Abstract
Objective
To assess the effect of pol replication capacity (RC) on the hazard ratio of progression to a composite endpoint of time to progression to <350 CD4+ cells/μL, initiation of therapy or death.
Methods
pol RC assays were performed after study closure in baseline samples obtained from 316 enrollees in a prospectively monitored cohort of treatment-naive adults with ≥450 CD4+ cells/μL and ≥1000 HIV-1 RNA copies/mL.
Results
The median RC was 79%. Patients with a lower RC had a lower median viral load (4.0 vs 4.2 Log HIV-1 RNA copies/mL, p=0.026) and a lower rate of protease inhibitor resistance 2% vs 8%, p=0.03). Otherwise, baseline demographic and laboratory characteristics were similar. The hazard ratio of progression to the composite endpoint was 0.73 (p=0.041) for persons with lower RC, 2.07 per 1.0 log10 higher viral load (p<0.001) and 0.86 per 50 cell/μL higher CD4+ cell count (p<0.001). The effect of lower RC was also significant in a separate analysis of time to initiation of therapy (p=0.04).
Conclusion
These results show that untreated patients with lower vs higher RC had a slower rate of progression as assessed by a composite outcome of time to CD4+ count ≤350 cells/μL, treatment initiation or death.
Keywords: disease progression, natural history, replication, HIV infections
Introduction
Recommendations regarding the timing of therapy for HIV-1 – infected patients are based primarily on CD4+ cell count measurements. Present guidelines recommend that therapy be started when the CD4+ cell count is below 350 CD4+ cells/μL. Other considerations include the rate of CD4+ cell loss, the magnitude of the viral load, and symptoms of HIV disease progression1. Notably, while the loss of CD4+ cells is largely determined by the plasma HIV-1 RNA concentration2, a recent analysis suggests that only 4 - 6% of the variability of the rate of CD4+ loss is attributable to the plasma HIV RNA level3. Other factors that influence the rate of HIV disease progression and CD4+ loss include patient age, HIV-directed immune responses, diminished CD8+ T-cell activation, host HLA genotype and chemokine receptor polymorphisms, as well as characteristics of viral isolates such as deletions in nef, syncitium formation (or the related property of co-receptor tropism) and replication capacity4-16.
Impairment of HIV-1 replication as determined by a single cycle assay that assesses the replication of a recombinant virus created with plasma HIV-1 derived pol genes (i.e., pol replication capacity) has been independently associated with a slower rate of CD4+ decline and disease progression in a cohort of chronically infected young hemophiliac patients, many of whom received prior therapy with nucleoside reverse transcriptase inhibitors17. In addition, impaired pol replication capacity has been reported to be associated with lower viral loads and higher CD4 counts in untreated patients during the first year of infection as well as in the aforementioned population of chronically infected, hemophiliac patients14;17. The goal of this study was to evaluate the relationship between HIV-1 pol replication capacity and disease progression in a large, demographically diverse cohort of chronically infected, treatment-naïve adults with early stage HIV disease.
Methods
Patient population
Inclusion criteria for enrollment in the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) Long Term Monitoring (LTM) treatment naïve cohort required that patients be HIV-infected, be ≥ 13 years old, provide written informed consent, and be antiretroviral-naïve, defined as having no previous use of protease inhibitor or non-nucleoside reverse transcriptase inhibitors or having received ≤1 week of treatment with lamivudine and ≤4 weeks of treatment with other nucleoside reverse transcriptase inhibitors. Patients were also required to have a life expectancy of at least 6 months as assessed on clinical grounds by the local study team. There were no exclusion criteria. The first participant was enrolled in April 1999. All participants were followed to a common closing date of July 1, 2006.
The association between baseline replication capacity and HIV disease progression was determined in the subset of LTM treatment-naïve patients who met the following additional eligibility criteria: a minimum of 4 months of follow-up during which antiretroviral therapy was not initiated, a baseline CD4+ count of ≥ 450 cells/μL, a baseline viral load ≥ 1,000 HIV-1 RNA copies/mL and the availability of sufficient plasma for laboratory analyses.
Study procedures
Baseline CD4+ cell count and viral loads were determined within 120 days prior to study enrollment. Plasma obtained from within 120 days prior to baseline was centrally stored for future CPCRA-approved, HIV-related research. Subsequent data collection visits occurred every 4 months after enrollment. At each visit, information was collected regarding the occurrence of new HIV-related diagnoses, current antiretroviral therapy, and CD4+ cell count and plasma HIV-1 RNA level. For consented patients, the National Death Index was searched to supplement mortality information collected from the local study sites. Clinical management decisions, i.e., whether to initiate antiretroviral therapy, were not specified by the study protocol but were instead left to the discretion of the treating physician. No information was collected regarding the reasons for the initiation of antiretroviral therapy.
