Abstract
Study Objectives:
Abdominal obesity, particularly common in centrally obese males, may have a negative impact on upper airway (UA) function during sleep. For example, cranial displacement of the diaphragm with raised intra-abdominal pressure may reduce axial tension exerted on the UA by intrathoracic structures and increase UA collapsibility during sleep.
Design:
This study aimed to examine the effect of abdominal compression on UA function during sleep in obese male obstructive sleep apnea patients.
Setting:
Participants slept in a sound-insulated room with physiologic measurements controlled from an adjacent room.
Participants:
Fifteen obese (body mass index: 34.5 ± 1.1 kg/m2) male obstructive sleep apnea patients (apnea-hypopnea index: 58.1 ± 6.8 events/h) aged 50 ± 2.6 years participated.
Interventions:
Gastric (PGA) and transdiaphragmatic pressures (PDI), UA closing pressure (UACP), UA airflow resistance (RUA), and changes in end-expiratory lung volume (EELV) were determined during stable stage 2 sleep with and without abdominal compression, achieved via inflation of a pneumatic cuff placed around the abdomen. UACP was assessed during brief mask occlusions.
Measurements and Results:
Abdominal compression significantly decreased EELV by 0.53 ± 0.24 L (P = 0.045) and increased PGA (16.2 ± 0.8 versus 10.8 ± 0.7 cm H2O, P < 0.001), PDI (11.7 ± 0.9 versus 7.6 ± 1.2 cm H2O, P < 0.001) and UACP (1.4 ± 0.8 versus 0.9 ± 0.9 cm H2O, P = 0.039) but not RUA (6.5 ± 1.4 versus 6.9 ± 1.4 cm H2O·L/s, P = 0.585).
Conclusions:
Abdominal compression negatively impacts on UA collapsibility during sleep and this effect may help explain strong associations between central obesity and obstructive sleep apnea.
Citation:
Stadler DL; McEvoy RD; Sprecher KE; Thomson KJ; Ryan MK; Thompson CC; Catcheside PG. Abdominal compression increases upper airway collapsibility during sleep in obese male obstructive sleep apnea patients. SLEEP 2009;32(12):1579-1587.
Keywords: Obstructive sleep apnea, abdominal loading, lung volume, caudal traction
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON SLEEP DISORDER CHARACTERIZED BY REPETITIVE PERIODS OF UPPER AIRWAY (UA) COLLAPSE DURING sleep. Male gender and obesity are 2 key predictors of OSA,1,2 but the mechanisms via which these factors contribute to OSA remain unclear. Central obesity, particularly common in obese male OSA patients, leads to increased intra-abdominal pressure (IAP). This pressure may have an important influence on diaphragm position, which may affect the degree of axial tension (caudal traction) exerted on the UA, and therefore the propensity for UA collapse. Such effects are likely to be most evident in the supine posture and during sleep, when other compensatory mechanisms are diminished and may at least partly explain gender and obesity influences in OSA.
While airway patency is importantly modulated by respiratory drive and negative airway pressure reflex activation of UA dilator muscles during inspiration, caudal traction is also likely to influence UA patency throughout the respiratory cycle. Studies in anesthetized animals3,4 show that decreased UA tension via cranial tracheal movement promotes UA collapse, which emphasizes the potential importance of tracheal traction for maintaining airway patency.
Investigating the influence of tracheal traction on UA function in humans is inevitably difficult. Changes in lung volume are well known to affect UA function and may reflect predominantly tracheal traction effects. Several studies have shown UA patency to decrease with compression of the abdominal and thoracic compartments via positive extrathoracic pressure.5–7 While data concerning the magnitude of lung volume changes during sleep with obesity are currently lacking, end-expiratory lung volume (EELV), which normally falls ~15% during sleep in healthy weight individuals,8,9 is profoundly reduced in obese patients during general anesthesia10 and likely during sleep. EELV reductions, at least in anesthesia, appear to be largely explained by cranial movement of the diaphragm.11 Consequently, changes in lung volume with obesity alone may substantially underpin the increased propensity for UA collapse in OSA.
Extrathoracic pressure induced changes in lung volume may have different effects on transdiaphragmatic forces, lung volume and tracheal traction than the thoracoabdominal compressive effects of obesity. While closely related to lung volume changes, intra-abdominal and transdiaphragmatic pressure changes are potentially stronger determinants of UA function than lung volume change per se. IAP, a key determinant of transdiaphragmatic force is approximately doubled in obese compared to healthy-weight individuals.12 Consequently, abdominal mass loading effects of obesity appear likely to influence UA function during sleep via influences on tracheal traction. However, direct evidence that abdominal compression has any impact on UA function during sleep is currently lacking.
The aim of this study was to examine the effect of experimental abdominal compression on UA function during sleep in obese male OSA patients, to test the hypothesis that acute abdominal compression would increase UA collapsibility and UA resistance (RUA) during sleep.
METHODS
Patient selection
Twenty-five obese (body mass index [BMI] 30-40 kg/m2) male OSA patients with moderate-to-severe OSA (apnea-hypopnea index [AHI] > 30 events/h) and between the ages of 18-65 years participated. Apart from OSA, patients were void of any other respiratory diseases and all demonstrated normal lung function (forced expiratory volume in 1 sec [FEV1] and forced vital capacity [FVC] > 80% predicted, JLab software version 4.53; