Abstract
The continuing rise in atmospheric CO2 causes closing of stomatal pores in leaves and thus globally affects CO2 influx into plants, water use efficiency and leaf heat stress1–4. However, the CO2-binding proteins that control this response remain unknown. Moreover, the cell type that responds to CO2, mesophyll or guard cells, and whether photosynthesis mediates this response are matters of debate5–8. We demonstrate that Arabidopsis double mutant plants in the β-carbonic anhydrases, βCA1 and βCA4, display impaired CO2-regulation of stomatal movements and increased stomatal density, but retain functional abscisic-acid and blue-light responses.βCA-mediated CO2-triggered stomatal movements are not, in-first-order, linked to leaf-photosynthesis and can function in guard cells. Furthermore, guard cell βCA-over-expression plants exhibit enhanced water use efficiency. Guard cell-expression of mammalian αCAII complements ca1ca4 shows that carbonic anhydrase-mediated catalysis is an important mechanism for βCA-mediated CO2-induced stomatal closing and patch clamp analyses indicate that CO2/HCO3−transfers the signal to anion channel regulation. These findings, together with ht1-29 epistasis analysis demonstrate that carbonic anhydrases function early in the CO2 signalling pathway that controls gas-exchange between plants and the atmosphere.
Guard cells form adjustable stomatal pores in the plant epidermis that allow CO2 influx for photosynthesis in exchange for transpirational water loss from plants to the atmosphere. The continuing rise in atmospheric [CO2] and the resulting increase in leaf intercellular [CO2] (Ci) is causing a reduction in stomatal apertures across diverse plant species2. To date, only a few Arabidopsis mutants have been characterized that show CO2 insensitivity in stomatal movement regulation10–14. However, these mutants also exhibit insensitivity to the hormone abscisic acid (ABA), consistent with present models that the encoded proteins function downstream of a convergence point of the CO2 and ABA stomatal closure signalling pathways10, 12–14. The only Arabidopsis protein proposed to function upstream of this convergence point is the negative regulator of CO2-induced stomatal closure, HIGH LEAF TEMPERATURE 1 kinase, for which mutations cause a constitutive high-[CO2] response9. The ABC transporter AtABCB14 identified as a malate uptake transporter in the guard cell plasma membrane also functions as a negative regulator of high [CO2]-induced stomatal closure15. Antisense repression of a MAP Kinase (NtMKP4) in tobacco reduces CO2 regulation of stomatal conductance but not ABA responses16. The CO2-/HCO3−-binding proteins, Rubisco and PEP carboxylases (PEPC), have been investigated for their putative participation in high CO2-induced stomatal closure5, 17, 18. However, it was shown that CO2-regulated stomatal conductance is independent of Rubisco activity5 and that PEPC levels had no direct effect on high CO2-triggered stomatal closing17, 18 (see also Supplementary Results). As no CO2-binding proteins have been identified in genetic screens of CO2-regulated stomatal signalling, we postulated that CO2-binding proteins that mediate this response may underlie a gene family with overlapping gene functions, similar to other stomatal movement control mechanisms19, 20.
We hypothesized that the CO2-binding proteins carbonic anhydrases that catalyze the reversible reaction of CO2 + H2O ⇔ HCO3− + H+, might function early in CO2 signaling (see also Supplementary Results). Transcriptome analyses of mesophyll and guard cells show that the β-carbonic anhydrases βCA1 (At3g01500), βCA4 (At1g70410) and βCA6 (At1g58180) are highly expressed in guard cells and/or mesophyll cells (Fig. 1a)21, 22 which was confirmed by quantitative RT-PCR (Fig. 1b). Consistent with these data, βCA1 and βCA4 were also detected in the guard cell proteome23.
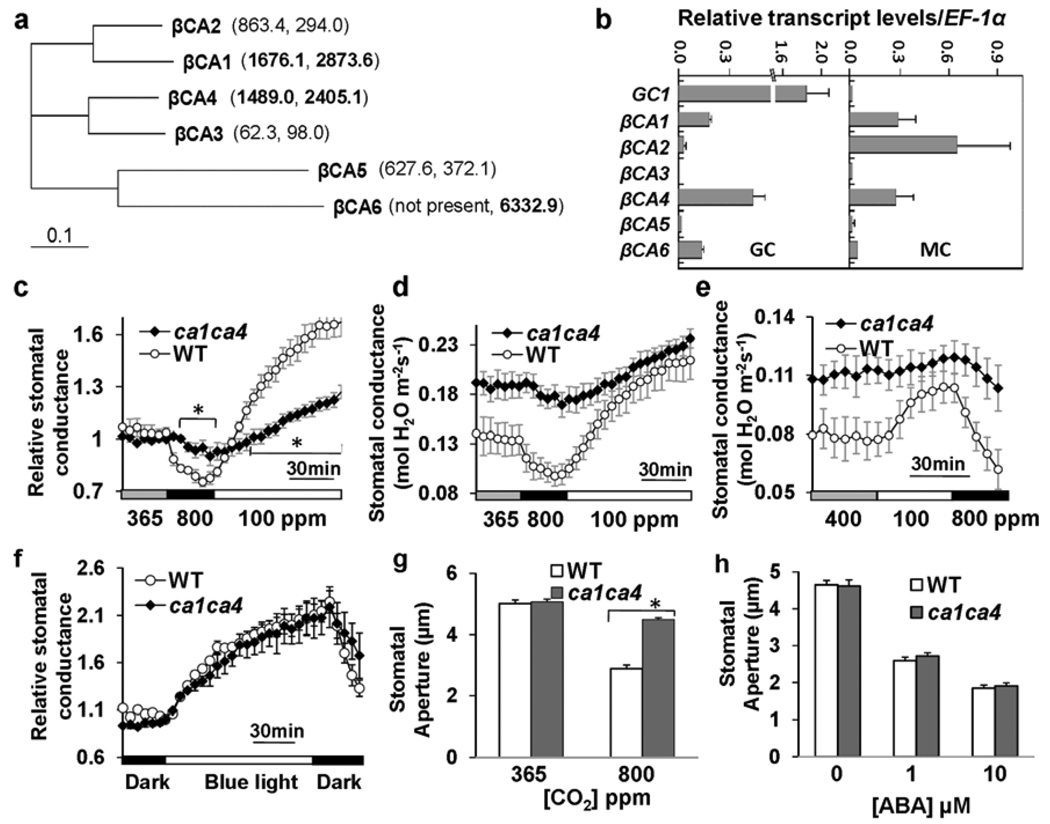
Figure 1. Disruption of the carbonic anhydrases βCA1 and βCA4 greatly impairs CO2-induced stomatal movements, but not responses to blue light and abscisic acid.