Replication capacity was measured by use of the PhenoSense HIV Assay (Monogram Biosciences, South San Francisco, CA) as previously described 14;18;19. In brief, this assay uses amplicons from patient-derived virus that include a region of the viral genome spanning the p7/p1 and p1/p6 cleavage sites in gag, all of protease, and the first 305 amino acids of reverse transcriptase. These amplicons are then inserted into an HIV-1 vector derived from a pNL4-3 infectious clone that contains a luciferase reporter gene in place of part of env. The recombinant HIV-1 clones are then co-transfected along with a plasmid that codes for expression of an amphotropic MLV (aMLV) envelope into HEK-293 cells18. The resultant pool of recombinant pseudotyped viruses is used to infect fresh HEK-293 cells and measurements of luciferase activity after a single cycle of replication serve as a surrogate measure of virus replication. The amount of replication (luciferase activity) of the NL4-3 based pseudotyped virus is set to 100%, which approximates the mean pol replication capacity of wild-type virus20. The results of the PhenoSense HIV Assay were also used to determine the susceptibility of HIV in the baseline samples to nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitor antiretroviral therapy agents; susceptibility results were not available for etravirine, darunavir and tipranavir as these agents were not in clinical use at the time these analyses were performed. All assays of replication capacity were done after closure of the study to follow-up.
Objectives
The primary objectives of these analyses, which were specified prior to data analysis, were to determine the distribution of HIV pol replication capacity at baseline, and the relationship between the baseline pol replication capacity and the time to progression to the composite outcome of a decrease in the CD4+ count to fewer than 350 cells/μL, initiation of antiretroviral therapy or death (whichever came first). The composite endpoint included initiation of therapy due to concerns that clinical indicators of HIV disease progression other than a decrease of the CD4+ count to < 350 cells/μL, i.e., increasing viral load, rapid declines in the CD4+ count or other clinical indications of disease progression cell loss may prompt the initiation of antiretroviral therapy1, thereby introducing informative censoring of the pure CD4+ endpoint.
Statistical analyses
Participants were divided into two groups based on median RC (79%). Four results equal to the median were included in the below-median group, resulting in 160 participants below the median and 156 above median RC. Demographic characteristics were compared between groups using Cochran-Mantel-Haenszel tests for proportions and linear regression for continuous variables. Both types of comparisons were stratified by clinical center. Differences in medians were compared using the Wilcoxon rank test. Differences between groups in terms of resistance to the number of classes of antiretroviral drugs and to each class of antiretroviral drugs were made using Fisher’s Exact Test without stratification.
Time-to-event data were analyzed using survival methods, including Kaplan-Meier graphs and the log-rank test for nonparametric analyses, and proportional hazards regression. The log-rank test for comparison of time to the combined endpoint of <350 CD4+ cells/μL, treatment initiation, or death was stratified by quartile of the baseline CD4+ cell count. Proportional hazards models for the same combined endpoint as well as its constituent events were adjusted for baseline CD4+ and baseline log HIV-RNA and stratified by clinical center. As a secondary analysis, the same proportional hazards models were also adjusted for risk factors of HIV acquisition (same sex contact, injection drug use), gender, ethnicity (Latino, Black and other), age, prior infection by hepatitis C, active hepatitis B infection, known duration of HIV infection, and prior AIDS-defining diagnoses. All time-to-event endpoints were censored for death, loss-to-follow-up or study closure. In addition, time to CD4+ ≤350 cells/μL as a secondary endpoint was censored for initiation of antiretroviral therapy.
Analyses of change in log10 HIV-RNA and CD4+ cell count, and slope of CD4+ cell count were performed using linear regression and random effects models, respectively. Viral load and CD4+ cell count were censored following the initiation of therapy; hence, these analyses are potentially biased as decisions to initiate therapy (which result in censuring to follow-up) are influenced by changes in the outcomes of interest (the CD4+ cell count and the viral load).
Models for change in CD4+ cell count (from Month 4) are adjusted for Month 4 CD4+ cell count and baseline log10 HIV-RNA. Models for change in log HIV-RNA (from baseline) are adjusted for baseline log10 HIV-RNA. Random effects models for slope of CD4+ cell count (from Month 4) contain individual random effects for intercept and slope, and fixed effects to adjust for baseline log HIV-RNA. Slope models are fit both on square-root transformed CD4+ data (as CD4+ data are not normally distributed) and on the untransformed scale.
Results
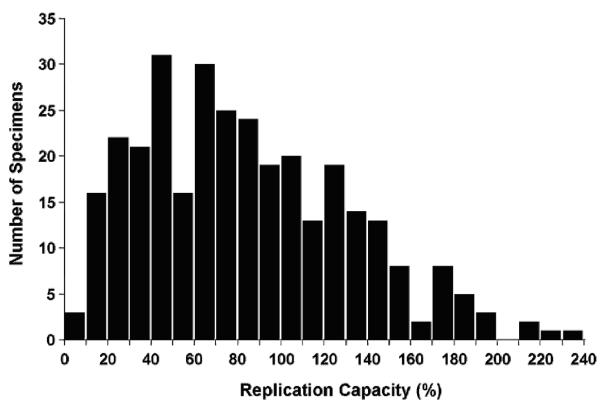
Patients were enrolled at 19 CPCRA sites in the United States. Enrollment began on March 16, 1999 and concluded on April 1, 2005. The study was closed to follow-up on July 1, 2006. Altogether the LTM study enrolled and followed a cohort of 1090 treatment naïve patients of whom 359 met the entry criteria for analysis of pol replication capacity. Patients were excluded for the following reasons: 35 were participants from clinical study units for which patient follow-up was terminated for administrative reasons, 519 had < 450 CD4+ cells/μL at baseline, 123 had < 1,000 HIV-1 RNA copies/mL at baseline, 41 started antiretroviral therapy within 4 months of study enrollment and 13 were lost to follow-up beyond baseline. Results were available for 316 patients; 18 participants did not have available baseline plasma specimens and replication capacity assays were unsuccessful in 25 other persons. The distribution of the baseline pol replication capacity results is shown in Figure 1; the median value was 79% (interquartile range 45 – 118%).
