Abstract
BACKGROUND:
Urinary tract infections (UTIs) are a common source of bacterial infection among young febrile children. The diagnosis of UTI is challenging because the clinical presentation is not specific.
OBJECTIVE:
To describe clinical predictors to identify young children needing urine culture for evaluation of UTI.
METHODS:
Retrospective cohort study of all children younger than two years of age (719 hospital visits for 545 patients) suspected of having a UTI during a 12-month period. The outcome was UTI, defined as a catheterized urine culture with pure growth of 104 colonies/mL or greater, or suprapubic aspiration culture with 103 colonies/mL or greater. Candidate predictors included demographic, historical and physical examination variables.
RESULTS:
The medical records of 545 children younger than two years of age were reviewed. Forty-six per cent were girls. Mean age was 9.1 months (SD 7 months). Four variables were found to predict UTI: absence of another source of fever on examination (odds ratio [OR]=41.6 [95% CI, 8.8 to 197.4]), foul smelling urine (OR=19.7 [95% CI, 5.7 to 68.2]), white blood cell count greater than 15,000/mm3 (OR=4.3 [95% CI, 2.0 to 9.3]), younger than six months old (OR=3.1 [95% CI, 1.3 to 7.1]). The sensitivity of an abnormal urine analysis was 0.77 (95% CI, 0.66 to 0.88) and the specificity was 0.31 (95% CI, 0.2 to 0.42).
CONCLUSION:
An incremental increase in risk for UTI is associated with younger age (younger than six months), having a white blood cell count higher than 15,000/mm3, parental report of malodorous or foul smelling urine and the absence of an alternative source of fever. In the present patient population, obtaining a urine culture from children with at least one of these clinical predictors would have resulted in missing one UTI (2%), and 111 negative cultures (20%) would have been avoided.
Keywords: Children, Diagnosis, Urinary tract infection
Abstract
HISTORIQUE :
Les infections urinaires (IU) sont une source courante d’infection bactérienne chez les jeunes enfants fiévreux. Le diagnostic d’IU est souvent difficile à cerner parce qu’à l’accoutumée, la présentation clinique n’est pas spécifique.
OBJECTIF :
Décrire les prédicteurs cliniques afin de repérer les jeunes enfants devant subir une uroculture pour évaluer une IU.
MÉTHODOLOGIE :
Étude rétrospective des cohortes de tous les enfants de moins de deux ans (719 consultations à l’hôpital par 545 patients) présumés avoir une IU sur une période de 12 mois. L’issue était une IU, définie comme une uroculture par cathéter avec croissance pure d’au moins 104 colonies/mL, ou comme une culture par ponction suspubienne d’au moins 103 colonies/mL. Les prédicteurs candidats incluaient des variables démographiques, historiques et physiques.
RÉSULTATS :
Ont été examinés les dossiers médicaux de 545 enfants de moins de deux ans. Quarante-six pour cent étaient des fillettes. L’âge moyen était de 9,1 mois (ÉT = 7 mois). On a découvert quatre variables prédictives d’une IU : l’absence d’une autre source de fièvre à l’examen (risque relatif [RR] = 41,6 [95 % IC, 8,8 à 197,4]), urine fétide (RR = 19,7 [95 % IC, 5,7 à 68,2]), numération leucocytaire supérieure à 15 000/mm3 (RR = 4,3 [95 % IC, 2,0 à 9,3]), âge inférieur à six mois (RR = 3,1 [95 % IC, 1,3 à 7,1]). La sensibilité d’une analyse d’urine anormale était de 0,77 (95 % IC, 0,66 à 0,88), et sa spécificité, de 0,31 (95 % IC, 0,2 à 0,42).
CONCLUSION :
Une augmentation incrémentielle du risque d’IU est associée à un très jeune âge (moins de six mois), à une numération leucocytaire supérieure à 15 000/mm3, à la présence d’une urine malodorante ou fétide déclarée par le parent et à l’absence d’autre source de fièvre. Au sein de la population actuelle de patients, une IU (2 %) aurait été ratée malgré l’obtention d’une uroculture auprès d’enfants présentant au moins l’un de ces prédicteurs cliniques, et 111 cultures négatives (20 %) auraient été évitées.
Urinary tract infection (UTI) is an old but important health problem for children, their parents and clinicians. It is estimated that about 1% of children will develop a UTI and become unwell with unpleasant, often febrile illness (1–3).
