Abstract
Aims
To investigate the expression of matrix metalloproteases (MMPs) and their inhibitors (TIMPs) in patients who develop local recurrence (LR) after mastectomy.
Methods
We analyzed the expressions of MMP-1, -2, -7, -9, -11, -13, -14, TIMP-1, -2, and -3, using immunohistochemical techniques, in primary tumors from patients without tumoral recurrence (n = 50), patients who developed distant metastasis (n = 50), and from patients who develop LRs (n = 25). LRs of the latter group were also analyzed for MMPs expression. All the patients underwent mastectomy.
Results
Score values for all MMPs and TIMPs were significantly higher in primary tumors of patients with distant metastasis. Primary tumors from patients with LR have lower expressions of MMPs and TIMPs compared with those from patients who developed distant metastasis, and with patients without recurrence for some MMPs. Remarkably, however, primary tumors from patients with LR showed significantly higher percentage of TIMP-1 and 2 expression in stromal cells compared to primary tumors from patients with distant metastasis or primary tumors from patients without tumoral progression. Furthermore, LRs had significantly higher MMP-9 expression than their corresponding primary tumors.
Conclusions
Our data indicate differences in MMPs/TIMPs expression between primary tumors of patients with LRs and of those with distant metastasis, both after mastectomy for breast cancer.
Keywords: Breast cancer, Local recurrence, MMPs, TIMPs, Tissue arrays
Introduction
Despite the increasing use of breast-conserving therapy, modified radical mastectomy remains as an important surgical technique in primary breast cancer. However, 10–18% of patients undergoing mastectomy will eventually develop a local recurrence (LR) (Lacour et al. 1983, 1987; Kaae and Johansen 1962; Carreno et al. 2007). LR is defined as the development of adenocarcinoma in one or more of the following locations: skin, subcutaneous tissues, or muscles of the ipsilateral chest wall. This is due, presumably, to the fact that scar tissue is a preferential site to lodge and grow circulating tumor cells. In fact, experimental studies have shown a preference of tumor cells to grow in scar tissue, this being either surgical scars (Alexander and Altemeier 1964; Fisher and Fisher 1959; Symmans et al. 2003; Durkan et al. 2003; Skipper et al. 1989) or radiated tissues (Dao and Yogo 1967; Fidler and Zeidman 1972). In addition, survival after a postmastectomy LR remains very poor, with 5-year survival rates ranging from 40 to 70% and disease-free survival ranging from 13 to 50% (Donegan et al. 1966; Spratt 1967; Karabali-Dalamaga et al. 1978; Bedwinek et al. 1981; Borner et al. 1994; Schuck et al. 2002), and is very often associated with distant metastases (Dao and Nemoto 1963). Hence, it would be extremely helpful to identify breast cancer patients at a higher risk of locoregional recurrence, which could be candidates for a more aggressive treatment, such as postmastectomy radiotherapy. There are a number of known parameters of the primary tumors, such as tumor size, nodal status, estrogen receptor status, and tumor grade (Orel et al. 1995; Pisansky et al. 1993; Stefanik et al. 1985; Sykes et al. 1989; Kamby et al. 1991; Willner et al. 1997; Barnes et al. 1991; Berstock et al. 1985; van Tienhoven et al. 1999; Kamby and Sengelov 1999), that directly correlate with the development of LR. However, there is a need to unravel new biological factors that could be implicated in the development of postmastectomy LR and in the outcome thereof.
Some of the best candidates for such a biological marker are the matrix metalloproteases (MMPs). Their role is well known in the degradation of connective tissue stroma and of basement membranes, which are key elements/barriers in tumor invasion and metastasis. In addition, recent data clearly challenge the classic dogma stating that MMPs promote metastasis solely by modulating the remodeling of extracellular matrix. Indeed, MMPs have also been attributed an impact on tumor cell behavior in vivo as a consequence of their ability to cleave growth factors, cell surface receptors, cell adhesion molecules, or chemokines/cytokines (Sternlicht and Werb 2001; Egeblad and Werb 2002; Turk et al. 2004; Rifkin et al. 1999; Manes et al. 1999; Noe et al. 2001). Furthermore, by cleaving of proapoptotic factors, MMPs are able to produce an aggressive phenotype by generating apoptotic resistant cells (Fingleton et al. 2001). MMPs may also regulate angiogenesis in cancer, both positively through their ability to mobilize or activate proangiogenic factors (Yu and Stamenkovic 2000; Stetler-Stevenson 1999), or negatively, via generation of angiogenesis inhibitors, such as angiostatin and endostatin, which are cleaved from large protein precursors (Dong et al. 1997; Cornelius et al. 1998; Ferreras et al. 2000). Consequently, several MMPs, in particular the gelatinases MMP-2 (Jones et al. 1999; Duffy et al. 2000; Talvensaari-Mattila et al. 2001, 2003; Baker et al. 2002; Grieu et al. 2004; Li et al. 2004; Sivula et al. 2005) and -9 (Li et al. 2004; Chantrain et al. 2004; Pellikainen et al. 2004; Vizoso et al. 2007), have been recently studied as prognostic factors in breast cancer, and, as a result, being associated with a poor outcome in various subsets of breast cancer patients. Likewise, it has been reported that several other MMPs, such as MMP-7 (Vizoso et al. 2007), -11 (Duffy et al. 2000; Vizoso et al. 2007), -14 (Jones et al. 1999; Vizoso et al. 2007; Mimori et al. 2001), and 13 (Nielsen et al. 2001), may be over-expressed and/or related to the clinical outcome in breast cancer. On the other hand, it is known that the activity of MMPs is specifically inhibited by tissue inhibitors of metalloproteases (TIMPs). Currently, four different TIMPs are known to exist: TIMPs 1, 2, 3, and 4 (Gonzalez et al. 2007). Nevertheless, it is now assumed that TIMPs are multifactorial proteins also involved in the induction of proliferation and the inhibition of apoptosis (Jiang et al. 2002; Baker et al. 1999). Thus, it has also been reported that some TIMPs, such as TIMP-1 (Vizoso et al. 2007; Ree et al. 1997; McCarthy et al. 1999; Nakopoulou et al. 2002; Schrohl et al. 2003, 2004) or TIMP-2 (Vizoso et al. 2007; Ree et al. 1997; Visscher et al. 1994; Remacle et al. 2000), may be over-expressed and/or related to the clinical outcome of breast cancer.
