Abstract
We have recently shown that deletion of constitutively expressed CYP1B1 is associated with attenuation of retinal endothelial cell (EC) capillary morphogenesis (CM) in vitro and angiogenesis in vivo. This was largely caused by increased intracellular oxidative stress and increased production of thrombospondin-2, an endogenous inhibitor of angiogenesis. Here, we demonstrate that endothelium nitric oxide synthase (eNOS) expression is dramatically decreased in the ECs prepared from retina, lung, heart, and aorta of CYP1B1-deficient (CYP1B1−/−) mice compared with wild-type (CYP1B1+/+) mice. The eNOS expression was also decreased in retinal vasculature of CYP1B1−/− mice. Inhibition of eNOS activity in cultured CYP1B1+/+ retinal ECs blocked CM and was concomitant with increased oxidative stress, like in CYP1B1−/− retinal ECs. In addition, expression of eNOS in CYP1B1−/− retinal ECs or their incubation with a nitric oxide (NO) donor enhanced NO levels, lowered oxidative stress, and improved cell migration and CM. Inhibition of CYP1B1 activity in the CYP1B1+/+ retinal ECs resulted in reduced NO levels and attenuation of CM. In contrast, expression of CYP1B1 increased NO levels and enhanced CM of CYP1B1−/− retinal ECs. Furthermore, attenuation of CYP1B1 expression with small interfering RNA proportionally lowered eNOS expression and NO levels in wild-type cells. Together, our results link CYP1B1 metabolism in retinal ECs with sustained eNOS activity and NO synthesis and/or bioavailability and low oxidative stress and thrombospondin-2 expression. Thus CYP1B1 and eNOS cooperate in different ways to lower oxidative stress and thereby to promote CM in vitro and angiogenesis in vivo.
Keywords: angiogenesis, retinal endothelial cells, oxidative stress, reactive oxygen species, thrombospondin-2
cytochrome P-450 (CYP) enzymes are expressed in vascular smooth muscle cells and endothelial cells (ECs) of various vascular beds. These enzymes utilize endogenous substrates, such as arachidonic acid, to generate intracellular second messengers with important roles in vascular function. Most studied CYPs with vascular function belong to the CYP4A, 2C, and 2J families (18). CYP4A enzymes generate 20-hydroxyeicosatetraenoic acid, a vasoconstrictor important in control of myogenic tone. In contrast, 2C and 2J epoxygenases generate epoxyeicosatrienoic acids (EETs) with vasodilatory, anti-inflammatory, and proangiogenic activities (18). 20-Hydroxyeicosatetraenoic acid protects pulmonary artery smooth muscle cells from proapoptotic stimulus and exhibits proangiogenic activity (59). An important role for CYP2C-derived EETs in angiogenesis of retinal ECs has been recently demonstrated (36). Bovine retinal ECs express CYP2C protein in culture and generate EETs. However, exposure to hypoxia resulted in enhanced CYP2C expression, EET production, and enhanced migration and capillary morphogenesis (CM) of retinal ECs. Thus hypoxia-mediated retinal angiogenesis may be dependent on CYP2C expression and production of EETs.
Recent studies conducted in our laboratory indicate that CYP1B1 is expressed in retinal ECs and plays a key role in regulating EC adhesion and migration in vitro and angiogenesis in vivo (54). CYP1B1 deficiency in mice resulted in attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy (OIR). In addition, retinal ECs prepared from CYP1B1−/− mice were less migratory, less adhesive to matrix proteins, and failed to undergo CM compared with CYP1B1+/+ retinal ECs. These defects were mainly attributed to the increased intracellular oxidative stress in the absence of CYP1B1. Attenuation of this oxidative stress by lowering oxygen levels (2%) or addition of N-acetylcysteine (NAC) reversed the effects of CYP1B1 deletion on EC function. Our data indicated that increased production of thrombospondin-2 (TSP2) under oxidative stress, at least in part, mediates these changes in EC adhesion and migration.
CYP1B1 is an unusual member of the CYP family as evidenced by the simple 2 intron gene structure, the highly extended 3.5-kbp 3′-UTR, and a promoter that is rich in GC islands (47, 52, 65). CYP1B1 is constitutively expressed in many hormonal responsive epithelia and in stromal cells, including fibroblasts (48) and vascular cells (15). CYP1B1 exhibits both hormonal regulation by cAMP (66) and induction through the aryl hydrocarbon receptor (48, 65). CYP1B1 ocular expression has also been implicated in the development of the trabecular meshwork, which determines flow of aqueous humor through the anterior chamber (7, 33). Most humans that are deficient in CYP1B1 develop early-onset glaucoma through aberrant development of the trabecular meshwork (3, 51). Similar defects have been shown in the eyes of CYP1B1−/− mouse, particularly when enhanced by tyrosinase deficiency (33).
These functions of CYPs overlap with regulation provided by endothelial nitric oxide synthase (eNOS), the predominant NOS expressed in EC (50). The formation of nitric oxide (NO) from l-arginine activates soluble guanylyl cyclase and initiates various signaling cascades, including the activation of mitogen-activated protein kinases (44). These roles of NO, along with its classical function as an inducer of vasodilatation, are thought to contribute to the migration and growth of ECs necessary for initiation of angiogenesis in vivo (21, 39, 41, 64). NO also is an effective anti-oxidant through the reaction with superoxide to form peroxynitrite (23), but this product has distinct activities (20). NO also inhibits CYP reactions through complex formation with the heme group (24, 37). In addition, inactivation of eNOS under oxidative stress conditions results in decreased NO bioavailability and increased levels of superoxide anions, resulting in EC dysfunction (60). Adenoviral infection of rats with CYP4A2 increases 20-hydroxyarachidonic acid production, which leads to decreased NO production and/or bioavailability and increased blood pressure (58). This cross talk between NO formed from eNOS and CYP metabolites, particularly those formed from arachidonic acid, affects angiogenesis in vivo and EC adhesion and migration in vitro through mechanisms that require further delineation.
Here, we demonstrate that CYP1B1 deficiency leads to a significant decrease in eNOS expression in cultured ECs from several tissues and in the retinal vasculature in vivo. We showed that inhibition of eNOS activity in CYP1B1+/+ retinal ECs resulted in increased oxidative stress and attenuation of CM. We hypothesized that reduced levels of eNOS in CYP1B1−/− retinal EC impacts their ability to undergo CM. In support of our hypothesis, we demonstrated that elevation of NO levels, using a NO donor or expression of eNOS, reduced oxidative stress and improved CM of CYP1B1−/− retinal ECs. Although the change in eNOS expression was not reproduced by acute expression of CYP1B1 in null cells or inhibition of CYP1B1 activity in wild-type cells, we observed a significant effect on NO levels. The stimulation of TSP2 expression in CYP1B1+/+ ECs was also unaffected by acute inhibition of CYP1B1 but, unlike eNOS, was partially reversed by restoration of CYP1B1 in null cells. These experiments, therefore, provide evidence that multiple changes occur during chronic downregulation of CYP1B1 that selectively affect NO synthesis and/or bioavailability and is associated with decreased expression of eNOS and increased production of TSP2. This notion was supported by recapitulation of null phenotype in CYP1B1+/+ cells, which stably expressed CYP1B1-specific small interfering RNAs (siRNAs). Together, our data describe overlapping CYP1B1 and eNOS effects on CM that are linked, in part, through their ability to enhance NO production and/or bioavailability and relieve oxidative stress. Thus coordinated expression of CYP1B1 and eNOS plays an important role in modulation of EC oxidative state and functions in vitro and angiogenesis in vivo.
MATERIALS AND METHODS
Cell lines.
Retinal, heart, lung, and aortic ECs were isolated from immorto-CYP1B1+/+ and -CYP1B1−/− mice as previously described (53) and cultured in DMEM containing 10% fetal bovine serum, 2 mM l-glutamine, 2 mM sodium pyrovate, 20 mM HEPES, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 U/ml penicillin (Invitrogen, Carlsbad, CA), freshly added heparin at 55 U/ml, endothelial growth supplement at 100 μg/ml (Sigma, St. Louis, MO), and murine recombinant interferon-γ (R&D, Minneapolis, MN) at 44 U/ml. For all experiments, cells were maintained at 33°C unless stated otherwise, with 5% CO2, propagated on 1% gelatin-coated 60-mm dishes, and used between passages 8 and 24 with minimal effect on experimental results.
Western blot analysis.
For cell lysates, cells were rinsed with PBS (Sigma) once and lysed with lysis buffer (10 mM Tris · HCl, pH 7.6, 1 mM EDTA, 1% Triton X-100, 1% Nonidet P-40, 0.1% SDS). For retina lysates, retinas were dissected out and placed in the lysis buffer (50 μl/retina). Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Samples (20 μg) were mixed with appropriate volumes of 6× SDS buffer and analyzed by 4–20% SDS-PAGE (Invitrogen). Proteins were transferred to nitrocellulose membrane and blocked in 20 mM Tri · HCl, pH 7.6, and 150 mM NaCl with 0.1% Tween 20 (TBST) containing 3% BSA and 3% nondairy creamer for 1 h at room temperature. After the blocking procedure was completed, blots were incubated with appropriate dilutions of primary antibodies prepared in TBST, washed with TBST, incubated with appropriate secondary antibody prepared in TBST, washed with TBST, and developed using enhanced chemiluminescence (Amersham, Piscataway, NJ). For reprobing, blots were washed once with TBST and stripped by two washes with 25 ml of prewarmed stripping solution (62.5 mM Tris · HCl, pH 6.8; 2% SDS; 100 mM β-mercaptoethanol) at 65°C. Blots were then washed twice with TBST (5 min each) at room temperature before proceeding with blotting as described above. The antibody to CYP1B1, developed in rabbits, reacts specifically with mouse CYP1B1. Western blot quantification was performed by calculating intensity of each band relative to control using Image J software (National Institutes of Health, Bethesda, MD).
Capillary morphogenesis.
Matrigel (10 mg/ml; BD Biosciences) was applied at 0.5 ml/35-mm tissue culture dish and incubated at 37°C for at least 30 min to allow hardening. Cells were removed from tissue culture dishes by trypsin-EDTA, washed once with growth medium, and resuspended at 1.5 × 105 cells per ml in growth medium. Cells (2 ml) were gently added to the Matrigel-coated plates, incubated at 37°C in a tissue culture incubator, monitored for 6–24 h, and photographed with a Nikon microscope in digital format. For quantitative assessment of the data, the mean number of branches, at points of intercepts per five representative high-power fields (×100) was determined.
Cell migration assays.
For scratch wound assays, cells (2 × 105) were plated on 60-mm tissue culture dishes and allowed to reach confluence (2–3 days). After we aspirated the medium, cell layers were wounded using a 1-ml micropipette tip. Plates were then rinsed with PBS, fed with growth medium containing 150 ng/ml of 5-fluorouracil to inhibit cell proliferation. This eliminates the potential contribution of differences in the rate of proliferation. We monitored wound closure with still photography in a digital format at 0, 24, 48, and 72 h after wounding. For quantitative assessment of the data, the percentage of wound closure, relative to time 0, was determined with Axio vision software (Zeiss).
Adenovirus infection of retinal EC.
Adenoviruses encoding a form of eNOS bearing a mutation of a key phosphorylation site serine 1179 to an aspartate residue (Ad-eNOS S1179D) were provided by Dr. William C. Sessa (Yale University, New Haven, CT). The phosphorylation of serine 1179 normally increases the basal synthesis of NO. The substitution of aspartate for serine mimics the negative charge imparted by the phosphate and renders eNOS constitutively active through increased rate of electron flux through the protein and increases basal NO production by severalfold (64). Adenoviruses expressing mouse CYP1B1 were prepared as previously described (53). Viruses were amplified in HEK 293 cells, tittered using a cytopathic effect assay (13), and stored in PBS containing 10% glycerol, 0.5 mM MgCl2, and 0.5 mM CaCl2. Retinal ECs were plated in 60-mm dishes and were 80–90% confluent by the next day. Cells were incubated with either recombinant adenovirus (10–30 pfu/cell) expressing the constitutively active eNOS (Ad-eNOS S1179D), CYP1B1 (Ad-CYP1B1), or vector control (Ad-GFP) in 1.5 ml of Opti-MEM (Invitrogen) with 10 μl of Lipofectin (Invitrogen) for 5 h in a tissue culture incubator. After incubation, cells were fed with EC growth medium and incubated for 2 days. The eNOS and CYP1B1 levels were determined by Western blot analysis of the cell lysates as described above.
CYP1B1 siRNA infection of retinal EC.
CYP1B1-specific siRNAs were prepared as previously described (54) (Table 1). Briefly, the mouse specific cDNA sequences were scanned for CYP1B1 for putative target sequences for siRNA using the siDESIGN center (www.dharmacon.com). The identified sequences were blasted against the mouse genome database to exclude sequences that match nonspecific mRNA. An oligonucleotide containing the 19-base specific sequence (Table 1), its reverse complementary sequence, a 9-base spacer sequence, and terminal sequences for ligation to the BglII and HindIII restriction sites were synthesized and cloned into pSUPER-retro vector digested with same enzymes (Invitrogen). The retroviral packaging cell line Phi-NX (Nolan Lab, Stanford, CA) was transfected with 5 μg of pSUPER-retro control siRNA vector or vector containing CYP1B1-specific siRNA inserts using Lipofectin (Invitrogen). The cell medium, which contained retroviral particles, was harvested and filtered through a 0.45-μm filter 48 h after transfection. Filtered virus-containing medium (1 ml) was added to retinal ECs (1 × 106 cells per 60-mm dish) in the presence of polybrene (4 μg/ml). The next day, cells were fed with fresh medium containing puromycin (2 μg/ml) and expanded. Stable cell populations expressing a specific siRNA or control vector were then evaluated for their ability to undergo CM or expression of eNOS and TSP2 as outlined above. In some experiments, retinal ECs were incubated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; an inducer of CYP1B1 at 10−7 M for 24 h) to enhance the levels of CYP1B1 expression for the evaluation of siRNA impact on CYP1B1 and eNOS expression.
Table 1.
CYP1B1 siRNAs
| Start | Region | siRNA Target Sequence |
|---|---|---|
| 1625 | ORF | CCAGTGGTCTGTGAATCAT |
| 1768 | ORF | TCGGTGAGGAACTGTCTAA |
| 2015 | 3′-UTR | AGGAATACCTATCTCATTA |
| 3271 | 3′-UTR | ACCTAACCTTTGCCTAAGA |
| Control | TTCTCCGAACGTGTCACGT |
siRNA, small interfering RNA.
Indirect immunofluorescence analysis.
Cells (2 × 104) were plated on glass coverslips and allowed to reach confluence. Cells were washed in PBS and then fixed with 4% paraformaldehyde containing 0.1% Triton X-100 for 15 min. Once fixed, cells were washed with PBS and incubated with antibody to eNOS (1:500 Santa Cruz) prepared in TBS with 1% ovalbumin at 37°C for 30 min. After they were washed with TBS, the coverslip was incubated with a 1:500 dilution of fluorescein isothiocyanate-conjugated appropriate secondary antibody (Pierce) in TBS with 1% ovalbumin at 37°C for 30 min. Cells were viewed on a Zeiss Axiophot fluorescence microscope and photographed in a digital format (Carl Zeiss, Chester, VA).
Immunohistochemical staining of eye frozen sections.
The immunostaining of eye frozen section was performed as previously describes (62). Rabbit anti-eNOS (Santa Cruz) and rat anti-endoglin (BD Biosciences) were used at 1:500 dilution prepared in blocking solution. Retina sections were viewed by fluorescence microscopy, and images were captured in digital format with a Zeiss microscope (Carl Zeiss).
Determination of reactive oxygen species in EC.
The level of cellular reactive oxygen species (ROS) was assessed using dihydroethidium (Molecular Probes, Eugene, OR) as previously described (54). Briefly, cells were plated on gelatin-coated coverslips at 1 × 105 cells/ml. After attachment, cells, with or without preincubation with nitro-l-arginine methyl ester (l-NAME) or nitro-d-arginine methyl ester (d-NAME) (3 mM) for 6 h, were rinsed twice with HBSS, and incubated with 5 μM dihydroethidium for 15 min at 37°C in 5% CO2. At the end of the incubation, cells were rinsed with HBSS and examined alive by fluorescence microscopy, and images were captured in digital format using a Zeiss microscope (Carl Zeiss). For quantitative assessments, the images were analyzed with Image J software (National Institutes of Health). Values were obtained from mean fluorescent intensity between CYP1B1+/+, CYP1B1+/+ with l-NAME or d-NAME, and CYP1B1−/− ECs from multiple cells captured within each section.
NO determinations.
The NO levels in the CYP1B1+/+, CYP1B1−/−, and CYP1B1+/+ siRNA retinal ECs were measured by using DAF-FM diacetate (Invitrogen). DAF-FM diacetate is cell permeable and essentially nonfluorescent until it reacts with NO and other NO derivatives such as N2O3 produced by autooxidation of NO to yield highly fluorescent benzotriazole. Briefly, 104 cells in 0.1 ml were plated in 96-well black/clear bottom plates (BD Falcon, Franklin Lakes, NJ) coated with 1% gelatin. The next day, cells were washed twice with serum-free EC growth medium and then incubated in serum-free EC growth medium containing 10 μM DAF-FM diacetate and 1.25 ng/ml of CellTracker red CMTPX (Invitrogen) for 45 min at 37°C. After incubation, the medium was gently removed, and 0.1 ml of fresh serum-free EC growth medium was added, and cells were incubated for an additional 20 min to allow complete de-esterification of the intracellular diacetates. Cells were then washed three times with TBS, 0.1 ml of TBS was added to each well, and DAF-FM fluorescence was measured using a fluorescent microplate reader (Victa2 1420 Multilabel Counter, Perkin Elmer) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Cell density was followed by CellTracker red CMTPX at an excitation wavelength of 545 nm and an emission wavelength of 610 nm. Images were captured using a Nikon RM-5 camera attached to an inverted fluorescent microscope (Axiovert 135, Zeiss). Measurements were repeated three times with three replicates each time and normalized for cell number. Adenovirus infections were performed in the 96-well plates as described above for 2 days before NO analysis. NO measurements were similarly performed in retinal ECs incubated with tetramethoxystilbene (TMS; 5 μM), NAC (1 mM), or diethylenetriamine-NO (DETA/NO; 500 μM) for 2 h before DAF-FM diacetate loading.