(a) Phylogenetic tree (ClustalX 1.83) of Arabidopsis β-carbonic anhydrases (βCAs) and corresponding average guard cell specific microarray expression data in brackets (Left, 8K AG Genechips21; Right, ATH1 Genechips22). βCA1, βCA4 and βCA6 showed the highest expression values among βCAs within guard cells as depicted in bold. βCA1 (At3g01500), βCA2 (At5g14740), βCA3 (At1g23730), βCA4 (At1g70410), βCA5 (At4g33580) and βCA6 (At1g58180)24. (b) Relative transcript levels (compared to EF-1α, At5g60390) of the six βCA genes in guard cells (GC) and mesophyll cells (MC) (qRT-PCR, n = 3 independent biological replicates, ± s.e.m.). qRT-PCR data confirmed high expression of βCA1, βCA4 and βCA6 in guard cells. GC1, At1g2269022. (c–e) Time-resolved stomatal conductance responses to [CO2] concentrations in wild-type (WT) and ca1ca4 mutant plants (c, d, n = 7; e, n = 5 leaves). (c) shows normalized responses of those shown in d. * means significant difference in the bracketed points between ca1ca4 and wild-type plants (P<0.05, unpaired Students t-test). For initial rates of stomatal conductance changes in d: for 800 ppm to 100 ppm shift, dConductance/dt = 0.028 ± 0. 005 in wild type and 0.008 ± 0.002 in ca1ca4; in e: for 100 ppm to 800 ppm shift, dConductance/dt = −0.034 ± 0. 004 in wild type and −0.005 ± 0.009 in ca1ca4, mmol H2O m−2s−1, means ± s.e.m., P<0.05, unpaired t-test. (f) Analyses of relative stomatal conductance responses to blue light and light-dark transitions in wild-type (WT) and ca1ca4 mutant plants (n = 4, ± s.e.m.). (g) High [CO2]-induced stomatal closing is impaired in ca1ca4 mutant leaf epidermes (n = 4 experiments, 80 stomata per condition), in which only guard cells and leaf pavement cells were alive and no mesophyll cells were in the vicinity. Leaf epidermes were treated with 800 ppm CO2 for 30 min. Data represent means ± s.e.m‥ (genotype blind analyses). * P<0.001, pairwise Student’s t-test. See also Supplementary Information Fig. S2d for a 60 min treatment. (h) Stomata in ca1ca4 leaves close in response to abscisic acid (n = 3 experiments, 30 stomata per experiment and condition). Data represent means ± s.e.m‥
Single T-DNA disruption mutant plants in βCA1, βCA4 and βCA6 did not display strong phenotypes in CO2 responses in mature leaves. ca1ca4, ca4ca6, ca1ca6 double mutants as well as ca1ca4ca6 triple mutant were subsequently generated for assessment of their CO2 sensitivities. Interestingly, ca1ca4 double and ca1ca4ca6 triple mutant plants showed strong insensitivities in CO2-induced stomatal conductance changes (Fig. 1c–e; Supplementary Information, S1d–h). However, ca1ca6 and ca4ca6 mutants did not exhibit an altered CO2 response, indicating no major role for the more distantly-related βCA6 (Supplementary Information, Fig. S1b, c). CO2-induced stomatal conductance changes in ca1ca4 were greatly impaired during ambient (365 ppm) to 800 ppm or 400 to 100 ppm [CO2] changes at both 55 µmol m−2s−1 and 1000 µmol m−2s−1 light fluence rates (Fig. 1c–e; Supplementary Information, Fig. S1e–h). However, more dramatic shifts from 800 to 100 ppm and from 100 to 800 ppm [CO2], triggered stomatal conductance changes in ca1ca4 plants, although at slower rates compared to wild-type plants (see captions of Fig. 1d, e). Furthermore, ca1ca4 plants consistently showed a higher stomatal conductance at ambient [CO2] (365–400 ppm) compared to wild-type plants (Fig. 1d, e; Supplementary Information, Fig. S1f). Interestingly, stomatal index (SI) and stomatal density (SD) were significantly higher in ca1ca4 (SI: 27 ± 0.9%; SD: 188 ± 13.2 per mm2) compared to wild type (SI: 22 ± 0.6%; SD: 142.5 ± 15.4 stomata per mm2, means ± s.e.m.), and this 1.3-fold increase in stomatal density could in part account for the higher stomatal conductance observed in ca1ca4 leaves.
In clear contrast to the impaired CO2 responses (Fig. 1c–e), and despite the higher starting stomatal conductance of the βca mutant plants (Fig. 1d, e), ca1ca4 and ca1ca4ca6 plants showed robust responses to blue light and blue light to dark transitions (Fig. 1f; Supplementary Information, Fig. S2a–c). The initial rates of stomatal conductance changes triggered by blue light were not significantly different between βca mutants and wild type (Supplementary information, captions to Fig. S2a, b). CO2 responses were also analyzed in leaf epidermes, which had been removed from their mesophyll cell environment. High [CO2]-induced stomatal closure was greatly impaired in ca1ca4 compared to wild type after 30 min (Fig. 1g) or 60 min (Supplementary Information, Fig. S2d) exposure. Thus, the impaired CO2 responses in intact leaves correlate with impaired CO2-induced stomatal movements. In sharp contrast to other known CO2-insensitive mutants12–14, ABA-induced stomatal closing remained clearly functional in ca1ca4 leaf epidermes (Fig. 1h), consistent with a model that βCA1 and βCA4 function early in CO2 signal transduction.
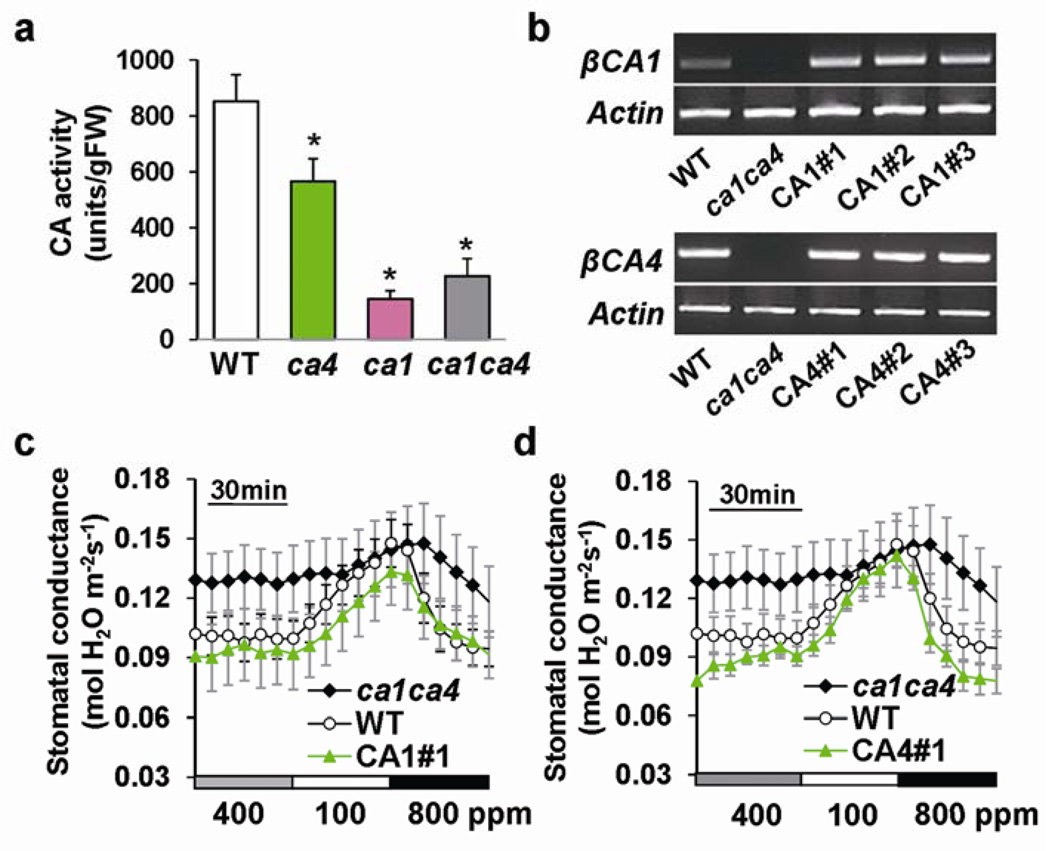
Biochemical carbonic anhydrase activities in mature leaves of 5–7 week-old ca1, ca4 single mutants and ca1ca4 were analyzed. The ca1ca4 mutant plants showed a ≈ 65 % reduction in CO2 hydration reactions compared to wild type (Fig. 2a). Introduction of a genomic copy of either βCA1 or βCA4 in ca1ca4 restored the expression of the respective gene (Fig. 2b) and complemented the CO2-responsive phenotype in six randomly selected transgenic lines (Fig. 2c, d; Supplementary Information, Fig. S3). Moreover, all transgenic lines showed wild-type-like stomatal densities and indices (Supplementary Information, Fig. S4). Thus, disruption of βCA1 and βCA4 is indeed responsible for the CO2 insensitive stomatal movement regulation and the stomatal density phenotypes observed in ca1ca4 and expression of either gene is sufficient for complementation of both phenotypes.