Figure 1.
Distribution of baseline replication capacity
The baseline characteristics of the subjects for whom baseline pol replication capacity results were above or below the median value are provided in Table 1. The demographic characteristics including age, gender, ethnicity, HIV risk factor, prior AIDS diagnoses, body mass index, chronic infection by hepatitis B or hepatitis C, duration of known HIV infection, and mean and median baseline CD4+ counts of patients were similar in patients with lower or higher replication capacity. However, both the mean and median viral loads were lower in patients with lower pol replication capacity results (mean 4.1 vs 4.2 Log HIV-1 RNA/mL, p = 0.01; median 4.0 vs 4.2 Log HIV-1 RNA/mL, p = 0.026). Phenotypic evidence of antiretroviral resistance was found in 20% of patients with pol replication capacity below the median and 19% of patients with higher pol replication capacity. Patients with lower pol replication capacity were less likely to have evidence of protease inhibitor resistance (2% vs 8%, p = 0.03). Otherwise there were no differences in the frequency or distribution of antiretroviral resistance. The baseline characteristics of the 43 patients from whom pol replication capacity were not available and the 316 patients from whom these results were available were similar except that the patients with missing results had a lower mean viral load (3.7 ± 0.6 vs 4.1 ± 0.6 Log HIV-1 RNA/mL; p < 0.01) and lower body mass index (25.7 vs 27.1 kg/m2; p = 0.04). .
Table 1.
Baseline Characteristics
|
pol Replicative Capacity |
|||||
|---|---|---|---|---|---|
| Characteristic | ≤79% | >79 | P | ||
| Patients | 160 | 156 | |||
| Female (%) | 40 | (25.0%) | 34 | (21.8%) | 0.48 |
| Ethnicity | 0.83 | ||||
| Latino/a (%) | 18 | (11.3%) | 14 | (9.0%) | 0.54 |
| Black (%) | 69 | (43.1%) | 69 | (44.2%) | 0.79 |
| White (%) | 68 | (42.5%) | 64 | (41.0%) | 0.66 |
| Other (%) | 5 | (3.1) | 9 | (5.8) | 0.24 |
| Age (years) | 38.1 | 38.5 | 0.89 | ||
| Injection drug use (%) | 34 | (21.3%) | 23 | (14.7%) | 0.32 |
| Same Sex Contact* (%) | 88 | (73.3%) | 99 | (81.1%) | 0.15 |
| Prior CDC class C diagnosis (%) | 6 | (3.8%) | 6 | (3.8%) | |
| Months known HIV+ | 45 | 51 | 0.22 | ||
| Median Follow-up (Months) | (52.7) | (49.6) | 0.74 | ||
| Body mass index (kg/m2) | 26.9 | 27.4 | 0.33 | ||
| CD4 (mean, STD) | 704.2 | (225.9) | 666.4 | (219.1) | 0.12 |
| CD4 (median, 25-75%) | 636 | (528, 822) | 598 | (525, 736) | 0.14 |
| Log HIV RNA/mL (mean, STD) | 4.1 | (0.6) | 4.2 | (0.6) | 0.01 |
| Log HIV RNA/mL (median, 25-75%) | 4.0 | (3.6, 4.5) | 4.2 | (3.8, 4.6) | 0.026 |
| Phenotypic antiretroviral resistance by class | |||||
| Any resistance (%) | 32 | (20) | 20 | (19) | 0.88 |
| Any NRTI (%) | 21 | (13) | 14 | (9) | 0.28 |
| Any NNRTI (%) | 14 | (9) | 6 | (4) | 0.10 |
| Any PI (%) | 4 | (2) | 13 | (8) | 0.03 |
| Phenotypic antiretroviral resistance by drug | 1.0 | ||||
| Resistance to 0 drugs (%) | 128 | (80) | 127 | (81) | |
| Resistance to 1 drug (%) | 26 | (16) | 25 | (16) | |
| Resistance to 2 drugs (%) | 5 | (3.1) | 4 | (2.6) | |
| Resistance to ≥ 3 drugs (%) | 1 | (0.6) | 0 | (0.0) | |
| Hepatitis B Antigen+ (%) | 9 | (5.6) | 8 | (5.1) | 0.21 |
| Hepatitis C Antibody+ (%) | 29 | (18.1) | 27 | (17.3) | 0.89 |
Men only
The median follow-up of enrolled eligible patients was 51 months, during which 190 patients reached the primary composite endpoint (i.e., reaching a CD4+ count of < 350 cells/μL (n=116), initiation of antiretroviral therapy (n=68) or death (n=6)). At the end of the study, 29 individuals out of 316 (9%) were lost-to-follow-up for final determination of the primary (combined) endpoint. In the below-median pol replication capacity group, the rate of loss was 16/156 (10.3%) as compared with 13/160 (8.1%) in the above-median group (p=0.56 by Fisher’s exact test). A Kaplan-Meier analysis of time to an ‘event’ of loss to follow-up was not significantly different for the above- vs below-median groups (log-rank = 0.815, p = 0.3666). Compared with persons with lower baseline pol replication capacity, persons with baseline pol replication capacity above the median reached the primary endpoint more rapidly (logrank = 5.04, p = 0.0247; Figure 2). Kaplan Meier logrank analyses of the component outcomes demonstrated that persons with lower pol replication capacity had a lower rate of initiation of antiretroviral therapy (logrank = 5.40, p = 0.02) and tended to have a lesser rate of progression to a CD4+ count of < 350 cells/μL (logrank = 2.63, p = 0.10).
