Abstract
The mechanical effects of varying the depth of cement penetration in the cement-bone interface was investigated using finite element analysis (FEA) and validated using companion experimental data. Two FEA models of the cement-bone interface were created from microcomputed tomography data and the penetration of cement into the bone was varied over six levels each. The FEA models, consisting of the interdigitated cement-bone constructs with friction between cement and bone, were loaded to failure in tension and in shear. The cement and bone elements had provision for crack formation due to excessive stress. The interfacial strength showed a strong relationship with the average interdigitation (r2=0.97 and r2=0.93 in tension and shear, respectively). Also, the interface strength was strongly related with the contact area (r2=0.98 and r2=0.95 in tension and shear, respectively). The FEA results compared favorably to the stiffness-strength relationships determined experimentally. Overall, the cement-bone interface was 2.5 times stronger in shear than in tension and 1.15 times stiffer in tension than in shear, independent of the average interdigitation. More cracks occurred in the cement than in the bone, independent of the average interdigitation, consistent with the experimental results. In addition, more cracks were generated in shear than in tension. In conclusion, achieving and maintaining maximal infiltration of cement into the bone to obtain large interdigitation and contact area is key to optimizing the interfacial strength.
Keywords: bone, bone cement, interface, finite element, mechanics
INTRODUCTION
In cemented total hip arthroplasty, the implant needs a mechanically stable cement-bone interface for long-term survival. Because there is no adhesive bonding between bone and conventional bone cement, such as polymethylmethacrylate (PMMA), fixation relies upon cement penetration to mechanically interlock the cement into the bone lacunar and trabecular spaces (Freeman et al., 1982; Skripitz and Aspenberg, 1999; Lucksanasombool et al., 2003; Janssen et al., 2009; Goto et al., 2009).
From a surgical perspective, the depth of cement penetration into the bone is dependent on several factors, including cement viscosity (Stone et al., 1996; Race et al., 2006), bone preparation technique (Majkowski et al., 1993; Berry, 2004) and degree of cement pressurization (Oates et al., 1995; Gozzard et al., 2005). These factors, combined with the quantity and morphology of the bone, contribute to a substantial variation in mechanical properties of the cement-bone interface (Bean et al., 1987; Bugbee et al., 1992).
Although previously the strength of the cement bone interface has been investigated as a single variable (Mann et al., 1998; Mann et al., 1999), the strength of the cement-bone interface has also been related to morphologic characteristics such as cement penetration depth and cement-bone contact area. While several studies found a positive relationship between the penetration depth and the strength of the cement-bone interface (Krause et al., 1982; Askew et al., 1984; MacDonald et al., 1993; Mann et al., 1997; Graham et al., 2003), others have not found such a relationship (Majkowski et al., 1994; Miller et al., 2007). On the other hand, a strong relationship between the cement-bone contact area and the interfacial tensile strength was reported (Mann et al., 2008).
A major limitation of destructive mechanical tests such as those described above is that measurement of the failure response of a specimen to different loading directions is not possible. Because the cement-bone interface in total joint replacements is not only loaded in tension, but also in shear (Perez et al., 2006), it would be very useful to determine if strength-penetration depth relationships were the same under different loading regimes. Furthermore, the effect of penetration depth on mechanical response is confounded in experiments by the specimen-to-specimen variability. Finally, the cement penetration depth as previously been measured experimentally was often restricted to the specimen’s outer surface, while it has recently been reported that the complete interdigitated volume should be analyzed instead of focusing on the outer surface only (Waanders et al., 2009).
Micro-mechanical finite element analysis (FEA) in which the level of cement penetration is varied within a single specimen of bone allows for removal of bone morphology as a confounding variable. In this study, we developed computed tomography (CT) based micro-mechanical FEA models of the cement-bone interface, in which we only varied the penetration depth of the cement. These models had provision for failure of the cement and bone constituents via cracking of the bulk components. Using this modeling approach, we asked the following four research questions: (1) Is there a relationship between the average interdigitation of the cement, contact area between cement and bone, and the interface strength and stiffness?; (2) Is the cement-bone interface stronger in shear than in tension and does this depend on the average interdigitation?; (3) How valid are the FEA models when the mechanical responses of the different interdigitation depths of the cement-bone interface are compared with experimental findings?; (4) Do the majority of cracks occur in the bone or in the cement in these models and is this consistent with experimental results?