Children with UTI present with a broad spectrum of signs and symptoms (2). In part, this is due to the fact that UTI presents at all ages. The specific signs of frequency and costovertebral angle tenderness, and the symptoms of dysuria and flank pain that are readily elicited from older children may not be evident on an assessment of children younger than two years of age. Accordingly, it is difficult to rely on a stereotypical presentation for suspicion of UTI. Although fever is often observed with UTI in children, it is not sensitive as a unique criterion for suspecting UTI (3–4). There has been increased recognition of UTI as the source of infection in otherwise well-appearing febrile children, even without signs and symptoms referable to the urinary tract and even in the presence of another possible source of fever (5). Hoberman et al (5) obtained urine cultures for 945 febrile children in the first year of life; there was no significant association between UTI and several of the clinical findings studied, including vomiting, diarrhea, lethargy and poor feeding. They have found that certain clinical factors are associated with a finding of UTI, including age, race and height of fever (5).
The aim of the present study was to identify clinical factors predictive of a positive urine culture in a cohort of children younger than two years of age suspected of having UTI by their physicians.
MATERIALS AND METHODS
Study design and subjects
The study was conducted in a university affiliated hospital. The general hospital is the only site for hospitalization of children in Sherbrooke and provides secondary and tertiary care for a population of 200,000. All children who presented to the emergency department, paediatric clinic, and/or were hospitalized were eligible. Children younger than two years of age who had a urine specimen collected for urine culture were eligible for the study. Test ordering is computerized in the authors’ setting. Each visit was treated individually, even in the presence of multiple urine culture. Medical records were reviewed retrospectively for 12 consecutive months (January 1999 to December 1999). The decisions to obtain a urine culture and the method of urine collection were made by the evaluating physician according to usual guidelines (4,6). No attempt was made to change current physician practice.
A urine culture was considered to be positive by standard criteria (7): greater than 103 colony-forming units (cfu)/mL from suprapubic aspirations, greater than or equal to 104 cfu/mL on catheterized specimens, and greater than or equal to 105 cfu/mL on clean voided specimens. A urine culture was considered to be negative if there were no cfu from suprapubic aspirations, catheterized specimens or bags.
Patients were excluded if they: were known to have an underlying renal and/or genitourinary abnormality (vesicoureteral reflux was not an exclusion criteria in the present study); had an underlying medical problem requiring repeated catheterizations (eg, neurogenic bladder); were known to have an immunodeficiency; had cultures considered to be contaminated (ie, the presence of more than one organism or nonpathogens [Acinetobacter species, Candida species, Gardnerella vaginalis, Micrococcus species, Streptococcus viridans, Staphilococcus nonaureus, Corynebacterium species]); and had a urine culture during antibiotic treatment.
A dipstick analysis was performed for leukocyte esterase (LE) and nitrite using Multistix (Bayer Corporation, USA). Degrees of LE found (1+, 2+, 3+) were considered to be positive results for the presence of LE. As part of our standard urine analysis (UA), the laboratory performed microscopic analysis only if the dipstick indicated the presence of LE, nitrite, blood or protein. Urine Gram stains were not routinely performed. A dipstick test result was considered to be positive if LE, nitrite, or both were present. Pyuria was defined as greater than or equal to five white blood cells (WBCs) per high-power field (original magnification ×40) on a spun specimen (centrifugated at 310 g for 5 min). A positive UA result was defined as a positive dipstick test result and/or pyuria.
Each chart was reviewed in a systematic fashion. The following variables were considered to be candidate predictor variables: age, triage temperature, history of fever, duration of fever, vomiting, loss of appetite, failure to thrive, diarrhea, foul smelling urine, past medical history of pyelonephritis or vesicoureteral reflux, constipation, circumcision status for boys, irritability during examination, general ill appearance, any urinary symptoms and laboratory test results. All data were transcribed onto a structured data sheet before investigator review of the laboratory test results. All urine cultures were reviewed for specimen type, colony counts and isolates. All patients who had a paired UA and urine culture were included for calculating the sensitivity and specificity of the UA.
Meningitis, pneumonia confirmed by chest radiograph, cellulitis, streptococcal pharyngitis, specific viral infection (eg, varicella, Coxsackie diseases, Herpetic stomatitis) and recognizable febrile diseases (eg, Kawasaki syndrome) were considered to be definitive sources of fever. Upper respiratory tract infection, bronchiolitis, croup, sinusitis and otitis media were considered to be possible sources of fever if present in the first clinical encounter (emergency department, paediatric clinic or admission note) before the urine culture was taken.