Statistical analysis.
Statistical differences between control and treated samples were evaluated with Student's unpaired t-test (2-tailed) or two-way ANOVA with Bonferroni correction for multiple comparisons when appropriate. Means ± SD are shown. P values ≤0.05 were considered significant.
RESULTS
Decreased expression of eNOS in CYP1B1−/− retinal vasculature and EC.
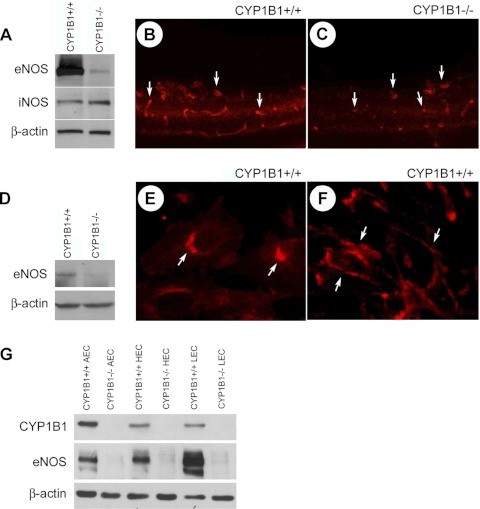
Figure 1A shows eNOS levels in lysates of retinal ECs prepared from CYP1B1+/+ and CYP1B1−/− mice. We observed near complete loss of eNOS protein in each of several CYP1B1−/− retinal EC strains. Immunofluorescence staining of frozen eye sections also showed much lower expression of eNOS in retinal vasculature of CYP1B1−/− mice compared with CYP1B1+/+ mice (Fig. 1, B and C). This was further confirmed by a substantial decrease in the level of eNOS observed in retinal lysates prepared from CYP1B1−/− mice compared with CYP1B1+/+ mice (Fig. 1D). CYP1B1+/+ and CYP1B1−/− retinal ECs expressed similar levels of iNOS (Fig. 1A), whereas nNOS expression was undetectable (not shown).
Fig. 1.
Decreased eNOS expression in the absence of CYP1B1. A: lysates prepared from CYP1B1+/+ and CYP1B1−/− retinal endothelial cells (ECs) were analyzed for eNOS and iNOS expression by Western blotting. Antibody to β-catenin was used as loading control. Please note a significant decrease in eNOS levels in retinal ECs from CYP1B1−/− mice. Frozen eye sections prepared from p17 CYP1B1+/+ (B) and CYP1B1−/− mice (C) during oxygen-induced ischemic retinopathy (OIR) were also stained with antibodies to eNOS. Eye sections were stained, and images were obtained under identical conditions. Please note the marked decrease in fluorescence staining of eNOS in CYP1B1−/− retinal vasculature compared with CYP1B1+/+ retinal vasculature. D: Western blot analysis of whole retinal extracts prepared from p21 CYP1B1+/+ and CYP1B1−/− mice. Antibody to β-actin was used as loading control. Please note a significant decrease in eNOS level in retina extracts from CYP1B1−/− mice compared with CYP1B1+/+ mice. These experiments were repeated twice with eyes from 4 different mice with similar results. E and F: eNOS localization in CYP1B1+/+ retinal ECs at subconfluent (E) and confluent (F) states. Cells were grown on glass coverslips and stained for eNOS as described in materials and methods. Please note cellular localization of eNOS (arrows), which changes with degree of confluence. Comparisons of CYP1B1 and eNOS expression in CYP1B1+/+ and CYP1B1−/− ECs from aorta, heart, and lung were made by Western blot analysis of cell lysates (G). Antibody to β-actin was used as loading control. Please note a significant decrease in eNOS level in cell extracts from CYP1B1−/− ECs compared with CYP1B1+/+ ECs.
Subcellular localization of eNOS in the endothelium plays an important role in its activation and NO production (19, 22, 25). We next examined eNOS subcellular localization in CYP1B1+/+ retinal EC. The eNOS was mainly localized in perinulear/Golgi region in subconfluent cultures (Fig. 1E). However, eNOS localized in plasma membranes, at sites of cell-cell contact in confluent cultures (Fig. 1F). The eNOS staining was undetectable in cultures of CYP1B1−/− retinal ECs (not shown).
To test the generality of the CYP1B1 and eNOS relationship, we examined expression of eNOS in ECs prepared from heart, lung, and aorta of CYP1B1+/+ and CYP1B1−/− mice. The CYP1B1 was expressed in these ECs, with highest level detected in ECs from aorta. The constitutive CYP1B1 levels were comparable in heart, lung, and retina (Fig. 1G) (54). As expected, the CYP1B1−/− ECs lacked CYP1B1 expression. All of the CYP1B1+/+ ECs expressed eNOS, with highest level in lung EC (Fig. 1, A and G). The eNOS level was greatly decreased in CYP1B1−/− ECs prepared from heart, lung, and aorta compared with CYP1B1+/+ ECs (Fig. 1, A and G). Thus the decreased eNOS expression in CYP1B1−/− ECs is not specific to retinal vasculature.
The eNOS activity is essential for CM of CYP1B1+/+ retinal EC.
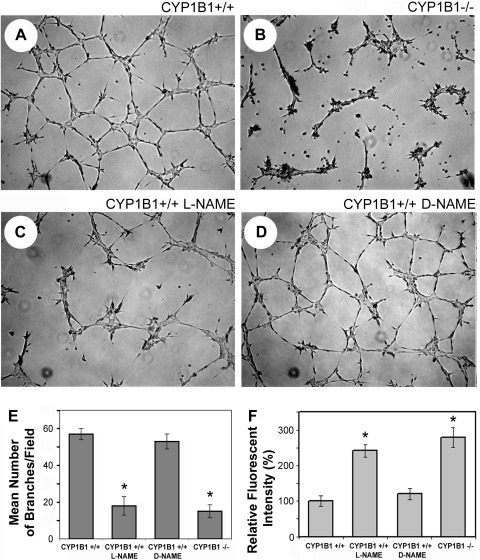
ECs rapidly organize and form a capillary-like network when plated on Matrigel. Plating of EC on this predominantly laminin extracellular matrix recapitulates the later stages of angiogenesis with minimal amounts of cell proliferation. This process, however, is influenced by the adhesion and migration characteristics of ECs. CYP1B1+/+ retinal ECs rapidly organized and formed a capillary-like network (Fig. 2A). The attenuation of CM in CYP1B1−/− retinal ECs is shown in Fig. 2B. CYP1B1−/− ECs from other tissues exhibited a similar defect in CM (not shown). Attenuation of CM has also been previously reported in eNOS-deficient ECs (31). Thus the defects in CYP1B1−/− ECs may be related to diminished eNOS acidity and/or NO synthesis and/or bioavailability.
Fig. 2.
eNOS activity is essential for capillary morphogenesis of CYP1B1+/+ retinal ECs in Matrigel. CYP1B1+/+ (A) and CYP1B1−/− (B) retinal ECs were plated in Matrigel without (A and B) or with (C) nitro-l-arginine methyl ester (l-NAME; 3 mM), an inhibitor of eNOS activity. After 17 h of incubation, CYP1B1+/+ retinal ECs formed a well-organized capillary-like network (A), whereas the ability of these cells to undergo capillary morphogenesis was attenuated upon incubation with l-NAME (C). These results are similar to what we observed in CYP1B1−/− retinal ECs, which express little or no eNOS (B). The inactive analog of eNOS inhibitor, nitro-d-arginine methyl ester (d-NAME; 3 mM), had no effect on CYP1B1+/+ retinal EC capillary morphogenesis (D). E: quantitative assessment of the data. Data in each bar are mean number of branches per 5 high-power fields (×100). Error bars indicate SD (*P < 0.05; n = 5). Reactive oxygen species (ROS) levels were determined in CYP1B1+/+ retinal ECs incubated with or without l-NAME or d-NAME as described in materials and methods (F). Please note a significant increase in oxidative stress of CYP1B1+/+ cells incubated with l-NAME compared with d-NAME, which was comparable with levels in CYP1B1−/− retinal ECs (*P < 0.05; n = 15).