METHODS
FEA models were created using micro-CT scans (12μm resolution) of two physical specimens containing the cement-bone interface that were sectioned (8×4×8mm3) from laboratory prepared cemented (PMMA) total hip replacements (Waanders et al., 2009). The FEA meshes (Figure 1) included the complex morphology of the cement-bone interface and were created by meshing the bone component and cement component using a custom algorithm to recreate an accurate representation of gaps between cement and bone (Waanders et al., 2009). Based on previous micro-FEA/experimental studies (Janssen et al., 2008), contact between bone and cement was modeled using a double-sided node-to-surface contact algorithm with a friction coefficient of 0.3 (MSC.MARC 2007r1, MSC Software Corporation, Santa Ana, CA, USA).
Figure 1.
Two finite element models were generated from micro-CT scans of the cement-bone interface. These two specimens were sectioned from total hip reconstructions, which were prepared using third generation cementing techniques using PMMA in a laboratory setting. From the initial FE-model of Specimen 1 (top), five other models were generated, each with a different penetration levels (the normal distance with respect to the transverse plane between the top of the bulk of the cement and the bottom of the bone). This resulted in six models of specimen 1 with penetration levels of 0.2, 0.6, 1.0, 1.4, 1.8 and 2.2mm. Same process was done for specimen 2 (bottom), resulting in levels of 0.2, 0.5, 0.8, 1.1, 1.4 and 1.7mm. The figures on the right are section views of the specimens for each penetration level.
The baseline (as cemented) models were constructed of four-noded tetrahedral elements (Specimen 1: 462,102 elements; Speciment 2: 219,664 elements). The initial material properties of the models were considered to be linear elastic. Young’s modulus and Poisson’s ratio (ν) of the cement was set to 3,000MPa and 0.3, respectively. The bone properties were based upon micro-CT grayscale values, which were converted to equivalent HA-densities using a calibration phantom. The assumption of a linear relationship between the HA-density and the Young’s modulus (Lotz et al., 1991) resulted in Young’s moduli ranging from 0.1 to 20,000MPa for the bone (ν =0.3).
Approach to Modify Penetration Level
To simulate less cement penetration into the bone, the baseline models were modified by removing elements of cement beyond specific limits. The baseline models had maximum cement penetration levels of 2.2 and 1.7mm for specimen 1 and 2, respectively. The penetration level was defined as the normal distance with respect to the transverse plane between the top of the cement and the bottom of the bone (Figure 1). Five additional penetration levels were generated for both specimens by removing all cement elements above that particular penetration level, resulting in 12 unique FEA models (Figure 1). For each model a CT-based stereology approach was used to document the local cement interdigitation through the whole cement-bone specimen (Miller et al., 2009) (Figure 2). Subsequently, the average interdigitation was determined by averaging all the local interdigitations. The average interdigitation was subsequently used as a global measure of cement penetration (Table 1).
Figure 2.
A grid (12×6; 0.65mm spacing (Miller et al., 2009)) was constructed on the micro-CT scans and projected vertically through the image sets (a). For each vertical grid line and cement penetration level, the local cement penetration depth was measured (b), resulting in different distributions of interdigitation (c). The average of the 72 local interdigitation measurements was used as a measure of cement penetration depth.
Table 1.
Morphology measures of penetration level, average interdigitation, and interface contact area and corresponding mechanical response of the cement-bone specimens.