Figure 2.
Rate of progression to composite endpoint of CD4+ ≤350, initiation of antiretroviral therapy or death for patients with pol replication capacity above vs below the mean (79%) at baseline. Results are stratified for quartile of baseline CD4+ cell count.
Proportional hazards regression analyses that were adjusted for the baseline viral load and CD4+ cell count demonstrated that the hazard ratio of progression to the composite endpoint was 0.73 (p = 0.041) for persons with circulating virus displaying a pol replication capacity below vs above the median value (Table 2). The effect of diminished pol replication capacity was stable across several proportional hazards regression models that in addition to the baseline CD4+ count and viral load, controlled for HIV risk factors, demographic factors, prior infection by hepatitis C, active hepatitis B infection, known duration of HIV infection, and prior AIDS-defining diagnoses (data not shown).
Table 2.
Proportional Hazards Regression of Time to Endpoints
| Covariate | HR* | 95% CI | p-value |
|---|---|---|---|
| Replication capacity below median | 0.73 | [0.54,0.99] | 0.041 |
| BL CD4+ [per 50 cells/μL increase] | 0.86 | [0.82,0.91] | <0.001 |
| Baseline Log10 HIV-1 RNA [per 1 log10 HIV-1 RNA/mL increase] | 2.07 | [1.57,2.72] | <0.001 |
Results are stratified by clinic center
3 other models showed similar relationships between pol replication capacity, CD4 count and viral load with progression to the composite outcome.
Hazard ratio
The rates of proceeding to separate endpoints of starting antiretroviral therapy, reaching a CD4+ count less than 350 cells/μL or death were also evaluated (Table 3). In analyses that adjusted for baseline viral load and CD4+ cell count, patients harboring virus with lower pol replication capacity were found to initiate therapy more slowly than did patients with higher pol replication capacity values (HR 0.68, p = 0.04). The rate of reaching the component event of reaching a CD4+ count lower than 350 cells/μL also tended to be lower in persons with lower pol replication capacity, but in adjusted analyses this was not statistically significant (HR 0.74, p = 0.13).
Table 3.
Progression to Endpoints
| Replication Capacity ≤ 79% |
Replication Capacity > 79% |
||||||
|---|---|---|---|---|---|---|---|
| Event | N | Rate*** | N | Rate*** | HR+ | CI | p |
| CD4+ < 350* | 52 | 10.4 | 64 | 15.1 | 0.74 | [0.50,1.09] | 0.13 |
| Initiation of therapy | 56 | 10 | 79 | 16 | 0.68 | [0.48,0.98] | 0.04 |
| Death* | 5 | 0.9 | 4 | 0.8 | 0.97 | [0.25,3.74] | 0.96 |
| Composite endpoint** | 84 | 17.8 | 106 | 26.8 | 0.73 | [0.54,0.99] | 0.04 |
| Total number of patients | 160 | 156 | |||||
Results are stratified by clinic center and adjusted for baseline CD4+ cell count and HIV plasma RNA level.
Hazard ratio, below vs above median pol replication capacity value
Censored for initiation of ART
Initiation of therapy or CD4+<350 or Death
Rates measured per hundred person-years of follow-up
Similar results were found in alternate models that also included HIV risk factors, demographic factors, prior infection by hepatitis C, active hepatitis B infection, known duration of HIV infection, and prior AIDS-defining diagnoses.
To better understand the relationship between pol replication capacity and disease progression, we used linear regression analyses to assess changes in the HIV-1 RNA plasma levels and CD4+ cell count in more detail (Table 4). After correction for the effects of baseline viral load, the relationship between baseline pol replication capacity and the change in viral load to the average of follow-up measurements through Month 24 was statistically significant, with the higher pol replication capacity group having a 0.22 log10 higher increase in viral load than those with lower pol replication capacity (β=0.22 log10, SE=0.08, p=0.0094). A similar, but non-significant trend is seen between baseline pol replication capacity and the change of viral load to Months 12 and 24.
Table 4.