We next incubated CYP1B1+/+ retinal ECs with 3 mM l-NAME, an eNOS inhibitor, to determine whether inhibition of NO synthesis and/or bioavailability is sufficient to attenuate CM. The inactive form of the inhibitor, d-NAME, was used as control. The inhibition of eNOS activity in CYP1B1+/+ retinal ECs by l-NAME resulted in attenuation of CM, similar to that observed in CYP1B1−/− retinal ECs, which express little or no eNOS (Fig. 2C). However, incubation of CYP1B1+/+ cells with the inactive analog of eNOS inhibitor (d-NAME) minimally affected CM (Fig. 2D). The quantitative assessment of the data is shown in Fig. 2E (P < 0.05; n = 5). Thus eNOS activity and NO synthesis and/or bioavailability are essential for CM of retinal ECs, as previously demonstrated for other ECs (41).
NO functions as an anti-oxidant in CYP1B1+/+ retinal EC.
Our group has previously shown that CYP1B1−/− ECs cultured under ambient oxygen (20%; hyperoxic) exhibit a substantial increase in ROS and that removal of this stress is sufficient to restore CM (54). Our group also observed a significant increase in lipidperoxide product, 4-hydroxy-2-nonenal (4-HNE), in eye sections from CYP1B1−/− mice and retinal ECs (54). 4-HNE promotes endothelial oxidative stress (55), endothelial barrier dysfunction (56), and apoptosis (32, 63). Furthermore, lipid peroxidation products increase ROS and promote eNOS uncoupling and NO production, further increasing superoxide anion radical in ECs (60). Thus 4-HNE likely increases ROS and oxidative stress in ECs by disrupting eNOS regulatory mechanisms and EC function. Alternatively, NO can react rapidly with superoxide anions to form peroxynitrate, thus interfering in the generation of hydrogen peroxide and hydroxyl radicals that initiate lipid peroxidation (16, 43). One possibility is that the very low level of eNOS in CYP1B1−/− retinal ECs is a major contributor to the chronic oxidative stress and attenuation of CM.
We next tested the effect eNOS inhibitor l-NAME on the oxidative stress in CYP1B1+/+ retinal ECs. We observed an increase in ROS similar to that observed normally in CYP1B1−/− ECs (Fig. 2F) (54). Our group has previously shown that the antioxidant NAC or low oxygen (2%) each lower ROS and restore CM of NO-deficient CYP1B1−/− retinal ECs (54). These results strongly support the notion that inhibition of ROS provides a major way in which eNOS participates in CM. Thus the attenuation of CM of CYP1B1−/− retinal ECs in Matrigel may be, in large part, due to decreased eNOS expression and NO synthesis and/or bioavailability (11). Incubation of CYP1B1−/− retinal EC with l-NAME or d-NAME had no effect on their CM in Matrigel or levels of ROS (Fig. 2F and not shown).
Improvement of CM in CYP1B1−/− retinal ECs in the presence of a NO donor.
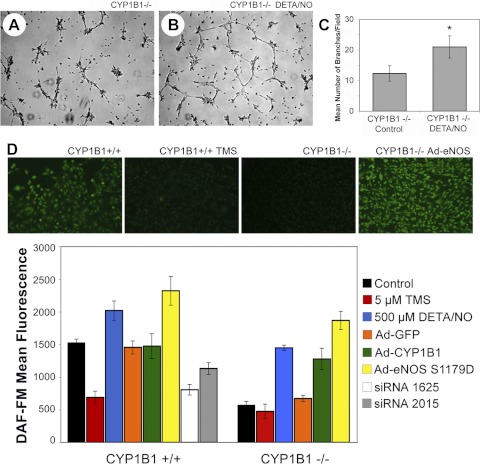
To further confirm that the attenuation of CM observed in CYP1B1−/− retinal ECs is due to lower eNOS expression and NO synthesis and/or bioavailability, we assessed the ability of the CYP1B1−/− ECs to undergo CM in the presence of the NO donor, DETA/NO. DETA/NO belongs to the class of direct NO donors. It is stable in 0.01 M NaOH and can be stored at 0°C for 24 h. In culture medium (pH 7.4), DETA/NO spontaneously releases NO with a half life of 20 h at 37°C in a strictly first-order reaction (30, 38). DETA/NO has been used by other investigators as an effective source of exogenous NO at concentrations ranging from 1 to 500 μM with minimal cytotoxicity (28, 38, 49, 61). Figure 3B shows that the ability of CYP1B1−/− retinal ECs to undergo CM in Matrigel was increased nearly twofold in the presence of 20 μM DETA/NO. The quantitative assessment of the CM is shown in Fig. 3C (P < 0.05; n = 5). CM was not significantly enhanced in the presence of higher concentrations of DETA/NO (up to 300 μM; not shown). The incubation of CYP1B1−/− retinal ECs with DETA/NO was concomitant with increased NO levels and/or bioavailability (Fig. 3D).
Fig. 3.
Restoration of capillary morphogenesis in CYP1B1−/− retinal ECs upon incubation with a nitric oxide (NO) donor, diethylenetriamine-NO (DETA/NO). CYP1B1−/− retinal ECs were preincubated with solvent control (A) or DETA/NO (20 μM; B) for 24 h, plated in Matrigel with freshly added DETA/NO (20 μM), and photographed after 17 h of incubation at 37°C. The capillary morphogenesis of CYP1B1−/− retinal ECs was significantly improved in the presence of the NO donor. C: quantitative assessment of the data. Data in each bar are mean number of branches per 5 high-power fields (×100). Error bars indicate SD (*P < 0.05; n = 5). D: NO levels were assessed in CYP1B1+/+ and CYP1B1−/− retinal ECs under various conditions as described in materials and methods. D, top: representative fluorescent images of retinal ECs incubated with DAF-FM. Please note reduced NO level in CYP1B1+/+ retinal ECs incubated with tetramethoxystilbene (TMS) and CYP1B1−/− retinal ECs. Expression of eNOS in CYP1B1−/− retinal ECs restored NO levels to near normal levels. NO levels were also assessed in retinal ECs incubated with TMS or DETA/NO or infected with viruses expressing GFP (control), CYP1B1, eNOS, or CYP1B1-specific small interfering RNAs (siRNAs). D, bottom: quantitative assessment of data. Data in each bar are mean fluorescence intensities of 3 separate experiments. Error bars indicate SD (P < 0.05 for wild-type cells expressing CYP1B1 siRNA or incubated with TMS compared with control or null cells expressing CYP1B1, eNOS, or incubated with DETA/NO compared with control).
Elevation of NO in CYP1B1−/− retinal ECs lowers ROS and restores CM.
We compared the effect of DETA/NO on ROS levels in CYP1B1−/− ECs at 2, 6, and 24 h to the effects of NAC. We found that DETA/NO was equally effective at 2 h but became less effective at later times (not shown) (54). We also compared the levels of NO in CYP1B1−/− ECs, with and without DETA/NO, to the levels seen in CYP1B1+/+ retinal ECs (Fig. 3D). Similar experiments were carried out with null retinal ECs expressing eNOS or CYP1B1 (Fig. 3D). Levels of NO were higher with the highest levels of eNOS in CYP1B1−/− ECs, and, unlike DETA/NO, they were sustained over many hours and lowered ROS in a more sustained manner (not shown). Thus the restoration of NO and lowering of oxidative stress correlated well with the restoration of CM.
Because superoxide may lower NO bioavailability, we tested whether NAC raised NO levels in CYP1B1−/− ECs in parallel with the effect on CM. We did not observe a significant increase in NO levels in CYP1B1−/− ECs incubated with NAC (not shown), although oxidative stress and TSP2 expression were reduced (54). Our results indicated that increased oxidative state is the critical negative regulator of CM in both CYP1B1+/+ ECs (parallel suppression by l-NAME /elevated O2/reversed by NAC) and CYP1B1−/− ECs (parallel restoration by DETA/NO/eNOS/NAC). However, the impact of NAC on CYP1B1−/− ECs appeared to be independent of significant changes in NO levels. This is not unexpected, since null cells express very little or no eNOS. In our previous study, we found NAC to be more effective than ascorbic acid, or vitamin E, as well as resveratrol, a phenolic antioxidant, to lower oxidative stress and TSP2 expression in CYP1B1−/− ECs (54). Ascorbic acid and vitamin E do not scavenge superoxide anions; rather, they scavenge OH radicals and lower 4-HNE levels (12). Thus our results suggest that superoxide anion is a key target for inhibition of CM and angiogenesis through upregulation of TSP2 expression and/or removal of eNOS and NO synthesis and/or bioavailability.
Reexpression of eNOS improved CM of CYP1B1−/− retinal ECs.