The present study, performed on women who had undergone mastectomy for breast cancer, had two aims: (1) to analyze the differences in the expression of MMPs and TIMPs between primary tumors: those of patients who developed a local lesion as the first event of tumoral recurrence, those of patients whose first sign of disease recurrence is the appearance of distant metastases, and, finally, those of patients without tumoral recurrence; and (2) to compare the expressions of MMPs and TIMPs in primary tumors and in paired isolated LRs.
Materials and methods
Patient characteristics
We analyzed the records of 1,087 women who were treated for breast cancer at Hospital de Jove (Gijón, Spain), Hospital de Cabueñes (Gijón, Spain), and at Hospital Central de Asturias (Oviedo, Spain), between 1990 and 2002. All the patients underwent a modified radical mastectomy. We identified a total of 98 patients with a first isolated breast cancer LR following primary mastectomy. None of the patients had tumoral involvement at the marginal resection in the mastectomy specimens. Patients with concomitant distant metastases at the time of the initial diagnosis were excluded from the study. None of the patients showed evidence of any other malignant tumor at the time of diagnosis. Specimens at the time of surgery from both primary tumors as well as from their corresponding recurrences were obtained from 25 out of the 98 patients of the study. The status of these 25 patients with respect to age, menopausal status, clinical tumoral stage, histological grade, hormonal receptor status, adjuvant radiotherapy, and/or systemic therapy, is listed in Table 1. The histological grade was determined according to criteria reported by the Nottingham modification of Bloom and Richardson score (Dixon et al. 1991), whereas nodal status was assessed histopathologically. LR was defined as any reappearance of tumor on the ipsilateral chest wall or mastectomy scar.
Table 1.
Basal characteristics from 25 patients with local recurrence and from 50 patients with distant metastasis, both as first manifestation after mastectomy, and from 50 patients without tumoral recurrence
| Characteristics | Patients without tumoral recurrence | Primary tumors of patients with local recurrence | Primary tumors of patients with distant metastasis |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total cases | 50 | 25 | 50 |
| Age (median) | |||
| ≤56 | 21 (42) | 13 (52) | 27 (54) |
| >56 | 29 (58) | 12 (48) | 23 (46) |
| Menopausal status | |||
| Premenopausal | 16 (32) | 9 (36) | 13 (26) |
| Postmenopausal | 34 (68) | 16 (64) | 37 (74) |
| T | |||
| T1–T2 | 28 (56) | 2 (8) | 21 (42) |
| T3–T4 | 22 (44) | 23 (92) | 29 (58) |
| Nodal status | |||
| Negative | 28 (56) | 12 (48) | 21 (42) |
| Positive | 22 (44) | 13 (52) | 29 (58) |
| SBR | |||
| I | 19 (38) | 2 (8) | 12 (24) |
| II | 24 (48) | 10 (40) | 25 (50) |
| III | 7 (14) | 7 (28) | 13 (26) |
| Unknown | 6 | ||
| RE | |||
| Negative | 15 (30) | 10 (40) | 32 (64) |
| Positive | 35 (70) | 15 (60) | 18 (36) |
| RP | |||
| Negative | 16 (32) | 13 (52) | 34 (68) |
| Positive | 34 (68) | 12 (48) | 16 (32) |
| Radiotherapy | |||
| No | 41 (82) | 8 (32) | 25 (50) |
| Yes | 9 (18) | 17 (68) | 25 (50) |
| Chemotherapy | |||
| No | 29 (58) | 9 (36) | 18 (36) |
| Yes | 21 (42) | 16 (64) | 32 (64) |
| Tamoxifen | |||
| No | 20 (40) | 6 (24) | 36 (72) |
| Yes | 30 (60) | 19 (76) | 14 (28) |
All these 25 patients were followed for disease recurrence and survival status by clinical and biochemical studies every 3 months for the first 2 years and then once a year. Radiological studies were performed once a year, or when considered necessary. The medium follow-up period was of 39 months (range 6–217 months). The end-point of the study was death, secondary to tumor progression.
This study also comprised one control group of patients who underwent mastectomy for invasive ductal breast cancer, and who develop distant metastasis as first manifestation of tumoral progression. An additional control group was that of patients without tumoral recurrence, who had a minimum of 5 years of follow-up. Both groups of patients were randomly selected among those treated between 1990 and 2001, and stratified by node status.
All patients were treated according to the guidelines used in our institutions, and the study adhered to national regulations, being approved by our institution’s Ethics and Investigation Committee.
Tissue arrays and immunohistochemistry
Breast carcinoma tissue samples were obtained at the time of surgery. Those used in the study were routinely fixed (overnight in 10% buffered formalin), paraffin-embedded tumoral samples stored in the Pathology laboratories. Histopathologically representative tumor areas were defined on hematoxylin and eosin-stained sections and marked on the slide. Tumor tissue array (TA) blocks were obtained by punching a tissue cylinder (core) with a diameter of 1.5 mm through a histologically representative area of each ‘donor’ tumor block, which was then inserted into an empty ‘recipient’ tissue array paraffin block using a manual tissue arrayer (Beecker Instruments, Sun Prairie, Wisconsin, USA) as described elsewhere (Parker et al. 2002). Collection of tissue cores was carried out under highly controlled conditions. Areas of non-necrotic cancerous tissue were selected for arraying by two experienced pathologists (L.O. González and A. M. Merino). Two cores (double redundancy) were employed for each case, as this method has been shown to correlate well with conventional immunohistochemical staining (Rimm et al. 2001). From the 50 tumor samples available, two TA blocks were prepared each containing 25 primary and secondary tumors samples, as well as internal controls including four normal breast tissue samples from two healthy women who underwent reductive mammary surgery. These latter samples contained epithelial components, in which the immunohistochemistry was negative with the antibodies of the study.
Two composite high-density TA blocks were designed, and serial 5-μm sections were consecutively cut with a microtome (Leica Microsystems GmbH, Wetzlar, Germany) and transferred to adhesive-coated slides. One section from each tissue array block was stained with H&E, and these slides were then reviewed to confirm that the sample was representative of the original tumor. Immunohistochemistry was done on these sections of TA fixed in 10% buffered formalin and embedded in paraffin using a TechMate TM50 autostainer (Dako, Glostrup, Denmark). Antibodies for MMPs and TIMPs were obtained from Neomarker (Lab Vision Corporation, Fremont, CA, USA). The dilution for each antibody was established based on negative and positive controls (1/50 for MMP-2, -7, -14 and TIMP-2; 1/100 for MMP-9, -13, TIMP-1 and -3; and 1/200 for MMP-1, -11). Negative control is DakoCytomation mouse serum diluted to the same mouse IgG concentration as the primary antibody. All the dilutions were made in Antibody Diluent, (Dako) and incubated for 30 min at room temperature.