Data analysis
Patient demographic characteristics are reported as mean ± SD. Clinical data were analyzed initially using univariate techniques. Univariate association was evaluated with χ2 test for nominal variables and Mann-Whitney U test for continuous variables. The authors attempted to develop a multivariate model. Factors with statistically nonsignificant (using a conservative significance level of P<0.25) univariate association with UTI were excluded from further consideration. The remaining factors were entered into a multiple logistic regression model, with UTI as the outcome variable. A backward elimination modelling technique was used with a significance limit for removal from the model of P>0.15 using maximum likelihood estimation. Those predictors remaining in the logistic regression model were then considered for the clinical decision rules. The goal was to create a simple linear score based on the presence or absence of each of the factors. A cutoff value would then be chosen to define a decision rule for identifying patients in whom urine testing should be performed. The overall discriminative ability of the resulting models was evaluated by the area under the receiver operating characteristic curve. Goodness-of-fit statistics were examined to determine the appropriateness of the final model.
All statistical tests were conducted based on two-tailed alternatives and P≤0.05 was considered significant. Statistical analyses were performed using StatView 5.0 (SAS institute, USA).
RESULTS
Seven hundred and nineteen visits were evaluated for 545 patients during the study period. Of these, 251 children (46%) were girls and the mean age was 9.1 months (SD 7 months) and the median age was eight months. Ninety-seven per cent were identified by their parents as white, 2% as black, 0.7% as hispanic, 0.3% as asian. The prevalence of circumcision was 0.7% (two of 294) in all males.
One hundred seventy-seven visits were excluded, with the most common reasons being contaminated urine culture (n=85), clinical data not in the chart (n=80), immunodeficiency (n=6), neurogenic bladder (n=3) and a medical problem requiring repeat catheterization (n=3).
Of those eligible for analysis, 55 of 545 cultures were positive (10%). The majority of patients had cultures positive for Escherichia coli (44 of 55).
Results of the univariate analysis for each of the candidate variables are shown in Table 1 for nominal variables and Table 2 for continuous variables. For the continuous variables, a cutoff point was selected to permit use of a dichotomous variable for prediction. Several clinical parameters were associated with a higher rate of UTI. Those children who did not have a potential source of fever, children whose caregivers reported foul smelling urine, younger children and boys had higher UTI prevalence.
TABLE 1.
Univariate analysis for predictors of urinary tract infection (UTI) (nominal variables)
| Variable | UTI (n=55) (n [%]) | No UTI (n=490) (n [%]) | P |
|---|---|---|---|
| Sex | |||
| Girl | 19 (35) | 232 (47) | |
| Boy | 36 (65) | 258 (53) | 0.07 |
| Race | |||
| White | 54 (98) | 476 (97) | |
| Other | 1 (2) | 14 (3) | 0.56 |
| Age <6 months | 35 (64) | 187 (38) | 0.0004 |
| Clinical parameters | |||
| Fever >24 h | 15 (27) | 157 (32) | 0.47 |
| Triage temperature ≥38.5°C (rectal) | 19 (35) | 192 (39) | 0.5 |
| Vomiting | 21 (38) | 196 (40) | 0.8 |
| Loss of appetite | 22 (40) | 197 (40) | 0.98 |
| Diarrhea | 9 (16) | 109 (22) | 0.31 |
| Constipation | 1 (2) | 21 (4) | 0.38 |
| Poor weight gain | 3 (5) | 25 (5) | 0.9 |
| Irritability | 10 (18) | 93 (19) | 0.9 |
| Foul smelling urine | 12 (25) | 25 (5) | <0.0001 |
| Past history of UTI | 4 (7) | 61 (12) | 0.26 |
| Absence of an alternative source of fever | 51 (93) | 202 (41) | <0.0001 |
| Febrile seizure | 1 (2) | 21 (4) | 0.22 |
TABLE 2.
Univariate analysis for predictors of urinary tract infection (UTI) (continuous variables)
| UTI (mean [SD]) | No UTI (mean [SD]) | P | |
|---|---|---|---|
| Duration of symptoms | 4.5 (8.2) | 4.4 (5.4) | 0.6 |
| Age (months) | 5.8 (6.4) | 9.5 (7.1) | 0.0002 |
| Heart rate (bpm) | 137 (22) | 141 (24) | 0.26 |
| WBC count (/mm3) | 14,700/mm3 (6.9) | 11,800/mm3 (5.7) | 0.002 |
| Duration of fever (days) | 2.2 (1.6) | 2.3 (2.2) | 0.57 |
bpm Beats per minute; WBC White blood cell
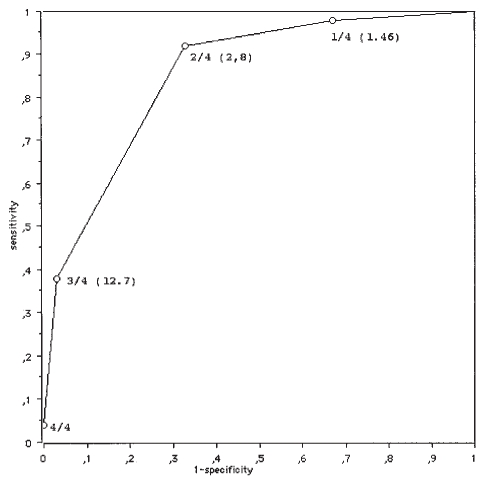
Candidate predictor variables remaining after the screening process were included in a logistic regression model, with a backward stepwise elimination. Four predictors remained in the final model: absence of an alternative source of fever (odds ratio [OR]=41.6 [95% CI, 8.8 to 197.4]), foul smelling urine (OR=19.7 [95% CI, 5.7 to 68.2]), WBC count greater than 15,000/mm3 (OR=4.3 [95% CI, 2.0 to 9.3]), age younger than six months (OR=3.1 [95% CI, 1.3 to 7.1]). Because many clinicians and parents place high value on sensitivity, particularly when the diagnostic test is relatively inexpensive and non-invasive, an attempt was made to define symptoms with the highest possible sensitivity. The presence of any one or more of these variables predicted a positive urine culture with a sensitivity of 0.98 (95% CI, 0.94 to 1.0), a specificity of 0.33 (95% CI, 0.28 to 0.38), and positive likelihood ratio of 1.46 (95% CI, 1.34 to 1.59) for a positive culture (Figure 1). Obtaining a urine culture from children with the presence of at least one predictor would have resulted in missing one UTI (2%), and 111 negative urine cultures would have been avoided (20% of the total).
Figure 1).
Receiver operating characteristic curve showing sensitivity versus 1 – specificity. The number next to each point represents the number of clinical predictors present, and the likelihood ratio at that cutoff value is shown in parentheses
A paired UA and urine culture from 518 patients was sent to evaluation. The sensitivity of an abnormal UA was 0.77 (95% CI, 0.66 to 0.88) and the specificity was 0.31 (95% CI, 0.2 to 0.42). Thirteen per cent of the patients had two urine cultures and 3% had three during their evaluations. For these patients, the sensitivity of the first culture was 0.98 (95% CI, 0.95 to 1).
DISCUSSION
In the present study, four easily observed clinical factors were identified that may permit a more selective approach to urine testing in young children. These predictors are clinically sensible and easy to use. Clinicians can use the information on likelihood ratios combined with the knowledge of the overall prevalence of disease in their patient populations to guide their decisions. The prevalence of UTI in children has been previously reported, but the study population, definitions of UTI, and urine collection methods have varied (5,8,9). Clinicians should consider the likelihood of UTI in a particular patient based on all of the clinical variables and then decide whether the patient has to be tested for a UTI.
Gorelick et al (10), in their study evaluating febrile young girls, identified five clinical predictors (less than 12 months old, white, temperature of 39.0°C or higher, fever for two days or more and absence of another source of fever) for UTI. In the present study we failed to show independent prediction for the duration or the importance of fever. Also, in our population (97% white) we didn’t find any predicting value for the race. Unlike Gorelick et al (10), we found a parental report of malodorous urine to be helpful.
Because the signs and symptoms of UTI are mostly non-specific or absent in young, nontoilet trained children, the decision to investigate must rely on the previous probability of UTI. Prompt recognition and treatment of children with UTI not only hasten resolution of the acute illness, but also may help to reduce the incidence of renal scarring and long term sequelae such as hypertension and renal failure associated with childhood pyelonephritis (11). Currently, there is no generally accepted strategy for screening children for UTI. Screening typically requires an invasive procedure such as urethral catheterization or suprapubic bladder aspiration (4). The prevalence of UTI is high in infants and young children 0 to two years of age who have no fever source evident from history or physical examination. Published guidelines for the management of young children with fever recommend obtaining urine culture only from boys younger than six months and from girls younger than two years with a temperature of 39.0°C or higher and no other source of fever (4,6). In our study population, following this recommendation would have led to detecting only 35% of the UTIs.
The retrospective design of our study does not allow for interpretation of why children had or did not have a urine culture. Also, the detected prevalence of UTI is an estimate of true prevalence of UTI because we used all patients with a urine culture as a denominator in this calculation regardless of whether the patient had an alternative source of fever. To be useful, predictors have to be validated prospectively in a different population. Such validation is crucial because predictors generally fail to perform as well when subsequently applied to different patients (12). The usual reason for this failure to validate is overfitting of a multiple regression model (10,13). Overfitting is minimized when the ratio of outcomes to predictors in the model is at least 10:1 (14). In our study we had 55 outcomes for four predictors.
Our study has some potential for work-up selection bias because the decision to obtain a urine specimen for culture was made by the care provider. Therefore, we may underestimate the prevalence of UTI because specimens were not obtained and cultured from all eligible patients. Such selection bias can affect the predictive ability of clinical variables. Overall, if the population selected for testing is at higher risk for the disease, the resulting study population will tend to have a higher prevalence of the disease, and both the true and false positive rates would be higher, leading to an overestimate of the sensitivity and an underestimate of the specificity. Studies trying to establish a clinical decision rule usually used unexplained fever as an eligibility criteria (10). Our prevalence rates and recommendations are based on one sample of children presenting to a university affiliated hospital and may not be generalized to all patient populations and clinical practices. The prevalence of circumcision is very low compared with other North American population. This may explain the high prevalence of UTI in boys in our study and should be taken into account before generalizing to population with higher rates of circumcision. In addition, the patient histories and physical examinations used in the analysis of data were performed by physicians with different specialty training and clinical experience. This variety of expertise likely reflects the true spectrum of practitioners evaluating children at risk for UTI.
CONCLUSIONS
The prevalence of UTI varies with age, sex, WBC count, specific symptoms and the presence of an alternative source of fever. An incremental increase in risk for UTI is associated with younger age (younger than six months), having a WBC count higher than 15,000/mm3, parental report of malodorous or foul smelling urine and the absence of an alternative source of fever in children suspected of having a UTI. In our patient population, a strategy of obtaining urine culture if any one of the four clinical factors are present would have resulted in the identification of 98% of affected children and the elimination of the need for substantial proportions of unnecessary tests.
REFERENCES
- 1.Hellstrom A, Hanson E, Hansson S, et al. Association between urinary symptoms at 7 years old and previous urinary tract infection. Arch Dis Child. 1991;66:232–4. doi: 10.1136/adc.66.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw KN, Gorelick MH. Urinary tract infection in the pediatric patient. Pediatr Clin North Am. 1999;46:1111–24. doi: 10.1016/s0031-3955(05)70177-2. [DOI] [PubMed] [Google Scholar]
- 3.Marild S, Jodal U. Incidence rate of first-time symptomatic urinary tract infection in children under 6 years of age. Acta Paediatr. 1998;87:549–52. doi: 10.1080/08035259850158272. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics, Committee on Quality Improvement, Subcommittee on Urinary Tract Infection The Diagnosis, Treatment, and Evaluation of the Initial Urinary Tract Infection in Febrile Infants and Young Children (SV9830) Pediatrics. 1999;103:843–52. doi: 10.1542/peds.103.4.843. [DOI] [PubMed] [Google Scholar]
- 5.Hoberman A, Chao HP, Keller DM, et al. Prevalence of urinary tract infection in febrile infants. J Pediatr. 1993;123:17–23. doi: 10.1016/s0022-3476(05)81531-8. [DOI] [PubMed] [Google Scholar]
- 6.Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without a source. Ann Emerg Med. 1993;22:1198–210. doi: 10.1016/s0196-0644(05)80991-6. [DOI] [PubMed] [Google Scholar]
- 7.Hellerstein S. Recurrent urinary tract infections in children. Pediatr Infect Dis. 1982;1:271–81. doi: 10.1097/00006454-198207000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics. 1998;102:e16. doi: 10.1542/peds.102.2.e16. [DOI] [PubMed] [Google Scholar]
- 9.Ansari BM, Jewkes F, Davies SG. Urinary tract infection in children, part I: Epidemiology, natural history, diagnosis and management. J Infect. 1995;30:3–6. doi: 10.1016/s0163-4453(95)92613-5. [DOI] [PubMed] [Google Scholar]
- 10.Gorelick MH, Shaw KN. Clinical decision rule to identify febrile young girls at risk for urinary tract infection. Arch Pediatr Adolesc Med. 2000;154:389–90. doi: 10.1001/archpedi.154.4.386. [DOI] [PubMed] [Google Scholar]
- 11.Hellerstein S. Urinary tract infections: Old and new concepts. Pediatr Clin North Am. 1995;42:1433–57. doi: 10.1016/s0031-3955(16)40092-1. [DOI] [PubMed] [Google Scholar]
- 12.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules: A review and suggested modifications of methodological standards. JAMA. 1997;277:488–94. [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: Advantages, problems, and suggested solutions. Cancer Treat Rep. 1985;69:1071–7. [PubMed] [Google Scholar]
- 14.Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118:201–10. doi: 10.7326/0003-4819-118-3-199302010-00009. [DOI] [PubMed] [Google Scholar]