| Tension | Shear | ||||||
|---|---|---|---|---|---|---|---|
| Penetration level [mm] | Average interdigitation [mm] | Contact area [mm2] | Stiffness [MPa/mm] | Strength [MPa] | Stiffness [MPa/mm] | Strength [MPa] | |
| Specimen 1 | 0.2 | 0.0001 | 0.45 | 2.21 | 0.02 | 3.96 | 0.07 |
| 0.6 | 0.0072 | 4.11 | 7.33 | 0.09 | 15.10 | 0.25 | |
| 1.0 | 0.0876 | 18.29 | 35.95 | 0.47 | 60.80 | 1.83 | |
| 1.4 | 0.2529 | 46.28 | 132.43 | 1.49 | 135.30 | 4.47 | |
| 1.8 | 0.4264 | 69.71 | 158.31 | 2.04 | 143.52 | 5.16 | |
| 2.2 | 0.4824 | 80.29 | 159.06 | 2.06 | 144.50 | 5.15 | |
| Specimen 2 | 0.2 | 0.0001 | 3.78 | 3.47 | 0.07 | 5.83 | 0.19 |
| 0.5 | 0.0032 | 8.95 | 15.47 | 0.27 | 20.44 | 0.80 | |
| 0.8 | 0.0597 | 15.13 | 26.73 | 0.51 | 33.61 | 1.42 | |
| 1.1 | 0.1207 | 20.55 | 29.87 | 0.63 | 42.33 | 1.91 | |
| 1.4 | 0.1378 | 25.83 | 30.55 | 0.66 | 43.65 | 1.95 | |
| 1.7 | 0.1444 | 26.81 | 30.56 | 0.66 | 44.37 | 2.02 | |
Cement and Bone Crack Formation
Previous experimental testing to failure in shear and tension indicates that cracks form in the cement and bone when loaded to failure (Mann et al., 2008). Crack formation in the bulk bone and cement due to excessive local stresses was implemented in the models using a custom-written FEA algorithm to simulate static failure. An in-house fatigue failure algorithm (Stolk et al., 2004) was adapted such that simulation of fatigue failure was disabled, while static failure was allowed to occur. Regardless of the type of load that was applied (tensile or shear), static failure was assumed when the local principal (tensile) stress of a cement or bone element exceeded its strength. A crack was simulated by setting the Young’s modulus to 0.1MPa, perpendicular to the corresponding principal stress direction. The principal strength of the cement was set to 40MPa (Lewis, 1997; Harper and Bonfield, 2000), while the strength of the bone (S) was based on its Young’s modulus (E) and was derived from equations defined previously (Keyak et al., 2005):
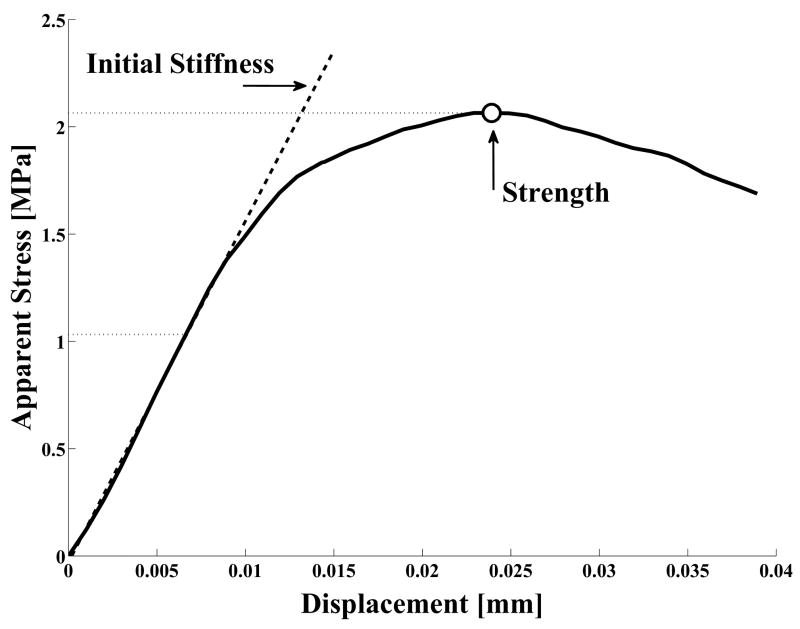
All models were loaded until failure with displacement increments of 0.001mm in shear or tension. The bottom part of the cement was fixed, while the top part of the bone was displaced uniformly such that the bone did not tilt. The resultant reaction force was calculated and the resulting apparent stress-displacement responses were determined, subsequently resulting in the apparent strength and initial stiffness, both in tension and shear (Figure 3).
Figure 3.
The stress-displacement curve of a cement-bone specimen. The strength was defined as the maximum applied load divided by the nominal cross sectional area of the cement-bone interface. The initial stiffness was determined by a least-squares fit through the stress versus displacement response for applied stress levels less than 50% of the strength (Mann et al., 1997). All cement-bone specimens were characterized by a linear slope followed by yielding till the strength was reached.