Figure 2. Introduction of wild-type genomic βCA complements the reduced CO2 sensitivity of ca1ca4.
(a) Catalytic carbonic anhydrase activity assays show a reduction by 65% in carbonic anhydrase activity of the ca1ca4 double mutant (n = 16) compared to wild-type plants (n = 16). Means ± s.e.m., *P<0.05 compared to wild type, unpaired Student’s t-test. Residual carbonic anhydrase activities were not significantly different between ca1 and ca1ca4 mutant plants (P>0.3, pairwise Student’s t-test). (b) RT-PCR analyses confirmed restoration of βCA1 and βCA4 expression in ca1ca4 leaves transformed with genomic βCA1 or βCA4 constructs. Three independent randomly selected transgenic lines per genomic construct were analyzed. Actin (At2g37620) was used as a control. (c) Complementation line with genomic βCA1 construct exhibits recovery of [CO2]-regulated stomatal conductance changes (n = 8 leaves for ca1ca4, n = 10 for WT and n = 4 for each complemented line). Means ± s.e.m‥ (d) Complementation line with genomic βCA4 construct exhibits recovery of [CO2]-regulated stomatal conductance changes (n = 8 leaves for ca1ca4, n = 10 for WT and n = 4 for each complemented line). Experiments in c and d were performed in the same experimental set with the same controls. Means ± s.e.m‥ Supplementary Information Fig. S3 shows four other independent transgenic lines analyzed in parallel.
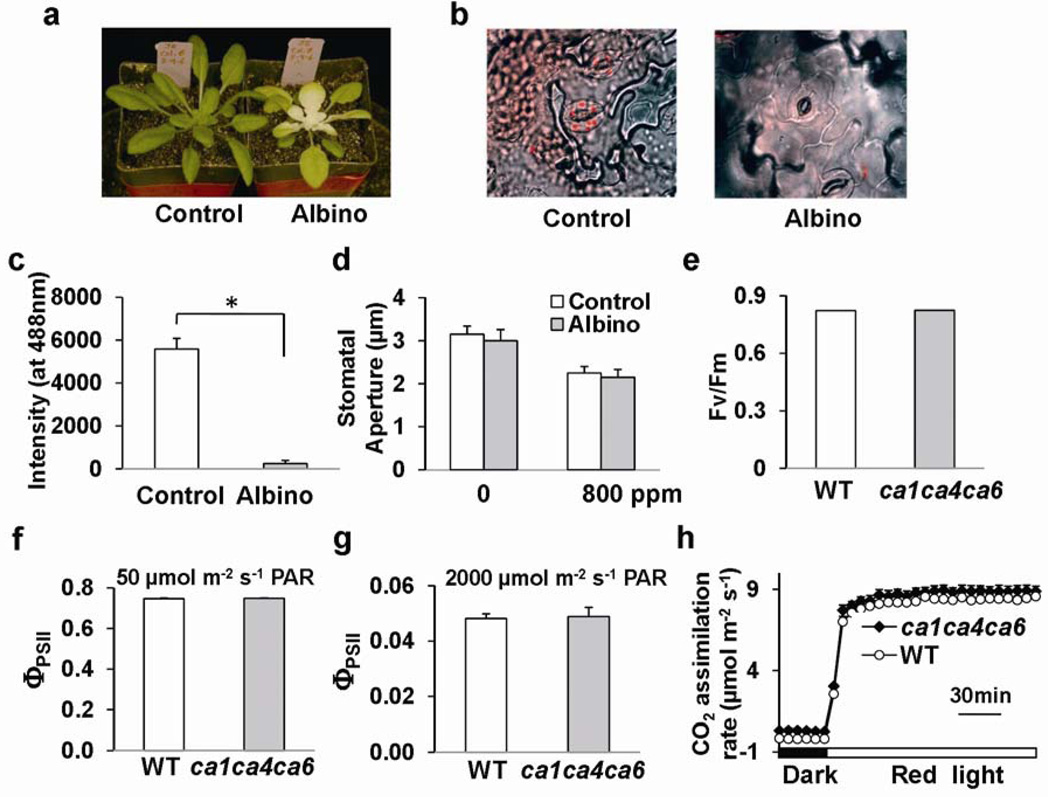
βCA1 and βCA4 have been identified in chloroplasts (βCA1) and at the plasma membrane in proteome and imaging analyses24–26. Consistent with these studies, βCA1-YFP and βCA4-YFP subcellular localization analyses suggest that βCA1 localizes to both chloroplasts and in the vicinity of the plasma membrane, while βCA4 localizes in the vicinity of the plasma membrane (Supplementary Information, Figs. S5, 6). As these subcellular localizations may indicate roles in transcellular carbon delivery to chloroplasts, as reported for aquaporins27, we analyzed whether the role of βCAs in CO2-induced stomatal closing is dependent on mature leaf photosynthesis. Photosynthesis was inhibited in newly emerging leaves of plants watered with the carotenoid biosynthesis inhibitor norflurazon (Fig. 3a–c). However CO2-induced stomatal closing remained functional in chlorophyll-deficient “albino” leaves (Fig. 3d), as previously reported in Vicia faba7. More direct genetic analyses in ca1ca4ca6 mutant plants showed no differences to wild-type plants in the maximum efficiency of photosystem II (Fv/Fm) (Fig. 3e, n = 10), or the quantum yield of photosystem II (ΦPSII) at low (50 µmol m−2s−1, n = 6) and high (2000 µmol m−2s−1, n = 6) light conditions (Fig. 3f, g). CO2 assimilation rates of ca1ca4ca6 plants in response to darkness to 300 µmol m−2s−1 red light shifts were also unaffected (Fig. 3h). Therefore, the stomatal CO2 response in Arabidopsis proceeds in the absence of chlorophyll (Fig. 3a–d) and mature leaf photosynthetic activities are not impaired in ca1ca4ca6 under the imposed growth conditions (Fig. 3e–h). Note that other growth conditions and other ca mutant combinations may lead to effects of CAs on photosynthetic activities, given the chloroplast location of βCA1 and proposed roles of CAs in photosynthetic activities.
Figure 3. Photosynthesis is not directly linked to βCA-mediated CO2-triggered stomatal responses.
(a) Chlorophyll-deficient albino wild-type leaves were generated by watering with the carotenoid biosynthesis inhibitor norflurazon. (b) Chlorophyll-deficiency in norflurazon-treated albino leaf guard cells compared to wild type was analyzed using confocal microscopy. (c) The absence of chlorophyll in albino guard cells was quantified by image analyses of chlorophyll fluorescence intensity (n = 3, 12 stomata/sample). *P<0.001, pairwise Student’s t-test. (d) The stomatal CO2 response to [CO2] changes was functional in intact albino leaf epidermes (n = 7 experiments, 50 stomata/sample). Means ± s.e.m‥ (e) The maximum efficiency of photosystem II (PSII)- Fv/Fm in dark-adapted leaves was unaffected in ca1ca4ca6 mutant plants (n = 10, ± s.e.m.). (f, g) No difference was observed between wild type (WT) and ca1ca4ca6 mutant plants with respect to the quantum yield of PSII (ΦPSII) in leaves pre-adapted (f) at 50 µmol m−2s−1 (n = 6) or (g) at 2000 µmol m−2s−1(n = 6) photosynthetically active radiation. Means ± s.e.m. (h) Red light (300 µmol m−2s−1) -induced CO2 assimilation of intact leaves was not impaired in ca1ca4ca6 plants (n = 6, ± s.e.m.).