Change in Viral Load vs Baseline pol Replication Capacity Change in CD4+ Count vs Baseline pol Replication Capacity
| Predictive Characteristic | Change from baseline to Month 12 |
Change from baseline to Month 24 |
Change from baseline to average of all values through Month 24 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | |
| Baseline Log10 HIV-1 RNA | −.36 | 0.09 | <0.001 | −.37 | 0.10 | 0.0005 | −0.40 | 0.08 | <0.001 |
| Baseline pol replication capacity* | 0.13 | 0.10 | 0.21 | 0.12 | 0.11 | 0.28 | 0.22 | 0.08 | .0094 |
| Predictive Characteristic | Change from Month 4 to Month 16 |
Change from Month 4 to Month 28 |
Change from Month 4to average of all values through Month 24 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | |
| Month 4 CD4+ count | −0.3 | 0.1 | <0.001 | −0.4 | 0.1 | <0.001 | −0.4 | 0.1 | <0.001 |
| Baseline Log10 HIV-1 RNA | −4.5 | 24.9 | 0.86 | 18.4 | 28.5 | 0.527 | 8.8 | 19.5 | 0.65 |
| Baseline pol replication capacity** | 8.9 | 27.9 | 0.75 | −19.4 | 31.8 | 0.54 | −15.4 | 21.7 | 0.48 |
Linear regression modeling was used with corrections for month 4 CD4+ and baseline viral load (change in CD4+ count) or the baseline viral load (change in viral load). Results are stratified by clinic center. Because participants were recruited on the basis of baseline CD4+ cell counts, models of both change and slope of decline of CD4+ cell counts are measured from Month 4 to the point of censoring to minimize bias from regression to the mean
pol Replication Capacity <79 vs <=79
In contrast, no statistically significant change was observed between those with lower pol replication capacity and the change in CD4+ cell count from Month 4 to Month 16, to Month 28, or the average of all available CD4+ cell measurements from Month 4 through Month 28. Finally, the slope of decline in CD4+ cell counts from Month 4 until censoring for initiation of therapy, death, or the end of study, was analyzed using random effects models. In order to satisfy model assumptions for normality, the CD4+ cell count was square root transformed. After adjusting for baseline log HIV-RNA, we did not find an association between higher baseline pol replication capacity group and the decline in CD4+ cell count on the square-root transformed scale (β=−0.19, 95%CI [−0.55, 0.17], p=0.31). Similarly no association was found in the decline of CD4+ cells on a non-transformed scale (data not shown).
Discussion
We studied an evaluable population of 316 persons with chronic untreated HIV-1 infection who did not meet indications for immediate initiation of antiretroviral therapy (median CD4+ count = 617 cells/μL). Among these subjects, persons with a baseline pol replication capacity less than the median of the study population (i.e., ≤ 79%), had a slower rate of HIV disease progression as assessed by time to progression to a composite outcome of CD4+ count <350 cells/μL, treatment initiation, or death. In analyses of the component outcomes, persons with lower pol replication capacity had a slower rate of initiating antiretroviral therapy (p = 0.04) and tended to have a slower rate of falling to fewer than 350 CD4+ cells/μL (p = 0.13). . Finally, persons with lower baseline pol replication capacity tended to have a slower rate of viral load increase (p = 0.0094 through 24 months). At baseline, having a pol replication capacity below the median was associated with a lower mean viral load (4.0 vs 4.2 log10 HIV-1 RNA copies/mL, p = 0.01) and a decreased prevalence of phenotypic evidence of resistance to protease inhibitor (2% vs 8%, p = 0.03). Otherwise there were no differences in the baseline characteristics of these patient groups.
This study is the first large scale study of the relationship between pol replication capacity and subsequent HIV disease progression in chronically infected, treatment-naïve adults with early HIV disease. The two previous studies of the prognostic import of pol replication capacity in persons not receiving combination antiretroviral therapy were performed in HIV-infected hemophiliac children and adolescents, many of whom received therapy with nucleoside reverse transcriptase inhibitors prior to the advent of combination antiretroviral therapy 17 or evaluated relatively short term outcomes in recently infected patients14. Both investigations showed decreased pol replication capacity to be associated with increased baseline CD4+ cell counts14;17. In addition, lower baseline pol replication capacity was associated with decreased baseline viral loads and decreased rates of subsequent CD4+ cell loss in the study of chronically infected hemophiliac patients17. The differences between the patient populations included in the current study and the reports of Daar et al.17 and Barbour et al.14 are summarized in Table 5. Other studies of the prognostic significance of impaired pol replication capacity have involved analyses of outcomes in treatment-experienced patients20-24.
Table 5.
Comparison with Prior Studies of pol replication capacity vs disease progression in Treatment-Naïve Patients
| Parameter | This study | Daar et al. 17 | Barbour et al.14 |
|---|---|---|---|
| N with follow-up data | 316 | 128 | 65* |
| Age (years, mean) | 38.1 | 13.1 | 34 |
| Female (%) | 37 | 0 | 7 |
| Ethnicity | |||
| Latino/a (%) | 10.1 | ND | ND |
| Black (%) | 43.6 | ND | ND |
| White (%) | 41.8 | ND | ND |
| Other (%) | 4.4 | ND | ND |
| HIV risk factor | |||
| Injection drug use (%) | 18.0 | 0 | ND |
| Same Sex Contact* (%) | 59.2@ | 0 | ND |
| Blood product (%) | 5 | 100 | ND |
| Other (%) | 18 | 0 | ND |
| Known duration of HIV infection (years) | 4 | 6.7 years% | < 1 year |
| Baseline laboratory studies | |||
| CD4+ count (median cells/μL) | 617 | 349 ^ | 519 |
| HIV-1 RNA copies/mL (median log10) | 4.1 | 3.7^ | 4.75 |
| Current antiretroviral therapy (%) | 0 | 50+ | 0 |
| Duration of follow-up (median) | 51.2 months | 3 - 4 years | 447 days |
| Evidence of prior antiretroviral resistance | |||
| Any agent (%) | 16.4 | 39.5# | 18.6 |
| Nucleoside reverse transcriptase inhibitor (%) | 11.1 | 39.5# | 11.2 |
| Non-nucleoside reverse transcriptase inhibitor (% | 6.3 | 0 | 10.3 |
| Protease inhibitor (% | 5.4 | 0 | 3.9 |
| pol replication capacity (%) (median, IQR) | 79 (45 – 118%) |
94 (69 – 118%) |
69 (43 – 101%) |
| pol replication capacity (%) threshold value& | 79 | 69 | 42 |
demographic data are for 191 subjects with baseline data; longitudinal outcome data were reported for 65 participants who were not otherwise characterized.