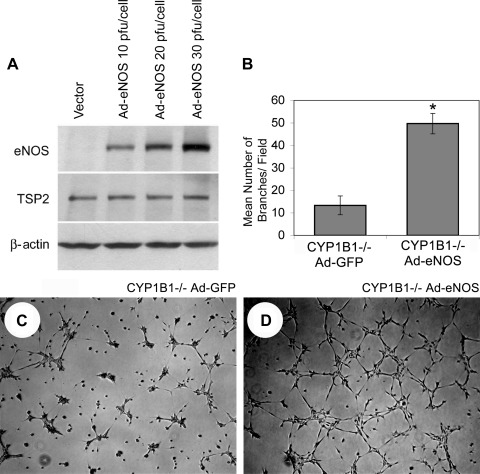
To overcome the relatively transient release of NO by DETA/NO, we infected CYP1B1−/− retinal ECs with an adenovirus that expresses a sustained level of constitutively active eNOS (Ad-eNOS S1179D) (64). We successfully expressed eNOS protein in the target cells in direct proportion to virus input level (Fig. 4A). eNOS expression in CYP1B1−/− retinal ECs substantially elevated eNOS protein and was 2.5 times more effective than DETA/NO in improving CM. The stimulated level of CM provided by reexpression of eNOS was comparable to the level seen in CYP1B1+/+ retinal ECs (compare Fig. 3, A and B, with Fig. 4, C and D). The quantitative assessment of the data is shown in Fig. 4B (P < 0.05; n = 5). The increased synthesis and/or bioavailability of NO in CYP1B1−/− cells expressing eNOS is shown in Fig. 3D.
Fig. 4.
Reexpression of eNOS restored capillary morphogenesis of CYP1B1−/− retinal ECs. A: Western blot analysis of whole cell lysates prepared from CYP1B1−/− retinal ECs infected with the adenoviruses expressing a constitutively active form of eNOS (CA-eNOS) or empty vector at different multiplicity of infection. Cells were lysed 2 days after infection, and eNOS levels were determined by Western blot. Antibody to β-actin was used to control for loading. Cells infected with control (C) or CA-eNOS (D) adenoviruses (20 pfu/cell) were plated in Matrigel. Images were obtained 17 h after incubation at 37°C. Expression of the CA-eNOS in CYP1B1−/− retinal ECs restored their ability to undergo capillary morphogenesis. B: quantitative assessment of the data. Data in each bar are mean number of branches per 5 high-power fields (×100). Error bars indicate SD (*P < 0.05; n = 5).
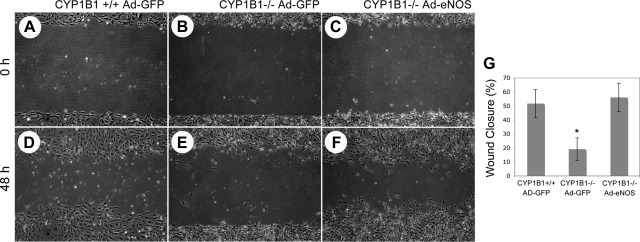
A key component activity in CM is the capacity of the cells to migrate. This was measured in the scratch wound assays in which cells migrate into a gap generated by the scratch in a confluent cell monolayer. Our group previously showed that CYP1B1−/− retinal ECs are less migratory in this assay than CYP1B1+/+ retinal ECs (54). We next determined the effects of eNOS expression on migration of CYP1B1−/− retinal ECs. Figure 5 shows that eNOS expression, which significantly restored CM, restored the migration defect observed in CYP1B1−/− cells compared with control cells. The quantitative assessment of the data is shown in Fig. 5G (P < 0.05; n = 5).
Fig. 5.
Reexpression of eNOS restored the migration of CYP1B1−/− retinal ECs. Confluent cultures of CYP1B1+/+ retinal ECs infected with control (A and D), CYP1B1−/− retinal ECs infected with control (B and E), and CYP1B1−/− retinal ECs infected with CA-eNOS (C and F) adenoviruses were wounded with a 1-ml micropipette tip 1 day after infection. Pictures were taken at 0 h (immediately after wounding) or 48 h after wounding. G: quantitative assessment of the data. Data in each bar are mean percentages of wound closed. Error bars indicate SD (*P < 0.05, n = 3). Please note that the CYP1B1−/− retinal ECs with restored eNOS expression/activity, similar to CYP1B1+/+ vector control cells, migrated significantly faster than CYP1B1−/− vector control cells.
The elevated TSP2 level in CYP1B1−/− retinal ECs is not affected by increased NO levels.
We previously showed that the extracellular matrix protein TSP2, which is essentially absent in CYP1B1+/+ ECs, becomes highly elevated in CYP1B1−/− retinal ECs and is a major contributor to suppression of CM. Here, we show that acute expression of eNOS, which restored CM, did not significantly reverse the elevated TSP2 levels in CYP1B1−/− retinal ECs (Fig. 4A). Thus our results suggest that elevated NO level, either directly or indirectly, can bypass the actions of TSP2 that suppress CM. It has been previously reported that the TSP2 closely related family member TSP1 blocks eNOS/NO effect on ECs mediated by cGMP (27). This suggests that restoration of NO level can overcome an equivalent block produced by TSP2. In addition, NAC, which lowered ROS in CYP1B1−/− ECs, also restored CM by lowering TSP2 with minimal effect on NO level (not shown) (54). Thus restoration of CM of CYP1B1−/− retinal ECs by increased NO synthesis and/or bioavailability may occur downstream of TSP2 and is independent of changes in TSP2 expression.
Acute reexpression of CYP1B1 lowered TSP2, increased NO level, and restored CM of CYP1B1−/− retinal ECs independent of changes in eNOS expression.
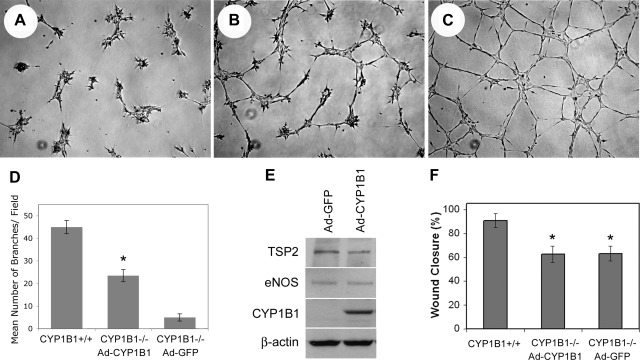
To demonstrate that CYP1B1 expression can restore CM in CYP1B1−/− retinal ECs, we expressed CYP1B1 in null retinal ECs using an adenovirus expressing murine CYP1B1 as previously described (54). Expression of CYP1B1 improved CM of CYP1B1−/− retinal ECs (Fig. 6, A–C) (54). The quantitative evaluation of the data is shown in Fig. 6D (P < 0.05; n = 5). Because TSP2 mediates CM suppression (34), we examined whether reexpression of CYP1B1 affects TSP2 expression in CYP1B1−/− retinal ECs. Figure 6E shows that reexpression of CYP1B1 was associated with a twofold decrease in TSP2 level, consistent with reduced oxidative stress and enhanced CM (Fig. 6B) (54). The acute expression of CYP1B1 in null cells, however, did not affect eNOS expression (Fig. 6E). Thus CYP1B1 promoted CM in the presence of low levels of eNOS. This may be attributed to decreased oxidative stress (54) and improved bioavailability of NO in CYP1B1-expressing null cells. We observed a significant increase in NO levels of CYP1B1−/− retinal ECs expressing CYP1B1 compared with Ad-GFP control (Fig. 3D). Thus the dramatic decrease in eNOS expression in null cells may be the result of chronic exposure to oxidative stress and decreased NO synthesis and/or bioavailability.
Fig. 6.
Reexpression of CYP1B1 in CYP1B1−/− retinal ECs restored capillary morphogenesis and lowered thrombospondin-2 (TSP2) level but did not affect eNOS level. Semiconfluent cultures of CYP1B1−/− retinal ECs were infected with control (A) or CYP1B1 expressing adenoviruses (B), and their ability to undergo capillary morphogenesis was assessed in Matrigel. C: capillary morphogenesis of CYP1B1+/+ cells. D: quantitative assessment of the date. Please note a significant enhancement of capillary morphogenesis in null cells expressing CYP1B1 (*P < 0.05; n = 5). E: Western blot of cell lysates prepared from CYP1B1 or vector control-infected cell. Please note decreased expression of TSP2 in null cells expressing CYP1B1 compared with vector control cells. No differences in the levels of eNOS were observed. β-Actin staining was used to control for loading. The effect of CYP1B1 expression on migration of null cells was assessed by scratch wound assays. F: quantitative assessment of the data. CYP1B1 expression had minimal effect on the migration of null cells. *P < 0.05; n = 5.
Selective effects of eNOS and CYP1B1 in restoring migration of CYP1B1−/− ECs.
The rate of cell migration over 48 h was measured by a scratch wound assay. We previously showed that CYP1B1−/− retinal ECs migrate at a slower rate than CYP1B1+/+ retinal ECs (Fig. 5) (54). We compared whether restoration of CYP1B1 expression would make comparable contributions to the migration of CYP1B1−/− ECs, as eNOS expression does (Fig. 5). In contrast to the stimulatory effect of eNOS, restoration of CYP1B1 was not as effective in improving the cell migration in CYP1B1−/− ECs (Fig. 6F). This may be contributed, at least in part, to the very low eNOS expression and lower NO level in CYP1B1−/− EC cells (Figs. 1 and 3). However, CYP1B1 expression did lower TSP2 expression in null cells by approximately twofold (Fig. 6E). Thus retinal EC migration may be more dependent on sufficient eNOS activity and NO synthesis and/or bioavailability than on CM, which was restored by expression of CYP1B1 in null cells. However, the significant reduction in TSP2 level by CYP1B1 expression in the null cells is likely to contribute to restoration of CM by removing the inhibitory effects of TSP2 on eNOS/NO activity. This is consistent with a higher level of NO detected in null cells expressing CYP1B1 (Fig. 3D), which may be sufficient to drive CM with minimal effect on restoration of migration. Thus a threshold level may exist for NO-mediated EC migration.