Tissue sections were deparaffinized in xylene and then rehydrated in graded concentrations of ethyl alcohol (100, 96, 80, 70%, then water). To enhance antigen retrieval only for some antibodies, TA sections were microwave treated in a H2800 Microwave Processor (EBSciences, East Granby, CT, USA) in citrate buffer (Target Retrieval Solution; Dako) at 99°C for 16 min. Endogenous peroxidase activity was blocked by incubating the slides in peroxidase-blocking solution (Dako) for 5 min. The EnVision Detection Kit (Dako) was used as the reactivity detection system. Sections were counterstained with hematoxylin, dehydrated with ethanol, and permanently coverslipped.
For each antibody preparation studied, the location of immunoreactivity, percentage of reactive area. and intensity were determined. All the cases were semiquantified for each protein-stained area. An image analysis system with the Olympus BX51 microscope and analysis software (analySIS®, Soft imaging system, Münster, Germany) was employed as follows. Tumoral sections were stained with antibodies according to the method explained above and counterstained with hematoxylin. There are different optical thresholds for both stains. Each core was scanned with a ×400 power objective in two fields per core. Fields were selected searching for the protein-reactive areas. The computer program selected and traced a line around antibody-reactive areas (higher optical threshold: red spots), with the remaining, non-stained areas (hematoxylin-stained tissue with lower optical threshold) standing out as a blue background. Any field has an area ratio of stained (red) versus non-stained areas (blue). A final area ratio was obtained after averaging two fields. To evaluate immunostaining intensity we used a numeric score ranging from 0 to 3, reflecting the intensity as follows: 0, no reactivity; 1, weak reactivity; 2, moderate reactivity; and 3, intense reactivity. This score was applied both to tumoral and to stromal cells. Using an Excel spreadsheet, the mean score was obtained by multiplying the intensity score (I) by the percentage of reactivity area (PA) and the results were added together (total score: I × PA). This overall score was then averaged with the number of cores that were done for each patient. If there was no tumor in a particular core, then no score was given. In addition, for each tumor the mean score of two core biopsy samples was calculated.
Data analysis and statistical methods
Differences in percentages were calculated with the chi-square test. Immunoreactivity scores for each protein were expressed as median (range). Comparison of immunoreactivity values between groups was made with the Mann–Whitney or Kruskal–Wallis tests. Comparison of immunoreactivity values between primary tumors and paired LRs were assessed by using the Wilcoxon test. Statistical results were corrected applying Bonferroni’s correction. In addition, a multivariate multinomial logistic regression model was used to evaluate simultaneously the influence of significant co-variables on the relationship between MMPs/TIMPs expressions and local recurrences. Probabilities of survival were calculated with the Kaplan–Meier method. Differences between curves were evaluated with the log rank test. The SPSS 17.00 program was used for all calculations. Statistical significance was considered at 5% probability level (p < 0.05).
Results
More than 3,000 determinations in cancer specimens, from 50 patients without tumoral recurrences, from 25 patients with LRs after mastectomy, and from 50 patients with distant metastasis after mastectomy were evaluated on TAs. Minimal internal variance of score data between duplicate tissue cores from the same patients was detected in the TAs, showing a high agreement for each protein (r > 0.95 and p < 0.0001, for each protein), in primary tumors as in LR tissues. Likewise, we have previously described a validation study for MMPs and TIMPs in invasive breast carcinomas (Vizoso et al. 2007).
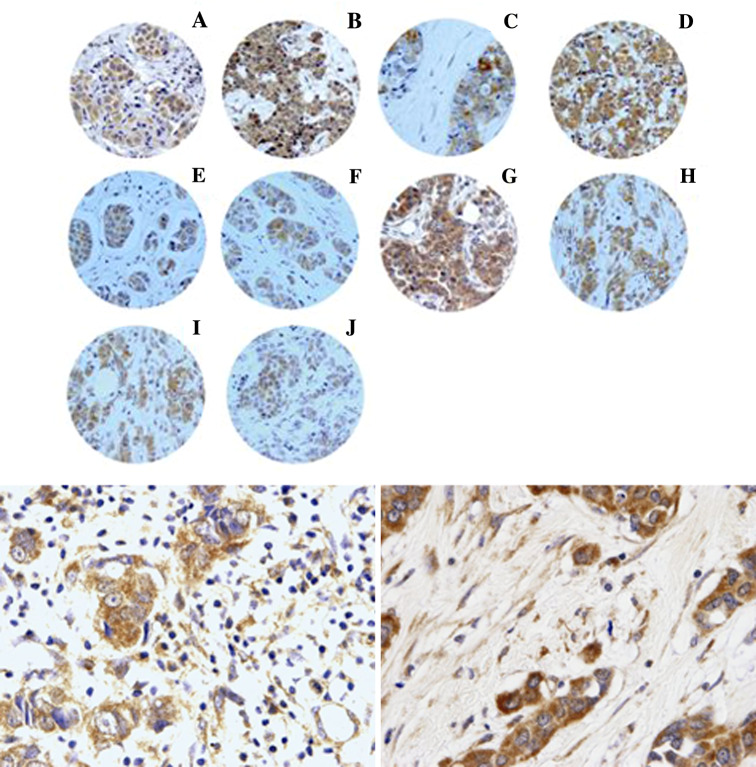
Figure 1 shows examples of TAs with immunoreactivity for each protein being evaluated. There was wide variability in the immunoreactivity score values for each protein (Table 2). Immunoreactivity for all the proteins studied was localized predominantly in tumor cells, but also, in a significant percentage of cases, in stromal cells.
Fig. 1.
Up Examples of tissue arrays with immunostaining for each protein evaluated. a MMP-1, b MMP-2, c MMP-7, d MMP-9, e MMP-11, f MMP-13, g MMP-14, h TIMP-1, i TIMP-2, j TIMP-3. Down (left) 400× Immunostaining for TIMP-2 in mononuclear inflammatory cells, (right) 400× Immunostaining for MMP-11 in fibroblastic cells
Table 2.
