercalation with DNA as a possible mechanism of antileukemic action. J. Med. Chem. 1976;19:1261–1263. doi: 10.1021/jm00232a020. [DOI] [PubMed] [Google Scholar]
- 21.Franceschin M, Rossetti L, D’Ambrosio A, Schirripa S, Bianco A, Ortaggi G, Savino M, Schultes C, Neidle S. Natural and syntehtic G-quadruplex interactive berberine derivatives. Bioorg. Med. Chem. Lett. 2006;16:1707–1711. doi: 10.1016/j.bmcl.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Gough AN, Jones RL, Wilson WD. Dimerization of Coralyne and its propyl analogue and their association with DNA. J. Med. Chem. 1979;22:1551–1554. doi: 10.1021/jm00198a025. [DOI] [PubMed] [Google Scholar]
- 23.García B, Ibeas S, Ruiz R, Leal JM, Biver T, Boggioni A, Secco F, Venturini M. Solvent effects on the thermodynamics and kinetics of Coralyne self-aggregation. J. Phys. Chem. B. 2009;113:188–196. doi: 10.1021/jp807894a. [DOI] [PubMed] [Google Scholar]
- 24.Islam MdM, Chowdhury SR, Kumar GS. Spectroscopic and calorimetric studies on the binding of alkaloids berberine, palmitine and coralyne to double stranded RNA polynucleotides. J. Phys. Chem. B. 2009;113:1210–1224. doi: 10.1021/jp806597w. [DOI] [PubMed] [Google Scholar]
- 25.Janik B. Physicochemical Characteristic of Oligonucleotides and Polynucleotides. IFI/Plenum, New York: Washington, London; 1971. [Google Scholar]
- 26.Maggini R, Secco F, Venturini M, Diebler H. Kinetic study of double-helix formation and double-helix dissociation of polyadenylic acid. J. Chem. Soc. Faraday Trans. 1994;90:2359–2363. [Google Scholar]
- 27.Maggini R, Secco F, Venturini M, Diebler H. Proton transfer involving nucleic acids: a temperature-jump study of the systems sulphonephthaleins-double stranded poly(A) and sulphonephthaleins-double stranded poly(C) Int. J. Chem. Kinet. 1998;30:161–169. [Google Scholar]
- 28.García B, Leal JM, Paiotta V, Ruiz R, Secco F, Venturini M. Role of the third strand in the binding of proflavine and Pt-proflavine to poly(rA)•2poly(rU): a thermodynamic and kinetic study. J. Phys. Chem. B. 2008;112:7132–7139. doi: 10.1021/jp800163n. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico ML, Paiotta V, Secco F, Venturini M. A kinetic study of the intercalation of ethidium bromide into Poly(A)Poly(U) J. Phys. Chem. B. 2002;41:12635–12641. [Google Scholar]
- 30.Riegler R, Rabl CB, Jovin TM. A temperature-jump apparatus for fluorescence measurements. Rev. Sci. Instrum. 1974;45:580–588. [Google Scholar]
- 31.Biver T, De Biasi A, Secco F, Venturini M, Yarmoluk S. Cyanine dyes as intercalating agents: kinetic and thermodynamic studies on the DNA/Cyan40 and DNA/CCyan2 systems. Biophys. J. 2005;89:374–383. doi: 10.1529/biophysj.105.059790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynes MJ, Diebler H. The binding of nickel(2+) to adenylyl-3′,5′-adenosine and to poly(adenylic acid) Biophys. Chem. 1982;16:79–88. doi: 10.1016/0301-4622(82)85010-2. [DOI] [PubMed] [Google Scholar]
- 33.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd edn. Kluwer Academic/Plenum Publishers, NY; 1999. [Google Scholar]
- 34.Stevens CL, Felsenfeld G. The conversion of two-stranded Poly(A+U) to three-strand Poly(A+2U) and PolyA by heat. Biopolymers. 1964;2:293–314. [Google Scholar]
- 35.Hammes GG. Investigation of Rates and Mechanism of Reactions in Techniques of Chemistry, Vol. VI, Part II. 3rd edn. Wiley-lnterscience; 1974. NY, 147 pp. [Google Scholar]
- 36.Jain SS, Polak M, Hud NV. Controlling nucleic acid secondary structure by intercalation: effects on DNA strand length on coralyne-driven duplex disproportionation. Nucleic Acids Res. 2003;31:4608–4615. doi: 10.1093/nar/gkg648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shchyolkina AK, Borisova OF. Stabilizing and destabilizing effects of intercalators on DNA triplexes. FEBS Lett. 1997;419:27–31. doi: 10.1016/s0014-5793(97)01417-8. [DOI] [PubMed] [Google Scholar]
- 38.Chaires JB. A thermodynamic signature for drug-DNA binding mode. Arch. Biochem. Biophys. 2006;453:26–31. doi: 10.1016/j.abb.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 39.Rich A, Davies DR, Crick FHC, Watson JD. The molecular structure of polyadenylic acid. J. Mol. Biol. 1961;3:71–86. doi: 10.1016/s0022-2836(61)80009-0. [DOI] [PubMed] [Google Scholar]
- 40.Steiner RF, Beers RF., Jr Polynucleotides. VI. Influence of various factors on the structural transition of polyriboadenylic acid at acid pH's; Biochim. Biophys. Acta. 1959;32:166–176. doi: 10.1016/0006-3002(59)90565-7. [DOI] [PubMed] [Google Scholar]
- 41.Giri P, Kumar GS. Self-structure induction in single stranded poly(A) by small molecules: studies on DNA intercalators, partial intercalators and groove binding molecules. Arch. Biochem. Biophys. 2008;474:183–192. doi: 10.1016/j.abb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Mergny JL, Lacroix L. Analysis of thermal melting curves. Oligonucleotides. 2003;13:515–537. doi: 10.1089/154545703322860825. [DOI] [PubMed] [Google Scholar]
- 43.Cetinkol ÖP, Hud NV. Molecular recognition of poly(A) by small ligands: an alternative method of analysis reveals nanomolar, cooperative and shape-selective binding. Nucleic Acids Res. 2009;37:611–621. doi: 10.1093/nar/gkn977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinha R, Kumar GS. Interaction of isoquinoline alkaloids with an RNA triplex: structural and thermodynamic studies of berberine, palmatine, and coralyne binding to poly(U)•poly(A)•poly(U) J. Phys. Chem. B. 2009;113:13410–13420. doi: 10.1021/jp9069515. [DOI] [PubMed] [Google Scholar]
- 45.Biver T, Secco F, Venturini M. Relaxation kinetics of the interaction between RNA and metal-intercalators: the Poly(A)Poly(U)/Platinum-proflavine system. Arch. Biochem. Biophys. 2005;437:215–223. doi: 10.1016/j.abb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Biver T, Boggioni A, Secco F, Turriani E, Venturini M, Yarmoluk S. Influence of cyanine dye structure on self-aggregation and interaction with nucleic acids: a kinetic approach to TO and BO binding. Arch. Biochem. Biophys. 2007;465:90–100. doi: 10.1016/j.abb.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 47.Record MT, Anderson CF, Jr, Lohaman TM. Thermodynamic analysis of ion effects on the binding and conformational equilibriums of proteins and nucleic acids: the roles of ion association of release, screening, and ion effects on water activity. Q. Rev. Biophys. 1978;11:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.