Each element had one integration point, in which three cracks could occur (one in each principal direction). Hence, the crack volume was defined as (Waanders et al., 2009):
In which ni and Vi are the number of cracks in a specific element and the element volume, respectively.
Outcome measures
As a validation, the results from the FEA simulations were compared with data obtained from experimental specimens (Mann et al., 2009; Miller et al., 2009). These cement-bone specimens were fabricated from lab-prepared cemented hip reconstructions and postmortem retrievals and were nominally the same size as the models. The acquired results comprised the apparent strength and stiffness of the specimens.
Because of interface and material discontinuities and differences in penetration levels, the apparent strain was not determined. Conversely, the stiffness was expressed as the ratio of the applied stress and the total deformation (MPa/mm) (Mann et al., 2008; Janssen et al., 2009).
The contact area, an estimation of the interfacial contact between bone and cement, was estimated for each model/penetration level. Segmentation of the specimen’s micro-CT data using MIMICS (MIMICS 11.1, Materialise, Leuven, Belgium) was followed by a dilation operation of the cement (Figure 4). Next, a Boolean intersection between the cement and bone resulted in the contact volume. This volume was subsequently divided by the dilation thickness giving the estimated contact area (Mann et al., 2008).
Figure 4.
Approach used to estimate the contact area between cement and bone (Mann et al., 2008). The micro-CT scan (a) represented the gaps and initial contact between the bone and cement. Subsequently, the micro-CT scan was segmented into two 3D objects (b): cement (grey) and bone (white). Next, the 3D cement object was dilated by two voxels (24μm) (c). The Boolean intersection between the dilated cement and bone object was calculated (d). This volume was subsequently divided by the amount of cement dilation (24μm), resulting in an estimation of the contact area between cement and bone.
Linear regression analysis was used to determine relationships between average cement interdigitation, contact area, and interface strength and stiffness, and to compare the strength in tension and shear.
RESULTS
At the greater penetration levels, specimen 1 had a much larger contact area than specimen 2 (Table 1), while at the lesser penetration levels, the contact area of specimen 2 was larger than specimen 1. Overall, there was a very strong correlation between average interdigitation and contact area for the twelve models (r2=0.99).
Very strong correlations were found between the tensile strength and the average interdigitation (r2=0.97) and the shear strength and average interdigitation (r2=0.93; Figure 5a–b). Surprising was the jump in strength specimen 2 with a small increase in average interdigitation at the lower average interdigitation level. The correlation between tensile and shear strength and the contact area was also very strong (r2=0.98 and r2=0.95, respectively; Figure 5c–d).
Figure 5.
Strong linear relationships existed between (a) the tensile strength and average interdigitation (r2=0.97) as well as between (b) shear strength and average interdigitation (r2=0.93). For specimen 2, there was a jump in strength with a small increase in the average interdigitation. The relationship between strength and contact area were also strong in tension (c) and shear (d) (r2=0.98 and r2=0.95, respectively. Note the different scales of the tensile and shear results.
A comparison between the tensile and shear results showed that the cement-bone interface was about 2.5 times stronger in shear than in tension (r2=0.98; Figure 6a).
Figure 6.
a. The bone-cement interface was 2.5 times stronger in shear than in tension, independent of the penetration depth of the cement (r2=0.98).
b. The bone-cement interface was 1.15 times stiffer in tension than in shear, independent of the penetration depth of the cement (r2=0.97).
The initial stiffness in tension and shear exhibited a similar behavior as the strength with regards to the effect of average interdigitation (Table 1). Penetration depth was less strongly correlated with tensile stiffness (r2=0.91) and shear stiffness (r2=0.89) than with contact area (r2=0.93 and r2=0.91, respectively). The cement-bone interface was 1.15 times stiffer in tension than in shear (r2=0.98; Figure 6b).
For all models, there was a strong stiffness-strength relationship in tension (r2=0.97) and shear (r2=0.98). The FEA results compared favorably to the experimental stiffness-strength relationships of the lab-prepared and post-mortem specimens; all FEA results presented here fell within the distribution of the experimental data (Figure 7).
Figure 7.