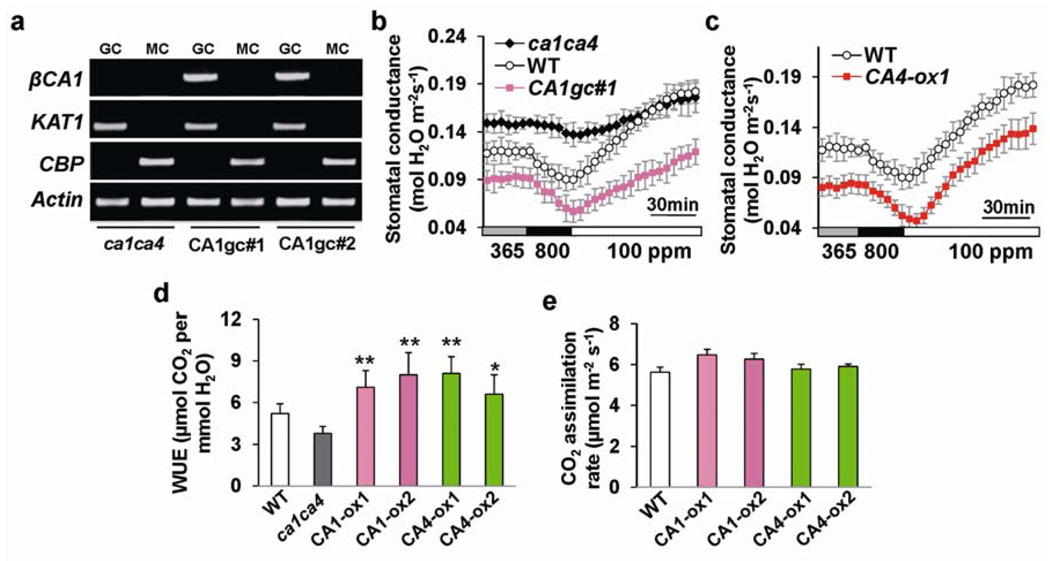
To analyze whether βCA expression targeted to guard cells is sufficient to complement the CO2 insensitive phenotype of ca1ca4, βCA1 or βCA4 cDNA driven by a strong guard cell promoter pGC122 was transformed into ca1ca4. In these stably transformed ca1ca4 lines, βCA1 and βCA4 transcripts were detected in guard cells but not in mesophyll cells (Fig. 4a; Supplementary Information, Fig. S7a). Four randomly selected independent transgenic lines expressing βCA1 or βCA4 in guard cells exhibited recovery of CO2 responsiveness (Fig. 4b; Supplementary Information, Fig. S7b–d). Interestingly, in contrast to ca1ca4, these transgenic lines displayed wild-type-like stomatal indices and densities (Supplementary Information, Fig. S7e, f). Thus, targeted expression of βCA1 or βCA4 in guard cells is sufficient to restore CO2 responsiveness and stomatal density.
Figure 4. βCA expression in ca1ca4 guard cells restores CO2 responses and βCA over-expression plants show improved instantaneous water use efficiency.
(a) RT-PCR analyses of βCA1 expression in guard cell (GC) and mesophyll cell (MC) protoplasts of two ca1ca4 lines expressing βCA1 driven by the pGC1 promoter. GC, guard cell; MC, mesophyll cell. KAT1, At5g46240, leaf guard cell marker; CBP, At4g33050, mesophyll cell marker. (b) βCA1 expression in guard cells restores CO2 responsiveness in intact leaves. CO2-induced stomatal conductance changes of guard cell-targeted line CA1gc#1and ca1ca4 and wild-type plants from the same experimental set (n = 4). Fig. S7 shows CO2 responses of other independent transgenic lines. Note that the starting stomatal conductance in guard cell-targeted lines was lower than that in wild type, probably because pGC1 drives stronger expression in guard cells22 than the native βCA promoters (Fig. 1b). (c) Stomatal conductance of βCA4 over-expressing lines and wild-type (WT) plants in response to the indicated [CO2] changes (n = 4, ± s.e.m.). Fig. S8 shows other independent transgenic lines analyzed in parallel. Experiments in (b) and (c) were performed in the same experimental set with the same controls. (d) βCA1 and βCA4 over-expressing lines show improved instantaneous water use efficiency (WUE, µmol CO2 assimilated per mmol H2O transpired). n = 5, error bars depict means ± s.e.m‥ P<0.01(**) and P<0.05(*), compared to wild type, pairwise Student’s t-test. (e) Rates of photosynthesis (CO2 assimilation) at ambient (365 ppm) [CO2] in wild type and the analyzed βCA1 and βCA4 guard cell over-expressing lines. Error bars depict means ± s.e.m‥
We then investigated whether over-expression of βCA1 or βCA4 in guard cells of wild-type plants can modulate intact plant gas exchange. Four randomly selected independent lines over-expressing βCA1 or βCA4 in the wild-type background (Supplementary Information, Fig. S8b) displayed a reduced stomatal conductance at all tested [CO2] (Fig. 4c; Supplementary Information, Fig. S8c–e). Interestingly, substantial increases in the instantaneous water use efficiency (WUE) of all analyzed guard cell-targeted over-expression lines were consistently found, with an average increase of 44% at ambient [CO2] (Fig. 4d), while CO2 assimilation rates were not significantly altered under the imposed growth conditions (Fig. 4e). All over-expressing lines exhibited reduced fresh weight loss from excised leaves compared to wild type (Supplementary Information, Fig. S8f), consistent with a reduced stomatal conductance at ambient [CO2] in all βCA-overexpressing lines (Fig. 4c; Supplementary Information, Fig. S8c–e).
No whole plant phenotypic growth differences and no reduction in total plant dry weight (growth penalty) were observed in βCA-overexpressing lines compared to wild-type plants under limited-watering or well-watered conditions (Supplementary Information, Fig. S8a, g, h). Guard cell-targeted overexpression lines displayed slightly lower average stomatal densities (−13%) and stomatal indices (−14%) compared to parallel grown wild-type plants (Supplementary Information, Fig. S9; P = 0.0312 to 0.0523 for these overexpression lines compared to wild type, Student’s t-test). The “single cell spacing phenotype” was not violated in ca1ca4 mutant and βCA-overexpression plants leaves28, 29. Therefore, guard cell-targeted over-expression of βCA is sufficient to modulate CO2 regulation of stomatal conductance and may provide an approach for improving the water use efficiency of C3 plants.