MMPs and TIMPs immunoreactivity values in primary tumors from patients without tumoral recurrences, from patients with local recurrences, from patients with distant metastasis and in local recurrences
| Factor | Primary tumors of patients without tumoral recurrence | Primary tumors of patients with tumoral recurrence | Primary tumors of patients with distant metastasis | Univariate p value* | Multivariate p value* | Local recurrences | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Median (range) | N | Median (range) | N | Median (range) | Median (range) | |||
| MMP-1 | 48 | 120 (0–277.5) | 24 | 48.9 (0–285) | 50 | 147.5 (0–285) | 0.002 | 0.006 | 0 (0–204.4) |
| MMP-2 | 48 | 0 | 25 | 0 (0–121.2) | 50 | 0 (0–246) | n.s. | n.s. | 0 (0–94.8) |
| MMP-7 | 47 | 140.9 (0–258.4) | 25 | 0 (0–130.8) | 50 | 120 (0–270) | 0.0001 | 0.0001 | 0 (0–53.8) |
| MMP-9 | 50 | 64.5 (0–156) | 25 | 0 (0–52.2) | 49 | 108 (0–273) | 0.0001 | 0.01 | 36.2 (0–120.2)† |
| MMP-11 | 49 | 134 (0–279) | 24 | 121.3 (0–257.1) | 49 | 173 (0–277.7) | 0.002 | 0.0001 | 150.9 (0–275.1) |
| MMP-13 | 50 | 65.7 (0–234) | 22 | 45.19 (0–133.8) | 50 | 60.6 (0–192.3) | 0.05 | 0.05 | 44.9 (0–142.1) |
| MMP-14 | 50 | 81 (0–261) | 24 | 73.4 (0.279.8) | 50 | 85.3 (0–258.5) | 0.05 | n.s. | 59.7 (0–159.8) |
| TIMP-1 | 50 | 132 (0–258) | 24 | 116.6 (21.8–235.7) | 49 | 158 (0–285) | 0.0001 | 0.01 | 127.8 (28.2–217.2) |
| TIMP-2 | 50 | 72 (0–220) | 24 | 80.2 (0–211.8) | 49 | 142 (34–243) | 0.0001 | 0.01 | 101.6 (39.4–215.9) |
| TIMP-3 | 50 | 101.7 (0–284.8) | 24 | 53.1 (0–213.6) | 50 | 126.9 (0–253.3) | 0.03 | n.s. | 61.3 (0–268.2) |
n.s. not significant
* p values for comparing the three groups of primary tumors primary tumors
†0.006: local recurrence versus primary tumors of local recurrences (Wilcoxon test)
First, we compared the expressions of MMPs and TIMPs between the respective primary tumors of patients with LR, patients with distant metastasis as first manifestation of tumoral recurrence, and patients without tumoral recurrence. As can be seen in Table 2, score values for the majority of MMPs and TIMPs were significantly higher in primary tumors of patients with distant metastasis compared to those of patients with LR or without tumoral recurrence. Nevertheless, it is worth mentioning that there were lower score values for MMP-1, -7, and -9, and TIMP-3, in patients with LR compared to patients without tumoral recurrence. Likewise, we found significant differences in the expressions of these factors between the primary tumors of these three groups of patients with regard to the cellular type (stromal cells (fibroblasts or MICs)) expressing each factor within the tumor. Indeed, as seen in Table 3, stromal cells of primary tumors from patients with distant metastasis showed more frequently positive immunoreactivity for MMP-1, -7, -9, -11, -13, and -14, than the other two groups of patients. In addition, primary tumors from patients with LR showed significantly lower expression of MMP-1, -7, and -14 by stromal cells than the other groups. However, primary tumors from patients with LR had significantly high percentages of expression of TIMP-1 and -2 in their stromal cells (Table 3). It is of note that the percentage of expression of these MMPs and TIMPs by stromal cells is similar in LRs and in their primary tumor counterparts (Table 3). On the other hand it is also remarkable to mention that we found no significant associations between MMPs or TIMPs expression and clinico-pathological parameters, such as tumor size, nodal status or histological grade in our study population of patients with breast cancer (data not shown).
Table 3.
Cell type expressing MMPs and TIMPs in primary tumors from patients without tumoral recurrence, from patients with local recurrences, from patients with distant metastasis, and in local recurrences
| Factor | Primary tumors of patients without recurrence | Primary tumors of patients with local recurrence | Primary tumors of patients with distant metastasis | Univariate p value* | Multivariate p value* | Local recurrences |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |||
| MMP-1 | ||||||
| Tumoral cell | 41 (85.4) | 16 (66.7) | 47 (94) | 0.008 | 0.02 | 11 (45.8) |
| Fibroblast | 38 (79.2) | 4 (16.7) | 45 (90) | 0.0001 | 0.001 | 3 (12.5) |
| MIC | 31 (64.6) | 3 (12.5) | 36 (72) | 0.0001 | 0.001 | 4 (16.7) |
| MMP-2 | ||||||
| Tumoral cell | 12 (25) | 5 (20) | 21 (42) | 0.08 | n.s. | 7 (28) |