Strength-stiffness relationships for tensile and shear loading. Lab-prepared specimens were loaded to failure in tension (Mann et al., 2008) and shear, while the post-mortem retrievals were loaded to failure only in tension (Miller et al., 2009). For the strength-stiffness relation in tension (a.), it is noted that even the higher penetrated models of specimen 2 have a strength-stiffness relation that corresponds with post-mortem interfaces. Like the strength-stiffness relation in tension, the strength-stiffness relation in shear compared satisfactorily with the experimental findings (b.).
At the point of structural failure of the models, more cracks occurred in the cement than in the bone (Figure 8a–b). Also, more cracks occurred in shear than in tension. In shear, the amount of bone cracks of specimen 1 and bone and cement cracks of specimen 2 did not increase beyond a penetration depth of 1.4mm and 1.1mm, respectively. All cracks occurred in the interdigitated area (Figure 9).
Figure 8.
a. Crack volume of the cement and bone for specimen 1 and 2 when the specimen’s strength was reached in tension.
b. Crack volume of the cement and bone for specimen 1 and 2 when the specimen’s strength was reached in shear.
Figure 9.
Crack patterns for a cross-section of specimen 1 loaded in tension when the apparent strength was reached. Although the figure only shows one specific cross-section of the interface, it can be seen that the cement in the 1.4mm penetration level envelops several bony spurs which increases the average interdigitation and subsequently the apparent strength (Figure 4a–b). In shear, cracks generally at the same locations, but progressed in a different direction compared to the ‘tensile cracks’. For all penetration levels and loading directions, all cracks were in the interdigitated area of the cement-bone interface and did not progress into the bulk of the bone or cement.
DISCUSSION
In this study, we investigated the difference in mechanical behavior of the cement-bone interface in response to tension and shear loading as a result of different cement penetration depths in a single bone morphology. The results show that the strength and stiffness of the cement-bone interface are linearly dependent on the average interdigitation and the contact area between the bone and cement, for both tensile and shear loading conditions. The cement-bone interface is 2.5 times stronger, but less stiff in shear than in tension, independent on the average interdigitation. As a validation, the FEA results were compared with experimental tests using post-mortem and lab-prepared cement-bone specimens. The FEA results compared favorably with these experiments. Finally, the majority of cracks occur in the cement which is consistent with what was found experimentally (Mann et al., 2008).
Our study was limited by the fact that the models of specimens with lower penetration depths may not have represented the actual physical morphology of that penetration depth. Due to the modeling approach of the different penetration depths, small cement fragments might have been present that enveloped bony spurs. It is unlikely that these situations occur physiologically.
The results of our analyses indicate that the strength of the cement-bone interface in the two models did not exceed 6MPa (Figure 5), while cracks occurred mainly in the cement, which had a tensile strength of 40MPa. This indicates that, although on an apparent level the applied loads were rather low, they had a substantial effect on the local stress distribution in the cement and bone.
As a validation, a direct comparison between the models and experiments is not possible because the cement penetration was varied numerically and failure was simulated in two loading directions. Therefore, we compared the FEA-simulation strength-stiffness relationships with experimental specimens that comprised a broad range of interdigitation levels. This approach provides a comparison between the models and experiments in terms of pre-yield (stiffness) and yielding behavior (strength). The crack patterns from the FE-simulations could also not be compared on a one-to-one basis. However, the finding that more cracks were present in the cement than the bone and all cracks occurred in the interdigitated region in these simulations is fully consistent to what has been reported for experimental specimens (Mann et al., 2008). Further, micromechanical FEA-simulations of the cement-bone interface with fatigue loading resulted in similar crack patterns to those found experimentally (Waanders et al., 2009).
Previously, it has been reported that the strength of the cement-bone interface does not increase when the cement penetration exceeded 3mm and 4mm (Majkowski et al., 1994; Askew et al., 1984). This finding could not be affirmed, since the models as used in this study had relative low penetration levels, also compared with previous specimens (2.59±0.85mm) (Waanders et al., 2009).
From previous experimental studies, an increase of penetration depth has been associated with an increase in strength of the cement-bone interface (Krause et al., 1982; Askew et al., 1984; MacDonald et al., 1993; Mann et al., 1997; Graham et al., 2003). A likely confounding factor in these studies was that during the fabrication of the cement-bone specimens, the bone was not warmed to body temperature what does not represent the operative situation (although MacDonald did an in-vivo study). The difference in temperature gradient would alter the polymerization front of the curing cement and could affect the cement-bone morphology. On the other hand, studies that did not find a relationship between penetration depth and strength (Majkowski et al., 1994; Miller et al., 2007) did warm up the bone to body temperature. This is not consistent with the current study, since our results show a strong relationship while keeping the bone at body temperature during specimen generation (Mann et al., 2008). However, the method used to measure penetration depth was different for the present study where the complete volume was sampled instead of measuring depth on a single exterior surface.