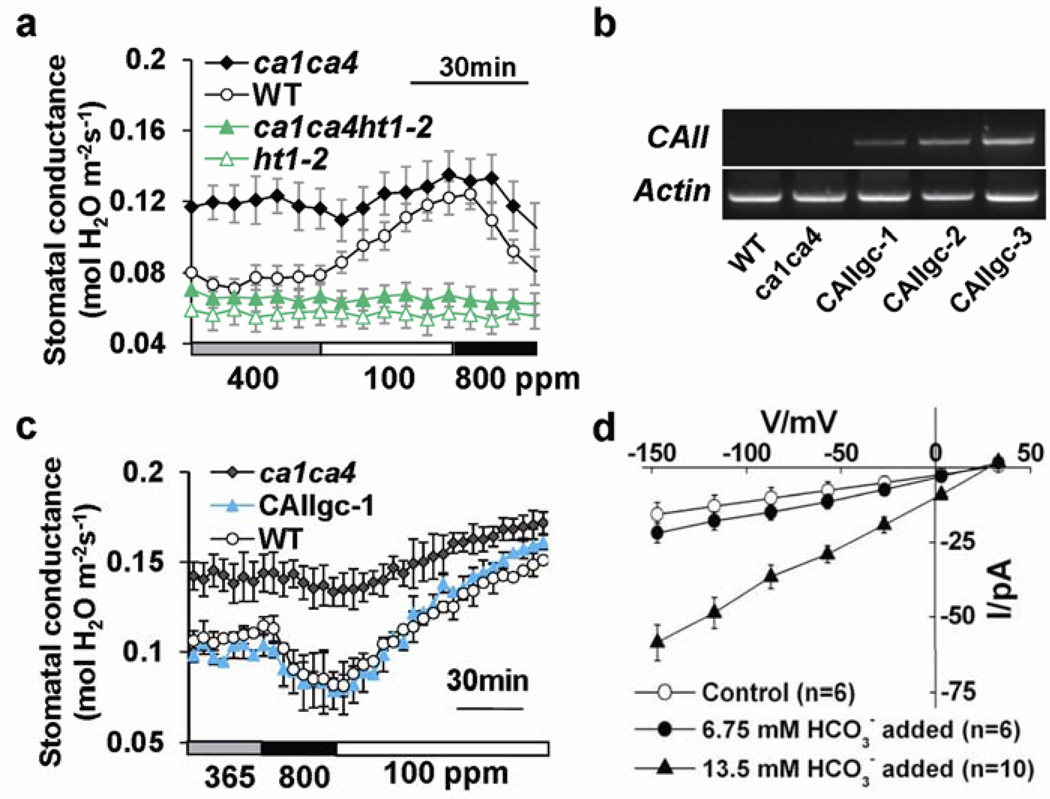
The earliest component of CO2 signalling in Arabidopsis guard cells identified thus far is the HT1 protein kinase, a negative regulator of the CO2 response pathway9. The recessive ht1-2 allele exhibits a constitutive high-[CO2] response9. Strikingly, ca1ca4ht1-2 triple mutant plants exhibited a constitutive high-[CO2] phenotype similar to ht1-2 (Fig. 5a), showing that HT1 is epistatic to βCA1 and βCA4 and supporting an early CO2 signalling role of βCAs. To determine whether enzymatic carbonic anhydrase activity mediates CO2 control of gas exchange, we expressed an unrelated α-carbonic anhydrase from human, αCAII 30, under the control of the guard cell promoter in ca1ca4 plants. Human αCAII shows only 9% identity to βCA1 and 12% to βCA4 at the amino acid level, respectively. Astonishingly, three randomly-selected independent human αCAII-expressing transgenic ca1ca4 lines (Fig. 5b) exhibited restoration of the CO2 insensitive phenotype, providing strong evidence that carbonic anhydrase activity in guard cells is required for CO2-mediated stomatal regulation (Fig. 5c; Supplementary Information, Fig. S10).
Figure 5. HT1 epistasis analysis, human αCAII expression in guard cells restores CO2 responsiveness and HCO3− regulation of anion channels.
(a) Time-resolved stomatal conductance analyses in ca1ca4 (n = 4), wild-type (n = 4), ht1-2 (n = 7) and ca1ca4ht1-2 triple mutant (n = 7) leaves in response to the indicated [CO2] changes, show that HT1 is epistatic to βCA1 and βCA4. (b) RT-PCR analyses show human αCAII expression in randomly selected human αCAII transgenic ca1ca4 plant leaves. (c) Stomatal conductance of guard cell-targeted human αCAII-expressing ca1ca4 lines, ca1ca4 and wild-type plants in response to the indicated [CO2] changes (n = 4, ± s.e.m.). Three human αCAII-expressing lines were randomly chosen for stomatal response experiments and all showed recovery of CO2 responsiveness. Fig. S10 shows two other independent transgenic lines analyzed in parallel. (d) Elevated bicarbonate activates S-type anion channel currents in Arabidopsis guard cells. Average current-voltage curves were recorded in wild-type guard cells at ambient conditions (open circles) or with intracellular addition of either 13.5 mM bicarbonate, buffered to 2 mM free CO2 (filled red triangles) or 6.75 mM bicarbonate, buffered to 1 mM free CO2 (filled circles). Error bars depict means ± s.e.m‥
To determine whether the carbonic anhydrase reaction product, intracellular bicarbonate, can participate in stomatal signalling, S-type anion channel regulation was analyzed. Addition of 6.75 mM bicarbonate and buffering free [CO2] to 1 mM at pH 7.1 resulted in typical small background currents (Fig. 5d, n = 6). In contrast, when 13.5 mM bicarbonate was added, which buffered free [CO2] to 2 mM at pH 7.1, strong S-type anion currents were observed in guard cells, demonstrating that plasma-membrane anion channels can be activated by CO2/HCO3−, though at high concentrations (Fig. 5d, n = 10). Furthermore, extracellularly applied bicarbonate buffered to 2 mM free [CO2] in the bath solution caused a smaller activation of S-type anion currents in guard cells compared to that of intracellular bicarbonate (P<0.01 at −147mV and −117 mV, pairwise Student’s t-test, Supplementary Information, Fig. S10c). These data indicate that HCO3- emanating from neighboring cells may be taken up by guard cells and might contribute to the stomatal response, albeit at a lower level. These results demonstrate for the first time that elevated intracellular bicarbonate and CO2 levels can contribute to activation of guard cell plasma-membrane anion channel currents and indicate that CO2/HCO3− flux may function in βCA-mediated CO2-induced stomatal closing.
The present data show that β-carbonic anhydrases function very early in the CO2-induced stomatal signal transduction cascade based on impaired CO2 responses yet robust responses to blue light, light-dark transitions and abscisic acid in ca1ca4 leaves and epistasis analyses with the ht1-2 mutant9. The differential CO2 response of wild-type and ca1ca4 in isolated leaf epidermes, together with guard cell-targeted complementation of ca1ca4 by βCA and even by the unrelated human aCAII as well as guard cell βCA over-expression plant phenotypes provide genetic and molecular evidence that guard cells are a major site for β-carbonic anhydrase-mediated CO2 regulation of stomatal movements. However, the existence of an additional mesophyll- and/or a photosynthesis-related pathway6, 8 contributing to the residual CO2-induced stomatal movements in ca1ca4 (Fig. 1c-e) cannot be strictly excluded. The residual and slowed CO2 responses in βca mutant leaves may result from a combination of: (a) additional carbonic anhydrases that are expressed in guard cells21, 22; (b) CO2 levels may be elevated in ca1ca4 guard cells compared to wild type, due to the reduced CO2 hydration activity found in ca1ca4; (c) non-guard cell tissues such as pavement cells and mesophyll cells may contribute to the residual CO2 response; (d) a parallel mechanism for CO2 signalling and (e) even in the absence of carbonic anhydrases a slow spontaneous reversible hydration of CO2 occurs.
Interestingly, an increased stomatal density in ca1ca4 and an opposite effect in βCA guard cell-overexpression plants were observed, indicating that βCA1/4 not only strongly affect CO2 control of stomatal movements but also modulate stomatal development at ambient [CO2]. Analyses of the pGC1 guard cell promoter activity during stomatal development22 further suggest that βCA-mediated control of stomatal development is non cell-autonomous, as stomatal development is defined prior to the guard cell stage28, 29. Previous studies have suggested non cell-autonomous long distance signalling from mature leaves to newly developing leaves as part of the mechanisms by which environmental cues regulate stomatal development31. Further research is needed to determine whether βCA1 and βCA4 function in environmental control of stomatal development.
Guard cell-targeted over-expression of βCA1 or βCA4 consistently increased instantaneous water use efficiency, indicating that: (i) βCA expression levels are not saturated in wild-type guard cells; and (ii) manipulation of carbonic anhydrases may provide an approach for engineering gas exchange and transpirational water loss or alternatively protection against heat-induced damage of plants in light of the continuing atmospheric [CO2] increase, climate change and limited global freshwater availability4, 32.