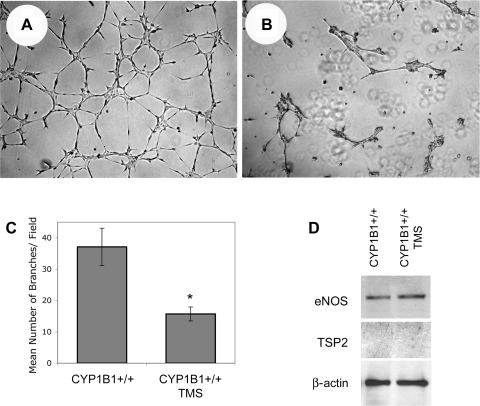
Acute TMS inhibition of CYP1B1 activity lowered NO level and blocked CM of CYP1B1+/+ retinal ECs, independent of changes in TSP2 and eNOS expression.
To provide a link between CYP1B1 activity and retinal EC CM, we incubated CYP1B1+/+ retinal ECs with 5 μM TMS (a specific inhibitor of CYP1B1 activity) (9). This concentration is sufficient to sustain inhibition of CYP1B1 activity for at least 24 h and increase oxidative stress (54). Figure 7, A and B, shows that incubation of CYP1B1+/+ retinal ECs with TMS resulted in attenuation of CM in Matrigel. The quantitative assessment of the data is shown in Fig. 7C (P < 0.05; n = 5). This inhibitory effect can be reversed with NAC, similar to CYP1B1−/− ECs (not shown) (54); therefore, it is unlikely to be a nonspecific effect of TMS.
Fig. 7.
Inhibition of CYP1B1 activity in CYP1B1+/+ retinal ECs blocked capillary morphogenesis without a significant effect on TSP2 and eNOS levels. Semiconfluent cultures of CYP1B1+/+ retinal ECs were incubated with DMSO (solvent control; A) or TMS (5 μM; B) overnight. The next day, cells were removed, and their ability to undergo capillary morphogenesis was evaluated in Matrigel as described in materials and methods (A and B). C: quantitative assessment of the data. Please note a significant decrease in capillary morphogenesis of cells incubated with TMS compared with control (*P < 0.05; n = 5). D: levels of eNOS and TSP2 were determined by Western blot analysis of cell lysates prepared from CYP1B1+/+ retinal ECs incubated with solvent control or TMS as described above. Please note TMS treatment had minimal effect on the level of eNOS or TSP2.
We next determined whether acute inhibition of CYP1B1 activity affected expression of TSP2 or eNOS in CYP1B1+/+ retinal ECs. Figure 7D shows that acute inhibition of CYP1B1 activity minimally affected the expression of TSP2 and eNOS in CYP1B1+/+ retinal ECs. However, increased oxidative stress in CYP1B1+/+ cells incubated with TMS (54) may promote eNOS uncoupling and NO synthesis and/or bioavailability without affecting eNOS or TSP2 expression. We observed a significant decrease in NO levels in CYP1B1+/+ cells incubated with TMS (Fig. 3D). Thus, although the prolonged effects of CYP1B1 depletion in the CYP1B1−/− ECs were not matched by the effects of short-term TMS inhibition on eNOS and TSP2 expression, the reduced level of NO was sufficient to inhibit CM. Therefore, suppression of eNOS and elevation of TSP2 levels each may require a long-term loss of CYP1B1 expression and a chronic oxidative stress state.
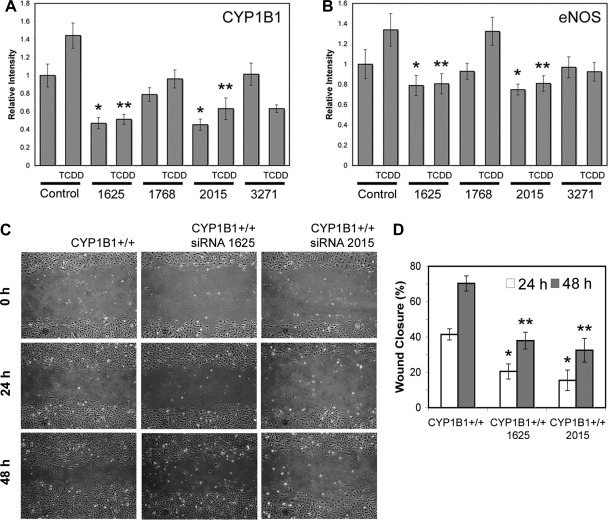
The siRNA knockdown of CYP1B1 lowered eNOS expression and NO level in CYP1B1+/+ ECs.
To determine whether a more prolonged suppression of CYP1B1 expression in CYP1B1+/+ cells lowers eNOS expression, we used CYP1B1-specific siRNAs. Stable lines were established from viruses that liberated four different siRNA sequences that targeted CYP1B1 after puromycin selection and expansion. This, therefore, may resemble the chronic effects derived from lack of CYP1B1 in null cells. Figure 8A shows that siRNA 1625 and 2015 significantly reduced CYP1B1 levels, by at least twofold, compared with a control virus that releases scrambled siRNA; siRNA 1768 was also largely ineffective. The suppression was consistently more pronounced after TCDD induction of CYP1B1 expression through Ah receptor (60–70% for all lines). We also observed consistent decreases in eNOS levels by CYP1B1-specific siRNAs and that there was a close correlation between CYP1B1 and eNOS expression (r = 0.982; P ≤ 0.001). Furthermore, knockdown of CYP1B1 in wild-type cells resulted in decreased NO level (Fig. 3D) and attenuation of cell migration (Fig. 8, C and D), as was seen in CYP1B1−/− cells (54).
Fig. 8.
Knockdown of CYP1B1 in CYP1B1+/+ retinal ECs resulted in decreased expression of eNOS and migration. Semiconfluent cultures of CYP1B1+/+ retinal ECs were infected with retroviruses encoding specific or control CYP1B1 siRNA and stable lines were established as described in materials and methods. A quantitative comparisons of Western blot of lysates prepared from CYP1B1+/+ cells expressing a specific CYP1B1 or control siRNA probed for CYP1B1 (A) or eNOS (B) are shown. Antibody to β-actin was used to control for loading. Please note significant knockdown of CYP1B1 relative to β-actin in cells expressing CYP1B1 siRNAs 1625 and 2015 (Table 1 and A) in the presence or absence of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; 10−7 M for 24 h) compared with control. A similar trend was observed when levels of eNOS were compared (B). Please note decreased levels of eNOS in cells expressing lower level of CYP1B1 (*P and **P < 0.05; n = 3). C: effect of CYP1B1 knockdown on migration of CYP1B1+/+ cells. D: quantitative assessment of data. Please note a significant decrease in migration of cells expressing siRNAs 1625 or 2015 compared with control (*P and **P < 0.05; n = 3).
DISCUSSION
CYPs catalyze NADPH-supported monooxygenation of diverse xenobiotics as well as many endogenous molecules, including polyunsaturated fatty acids, eicosanoids, and steroids (4–6, 8). Our group recently showed that CYP1B1, whose deficiency causes congenital glaucoma, is constitutively expressed in retinal ECs and plays an important role in postnatal retinal vascular development and neovascularization during OIR. Retinas from CYP1B1−/− mice exhibited reduced vascular density and failed to undergo neovascularization during OIR (54). CYP1B1 deficiency also resulted in increased oxidative stress and attenuation of retinal EC adhesion, migration, and CM in Matrigel. Here, we demonstrate that CYP1B1 and eNOS are coexpressed in retinal, heart, lung, and aortic ECs and that the eNOS expression is substantially reduced in each of these CYP1B1−/− ECs compared with CYP1B1+/+ ECs (Fig. 1). We also observed decreased eNOS staining in retinal blood vessels of CYP1B1−/− mice compared with CYP1B1+/+ mice, especially during OIR (Fig. 1, B and C). These observations are consistent with our hypothesis that decreased eNOS expression and NO synthesis and/or bioavailability in the absence of CYP1B1 contributes to the attenuation of angiogenesis both in vivo and in vitro.
The eNOS shares many enzymatic characteristics of CYPs. Recent studies also indicated that CYP2C agonists, cerivastatin and fluvastatin, induce eNOS expression in ECs (17, 26). In addition, both CYP1B1 and eNOS show increased activity in response to shear stress (10, 15, 40), suggesting the presence of common regulatory mechanisms. We observed downregulation of eNOS expression in retinas from CYP1B1−/− mice, as well as in CYP1B1−/− retinal, lung, heart, and aortic ECs. We hypothesized that eNOS expression is coregulated with that of CYP1B1. This hypothesis was further supported by the fact that eNOS expression was increased coordinately on incubation of retinal ECs with TCDD (an inducer of CYP1B1 expression). Furthermore, the knockdown of CYP1B1 level was concomitant with downregulation of eNOS levels in CYP1B1+/+ retinal ECs and reduced NO synthesis and/or bioavailability (Figs. 3D and 8, A and B). Thus a direct relationship between CYP1B1 and eNOS expression may exist in retinal endothelium with significant impact on angiogenesis.