The results of the current study, in which the bone morphology was constant for each specimen, showed a strong relationship between the average interdigitation and tensile strength. However, in addition to the experimental literature detailed above, previous experiments of lab-prepared cement-bone specimens (Mann et al., 2008) showed no correlation (r2=0.06) between stereology based measures of average interdigitation and tensile strength (unpublished data from co-author). This suggests that bone morphology plays an important role in the interface strength and one could expect a wide variety of interface strengths for the same average interdigitation in the in vivo environment. This finding is consistent with micro-CT based measurements of 21 different experimental specimens (Mann et al., 2008); there was a poor correlation (r2=0.06) between penetration depth, measured as the maximum distance between the bone and cement, and tensile strength of the cement-bone interface. The very high correlation between contact area and interface strength found for the models performed here has also been noted in experimental studies (Mann et al., 2008) of cement-bone specimens loaded to failure. Combining the FEA-findings with previous experimental tests suggests that achieving a maximal infiltration between the bone and cement is essential for increasing the interfacial strength. This suggests that efforts to maximize and maintain apposition between the cement and bone would be beneficial for implant fixation.
A large average interdigitation and contact area in trabecular bone can be achieved by preparing the bone with pulsatile lavage to allow for cement infiltration, but will be more difficult to achieve in cortical bone. Therefore, it might be beneficial to brush the cortical bone before cement insertion to increase the cement-bone contact area. The contact area can also be enlarged by reducing interfacial gaps, which can be obtained by reducing polymerization shrinkage and air and fluid inclusions (Wang et al., 1999; Race et al., 2005).
Although the results of this study showed that the strength and stiffness of the cement-bone interface increased linearly with the average interdigitation, using excessive pressurization to obtain a large interdigitation may be deleterious for the surgical patient. Over pressurizing of the femoral canal can lead to a fat and bone-marrow embolism syndrome, which can cause complications and can sometimes even be fatal (Pitto et al., 1999; Sierra et al., 2009). Secondly, as noted in several laboratory studies, excessive pressurization to achieve a large penetration has limited value (Askew et al., 1984; Majkowski et al., 1994).
An obvious limitation of this study was that that only the direct post-operative situation was considered. Over the long term, bone resorption may occur at the interface, which considerably weakens the interface (Miller et al., 2009). From that point of view it might be advantageous to use a larger penetration depth, so that in the long term a large contact area can still be achieved to distribute the loads over the cement-bone interface.
In conclusion: (1) There are very strong positive relationships between the average interdigitation depth of the cement-bone interface and the strength and stiffness as well as the contact area and strength and stiffness. It is likely that this relationship depends on the morphology of the bone.; (2) The cement-bone interface is stronger in shear than in tension, independent of the average interdigitation.; (3) The stiffness-strength relationships of the FEA simulations compared satisfactorily with experimental results.; (4) Upon structural failure of the cement-bone interface, the majority of cracks occurred in the cement.
Acknowledgments
This work was funded by the NIH grant AR42017.
