Abstract

A synthetic procedure towards 1,3-diazepane scaffolds of natural product-like complexity was developed for the construction of RNA-directed ligand libraries. A molecular building block was designed that combines the characteristics of RNA-binding natural products, including a high density of hydrogen bond donors and acceptors around a rigid, non-planar scaffold with straightforward total-synthetic accessibility that permits extensive control over the chemical space. The synthesis of the 1,3-diazepane scaffold was achieved via an unprecedented cyanamide-induced rearrangement of epoxy-δ-lactams.
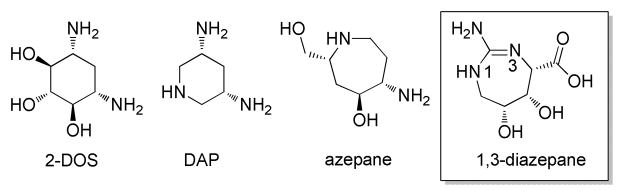
Seven-membered rings are largely unexplored in RNA-binding ligands despite the fact that they provide attractive scaffolds for the assembly of RNA-directed functional groups within a rigid non-planar framework of high atom economy. The structural variety of natural product antibiotics that target the bacterial ribosomal RNA (rRNA), and thereby interfere with protein synthesis, has inspired synthetic approaches towards novel RNA binders, however, with emphasis on five- and six-membered rings, which are found in oxazolidinones, tetracyclins and aminoglycosides, as well as macrocycles, which occur in macrolides and their congeners.1,2,3 Dissection of the molecular properties that predispose antibacterial translation inhibitors for binding to RNA folds suggest that a dense arrangement of hydrogen bond donors and acceptors at the periphery of non-planar and rigid molecular scaffolds provides the basis for both target affinity and selectivity in these natural products.4 A prime example for a privileged scaffold for RNA recognition is the 2-deoxystreptamine ring (2-DOS) found in the aminoglycoside antibiotics (Figure 1).1,5
Figure 1.
Scaffolds for RNA recognition and the novel 1,3-diazepane building block for the synthesis of RNA-targeted ligands. 2-Deoxystreptamine (2-DOS) is the pharmacophore of many natural aminoglycoside antibiotics.5 3,5-Diaminopiperidine (DAP) has been designed as a 2-DOS mimetic.6 The azepane scaffold has been investigated as a replacement for 2-DOS in synthetic azepane-glycoside antibacterials.7 In this communication, we describe the synthesis of 1,3-diazepane scaffolds, including the carboxylic acid shown here, for the construction of RNA-targeting small molecule libraries.
While natural RNA-binding antibiotics often violate rules for drug-like molecules,8 their structural complexity reflects an exquisite optimization of functional group density and arrangement for selective interaction with the RNA target. The structural complexity of the natural products is often limiting access to the chemical space around RNA-targeting scaffolds in antibiotics. For practical and economical reasons, semi-synthetic procedures starting from the natural products dominate the medicinal chemistry of ribosome-directed antibiotics and thus bias the chemical diversity of RNA-binding ligands.9 Novel chemical classes of RNA binders may emerge from the screening of synthetic compounds and from de novo or structure-guided ligand design. For example, the 3,5-diaminopiperidine ring (DAP) has been described as a structural mimetic of the RNA-recognizing pharmacophore of the 2-DOS scaffold (Figure 1).6 For RNA-directed small molecules that contain a seven-membered heterocycle as a central motif, only few examples have been described, including micromolar binders of the ribosomal decoding site which were discovered by screening or structure-guided design (Figure 1).7,10,11 Seven-membered rings, however, are conspicuously absent from a recently reported fragment library directed at RNA targets.12
Here, we report a novel synthetic approach to substituted 1,3-diazepane scaffolds of natural product-like complexity for the construction of small molecules directed at RNA targets (Figure 1). We sought to develop a versatile molecular building block that combines the characteristics of RNA-binding natural products, including a high density of hydrogen bond donors and acceptors around a rigid, non-planar scaffold with straightforward total-synthetic accessibility that permits extensive control over the chemical space around the central scaffold. Ideally this would be achieved within a compact molecular architecture of high atom economy. This communication describes the synthesis of such building blocks via an unprecedented cyanamide-induced rearrangement of epoxy-δ-lactams (6, 7), which are readily prepared from commercially available sugars (Scheme 1).
Scheme 1.
Preparation of epoxy-δ-lactams 6 and 7.
The starting material for the rearrangement, silyl-protected epoxy-δ-lactams 6 and 7, was prepared from D-ribono-1,4-lactone (1) through bromination13 and epoxide formation,14 followed by opening of the epoxy-γ-lactones and cyclization to the lactams which were then silyl protected.15 Bromination of 1 and epoxidation furnished two diastereomers13 which were carried forward as a mixture. Silyl protection gave the less polar diastereomers 6 and 7 which were readily separated by flash chromatography.