Most ECs, including CYP1B1+/+ retinal ECs, rapidly undergo CM, forming a capillary-like network when plated in Matrigel. This recapitulates the later stages of angiogenesis when ECs organize into capillaries with minimal cell proliferation. l-NAME, an eNOS substrate l-arginine analog, inhibits eNOS activity and NO synthesis and/or bioavailability (50). Incubation of CYP1B1+/+ retinal ECs with l-NAME resulted in increased oxidative stress and attenuation of CM, very similar to that observed in CYP1B1−/− retinal ECs, which express little or no eNOS (Figs. 1 and 2). The inactive analog of l-NAME, d-NAME, had a minimal effect on CM and oxidative stress in CYP1B1+/+ retinal ECs. Thus eNOS expression and NO synthesis and/or bioavailability are essential for CM and relief of oxidative stress in retinal ECs under ambient oxygen conditions (31, 41).
Low eNOS in the CYP1B1−/− retinal ECs may lead to insufficient NO production in these cells. NO modulates blood flow and many aspects of angiogenesis, including cell migration and CM (2). The supplementation of an NO donor, DETA/NO, significantly improved CM of the CYP1B1−/− retinal ECs (Fig. 3), further supporting the important role of NO in angiogenesis. To further elucidate the role of eNOS in retinal EC angiogenic properties, we also used an adenovirus, which expresses a constitutively active form of eNOS. Re-expression of eNOS was sufficient to enhance NO synthesis and/or bioavailability, relieve oxidative stress, and promote CM and migration of CYP1B1−/− retinal ECs (Figs. 3–5).
We have shown that CYP1B1−/− retinal ECs have increased oxidative stress and fail to undergo CM under ambient oxygen (20%; hyperoxic) conditions (54). Incubation under low oxygen concentration (2%; hypoxic) or in the presence of an antioxidant (NAC) restored CM of CYP1B1−/− retinal ECs. Increased oxidative stress resulted in increased production of TSP2, a potent inhibitor of angiogenesis, and attenuation of CM. We observed a significant increase in TSP2 production in CYP1B1−/− retinal ECs, which was decreased in the presence of NAC (54). A similar result was also observed in vivo (54). Thus a direct relationship between oxidative stress and TSP2 expression may exist in the retinal endothelium, as shown for aortic ECs (34).
We showed that downregulation of TSP2 using a specific siRNA improved CM of CYP1B1−/− retinal ECs with minimal effect on their migration (54). In addition, expression of CYP1B1 improved CM of CYP1B1−/− retinal ECs. This was concomitant with decreased TSP2 expression with minimal effect on migration. Furthermore, expression of CYP1B1 decreased oxidative stress in CYP1B1−/− retinal ECs and increased NO levels independent of changes in eNOS expression (Figs. 3D and 6, E and F) (54). However, reexpression of eNOS reduced oxidative stress and restored CM and migration of CYP1B1−/− retinal ECs with minimal effects on TSP2 expression (Fig. 4). These results are consistent with the role of NO in EC migration and the possibility that TSP2 functions as an inhibitor of NO activity rather than synthesis in attenuating angiogenesis, as shown for TSP1, a closely related family member with antiangiogenic activity (27). Thus CYP1B1 expression under hyperoxic conditions promotes a proangiogenic phenotype by reducing oxidative stress and TSP2 and maintaining eNOS expression and NO synthesis and/or bioavailability (Fig. 9). However, under hypoxic conditions, the contribution of CYP1B1 to modulation of oxidative stress and NO synthesis and/or bioavailability is minimal.
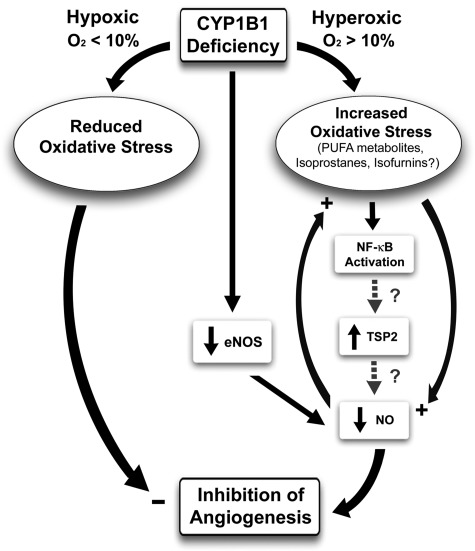
Fig. 9.
CYP1B1 and modulation of angiogenesis. In the absence of CYP1B1, especially under ambient/hyperoxic (>10%) oxygen conditions, there is an increase in oxidative stress. The identity of the CYP1B1 metabolites/substrates, whose accumulation in the absence of CYP1B1 is responsible for increased oxidative stress, remains elusive. However, there is a significant decline in eNOS expression, resulting in decreased EC migration, and inhibition of capillary morphogenesis in vitro and angiogenesis in vivo. The increase in TSP2 expression in the absence of CYP1B1 is mediated, at least in part, through increased oxidative stress. Increased oxidative stress may activate NF-κB transcription factor, which drives TSP2 expression. CYP1B1−/− retinal ECs exhibit enhanced NF-κB activity in transient transfection with NF-κB reporter plasmids compared with wild-type cells (our unpublished date). Whether increased activity of NF-κB drives expression of TSP2 is currently under investigation. Our data indicated that downregulation of TSP2 in CYP1B1−/− retinal ECs was sufficient to improve capillary morphogenesis of CYP1B1−/− retinal ECs independent of changes in eNOS expression. Although TSP1, a TSP2 closely related family member with antiangiogenic activity, is shown to inhibit NO-mediated angiogenesis, the role of TSP2 in this process needs further investigation. The lack of CYP1B1 minimally affects angiogenesis under low-oxygen (<10%; hypoxic) conditions.
Acute inhibition of CYP1B1 activity in CYP1B1+/+ retinal ECs reproduced the oxidant stress-mediated loss of CM (blocked by NAC) and decreased NO synthesis and/or bioavailability, although without changes in eNOS or TSP2 expression (Figs. 3D and 7D). These results suggest that chronic exposure to oxidative stress is essential for changes in TSP2 and eNOS expression. We have, in fact, observed a reciprocal relationship between TSP2 and eNOS expression in our CYP1B1−/− retinal EC isolations, which vary in their extent with different times in culture (not shown). Thus, in the adapted state, the substantial oxidant-sensitive elevation of TSP2 and depressed eNOS expression may be necessary to restrain CM in vitro and angiogenesis in vivo.
This notion was further supported by the suppression of eNOS expression and NO levels following chronic siRNA knockdown of CYP1B1 in wild-type cells. This involved a process of passaging and selection in vitro, which reproduces the adaptation that occurs after the initial isolation and culture of CYP1B1−/− ECs. Although we did not observe a significant difference in the level of active eNOS (phopho-eNOS) in CYP1B1+/+ cells incubated with TMS or solvent control (not shown), we cannot rule out the possibility of the adverse effects of increased ROS on NO synthesis and/or bioavailability (Fig. 3D) (11, 50), as was seen in CYP1B1+/+ cells incubated with l-NAME (Fig. 2). This could affect angiogenesis independent of changes in eNOS and TSP2 levels (Fig. 9). Thus coordinated constitutive expression and/or activities of CYP1B1 and eNOS play important roles in regulation of EC function through modulation of the intracellular oxidative state and are subject of future investigation.
The dramatic loss of eNOS in CYP1B1−/− retinal ECs removes three distinct contributions to EC regulation: 1) direct signaling mediated by NO (1, 29, 41), 2) combination with superoxide to form peroxynitrite, which produces distinct signaling events (14, 35, 42, 45, 46), and 3) intervention in oxidative stress processes mediated by hydrogen peroxide and hydroxyl radicals (35, 45, 46, 57). Thus modulation of eNOS expression and NO synthesis and/or bioavailability is an important target of CYP1B1-mediated EC function. Understanding the mechanisms involved will provide new insights into the regulatory mechanisms that keep angiogenesis in check.
In summary, we demonstrate that expression of eNOS is dramatically downregulated in the retinal ECs and retinal vasculature of CYP1B1−/− mice. Inhibition of eNOS activity and NO bioavailability in CYP1B1+/+ retinal ECs attenuated their migration and CM and increased oxidative stress. In addition, reexpression of eNOS or incubation with a NO donor improved migration and CM of CYP1B1−/− retinal ECs and reduced oxidative stress, concomitant with enhanced NO levels. Furthermore, eNOS levels directly correlated with CYP1B1 levels in CYP1B1+/+ retinal ECs. Thus coordinated CYP1B1 and eNOS constitutive expression and activity may affect retinal EC migration and angiogenesis, through modulation of intracellular oxidative stress, NO synthesis and/or bioavailability, and TSP2 expression.