3 patients on dual nucleoside reverse transcriptase inhibitor therapy, 61 patients with prior mono-nucleoside reverse transcriptase inhibitor therapy. 5 patients had received prior triple drug therapy including a protease inhibitor.
Resistance defined as a 2.5 cut-off for phenotypic drug resistance.
Men only
estimated duration of infection 40
Mean value
pol replication capacity value used in determinations of effect of replication capacity on outcomes of interest.
The Monogram Biosciences PhenoSense HIV assay utilizes a single-replication-cycle format to compare the pol replication rate of a recombinant pseudotyped virus derived from clinical specimens with that of a reference pseudotyped virus for which the replication capacity is set at 100%18. Although env, gag, protease and reverse transcriptase activity are known to affect viral fitness25-28, the Monogram assay only assesses contributions to viral replication of areas of the viral genome that span the 3′ end of gag, protease and the first 305 codons of reverse transcriptase26. Other studies using multiple-cycle, whole virus, parallel infection assays to assess viral replication have shown decreased viral replication in long-term non-progressors29-31 as well as an association between decreased replication and lower viral loads30 in treatment naïve patients29;30;32;33. The Monogram Biosciences PhenoSense HIV provides the only commercially available information on HIV-1 replication capacity.
Although impairment of pol replication capacity is largely associated with resistance to antiretroviral agents20-24, substantial variations have been previously reported in treatment-naïve patients14;27 as demonstrated in a study of 191 recently infected, treatment-naïve patients, wherein the median initial pol replication capacity value was 69%14. In this study only 6% of the variance was explained by drug-resistance mutations. Similarly, we found no evidence of antiretroviral resistance in 80% of our cohort and no association between pol replication capacity and the presence of antiretroviral resistance. Of note, while the presence of primary protease inhibitor mutations has been associated with decreased pol replication capacity (p = 0.01) at baseline in recently infected patients14, paradoxically we found that the prevalence of protease inhibitor mutations (8%) was less in persons with lower pol replication capacity. Similar impairment of pol replication capacity has previously been observed in wild type HIV populations14;27 and, at least in part, may be explained in part by gag mutations27 or by incompatibilities between the patient-derived protease and reverse transcriptase and the gag proteins derived from the test vector14;34. Alternatively, the presence of minor protease inhibitor mutations that do not directly contribute to antiretroviral resistance may have compensated for the presence of major protease inhibitor mutations and increased the pol replication capacity in patients harboring these mutations 21.
The strengths of this study include the demographic diversity, prospective data collection and long duration of follow-up. The findings of slower progression to the composite endpoint (i.e., progression to <350 CD4+ cells/μL, death or initiation of antiretroviral therapy) in persons with lower pol replication capacity are supported by the observation that in analyses of the component outcomes, persons with lower pol replication capacity had a slower rate of initiating antiretroviral therapy and tended to have a slower rate of decline to fewer than 350 CD4+ cells/μL. Of note, the CD4+ outcome is based on fewer events than for the composite endpoint or initiation of therapy and thus has less statistical power; there were too few deaths to for meaningful analysis. While we cannot rule out potential bias due to losses-to-follow-up in the study, given that the difference in the proportion of individuals in each group that experienced the combined endpoint (68% versus 52%) and that losses are fairly evenly distributed between the two groups, it is unlikely that any potential bias produced a qualitative change in the study results.
Weaknesses include uncertainty regarding the stability and reproducibility of pol replication capacity over time. Most data regarding the evolution of pol replication capacity are derived from studies of patients receiving a stable antiretroviral regimen despite ongoing viral replication21;35. The relevance of these data to untreated patients with impaired replication capacity is not known. Also, we did not account for other factors associated with disease progression such as genetic determinants in other parts of the viral genome, host genetic factors or innate or acquired immunological responses that may affect the rate of disease progression29;36. In addition, analyses of the relationship between pol replication capacity and changes in the CD4+ cell count and viral load are potentially biased by missing CD4+ data and censoring of observations due to the initiation of therapy and thus statistical inference must therefore be approached with caution. Finally, the mechanisms by which impaired pol replication capacity may result in decreased HIV disease progression are uncertain. However, it is noteworthy that protease inhibitor-resistant HIV-1 isolates with impaired pol replication capacity replicate poorly in human thymus tissue 37 but replicate well in activated cells that are permissive for HIV-1 replication 38;39. Such a limitation of the replication of virus with impaired pol replication capacity to cells that contribute to plasma HIV-1 RNA levels while sparing cells that are responsible for repletion of the CD4+ cell pool could account for CD4+ cell count stability in the face of high viral loads in persons with circulating HIV-1 with impaired pol replication capacity 14. Moreover, it is notable that persons with higher baseline pol replication capacity had a greater subsequent increase in the viral load, which may in turn have contributed to higher rates of treatment initiation.