Restoration of CO2 regulation of stomatal movements by guard cell expression of the structurally unrelated human αCAII provides strong evidence that catalytic CA activity and bicarbonate and/or proton production function in mediation of this CO2 response and indicates that CO2 regulation of stomatal conductance in plants underlies flux control33. A previous study has suggested that the CO2 response is not mediated through changes in cytosolic pH34. Moreover, the activation of large S-type anion channel currents by high intracellular bicarbonate levels provides evidence that CO2/HCO3− may act as messengers contributing to guard cell CO2 signal transduction. Note that although these CO2/HCO3− are much higher than CO2 concentrations used in gas exchange experiments, high CO2 concentrations have been used in other electrophysiological studies (e. g. 30) and whole-cell dialysis during patch clamping may reduce the CO2/HCO3− sensitivity of downstream signaling mechanisms. Together our findings reveal an essential function of guard cell-expressed carbonic anhydrases in CO2 regulation of plant water transpiration and CO2 influx and are consistent with a model in which βCA1 and βCA4 function in the early CO2 response machinery in guard cells.
METHODS
Plant growth conditions and mutant genotyping
All Arabidopsis thaliana plants used in this study were of the Columbia ecotype (Col 0). Wild-type, ht1-2 and carbonic anhydrase mutant plants were grown in a Conviron growth chamber (Winnipeg, Canada) (20°C, 60 to 80% humidity with a 16-h-light/8-h-dark photoperiod regime at ~75 µmol m−2s−1). The β-carbonic anhydrase T-DNA insertional mutants ca1 (SALK_106570; insertion in Exon IX at nucleotide +2631), ca4 (WiscDsLox508D11; insertion in Intron II at nucleotide +618) and ca6 (SALK_044658; insertion in Exon V at nucleotide +691) were obtained from The Arabidopsis Biological Resource Center (ABRC). Genotyping PCR reactions were performed using the primer pairs CA1F-RT/CA1R-RT and CA1R-RT/LBa1 for ca1; CA4F-RT/CA4R-RT, and CA4R-RT/LB-Wisc for ca4 and finally CA6F-RT/CA6R-RT and CA6F-RT/LBa1 for ca6 (Supplementary Table 1). To confirm the ht1-2 point mutation, the primers HT1-F and HT1-R were used to amplify a 300bp PCR fragment from plant genomic DNA which was then sequenced.
Time-resolved intact leaf stomatal conductance experiments with [CO2] shifts
Stomatal conductance recordings from intact, mature non senescent leaves of 5 to 7 week-old plants were conducted starting 1 to 2 hrs after growth chamber light onset during mornings using a Li-6400 infrared (IRGA)-based gas exchange analyzer system with a fluorometer chamber (Li-Cor Inc., Lincoln, NE). Temperature and relative humidity were held at 20°C and approximately 60% to 70% respectively, while photon flux density was 55 µmol m−2s−1 except for high light experiments (Supplementary Information, Fig. S1e, f) where temperature was 22°C and photon flux density was 1000 µmol m−2s−1. Analyzed leaves always covered the whole surface of the gas exchange analyzer chamber so that all measurements would be dependent on the stomatal density and the stomatal aperture responses in the chamber.
For stomatal closing experiments, stomatal conductance was stabilized at ambient [CO2] (365 ppm) for 30 min then [CO2] was shifted to 800 ppm for 30 min or 60 min then changed to 100 ppm for at least 30 min. For additional experiments, stomatal conductance was stabilized at 400 ppm [CO2] for 30 min, then [CO2] was shifted to 100 ppm for 30 min and then changed to 800 ppm. The data presented are means of at least 3 leaves per genotype per treatment ± s.e.m. Relative stomatal conductance values were determined by normalization relative to the last data point prior to the 365 to 800 ppm [CO2], 400 to 100 ppm [CO2] transitions or the dark to blue light transitions.
Instantaneous water use efficiency (WUE) defined as the ratio of CO2 assimilated to water lost during transpiration (µmol CO2 mmol−1 H2O−1) was calculated from data collected with the Li-6400 gas exchange analyzer at ambient [CO2]. Three time points (first, medium and last point under ambient conditions) were chosen for each leaf. P values were calculated using Students t-test using two-tailed distribution and two-sample equal variance.
To calculate the initial rate of stomatal conductance changes in response to [CO2] shifts (n = 7; Fig. 1d) or dark/blue light transitions (n = 4, Fig. S2a; n = 5, Fig. S2b) in wild-type and ca1ca4 or ca1ca4ca6 plants, Li-6400 data collected during the first 20 minutes following [CO2] or blue light shifts were plotted and regression analyses were performed. These data are the average of the slopes of 4, 5 and 7 fits ± s.e.m‥ P values were calculated using unpaired t-test with two-tailed distribution and two-sample equal variance. Note that in the ca1ca4ca6 triple mutant an additional later slow rate of stomatal conductance increase was observed (Fig. S2b), whereas in the ca1ca4 mutant, normalized data show similar rates in wild-type and mutant plants (Fig. 1f, Fig. S2a).
Stomatal measurements
For responses to buffers pre-equilibrated with high CO2 (800 ppm) in balance with air or control ambient air, intact submerged leaf epidermal layers were prepared with intact guard cells and leaf pavement cells12. Stomatal apertures were analyzed only in stomatal complexes with no mesophyll cells in their vicinity. Leaf epidermal layers were pre-incubated for 1.5 h in pre-incubation buffer (10 mM MES, 10 mM KCl, 50 µM CaCl2, pH 6.15) and exposed continuously to the indicated CO2 conditions for 30 min or 60 min as described previously12. As leaf epidermes were submerged in a solution volume of 7 ml, the likelihood of diffusible signals emanating from distant cells was remote. Fig. 1g and Fig. S2d correspond to 30 and 60 min CO2 exposure times respectively at pH 6.15. For responses to ABA, epidermal layer were incubated in stomatal opening buffer (5 mM MES, 10 mM KCl, 50 µM CaCl2, pH 6.15) for 3 h and exposed to the indicated ABA concentrations for 60 min. Thereafter stomatal apertures were measured. Data shown in Fig. 1g and Fig. S2d were genotype blind analyses and in Fig. 1h were genotype and [ABA] blind analyses (n = 3 experiments, 30 stomata per experiment and condition). For stomatal index and density analyses, 16 leaves for each genotype were analyzed per experiment from 4–5 week-old plants of similar plant sizes grown in an AR-22L Arabidopsis growth chamber (Percival, Iowa, 21°C, ~75% relative humidity, 320–340 ppm CO2, 16-h-light/8-h-dark photoperiod regime at ~120 µmol m−2 s−1) and two areas (0.039mm2 ) in the middle region of abaxial epidermes of each leaf were measured. Stomatal index was defined as 100% * number of stomata / (number of stomata + number of epidermal cells) in each area.
Genomic and guard cell-targeted complementation of ca1ca4
For genomic complementation, the 4.5 Kb βCA1 (including 2077 bp-long 5’ region upstream of the start codon) and 4.3 Kb βCA4 (including 1677 bp-long 5’ region upstream of the start codon) genomic DNA fragments containing the βCA1 and βCA4 genes with their flanking sequences were PCR-amplified from the BACs F4P13 (accession number AC009325) and F17O7 (AC003671) and were recombined into the binary Gateway vector pHGY (RIKEN Plant Science Center, Japan) by LR reaction (Invitrogen). For guard cell-targeted expression of βCA1 and βCA4 cDNAs in wild type and ca1ca4, βCA1 and βCA4 full length cDNAs were amplified and recombined into the binary vector derivated from pXCSG-Strep35, where the 35S promoter was replaced by the guard cell-targeted promoter pGC122. For human αCAII cDNA expression in guard cells of ca1ca4 plants, the full-length cDNA was amplified from the cDNA clone (SC107902) which was purchased from the OriGENE (Rockville, MD) with primer pairs HmCAIIF/HmCAIIR and subcloned into the modified binary vector pGreenII0179 with the pGC1 promoter.