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have financial or personal relationships with other people or organizations that could inappropriately influence or bias the currently presented work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askew MJ, Steege JW, Lewis JL, Ranieri JR, Wixson RL. Effect of cement pressure and bone strength on polymethylmethacrylate fixation. J Orthop Res. 1984;1:412–420. doi: 10.1002/jor.1100010410. [DOI] [PubMed] [Google Scholar]
- Bean DJ, Convery FR, Woo SL, Lieber RL. Regional variation in shear strength of the bone-polymethylmethacrylate interface. J Arthroplasty. 1987;2:293–298. doi: 10.1016/s0883-5403(87)80062-1. [DOI] [PubMed] [Google Scholar]
- Berry DJ. Cemented femoral stems: what matters most. J Arthroplasty. 2004;19:83–84. doi: 10.1016/j.arth.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Bugbee WD, Barrera DL, Lee AC, Convery FR. Variations in shear strength of the bone-cement interface in the proximal femur. Trans Orthop Res Soc. 1992;17:22. [Google Scholar]
- Freeman MA, Bradley GW, Revell PA. Observations upon the interface between bone and polymethylmethacrylate cement. J Bone Joint Surg Br. 1982;64:489–493. doi: 10.1302/0301-620X.64B4.7096429. [DOI] [PubMed] [Google Scholar]
- Goto K, Kawanabe K, Kowalski R, Baker D, Nakamura T. Bonding ability evaluation of bone cement on the cortical surface of rabbit’s tibia. J Mater Sci Mater Med. 2009 doi: 10.1007/s10856-009-3861-7. in press. [DOI] [PubMed] [Google Scholar]
- Gozzard C, Gheduzzi S, Miles AW, Learmonth ID. An in-vitro investigation into the cement pressurization achieved during insertion of four different femoral stems. Proc Inst Mech Eng [H] 2005;219:407–413. doi: 10.1243/095441105X34400. [DOI] [PubMed] [Google Scholar]
- Graham J, Ries M, Pruitt L. Effect of bone porosity on the mechanical integrity of the bone-cement interface. J Bone Joint Surg Am. 2003;85-A:1901–1908. doi: 10.2106/00004623-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Harper EJ, Bonfield W. Tensile characteristics of ten commercial acrylic bone cements. J Biomed Mater Res. 2000;53:605–616. doi: 10.1002/1097-4636(200009)53:5<605::aid-jbm22>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Janssen D, Mann KA, Verdonschot N. Micro-mechanical modeling of the cement-bone interface: The effect of friction, morphology and material properties on the micromechanical response. J Biomech. 2008;41:3158–3163. doi: 10.1016/j.jbiomech.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D, Mann KA, Verdonschot N. Finite element simulation of cement-bone interface micromechanics: a comparison to experimental results. J Orthop Res. 2009;27:1312–1318. doi: 10.1002/jor.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyak JH, Kaneko TS, Tehranzadeh J, Skinner HB. Predicting proximal femoral strength using structural engineering models. Clin Orthop Relat Res. 2005;437:219–228. doi: 10.1097/01.blo.0000164400.37905.22. [DOI] [PubMed] [Google Scholar]
- Krause WR, Krug W, Miller J. Strength of the cement-bone interface. Clin Orthop Relat Res. 1982;163:290–299. [PubMed] [Google Scholar]
- Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38:155–182. doi: 10.1002/(sici)1097-4636(199722)38:2<155::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Gerhart TN, Hayes WC. Mechanical properties of metaphyseal bone in the proximal femur. J Biomech. 1991;24:317–329. doi: 10.1016/0021-9290(91)90350-v. [DOI] [PubMed] [Google Scholar]
- Lucksanasombool P, Higgs WA, Ignat M, Higgs RJ, Swain MV. Comparison of failure characteristics of a range of cancellous bone-bone cement composites. J Biomed Mater Res A. 2003;64:93–104. doi: 10.1002/jbm.a.10361. [DOI] [PubMed] [Google Scholar]
- MacDonald W, Swarts E, Beaver R. Penetration and shear strength of cement-bone interfaces in vivo. Clin Orthop Relat Res. 1993;286:283–288. [PubMed] [Google Scholar]
- Majkowski RS, Bannister GC, Miles AW. The effect of bleeding on the cement-bone interface. An experimental study. Clin Orthop Relat Res. 1994;299:293–297. [PubMed] [Google Scholar]
- Majkowski RS, Miles AW, Bannister GC, Perkins J, Taylor GJ. Bone surface preparation in cemented joint replacement. J Bone Joint Surg Br. 1993;75:459–463. doi: 10.1302/0301-620X.75B3.8496223. [DOI] [PubMed] [Google Scholar]