In summary, we have reported the first large scale study of the relationship between pol replication capacity and subsequent HIV disease progression in chronically infected, treatment-naïve adults with early HIV disease. In this cohort, decreased pol replication capacity was associated with delayed progression to a primary composite endpoint of reaching a CD4+ count of < 350 cells/μL, initiating antiretroviral therapy, or death. These data are consistent with the relationship between diminished pol replication capacity and decreased progression to AIDS in hemophiliac children and adolescents in the pre-cART era17 and extend these observations to heterogeneous population of treatment-naïve adults. The impact of having a baseline pol replication capacity less than the median value of 79% on the hazard ratio for the primary composite endpoint was similar to that of a 104 cell/μL increase in baseline CD4 count or a 0.43 log10/mL lower baseline viral load. These results also build upon the findings in treatment-experienced patients wherein impaired pol replication capacity has been associated with the preservation of a beneficial immunologic response and a slow rate of viral load increase despite failure of antiretroviral therapy to maximally suppress plasma viremia10;20;21;24. Finally, the observation that persons with decreased pol replication capacity have a slower rate of HIV disease progression suggests that measurement of pol replication capacity, along with other measures such as co-receptor tropism16, may be useful as an additional indicator in determining the timing of the initiation of cART in persons with greater than 350 CD4+ cells/μL. However, such use of pol replication capacity results will require further validation of this measure in other patient populations.
Acknowledgements
Sources of funding: NIAID grants U01 AI042170, U01 AI046362 and U01 AI068641 to the CPCRA and SBIR R44AI050321 to Monogram Biosciences.
Footnotes
Author participation: All authors gave final approval for this manuscript. Matthew B. Goetz contributed to the conception of the study, interpretation of the data and drafting the article. Robert Leduc contributed to the conception and design of the study, analysis and interpretation of data, and drafting the article. Nicole Wyman contributed to statistical and analytical plan. Jay R. Kostman, Ann M. Labriola, and Roberta Luskin-Hawk, contributed to the conception and design of the study and revising the article for critical intellectual content. Yolanda Lie, Jodi Weidler, Eoin Coakley and Michael Bates contributed to acquisition of the data, interpretation of the data, critical revisions of the intellectual content of the manuscript.
These data have been presented previously in part at the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention. July 22 – 25, 2007; Sydney, Australia; Abstract WEPDB07
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. 2008 November 4; Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 2.Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–80. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 4.Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340:1715–22. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- 5.Alexander L, Weiskopf E, Greenough TC, et al. Unusual polymorphisms in human immunodeficiency virus type 1 associated with nonprogressive infection. J Virol. 2000;74:4361–76. doi: 10.1128/jvi.74.9.4361-4376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grovit-Ferbas K, Ferbas J, Gudeman V, et al. Potential contributions of viral envelope and host genetic factors in a human immunodeficiency virus type 1-infected long-term survivor. J Virol. 1998;72:8650–8658. doi: 10.1128/jvi.72.11.8650-8658.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito JM, Lopez M, Soriano V. The role of CD8+ T-cell response in HIV infection. AIDS Rev. 2004;6:79–88. [PubMed] [Google Scholar]
- 8.Ioannidis JP, Rosenberg PS, Goedert JJ, et al. Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3′A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med. 2001;135:782–95. doi: 10.7326/0003-4819-135-9-200111060-00008. [DOI] [PubMed] [Google Scholar]
- 9.Mulherin SA, O’Brien TR, Ioannidis JP, et al. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. AIDS. 2003;17:377–87. doi: 10.1097/01.aids.0000050783.28043.3e. [DOI] [PubMed] [Google Scholar]
- 10.Sufka SA, Ferrari G, Gryszowka VE, et al. Prolonged CD4+ cell/virus load discordance during treatment with protease inhibitor-based highly active antiretroviral therapy: immune response and viral control. J Infect Dis. 2003;187:1027–37. doi: 10.1086/368359. [DOI] [PubMed] [Google Scholar]
- 11.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 12.Edward J.Hines, Jr.VA Hospital . VIReC Research User Guide: VHA Pharmacy Prescription Data. Veterans Affairs Information Resource Center; Hines, IL: 2003. Ref Type: Generic. [Google Scholar]
- 13.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. 2008 February 9;
- 14.Barbour JD, Hecht FM, Wrin T, et al. Higher CD4+ T cell counts associated with low viral pol replication capacity among treatment-naive adults in early HIV-1 infection. J Infect Dis. 2004;190:251–56. doi: 10.1086/422036. [DOI] [PubMed] [Google Scholar]
- 15.Daar ES, Kesler KL, Petropoulos CJ, et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis. 2007;45:643–49. doi: 10.1086/520650. [DOI] [PubMed] [Google Scholar]