RT-PCR and qRT-PCR analyses
Protoplasts of guard cells and mesophyll cells from wild-type and complementation plant leaves were isolated as described previously 21 and total RNA samples from protoplasts and leaves were isolated as described 36. RT-PCR shown in Fig. 4a; 5b; and Fig. S1a, S8b were carried out for 30 cycles to amplify target sequences βCA1 (CA1F/CA1R), βCA4 (CA4F/CA4R), βCA6 (CA6F/CA6R) and human αCAII (NM_000067) (HmCAIIF/HmCAIIR). For Fig. 2b, RT-PCR experiments were carried out for 29 cycles with the primer pairs CA1F-RT/CA1R-RT and CA4F-RT/CA4R-RT to amplify βCA1 and βCA4 respectively. For quantitative real-time PCR (qRT-PCR), the cDNAs obtained as above were diluted five times. Then, qRT-PCR was performed by using a LightCycler (Roche) with the SYBR Green I detection system, under the following conditions: 95°C for 10 min; 45 cycles of 95°C for 5 s, 55°C for 5 s, and 72°C for 13 s; followed by melting curve analysis. EF-1α (At5g60390) was selected as the reference gene according to 37. The primers for the six βCAs genes as well as GC1 were designed according to 38 and are shown in Supplemental Table 1. PCR mixture at a final volume of 10 µL contained 2 µL of cDNA, 0.5 µM of each primer, 4 mM Mg2+, and 1 µL of LightCycler-FastStart DNA Master SYBR Green I mixture (Roche). Quantitative data analyses were performed with the LightCycler software 4.0 (Roche).
Water loss measurements
For water-loss measurements, the weight of detached leaves, incubated abaxial side up under laboratory conditions was measured at the illustrated time points. Water loss was calculated as the percentage of initial fresh weight. For whole plant dry weight analyses, germinated plants were transferred to soil, 5 plants per pot (8.5×8.5×8.5 cm3). The soil filled in each pot before planting was the same and adjusted by weighing. Plants were grown in a growth room with 16-h-light/8-h-dark; the same amounts of water were applied to each pot. For limited watering 5 ml water was added every 2 days to each pot. For well-watered plants 8 ml water was added every day to each pot. Four-week old plants were carefully removed from the soil and washed, dried at 37°C for 5 days and dry weights were measured. Plant genotypes were blinded to the experimenter.
Carbonic anhydrase activity analyses
0.5g of mature leaf samples from 5–7 week-old and non-senescent Arabidopsis plants were ground in liquid nitrogen and immediately resuspended in 1 mL of extraction buffer (100 mM N, N-Bis 2-hydroxymethyl Gly)-NaOH buffer (pH 8.5), 20mM MgCl2, 1mM EDTA). The lysate was cleared by centrifugation at 18,400 g for 10 min at 4°C. CA activity was measured by the potentiometric method39 with some modifications. 50 µL of cell suspension was added to 3 mL of 20 mM Tris-Sulfate buffer (pH 8.3) in a scintillation vial maintained at 2°C. Addition of 2 mL of ice-cold CO2-saturated water initiated the reaction and the time required for the pH change from 8.3 to 6.3 was measured.
Norflurazon-treated plants and analyses
Three to four week-old plants were watered once with a solution of ~67 µM norflurazon which was fully absorbed into the plant soil system followed by normal watering. Newly formed leaves showed chlorosis (bleaching) after one week. Norflurazon slowed the growth of plants; therefore control plants used in experiments were 4–5 weeks old, but at the same developmental stage as 6–7 week-old albino plants. Intact epidermal layers of control and norflurazon-treated albino leaves were analyzed using confocal imaging, with an excitation wavelength of 488 nm to measure chlorophyll fluorescence. The fluorescence intensity was quantified (Fig. 3c) following background subtraction using ImageJ (freeware National Institutes of Health, MD).
Photosynthetic activity measurements
Chlorophyll fluorescence (F) was measured using the fluorometer chamber of the Li-6400 system (LI-COR Inc, Lincoln, NE) with default settings. The Fv/Fm of pre-darkened leaves (6 weeks old) was calculated as (Fm-F0)/Fm 40 (n = 10). The photochemical efficiency of photosynthesis (ΦPSII) was determined by measuring steady-state fluorescence (Fs) and maximum fluorescence during a light saturating pulse (F’m) 40 on fully expanded attached leaves (n = 6). The leaves were adapted to low (50 µmol m−2 s−1) or high (2000 µmol m−2 s−1) illumination consisting of 90% red light (630 nm) and 10% blue light (470 nm). In addition, the onset of photosynthetic activity was measured as CO2 assimilation rate (µmol m−2 s−1) in pre-darkened leaves exposed to red light. Leaves were pre-darkened until they showed stable stomatal conductance levels for a period of 30 minutes and then exposed to 300 µmol m−2 s−1 red light for 2 hrs (n = 6, 5–7 weeks old). Data presented as mean ± s.e.m‥
Patch clamp analyses
Arabidopsis guard cell protoplasts were isolated from rosette leaves of 4 to 6 week-old plants using a protoplast isolation solution containing 1.0% Cellulase R10, 0.5% Macerozyme R10 (Yakutt Horisha Co. Ltd, http://www.yakutt.co.jp/ypi/en/product.html), 0.5% bovine serum albumin, 0.1% kanamycin, 10 mM ascorbic acid, 0.1 mM KCl, 0.1 mM CaCl2 and 500 mM D-mannitol (buffered to pH 5.5 using KOH). Whole-cell patch-clamp experiments were performed as described previously 41. For analyses of S-type anion currents, the pipette solution contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM EGTA, 5 mM Mg-ATP, 5 mM Tris-GTP, 1 mM HEPES/Tris pH 7.1, and CaCl2 was added to 2µM free Ca2+. In analyses of intracellular bicarbonate/CO2 activation of S-type anion currents, bicarbonate was freshly added to the pipette solution and the pH was adjusted to the indicated value with Tris-HCl. The addition of 13.5 mM bicarbonate buffered to 2 mM free CO2 and 6.75 mM bicarbonate buffered to 1 mM free CO2 in the pipette solution are calculated at pH 7.1 according to 42, 43. The bath solution contained 30 mM CsCl, 2 mM MgCl2, 5 mM CaCl2 and 10 mM Mes/Tris pH 5.6. In analyses of S-type anion channels activated by extracellular bicarbonate/CO2, the addition of 2.4 mM bicarbonate buffered to 2 mM free CO2 and1.2 mM bicarbonate buffered to 1 mM free CO2 were added to the bath solution at pH 5.6 adjusted with Tris-HCl. Before patch clamping, the guard cells were incubated in CsHCO3-containing solution for 30–90 min. The bath solution contained 30 mM CsCl, 2 mM MgCl2, 5 mM CaCl2, Osmo 500 mmol/kg and 10 mM MES/Tris pH 5.6 and the pipette solution contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM EGTA, 5 mM Mg-ATP, 5 mM Tris-GTP, 6.03 CaCl2 ( 2µM free Ca2+), 1 mM HEPES-Tris and Osmo 500 mmol/kg, pH 7.1.
Subcellular localization of βCA-YFP protein
To generate the βCA1-YFP and βCA4-YFP constructs, 1061 bp βCA1 and 836 bp βCA4 cDNAs were amplified with the primer pairs CA1F/CA1YFPR and CA4F/CA4YFPR respectively and cloned into the binary pXCSG-YFP44. The pXCSG-YFP vector containing the plasma membrane targeted FLS2-YFP fusion was used as a positive control for membrane localization and provided by Dr. Silke Robatzek (Max Planck Institute for Plant Breeding Research, Cologne) (Robatzek et al., 2006). The pH35YG vector45 containing the 35S-YFP was used as a positive control for cytosol and nuclear localizations. Protoplasts were prepared from infiltrated leaves as described46. Protoplasts were stained with 2µM FM4-64 dye for 5 min to only stain the plasma membrane. Fluorescence imaging was acquired by spinning-disc confocal microscopy. Images were captured with an electron multiplying charge-coupled device (EMCCD) camera (Cascade II: 512, Photometrics, Tucson, AZ, USA) using Metamorph software (Universal Imaging, Downington, PA, USA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mohammad Maktabi, Jared Young and Cawas Engineer for preliminary analyses of βca mutants and Roger Xu for assistance. We thank Sam Zeeman (ETH Zűrich) for suggestions and Koh Iba (Kyushu University) for providing ht1-2 seeds. This research was supported by NSF (MCB0918220), NIH (GM060396) and in part DOE (DE-FG02-03ER15449) grants (to J.I.S.) and by fellowships from the Swedish Research Council Formas (to M. I.-N.), the Deutsche Forschungsgemeinschaft (to M. B.), EMBO (to J. M. K.) and in part from the King Abdullah University of Science and Technology (KAUST) (No. KUS-F1-021-31 to H. H.).