| Fibroblast | 8 (16.7) | 4 (16) | 15 (30) | n.s. | n.s. | 6 (24) |
| MIC | 0 | 0 | 1 (2) | n.s. | n.s. | 0 |
| MMP-7 | ||||||
| Tumoral cell | 39 (83) | 6 (24) | 46 (92) | 0.0001 | 0.0001 | 3 (12) |
| Fibroblast | 29 (61.7) | 1 (4) | 41 (82) | 0.0001 | 0.001 | 1 (4) |
| MIC | 20 (42.6) | 1 (4) | 32 (64) | 0.0001 | 0.0001 | 0 |
| MMP-9 | ||||||
| Tumoral cell | 29 (58) | 9 (36) | 44 (89.8) | 0.0001 | 0.012 | 16 (64) |
| Fibroblast | 0 | 1 (4) | 27 (34.7) | 0.0001 | 0.01 | 1 (4) |
| MIC | 0 | 5 (20) | 8 (16.3) | 0.007 | 0.0001 | 4 (16) |
| MMP-11 | ||||||
| Tumoral cell | 39 (79.6) | 18 (75) | 46 (93.9) | 0.05 | 0.005 | 19 (79.2) |
| Fibroblast | 19 (38.8) | 15 (62.5) | 43 (87.8) | 0.0001 | 0.05 | 19 (79.2) |
| MIC | 0 | 13 (54.2) | 27 (55.1) | 0.0001 | 0.0001 | 15 (62.5) |
| MMP-13 | ||||||
| Tumoral cell | 37 (74) | 15 (68.2) | 40 (80) | n.s. | n.s. | 14 (63.6) |
| Fibroblast | 14 (28) | 3 (13.6) | 36 (72) | 0.0001 | 0.0001 | 7 (31.8) |
| MIC | 8 (16) | 0 | 28 (56) | 0.0001 | 0.01 | 2 (9.1) |
| MMP-14 | ||||||
| Tumoral cell | 45 (90) | 14 (60.9) | 45 (90) | 0.002 | n.s. | 13 (56.6) |
| Fibroblast | 40 (80) | 4 (17.4) | 39 (78) | 0.0001 | 0.001 | 2 (8.7) |
| MIC | 11 (22) | 3 (13) | 35 (70) | 0.0001 | 0.01 | 7 (30.4) |
| TIMP-1 | ||||||
| Tumoral cell | 45 (90) | 24 (100) | 48 (96) | n.s. | n.s. | 24 (100) |
| Fibroblast | 24 (48) | 19 (79.2) | 26 (52) | 0.03 | n.s. | 21 (87.5) |
| MIC | 3 (6) | 20 (83.3) | 24 (48) | 0.0001 | 0.0001 | 22 (91.7) |
| TIMP-2 | ||||||
| Tumoral cell | 39 (78) | 23 (95.8) | 47 (94) | 0.02 | n.s. | 24 (100) |
| Fibroblast | 6 (12) | 21 (87.5) | 33 (66) | 0.0001 | 0.0001 | 20 (83.3) |
| MIC | 5 (10) | 20 (83.3) | 27 (54) | 0.0001 | 0.0001 | 20 (83.3) |
| TIMP-3 | ||||||
| Tumoral cell | 44 (88) | 18 (72) | 41 (82) | n.s. | n.s. | 19 (76) |
| Fibroblast | 19 (38) | 16 (64) | 36 (72) | 0.002 | 0.01 | 10 (40) |
| MIC | 20 (40) | 2 (8) | 28 (56) | 0.0001 | 0.008 | 3 (20) |
n.s. not significant
* p value for comparing the three groups of primary tumors
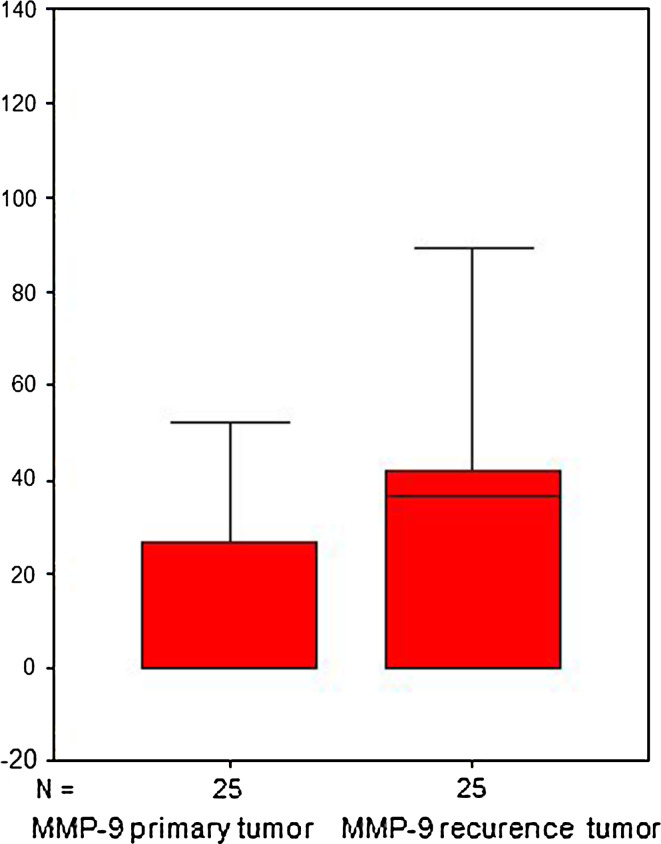
We also investigated possible differences of MMPs and TIMPs expression between primary tumors and their corresponding LRs. As can be seen in Table 2, we only found significant differences for MMP-9 score values. LRs had significantly higher MMP-9 expression than their corresponding primary tumors (p = 0.006; Fig. 2). It is also remarkable that multivariate analysis adjusted for potential confounders confirmed the more above point out as significant for the majority associations between MMPs/TIMPs expression and LR (Tables 2, 3). However, for each cellular type, non-significant differences were found between expressions of MMPs or TIMPs in primary tumors and in LRs (Table 3).
Fig. 2.
Comparative expression of MMP-9 in primary tumors and local recurrence
Our results showed a significantly negative, although low, correlation between MMP-11 expression in primary tumors and paired LRs (r = −0.438; p = 0.032); whereas there was a significant and positive correlation for TIMP-2 between these two paired sets (r = 0.438; p = 0.032). However, no significant correlations for other MMPs or TIMPs, between primary tumors and LRs were found (data not shown).
We also investigated the possible prognostic value of the expressions of MMPs and TIMPs, both in primary tumors as in their corresponding LRs. However, no significant prognostic value of MMPs or TIMPs expression was found (data not shown).
Discussion
To our knowledge, this is the first study comparing the MMPs/TIMPs expression in the primary tumors of breast cancer patients after mastectomy. The patients were grouped in three cohorts: patients who developed LR, patients who developed distant metastasis (both LR and metastasis as first manifestation of tumoral recurrence), and patients without tumoral recurrence. Our results demonstrate significant differences in MMPs and TIMPs expressions between these three groups. Primary tumors from patients with LR have lower expression of MMPs and TIMPs compared to those with distant metastasis, and even, for some MMPs, to those without recurrence. However, it was remarkable that primary tumors in the LR group showed significantly higher percentage of stromal cell expression of TIMP-1 and 2, compared to the other two groups. In addition, we also found evolutionary changes in MMP-9 expression of primary tumors versus their corresponding LRs, which may be of biological and clinical importance.