GRANTS
This work was supported by National Eye Institute Grants EY-16995 and EY-18179 (N. Sheibani), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-67120 (C. M. Sorenson), National Cancer Institute Grant CA-81493 (C. R. Jefcoate), University of Wisconsin Paul P. Carbone Cancer Center Support Grants P30 CA-014520 and P30 EY-16665, and an unrestricted departmental award from Research to Prevent Blindness. N. Sheibani is a recipient of a Research Award from American Diabetes Association (1-06-RA-123) and Retina Research Foundation. Y. Tang is supported by an American Heart Association Predoctoral Fellowship (0810200Z).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, Saikia J, Kim M, Melia M, Fishman M, Huang P, Campochiaro PA. Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol 191: 116–124, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Beauchamp MH, Sennlaub F, Speranza G, Gobeil JF, Checchin D, Kermorvant-Duchemin E, Abran D, Hardy P, Lachapelle P. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med 37: 1885–1894, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bejjani BA, Stockton DW, Lewis RA, Tomey KF, Dueker DK, Jabak M, Astle WF, Lupski JR. Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus. Hum Mol Genet 9: 367–374, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya KK, Brake PB, Eltom SE, Otto SA, Jefcoate CR. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem 270: 11595–11602, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Int 72: 683–689, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development 134: 1369–1383, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Choudhary D, Jansson I, Sarfarazi M, Schenkman JB. Physiological significance and expression of P450s in the developing eye. Drug Metab Rev 38: 337–352, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32: 840–847, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chun YJ, Kim S, Kim D, Lee SK, Guengerich FP. A New selective and potent inhibitor of human cytochrome P450 1B1 and its application to antimutagenesis. Cancer Res 61: 8164–8170, 2001. [PubMed] [Google Scholar]
- 10.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai S, He Y, Zhang H, Yu L, Wan T, Xu Z, Jones D, Chen H, Min W. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol 29: 495–502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De K, Roy K, Sengupta C. Inhibition of lipid peroxidation induced by hydroxyprogesterone caproate by some conventional antioxidants in goat liver homogenates. Acta Pol Pharm 64: 201–210, 2007 [PubMed] [Google Scholar]
- 13.Ehrengruber MU, Lanzrein M, Xu Y, Jasek MC, Kantor DB, Schuman EM, Lester HA, Davidson N. Recombinant adenovirus-mediated expression in nervous system of genes coding for ion channels and other molecules involved in synaptic function. Methods Enzymol 293: 483–503, 1998 [DOI] [PubMed] [Google Scholar]
- 14.El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, Tsai N, Motamed K, Caldwell RB. Peroxynitrite mediates VEGF's angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J 21: 2528–2539, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: up-regulation by shear stress. Endothelium 11: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Fisslthaler B, Michaelis UR, Randriamboavonjy V, Busse R, Fleming I. Cytochrome P450 epoxygenases and vascular tone: novel role for HMG-CoA reductase inhibitors in the regulation of CYP 2C expression. Biochim Biophys Acta 1619: 332–339, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med 18: 20–25, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98: 2604–2609, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulton D, Babbitt R, Zoellner S, Fontana J, Acevedo L, McCabe TJ, Iwakiri Y, Sessa WC. Targeting of endothelial nitric-oxide synthase to the cytoplasmic face of the Golgi complex or plasma membrane regulates Akt- versus calcium-dependent mechanisms for nitric oxide release. J Biol Chem 279: 30349–30357, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Gao YT, Roman LJ, Martasek P, Panda SP, Ishimura Y, Masters BSS. Oxygen metabolism by endothelial nitric-oxide synthase. J Biol Chem 282: 28557–28565, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Gergel D, Misik V, Riesz P, Cederbaum AI. Inhibition of rat and human cytochrome P4502E1 catalytic activity and reactive oxygen radical formation by nitric oxide. Arch Biochem Biophys 337: 239–250, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Govers R, Bevers L, de Bree P, Rabelink TJ. Endothelial nitric oxide synthase activity is linked to its presence at cell-cell contacts. Biochem J 361: 193–201, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori Y, Nakanishi N, Akimoto K, Yoshida M, Kasai K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol 23: 176–182, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isenberg JS, Ridnour LA, Thomas DD, Wink DA, Roberts DD, Espey MG. Guanylyl cyclase-dependent chemotaxis of endothelial cells in response to nitric oxide gradients. Free Radic Biol Med 40: 1028–1033, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest 115: 1816–1827, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keefer LK, Nims RW, Davies KM, Wink DA. “NONOates” (1-substituted diazen-1-ium-1,2-diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol 268: 281–293, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC, Gimbrone MA, Jr, Ertl G, Huang PL. .Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol 286: C1195–C1202, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Sharma R, Patrick B, Sharma A, Jeyabal PV, Reddy PM, Saini MK, Dwivedi S, Dhanani S, Ansari NH, Zimniak P, Awasthi S, Awasthi YC. Regulation of CD95 (Fas) expression and Fas-mediated apoptotic signaling in HLE B-3 cells by 4-hydroxynonenal. Biochemistry (Mosc) 45: 12253–12264, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Libby RT, Smith RS, Savinova OV, Zabaleta A, Martin JE, Gonzalez FJ, John SW. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science 299: 1578–1581, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Lopes N, Gregg D, Vasudevan S, Hassanain H, Goldschmidt-Clermont P, Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol Cell Biol 23: 5401–5408, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR, Fleming I. Role of cytochrome P450 2C epoxygenases in hypoxia-induced cell migration and angiogenesis in retinal endothelial cells. Invest Ophthalmol Vis Sci 49: 1242–1247, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. J Pharmacol Exp Ther 283: 1479–1485, 1997 [PubMed] [Google Scholar]
- 38.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol 25: 674–678, 1995 [PubMed] [Google Scholar]
- 39.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res 79: 857–863, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 100: 3131–3139, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt DH, Bartoli M, El-Remessy AB, Al-Shabrawey M, Lemtalsi T, Fulton D, Caldwell RB. Peroxynitrite increases VEGF expression in vascular endothelial cells via STAT3. Free Radic Biol Med 39: 1353–1361, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30: 277–290, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol 289: L371–L381, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Pritchard KA, Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res 77: 510–518, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem 269: 14905–14911, 1994 [PubMed] [Google Scholar]
- 48.Savas U, Carstens CP, Jefcoate CR. Biological oxidations and P450 reactions. Recombinant mouse CYP1B1 expressed in Escherichia coli exhibits selective binding by polycyclic hydrocarbons and metabolism which parallels C3H10T1/2 cell microsomes, but differs from human recombinant CYP1B1. Arch Biochem Biophys 347: 181–192, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Schmidt A, Bilgasem S, Lorkowski S, Vischer P, Volker W, Breithardt G, Siegel G, Buddecke E. Exogenous nitric oxide regulates activity and synthesis of vascular endothelial nitric oxide synthase. Eur J Clin Invest 38: 476–485, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Sessa WC. Regulation of endothelial derived nitric oxide in health and disease. Mem Inst Oswaldo Cruz 100, Suppl 1: 15–18, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet 6: 641–647, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem 269: 13092–13099, 1994 [PubMed] [Google Scholar]
- 53.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113: 744–754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113: 744–754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-Hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 274: 2234–2242, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Usatyuk PV, Parinandi NL, Natarajan V. Redox regulation of 4-hydroxy-2-nonenal-mediated endothelial barrier dysfunction by focal adhesion, adherens, and tight junction proteins. J Biol Chem 281: 35554–35566, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264: 85–97, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, Laniado-Schwartzman M. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res 98: 962–969, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Tang X, Li Y, Leu C, Guo L, Zheng X, Zhu D. 20-Hydroxyeicosatetraenoic acid inhibits the apoptotic responses in pulmonary artery smooth muscle cells. Eur J Pharmacol 588: 9–17, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Whitsett J, Picklo MJ, Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol 27: 2340–2347, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Winter S, Konter J, Scheler S, Lehmann J, Fahr A. Permeability changes in response to NONOate and NONOate prodrug derived nitric oxide in a blood-brain barrier model formed by primary porcine endothelial cells. Nitric Oxide 18: 229–239, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn 235: 1908–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4–4 protects against oxidative stress and modulates iNOS expression through NF-κB translocation. Toxicol Appl Pharmacol 230: 187–196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA 102: 10999–11004, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Savas U, Alexander DL, Jefcoate CR. Characterization of the mouse Cyp1B1 gene. Identification of an enhancer region that directs aryl hydrocarbon receptor-mediated constitutive and induced expression. J Biol Chem 273: 5174–5183, 1998 [DOI] [PubMed] [Google Scholar]
- 66.Zheng W, Jefcoate CR. Steroidogenic factor-1 interacts with cAMP response element-binding protein to mediate cAMP stimulation of CYP1B1 via a far upstream enhancer. Mol Pharmacol 67: 499–512, 2005. [DOI] [PubMed] [Google Scholar]