Footnotes
AUTHOR CONTRIBUTIONS
J.I.S. conceived of the project and proposed the experimental design. H.H., A. B.-D. and M.I.-N. performed most of the experiments and contributed equally to the work. M.B. performed CA activity analyses. S. X. performed patch clamp experiments. A.R. contributed to stomatal movement and stomatal index measurements. J. G. performed norflurazon experiments. J.M.K. analyzed CO2-/HCO3−-binding protein-encoding gene expression patterns and isolated the initial CA, PEPC and Rubisco T-DNA insertion lines. J.I.S., H.H., A.B.-D. and M.I.-N. wrote the paper.
REFERENCES
- 1.Sellers PJ, et al. Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science. 1997;275:502–509. doi: 10.1126/science.275.5299.502. [DOI] [PubMed] [Google Scholar]
- 2.Medlyn BE, et al. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol. 2001;149:247–264. doi: 10.1046/j.1469-8137.2001.00028.x. [DOI] [PubMed] [Google Scholar]
- 3.LaDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292:95–98. doi: 10.1126/science.1057547. [DOI] [PubMed] [Google Scholar]
- 4.Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- 5.von Caemmerer S, et al. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J. Exp. Bot. 2004;55:1157–1166. doi: 10.1093/jxb/erh128. [DOI] [PubMed] [Google Scholar]
- 6.Messinger SM, Buckley TN, Mott KA. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol. 2006;140:771–778. doi: 10.1104/pp.105.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roelfsema MR, et al. Guard cells in albino leaf patches do not respond to photosynthetically active radiation, but are sensitive to blue light, CO2 and abscisic acid. Plant Cell Environ. 2006;29:1595–1605. doi: 10.1111/j.1365-3040.2006.01536.x. [DOI] [PubMed] [Google Scholar]
- 8.Mott KA, Sibbernsen ED, Shope JC. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 2008;31:1299–1306. doi: 10.1111/j.1365-3040.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, et al. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 2006;8:391–397. doi: 10.1038/ncb1387. [DOI] [PubMed] [Google Scholar]
- 10.Webb AA, Hetherington AM. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol. 1997;114:1557–1560. doi: 10.1104/pp.114.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leymarie J, Vavasseur A, Lasceve G. CO2 sensing in stomata of abi1-1 and abi2-1 mutants of Arabidopsis thaliana. Plant Physiol. Biochem. 1998;36:539–543. [Google Scholar]
- 12.Young JJ, et al. CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. USA. 2006;103:7506–7511. doi: 10.1073/pnas.0602225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahisalu T, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–491. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negi J, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- 15.Lee M, et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat. Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- 16.Marten H, et al. Silencing of NtMPK4 impairs CO2-induced stomatal closure, activation of anion channels and cytosolic Ca2+ signals in Nicotiana tabacum guard cells. Plant J. 2008;55:698–708. doi: 10.1111/j.1365-313X.2008.03542.x. [DOI] [PubMed] [Google Scholar]
- 17.Gehlen J, et al. Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol. Biol. 1996;32:831–848. doi: 10.1007/BF00020481. [DOI] [PubMed] [Google Scholar]
- 18.Cousins AB, et al. The role of phosphoenolpyruvate carboxylase during C4 photosynthetic isotope exchange and stomatal conductance. Plant Physiol. 2007;145:1006–1017. doi: 10.1104/pp.107.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinoshita T, et al. PHOT1 and PHOT2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 20.Mori IC, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:1749–1762. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonhardt N, et al. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell. 2004;16:596–615. doi: 10.1105/tpc.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential role as a research tool. Pl. Methods. 2008;4:1–15. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Zhang W, Stanley BA, Assmann SM. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell. 2008;20:3210–3226. doi: 10.1105/tpc.108.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fabre N, Reiter IM, Becuwe-Linka N, Genty B, Rumeau D. Characterization and expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant Cell Environ. 2007;30:617–629. doi: 10.1111/j.1365-3040.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura Y, Uemura M. Mass spectrometric approach for identifying putative plasma membrane proteins of Arabidopsis leaves associated with cold acclimation. Plant J. 2003;36:141–154. doi: 10.1046/j.1365-313x.2003.01864.x. [DOI] [PubMed] [Google Scholar]
- 26.Froehlich JE, et al. Proteomic study of the Arabidopsis thaliana chloroplastic envelope membrane utilizing alternatives to traditional two-dimensional electrophoresis. J. Proteome Res. 2003;2:413–425. doi: 10.1021/pr034025j. [DOI] [PubMed] [Google Scholar]
- 27.Uehlein N, et al. Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell. 2008;20:648–657. doi: 10.1105/tpc.107.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergmann DC, Sack FD. Stomatal development. Annu. Rev. Plant Biol. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- 29.Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- 30.Lu J, et al. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na+/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J. Biol. Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- 31.Lake JA, Quick WP, Beerling DJ, Woodward FI. Plant development. Signals from mature to new leaves. Nature. 2001;411:154. doi: 10.1038/35075660. [DOI] [PubMed] [Google Scholar]
- 32.Jacob EJ. Water: under pressure. Nature. 2008;452:269. doi: 10.1038/452269a. [DOI] [PubMed] [Google Scholar]
- 33.Price ND, Reed JL, Palsson BO. Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat. Rev. Microbiol. 2004;2:886–897. doi: 10.1038/nrmicro1023. [DOI] [PubMed] [Google Scholar]
- 34.Brearley J, Venis MA, Blatt MR. The effect of elevated CO2 concentrations on K+ and anion channels of Vicia faba L. guard cells. Planta. 1997;203:145–154. [Google Scholar]
- 35.Witte CP, Noel LD, Gielbert J, Parker JE, Romeis T. Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol. Biol. 2004;55:135–147. doi: 10.1007/s11103-004-0501-y. [DOI] [PubMed] [Google Scholar]
- 36.Boisson-Dernier A, Frietsch S, Kim TH, Dizon MB, Schroeder JI. The peroxin loss-of-function mutation abstinence by mutual consent disrupts male-female gametophyte recognition. Curr. Biol. 2008;18:63–68. doi: 10.1016/j.cub.2007.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Udvardi MK, Czechowski T, Scheible WR. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 1948;11 [PubMed] [Google Scholar]
- 40.Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochem. Biophys. Acta. 1989;990:87–92. [Google Scholar]
- 41.Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. Plant Cell. 2002;14:1649–1662. doi: 10.1105/tpc.010448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natelson S, Nobel D. More on blood bicarbonate measurement. Clinical Chem. 1978;24:1082–1083. [PubMed] [Google Scholar]
- 43.Pigott JD. Coupled ion-selective electrode measurement of aqueous carbonate and bicarbonate ion activities. Anal. Chem. 1989;61:638–640. [Google Scholar]
- 44.Feys BJ, et al. Arabidopsis senescence-associated gene101 stabilizes and signals within an enhanced disease susceptibility1 complex in plant innate immunity. Plant Cell. 2005;17:2601–2613. doi: 10.1105/tpc.105.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kubo M, et al. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005;19:1855–1860. doi: 10.1101/gad.1331305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter M, et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004;40:428–438. doi: 10.1111/j.1365-313X.2004.02219.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.