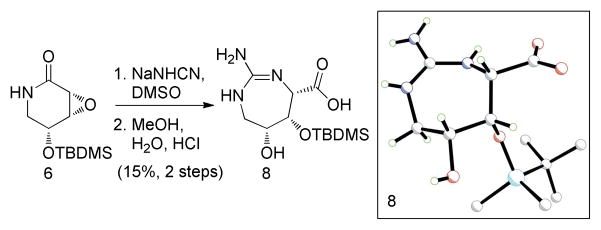
Treatment of the silyl-protected epoxy-δ-lactam 6 with sodium cyanamide in dry DMSO for 24 h at room temperature followed by addition of aqueous methanol and acidic workup furnished the highly substituted 1,3-diazepane carboxylic acid 8 as a major product (Scheme 2 and Supporting Information).
Scheme 2.
One pot synthesis of the silyl-protected 1,3-diazepane carboxylic acid 8.
To gain insight into this intriguing synthetic transformation, which formally corresponds to an insertion of the cyanamide nitrile group into the δ-lactam, we investigated the mechanism and intermediates of the reaction (Figure 2). When a solution of 6 and sodium cyanamide in dry DMSO was quenched with aqueous acid after 24 h at room temperature, a mixture of three compounds, 9, 10 and 11, was isolated with the cyanamides being the major products (7:10 ratio of 9:11). Eventually, a modified procedure was devised to produce 10 as the major product of the reaction of 6 with cyanamide (see Supporting Information). The intermediates 9, 10 and 11 were sufficiently stable to allow for their purification and spectroscopic characterization at room temperature (Supplementary Figure 1 and Supporting Information). Crystallization and X-ray structure determination succeeded for 9 and 10, which established their constitution unequivocally (Figure 2). Compound 11 was isolated as a green oil that resisted crystallization.
Figure 2.
Proposed mechanism for the formation of the 1,3-diazepane carboxylic acid 8 from epoxy-δ-lactam 6. Crystal structures are shown for the isolated key intermediates 9 and 10 (boxed). All intermediates, except 14, have been isolated and characterized by MS and NMR spectroscopy. For clarity, the corresponding bases (deprotonated forms) are shown for compounds 8, 9, 10, 11 and 13. Hydrogen atoms for the TBDMS protection group are omitted in the crystal structures. The conformation of 11 (dashed box) is proposed on the basis of sterical considerations.
We suggest that, in the absence of a proton source that would stabilize the end products, the equilibration of 9 to 10 and 11 is slow. The crystal structure of 9 reveals the bulky TBDMS group in an axial conformation which fixes the neighboring alkoxide and cyanamide substituents in a trans-equatorial orientation with the nitrile functionality directed away from the alkoxide nucleophile and disfavoring the cyclization towards the oxazole 10 (route 1 in Figure 2). Migration of the axial TBDMS group to the equatorial neighboring alcohol, which leads to the interconversion of 9 to 11, seems to be slow as well (route 2 in Figure 2). Treatment of pure 11 with aqueous hydroxide for 45 minutes followed by quenching with acid (see Supporting Information) furnished the regioisomer 9, through re-equilibration, along with the oxazoles 12 and 13.
While the alkoxide in 11 adopts an unfavorable orientation for an intramolecular attack, the carbonyl oxygen of the lactam is in an optimal position for a nucleophilic addition at the nitrile of the cyanamide group. The ensuing ring closure to the amino-oxazole 12 might be facilitated by concurrent deprotonation of the lactam nitrogen when aqueous hydroxide is added to the reaction (step b in Scheme 2). Following cyclization, a hydroxide nucleophile adds to the electrophilic carbon center in 12 which furnishes the nitrogen analog of an orthoester (13). The intermediate 13 undergoes further rearrangement to the 1,3-diazepane final product 8, perhaps via the bicyclo[3.2.1] derivative 14, which we have not been able to isolate. The intermediates 12 and 13 could not be crystallized but were isolated and are supported by spectroscopic evidence (see Supporting Information).
The proposed mechanism of the rearrangement suggested that instead of aqueous hydroxide other nucleophiles and proton sources might be added after the reaction with cyanamide to furnish synthetically useful derivatives of 8. Indeed, when the isolated intermediate 9, or a mixture of the regioisomers 9 and 11, was treated with sodium methoxide in methanol the 1,3-diazepane methylester 15 was obtained in good yield (Scheme 3 and Supporting Information). Though the methyl ester could also be formed in the one-pot version of the transformation, by adding methoxide to the reaction of 6 with cyanamide (compare Scheme 2 and Supporting Information), purification of the product from DMSO was difficult due to facile hydrolysis of the ester to the carboxylic acid 8 and better yields are obtained in the two-step route via the isolation of intermediate 9 (Scheme 3).
Scheme 3.

Synthesis of the 1,3-diazepane methyl ester 15.
When higher alcohols and alkoxides were used in the reaction with cyanamide 9 the esters were not obtained but racemisation occurred, furnishing a diastereomer (17) of the oxazole 10 as the major product (Supplementary Figure 3). We speculate that the sterical requirements for higher alkoxides hinder the nucleophilic attack on 12 which may favor base-catalyzed epimerisation of the cyanamide or another intermediate. In contrast, the oxazole diastereomer 17 was isolated only as a minor side product from the reaction with methoxide.