- Mann KA, Allen MJ, Ayers DC. Pre-yield and post-yield shear behavior of the cement-bone interface. J Orthop Res. 1998;16:370–378. doi: 10.1002/jor.1100160314. [DOI] [PubMed] [Google Scholar]
- Mann KA, Ayers DC, Werner FW, Nicoletta RJ, Fortino MD. Tensile strength of the cement-bone interface depends on the amount of bone interdigitated with PMMA cement. J Biomech. 1997;30:339–346. doi: 10.1016/s0021-9290(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Cleary RJ, Janssen D, Verdonschot N. Experimental micromechanics of the cement-bone interface. J Orthop Res. 2008;26:872–879. doi: 10.1002/jor.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Miller MA, Race A, Verdonschot N. Shear fatigue micromechanics of the cement-bone interface: An in vitro study using digital image correlation techniques. J Orthop Res. 2009;27:340–346. doi: 10.1002/jor.20777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KA, Werner FW, Ayers DC. Mechanical strength of the cement-bone interface is greater in shear than in tension. J Biomech. 1999;32:1251–1254. doi: 10.1016/s0021-9290(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Miller MA, Eberhardt AW, Cleary RJ, Verdonschot N, Mann KA. Micro-mechanics of Post-mortem Retrieved Cement-Bone Interface. J Orthop Res. 2009 doi: 10.1002/jor.20893. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Race A, Gupta S, Higham P, Clarke MT, Mann KA. The role of cement viscosity on cement-bone apposition and strength: an in vitro model with medullary bleeding. J Arthroplasty. 2007;22:109–116. doi: 10.1016/j.arth.2006.02.076. [DOI] [PubMed] [Google Scholar]
- Oates KM, Barrera DL, Tucker WN, Chau CC, Bugbee WD, Convery FR. In vivo effect of pressurization of polymethyl methacrylate bone-cement. Biomechanical and histologic analysis. J Arthroplasty. 1995;10:373–381. doi: 10.1016/s0883-5403(05)80188-3. [DOI] [PubMed] [Google Scholar]
- Perez MA, Garcia-Aznar JM, Doblare M, Seral B, Seral F. A comparative FEA of the debonding process in different concepts of cemented hip implants. Med Eng Phys. 2006;28:525–533. doi: 10.1016/j.medengphy.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Pitto RP, Koessler M, Kuehle JW. Comparison of fixation of the femoral component without cement and fixation with use of a bone-vacuum cementing technique for the prevention of fat embolism during total hip arthroplasty. A prospective, randomized clinical trial. J Bone Joint Surg Am. 1999;81:831–843. doi: 10.2106/00004623-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Race A, Miller MA, Clarke MT, Mann KA. Cement-implant interface gaps explain the poor results of CMW3 for femoral stem fixation: A cadaver study of migration, fatigue and mantle morphology. Acta Orthop. 2005;76:679–687. doi: 10.1080/17453670510041763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race A, Miller MA, Clarke MT, Mann KA, Higham PA. The effect of low-viscosity cement on mantle morphology and femoral stem micromotion: a cadaver model with simulated blood flow. Acta Orthop. 2006;77:607–616. doi: 10.1080/17453670610012683. [DOI] [PubMed] [Google Scholar]
- Sierra RJ, Timperley JA, Gie GA. Contemporary cementing technique and mortality during and after exeter total hip arthroplasty. J Arthroplasty. 2009;24:325–332. doi: 10.1016/j.arth.2008.01.301. [DOI] [PubMed] [Google Scholar]
- Skripitz R, Aspenberg P. Attachment of PMMA cement to bone: force measurements in rats. Biomaterials. 1999;20:351–356. doi: 10.1016/s0142-9612(98)00175-6. [DOI] [PubMed] [Google Scholar]
- Stolk J, Verdonschot N, Murphy BP, Prendergast PJ, Huiskes R. Finite element simulation of anisotropic damage accumulation and creep in acrylic bone cement. Engineering Fracture Mechanics. 2004;71:513–528. [Google Scholar]
- Stone JJ, Rand JA, Chiu EK, Grabowski JJ, An KN. Cement viscosity affects the bone-cement interface in total hip arthroplasty. J Orthop Res. 1996;14:834–837. doi: 10.1002/jor.1100140523. [DOI] [PubMed] [Google Scholar]
- Waanders D, Janssen D, Miller MA, Mann KA, Verdonschot N. Fatigue creep damage at the cement-bone interface: An experimental and a micro-mechanical finite element study. J Biomech. 2009 doi: 10.1016/j.jbiomech.2009.07.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, Franzen H, Lidgren L. Interface gap after implantation of a cemented femoral stem in pigs. Acta Orthop Scand. 1999;70:234–239. doi: 10.3109/17453679908997799. [DOI] [PubMed] [Google Scholar]