There are two main hypothesis to explain the origin and significance of LRs: the first one states that LR is caused by an incomplete initial removal of the tumor (Donegan et al. 1966; Auchincloss 1958; Scanlon 1985; Toonkel et al. 1983); the second one proposes that LR is a sign (the first one) of the disease being already disseminated (Crile 1972; Baral et al. 1985; Fisher et al. 1980; Gilliland et al. 1983; Papaioannou 1985; Valagussa et al. 1978). Although, at the present time, it is not possible to determine the cause of isolated LRs, our data shows a significant lower MMPs profile in the primary tumors of patients who develop LR as first manifestation of tumoral progression, suggesting that an incomplete removal could be the cause behind local recurrence. Indeed, the putative relationship between MMPs expression and tumor invasion and distant metastasis is widely accepted. In accordance with our previous reports (Gonzalez et al. 2007; Vizoso et al. 2007), our work demonstrates a high global expression of MMPs (scores values), but additionally we also show a high expression rates of MMPs in stromal cells from primary tumors of patients who develop distant metastasis as first manifestation of tumoral progression. This supports the notion that stromal cells, such as fibroblasts and MICs, at least, could play an active role in tumoral progression. In addition, it is also of note our finding of high expressions of TIMP-1 and -2 in LR fibroblasts. It is well known that TIMPs are multipotential proteins: in addition to their role inhibiting the MMPs, it is now assumed that TIMPs are multifactorial proteins involved in the induction of proliferation as well as in the inhibition of apoptosis (Jiang et al. 2002; Baker et al. 1999). It is remarkable that LR stromal cells have high expression of TIMP-1 and -2. This make us hypothesize that the properties of host stromal cells may contribute to the survival and proliferation of those tumor cells that are incompletely removed in the initial treatment of tumors.
It seems reasonable to consider that the detection of a first isolated LR may be, in part, a consequence of the absence of prior distant tumor development, which might be a selection criteria of low tumoral aggressiveness. Nevertheless, although certain subgroups with more favorable prognosis are believed to exist, the outcome of patients with local or regional breast cancer recurrence after mastectomy is often described as fatal (Veronesi et al. 1995), because many patients develop distant metastases within a short period of time (Aberizk et al. 1986; Bedwinek 1994). To identify subsets of patients differing in the clinical course of the disease, different well-known prognostic factors for primary tumors and/or locoregional tumoral recurrences have been described, such as large size of primary tumors, node-positive status and poorly differentiated grade (Orel et al. 1995; Pisansky et al. 1993; Stefanik et al. 1985; Sykes et al. 1989; Kamby et al. 1991; Willner et al. 1997; Barnes et al. 1991; Berstock et al. 1985; van Tienhoven et al. 1999; Kamby and Sengelov 1999; Pineiro et al. 2004). Nevertheless, it is remarkable that these same parameters are not of value in predicting the risk of LR versus distant metastasis after mastectomy for invasive breast cancer. Because of that, it seems reasonable to consider the possibility that prognostic factors drawn from the recurrence itself might predict the final outcome in a more accurately way. Although the present study does not include enough to reach a prognostic value, it is worth commenting our comparative evaluation of MMPs/TIMPs expression in primary tumors and in their corresponding LRs after mastectomy. Of the 10 parameters analyzed, we only found significant and weak correlations between the primary tumors and the paired LRs for MMP-11 and TIMP-2. This could be the sign of evolutional changes happening in LRs versus their corresponding primary tumors. Similarly, in a prior report, we found no significant concordance for androgen receptors, c-erbB-2 or ki67 (Carreno et al. 2007). Thus, all these data suggest that LRs evolve some of the biological features of their corresponding primary tumors.
Our data also demonstrate that LRs express significantly higher MMP-9 score values than their corresponding primary tumors. MMP-9 was mainly expressed by tumoral cells, which seems to reflect evolutionary biological changes in the LR tumoral cells, which could be of prognostic importance. MMP-9 (Gelatinase B) is related to tumor invasion and metastasis through its special capacity to degrade the type IV collagen found in basement membranes (Jones and Walker 1997), as well as to induce angiogenesis (Egeblad and Werb 2002). There are several reports showing that a high MMP-9 expression correlates significantly with tumoral aggressiveness and poor prognosis in breast cancer (Li et al. 2004; Chantrain et al. 2004; Pellikainen et al. 2004). It has also been described that as breast cancer progresses, activation of MMP-9 occurs during the late cancerous stage (Liotta and Kohn 2001).
In summary, our data indicate differences in MMPs/TIMPs expression between primary tumors of patients with LRs and of those with distant metastasis, both after mastectomy for breast cancer. Further studies are necessary to evaluate if high MMP-9 in local recurrences may be a valuable prognostic marker. We postulate that this marker could be potentially used to select candidates for further therapeutic strategies when local recurrence is the first tumor manifestation after mastectomy for breast cancer.