The methyl ester 15 is a useful building block that contains orthogonal functional groups for the construction of RNA-targeted compound libraries (Figure 3). The 1,3-diazepane is an ideal scaffold for the rigid spatial orientation of hydrogen bonding groups and substituents for interactions with RNA targets. A cluster of hydrogen bond donors on the heterocycle provides an interface for molecular recognition of unpaired bases in RNA loops and bulges4,16 while the carboxylic acid and hydroxyl groups provide handles for the attachment of additional RNA-interacting moieties. The distinct reactivity of the functional groups in the ester 15 allows straightforward derivatization towards RNA-targeted libraries (Figure 3). Substitution at the carboxylic acid (R1) followed by substitution at the free alcohol (R2) and final removal of the silyl protection group will provide entry into a wide chemical diversity space around the diazepane scaffold. We are currently exploring the synthesis of such libraries.
Figure 3.
The methyl ester 15 is a versatile advanced intermediate for the synthesis of RNA-targeted compound libraries. The structure of the 1,3-diazepane ring (bottom, from the crystal structure of 8) highlights the clustering of hydrogen bond donors and acceptors on one face of the seven-membered scaffold as well as the spatial separation of the attachment points for R1 and R2 as handles for additional RNA-interacting substituents.
In summary, we have developed an effective synthetic approach towards substituted 1,3-diazepane scaffolds of natural product-like complexity for the construction of RNA-directed small molecule libraries. While the one pot procedure described here leads from a readily accessible epoxy-δ-lactam, via an unprecedented rearrangement, directly to the 1,3-diazepane in 15% yield, improved syntheses for the preparation of the diazepanes can be envisaged. For example, isolation of the cyanamide intermediate 9 followed by reaction with a nucleophile (as outlined in Scheme 3) gave increased yields over the one pot procedure. The novel 1,3-diazepane building block contains a surface made from a cluster of hydrogen bond donors and acceptors in an ideal orientation for interaction with RNA as well as spatially separated handles for further synthetic diversification. The facile synthesis of substituted diazepane scaffolds described here is a first step towards unlocking the potential of rigid seven-membered heterocycles as frameworks of high atom economy for RNA targeting by small molecule ligands.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health, grants AI72012 and CA132753.
Footnotes
Supporting Information Available: Experimental procedures for transformations and spectroscopic as well as crystallographic data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hermann T. Curr Opin Struct Biol. 2005;15:355. doi: 10.1016/j.sbi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Poehlsgaard J, Douthwaite S. Nat Rev Microbiol. 2005;3:870. doi: 10.1038/nrmicro1265. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JR, Hergenrother PJ. Chem Rev. 2008;108:1171. doi: 10.1021/cr0681546. [DOI] [PubMed] [Google Scholar]
- 4.Hermann T. Angew Chem Int Ed Engl. 2000;39:1890. doi: 10.1002/1521-3773(20000602)39:11<1890::aid-anie1890>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Busscher GF, Rutjes FP, van Delft FL. Chem Rev. 2005;105:775. doi: 10.1021/cr0404085. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Gregor VE, Sun Z, Ayida BK, Winters GC, Murphy D, Simonsen KB, Vourloumis D, Fish S, Froehlich JM, Wall D, Hermann T. Antimicrob Agents Chermother. 2005;49:4942. doi: 10.1128/AAC.49.12.4942-4949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barluenga S, Simonsen KB, Littlefield ES, Ayida BK, Vourloumis D, Winters GC, Takahashi M, Shandrick S, Zhao Q, Han Q, Hermann T. Bioorg Med Chem Lett. 2004;14:713. doi: 10.1016/j.bmcl.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Foloppe N, Matassova N, Aboul-Ela F. Drug Discov Today. 2006;11:1019. doi: 10.1016/j.drudis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Hermann T. Biopolymers. 2003;70:4. doi: 10.1002/bip.10410. [DOI] [PubMed] [Google Scholar]
- 10.Foloppe N, Chen IJ, Davis B, Hold A, Morley D, Howes R. Bioorg Med Chem. 2004;12:935. doi: 10.1016/j.bmc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Maddaford SP, Motamed M, Turner KB, Choi MS, Ramnauth J, Rakhit S, Hudkins RR, Fabris D, Johnson PE. Bioorg Med Chem Lett. 2004;14:5987. doi: 10.1016/j.bmcl.2004.09.088. [DOI] [PubMed] [Google Scholar]
- 12.Bodoor K, Boyapati V, Gopu V, Boisdore M, Allam K, Miller J, Trealeaven WD, Weldeghiorghis T, Aboul-ela F. J Med Chem. 2009;52:3753. doi: 10.1021/jm9000659. [DOI] [PubMed] [Google Scholar]
- 13.Bock K, Lundt I, Pedersen C. Carbohydrate Res. 1981;90:17. [Google Scholar]
- 14.Bols M, Lundt I. Acta Chem Scand. 1990;44:252. [Google Scholar]
- 15.Jespersen TM, Bols M, Sierks MR, Skrydstrup T. Tetrahedron. 1994;50:13449. [Google Scholar]
- 16.Hermann T, Patel DJ. Structure. 2000;8:R47. doi: 10.1016/s0969-2126(00)00110-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.