- 16.Goetz MB, Leduc R, Kostman JR, et al. Relationship Between HIV Coreceptor Tropism and Disease Progression in Persons With Untreated Chronic HIV Infection. J Acquir Immune Defic Syndr. 2009;50:259–66. doi: 10.1097/QAI.0b013e3181989a8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daar ES, Kesler KL, Wrin T, et al. HIV-1 pol replication capacity predicts disease progression. AIDS. 2005;19:871–77. doi: 10.1097/01.aids.0000171400.15619.e1. [DOI] [PubMed] [Google Scholar]
- 18.Petropoulos CJ, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agent Chemother. 2000;44:920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates M, Wrin T, Huang W, et al. Practical applications of viral fitness in clinical practice. Curr Opin Infect Dis. 2003;16:11–18. doi: 10.1097/00001432-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Deeks SG, Wrin T, Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344:472–80. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 21.Barbour JD, Wrin T, Grant RM, et al. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J Virol. 2002;76:11104–12. doi: 10.1128/JVI.76.21.11104-11112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White KL, Margot NA, Wrin T, et al. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob Agents Chemother. 2002;46:3437–46. doi: 10.1128/AAC.46.11.3437-3446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang W, Gamarnik A, Limoli K, et al. Amino acid substitutions at position 190 of human immunodeficiency virus type 1 reverse transcriptase increase susceptibility to delavirdine and impair virus replication. J Virol. 2003;77:1512–23. doi: 10.1128/JVI.77.2.1512-1523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haubrich R, Hernandez J, Bates M, et al. Determinants of Replication Capacity (RC) in HIV-1 Isolates from ART-Experienced Adults Failing a PI-Based Regimen, and Relationship of RC with HIV-1 RNA and CD4 Counts. Antivir Ther. 2004;9:S162. Abstract 146. [Google Scholar]
- 25.Marozsan AJ, Moore DM, Lobritz MA, et al. Differences in the fitness of two diverse wild-type human immunodeficiency virus type 1 isolates are related to the efficiency of cell binding and entry. J Virol. 2005;79:7121–34. doi: 10.1128/JVI.79.11.7121-7134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dykes C, Demeter LM. Clinical significance of human immunodeficiency virus type 1 replication fitness. Clin Microbiol Rev. 2007;20:550–578. doi: 10.1128/CMR.00017-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates M, Chappey C, Parkin N. Mutations in p6 Gag Associated with Alterations in Replication Capacity in Drug Sensitive HIV-1 Are Implicated in the Budding Process Mediated by TSG101 and AIP1. Conf Retroviruses Opportunistic Infect. 2004;11 Abstract 121. [Google Scholar]
- 28.Rangel HR, Weber J, Chakraborty B, et al. Role of the human immunodeficiency virus type 1 envelope gene in viral fitness. J Virol. 2003;77:9069–73. doi: 10.1128/JVI.77.16.9069-9073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Qin L, Zhang L, et al. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 30.Blaak H, Brouwer M, Ran LJ, et al. In vitro replication kinetics of human immunodeficiency virus type 1 (HIV-1) variants in relation to virus load in long-term survivors of HIV-1 infection. J Infect Dis. 1998;177:600–610. doi: 10.1086/514219. [DOI] [PubMed] [Google Scholar]
- 31.Quinones-Mateu ME, Ball SC, Marozsan AJ, et al. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J Virol. 2000;74:9222–33. doi: 10.1128/jvi.74.19.9222-9233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell TB, Schneider K, Wrin T, et al. Relationship between in vitro human immunodeficiency virus type 1 replication rate and virus load in plasma. J Virol. 2003;77:12105–12. doi: 10.1128/JVI.77.22.12105-12112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trkola A, Kuster H, Leemann C, et al. Human immunodeficiency virus type 1 fitness is a determining factor in viral rebound and set point in chronic infection. J Virol. 2003;77:13146–55. doi: 10.1128/JVI.77.24.13146-13155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mammano F, Petit C, Clavel F. Resistance-associated loss of viral fitness in human immunodeficiency virus type 1: phenotypic analysis of protease and gag coevolution in protease inhibitor-treated patients. J Virol. 1998;72:7632–37. doi: 10.1128/jvi.72.9.7632-7637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goetz MB, Moatamed F, Wrin T, et al. Long term durability of impaired HIV replication capacity in patients maintained on a stable treatment regimen despite low-level virological failure. Antivir Ther. 2003;8:S250. [Google Scholar]
- 36.O’Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet. 2004;36:565–74. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- 37.Stoddart CA, Liegler TJ, Mammano F, et al. Impaired replication of protease inhibitor-resistant HIV-1 in human thymus. Nat Med. 2001;7:712–18. doi: 10.1038/89090. [DOI] [PubMed] [Google Scholar]
- 38.Liegler TJ, Hayden MS, Lee KH, et al. Protease inhibitor-resistant HIV-1 from patients with preserved CD4 cell counts is cytopathic in activated CD4 T lymphocytes. AIDS. 2001;15:179–84. doi: 10.1097/00002030-200101260-00006. [DOI] [PubMed] [Google Scholar]
- 39.Penn ML, Myers M, Eckstein DA, et al. Primary and recombinant HIV type 1 strains resistant to protease inhibitors are pathogenic in mature human lymphoid tissues. AIDS Res Hum Retroviruses. 2001;17:517–23. doi: 10.1089/08892220151126580. [DOI] [PubMed] [Google Scholar]
- 40.Hilgartner MW, Donfield SM, Willoughby A, et al. Hemophilia growth and development study. Design, methods, and entry data. Am J Pediatr Hematol Oncol. 1993;15:208–18. doi: 10.1097/00043426-199305000-00009. [DOI] [PubMed] [Google Scholar]