References
- Aberizk WJ, Silver B, Henderson IC, Cady B, Harris JR (1986) The use of radiotherapy for treatment of isolated locoregional recurrence of breast carcinoma after mastectomy. Cancer 58:1214–1218 [DOI] [PubMed] [Google Scholar]
- Alexander JW, Altemeier WA (1964) Susceptibility of injured tissues to hematogenous metastases: an experimental study. Ann Surg 159:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss H Jr (1958) The nature of local recurrence following radical mastectomy. Cancer 11:611–619 [DOI] [PubMed] [Google Scholar]
- Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC (1999) Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer 79:1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EA, Stephenson TJ, Reed MW, Brown NJ (2002) Expression of proteinases and inhibitors in human breast cancer progression and survival. Mol Pathol 55:300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral E, Ogenstad S, Wallgren A (1985) The effect of adjuvant radiotherapy on the time of occurrence and prognosis of local recurrence in primary operable breast cancer. Cancer 56:2779–2782 [DOI] [PubMed] [Google Scholar]
- Barnes R, Masood S, Barker E et al (1991) Low nm23 protein expression in infiltrating ductal breast carcinomas correlates with reduced patient survival. Am J Pathol 139:245–250 [PMC free article] [PubMed] [Google Scholar]
- Bedwinek J (1994) Natural history and management of isolated local-regional recurrence following mastectomy. Semin Radiat Oncol 4:260–269 [DOI] [PubMed] [Google Scholar]
- Bedwinek JM, Lee J, Fineberg B, Ocwieza M (1981) Prognostic indicators in patients with isolated local-regional recurrence of breast cancer. Cancer 47:2232–2235 [DOI] [PubMed] [Google Scholar]
- Berstock DA, Houghton J, Haybittle J, Baum M (1985) The role of radiotherapy following total mastectomy for patients with early breast cancer. World J Surg 9:667–670 [DOI] [PubMed] [Google Scholar]
- Borner M, Bacchi M, Goldhirsch A et al (1994) First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. Swiss Group for Clinical Cancer Research. J Clin Oncol 12:2071–2077 [DOI] [PubMed] [Google Scholar]
- Carreno G, Del Casar JM, Corte MD et al (2007) Local recurrence after mastectomy for breast cancer: analysis of clinicopathological, biological and prognostic characteristics. Breast Cancer Res Treat 102:61–73 [DOI] [PubMed] [Google Scholar]
- Chantrain CF, Shimada H, Jodele S et al (2004) Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res 64:1675–1686 [DOI] [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E et al (1998) Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol 161:6845–6852 [PubMed] [Google Scholar]
- Crile G Jr (1972) Low incidence and morbidity of local recurrence after conservative operations for cancer of the breast. Ann Surg 175:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao TL, Nemoto T (1963) The clinical significance of skin recurrence after radical mastectomy in women with cancer of the breast. Surg Gynecol Obstet 117:447–453 [PubMed] [Google Scholar]
- Dao TL, Yogo H (1967) Enhancement of pulmonary metastases by x-irradiation in rats bearing mammary cancer. Cancer 20:2020–2025 [DOI] [PubMed] [Google Scholar]
- Dixon AR, Ellis IO, Elston CW, Blamey RW (1991) A comparison of the clinical metastatic patterns of invasive lobular and ductal carcinomas of the breast. Br J Cancer 63:634–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan WL, Perez-Mesa CM, Watson FR (1966) A biostatistical study of locally recurrent breast carcinoma. Surg Gynecol Obstet 122:529–540 [PubMed] [Google Scholar]
- Dong Z, Kumar R, Yang X, Fidler IJ (1997) Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell 88:801–810 [DOI] [PubMed] [Google Scholar]
- Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkan GC, Nutt JE, Marsh C et al (2003) Alteration in urinary matrix metalloproteinase-9 to tissue inhibitor of metalloproteinase-1 ratio predicts recurrence in nonmuscle-invasive bladder cancer. Clin Cancer Res 9:2576–2582 [PubMed] [Google Scholar]
- Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174 [DOI] [PubMed] [Google Scholar]
- Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J (2000) Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett 486:247–251 [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Zeidman I (1972) Enhancement of experimental metastasis by X-ray: a possible mechanism. J Med 3:172–177 [PubMed] [Google Scholar]
- Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM (2001) Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Fisher ER (1959) Experimental studies of factors influencing hepatic metastases. III. Effect of surgical trauma with special reference to liver injury. Ann Surg 150:731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Redmond C, Fisher ER (1980) The contribution of recent NSABP clinical trials of primary breast cancer therapy to an understanding of tumor biology—an overview of findings. Cancer 46:1009–1025 [DOI] [PubMed] [Google Scholar]
- Gilliland MD, Barton RM, Copeland EM 3rd (1983) The implications of local recurrence of breast cancer as the first site of therapeutic failure. Ann Surg 197:284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LO, Pidal I, Junquera S et al (2007) Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer 97:957–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieu F, Li WQ, Iacopetta B (2004) Genetic polymorphisms in the MMP-2 and MMP-9 genes and breast cancer phenotype. Breast Cancer Res Treat 88:197–204 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Goldberg ID, Shi YE (2002) Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene 21:2245–2252 [DOI] [PubMed] [Google Scholar]
- Jones JL, Walker RA (1997) Control of matrix metalloproteinase activity in cancer. J Pathol 183:377–379 [DOI] [PubMed] [Google Scholar]
- Jones JL, Glynn P, Walker RA (1999) Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol 189:161–168 [DOI] [PubMed] [Google Scholar]
- Kaae S, Johansen H (1962) Breast cancer; five year results: two random series of simple mastectomy with postoperative irradiation versus extended radical mastectomy. Am J Roentgenol Radium Ther Nucl Med 87:82–88 [PubMed] [Google Scholar]
- Kamby C, Sengelov L (1999) Survival and pattern of failure following locoregional recurrence of breast cancer. Clin Oncol (R Coll Radiol) 11:156–163 [DOI] [PubMed] [Google Scholar]
- Kamby C, Andersen J, Ejlertsen B et al (1991) Pattern of spread and progression in relation to the characteristics of the primary tumour in human breast cancer. Acta Oncol 30:301–308 [DOI] [PubMed] [Google Scholar]
- Karabali-Dalamaga S, Souhami RL, O’Higgins NJ, Soumilas A, Clark CG (1978) Natural history and prognosis of recurrent breast cancer. Br Med J 2:730–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour J, Le M, Caceres E, Koszarowski T, Veronesi U, Hill C (1983) Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten year results of an international cooperative trial in breast cancer. Cancer 51:1941–1943 [DOI] [PubMed] [Google Scholar]
- Lacour J, Le MG, Hill C, Kramar A, Contesso G, Sarrazin D (1987) Is it useful to remove internal mammary nodes in operable breast cancer? Eur J Surg Oncol 13:309–314 [PubMed] [Google Scholar]
- Li HC, Cao DC, Liu Y et al (2004) Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat 88:75–85 [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411:375–379 [DOI] [PubMed] [Google Scholar]
- Manes S, Llorente M, Lacalle RA et al (1999) The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem 274:6935–6945 [DOI] [PubMed] [Google Scholar]
- McCarthy K, Maguire T, McGreal G, McDermott E, O’Higgins N, Duffy MJ (1999) High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer 84:44–48 [DOI] [PubMed] [Google Scholar]
- Mimori K, Ueo H, Shirasaka C, Mori M (2001) Clinical significance of MT1-MMP mRNA expression in breast cancer. Oncol Rep 8:401–403 [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Giannopoulou I, Stefanaki K et al (2002) Enhanced mRNA expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) in breast carcinomas is correlated with adverse prognosis. J Pathol 197:307–313 [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Rank F, Lopez JM et al (2001) Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res 61:7091–7100 [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K et al (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114:111–118 [DOI] [PubMed] [Google Scholar]
- Orel SG, Schnall MD, Powell CM et al (1995) Staging of suspected breast cancer: effect of MR imaging and MR-guided biopsy. Radiology 196:115–122 [DOI] [PubMed] [Google Scholar]
- Papaioannou AN (1985) Hypothesis: increasingly intensive locoregional treatment of breast cancer may promote recurrence. J Surg Oncol 30:33–41 [DOI] [PubMed] [Google Scholar]
- Parker RL, Huntsman DG, Lesack DW et al (2002) Assessment of interlaboratory variation in the immunohistochemical determination of estrogen receptor status using a breast cancer tissue microarray. Am J Clin Pathol 117:723–728 [DOI] [PubMed] [Google Scholar]
- Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM (2004) Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res 10:7621–7628 [DOI] [PubMed] [Google Scholar]
- Pineiro A, Salinas J, Illana J et al (2004) Locoregional recurrence and metastasis in the long-term follow-up of postmastectomy breast cancer patients with T1–T2 tumors and one to three positive lymph nodes. Rev Oncol 6:341–346 [Google Scholar]
- Pisansky TM, Ingle JN, Schaid DJ et al (1993) Patterns of tumor relapse following mastectomy and adjuvant systemic therapy in patients with axillary lymph node-positive breast cancer. Impact of clinical, histopathologic, and flow cytometric factors. Cancer 72:1247–1260 [DOI] [PubMed] [Google Scholar]
- Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O (1997) High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 3:1623–1628 [PubMed] [Google Scholar]
- Remacle A, McCarthy K, Noel A et al (2000) High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer 89:118–121 [DOI] [PubMed] [Google Scholar]
- Rifkin DB, Mazzieri R, Munger JS, Noguera I, Sung J (1999) Proteolytic control of growth factor availability. Apmis 107:80–85 [DOI] [PubMed] [Google Scholar]
- Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, Reiss M (2001) Tissue microarray: a new technology for amplification of tissue resources. Cancer J 7:24–31 [PubMed] [Google Scholar]
- Scanlon EF (1985) Local recurrence in the pectoralis muscles following modified radical mastectomy for carcinoma. J Surg Oncol 30:149–151 [DOI] [PubMed] [Google Scholar]
- Schrohl AS, Christensen IJ, Pedersen AN et al (2003) Tumor tissue concentrations of the proteinase inhibitors tissue inhibitor of metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitor type 1 (PAI-1) are complementary in determining prognosis in primary breast cancer. Mol Cell Proteomics 2:164–172 [DOI] [PubMed] [Google Scholar]
- Schrohl AS, Holten-Andersen MN, Peters HA et al (2004) Tumor tissue levels of tissue inhibitor of metalloproteinase-1 as a prognostic marker in primary breast cancer. Clin Cancer Res 10:2289–2298 [DOI] [PubMed] [Google Scholar]
- Schuck A, Konemann S, Matthees B et al (2002) Radiotherapy in the treatment of locoregional relapses of breast cancer. Br J Radiol 75:663–669 [DOI] [PubMed] [Google Scholar]
- Sivula A, Talvensaari-Mattila A, Lundin J et al (2005) Association of cyclooxygenase-2 and matrix metalloproteinase-2 expression in human breast cancer. Breast Cancer Res Treat 89:215–220 [DOI] [PubMed] [Google Scholar]
- Skipper D, Jeffrey MJ, Cooper AJ, Alexander P, Taylor I (1989) Enhanced growth of tumour cells in healing colonic anastomoses and laparotomy wounds. Int J Colorectal Dis 4:172–177 [DOI] [PubMed] [Google Scholar]
- Spratt JS (1967) Locally recurrent cancer after radical mastectomy. Cancer 20:1051–1053 [DOI] [PubMed] [Google Scholar]
- Stefanik D, Goldberg R, Byrne P et al (1985) Local-regional failure in patients treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 3:660–665 [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG (1999) Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest 103:1237–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes HF, Sim DA, Wong CJ, Cassady JR, Salmon SE (1989) Local-regional recurrence in breast cancer after mastectomy and adriamycin-based adjuvant chemotherapy: evaluation of the role of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 16:641–647 [DOI] [PubMed] [Google Scholar]
- Symmans WF, Ayers M, Clark EA et al (2003) Total RNA yield and microarray gene expression profiles from fine-needle aspiration biopsy and core-needle biopsy samples of breast carcinoma. Cancer 97:2960–2971 [DOI] [PubMed] [Google Scholar]
- Talvensaari-Mattila A, Paakko P, Blanco-Sequeiros G, Turpeenniemi-Hujanen T (2001) Matrix metalloproteinase-2 (MMP-2) is associated with the risk for a relapse in postmenopausal patients with node-positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Res Treat 65:55–61 [DOI] [PubMed] [Google Scholar]
- Talvensaari-Mattila A, Paakko P, Turpeenniemi-Hujanen T (2003) Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br J Cancer 89:1270–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toonkel LM, Fix I, Jacobson LH, Wallach CB (1983) The significance of local recurrence of carcinoma of the breast. Int J Radiat Oncol Biol Phys 9:33–39 [DOI] [PubMed] [Google Scholar]
- Turk V, Kos J, Turk B (2004) Cysteine cathepsins (proteases)—on the main stage of cancer? Cancer Cell 5:409–410 [DOI] [PubMed] [Google Scholar]
- Valagussa P, Bonadonna G, Veronesi U (1978) Patterns of relapse and survival following radical mastectomy. Analysis of 716 consecutive patients. Cancer 41:1170–1178 [DOI] [PubMed] [Google Scholar]
- van Tienhoven G, Voogd AC, Peterse JL et al (1999) Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Eur J Cancer 35:32–38 [DOI] [PubMed] [Google Scholar]
- Veronesi U, Marubini E, Del Vecchio M et al (1995) Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst 87:19–27 [DOI] [PubMed] [Google Scholar]
- Visscher DW, Hoyhtya M, Ottosen SK et al (1994) Enhanced expression of tissue inhibitor of metalloproteinase-2 (TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer 59:339–344 [DOI] [PubMed] [Google Scholar]
- Vizoso FJ, Gonzalez LO, Corte MD et al (2007a) Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer 96:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso FJ, Rodriguez M, Altadill A et al (2007b) Liver expression of steroid hormones and apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol 13:3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner J, Kiricuta IC, Kolbl O (1997) Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys 37:853–863 [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I (2000) Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14:163–176 [PMC free article] [PubMed] [Google Scholar]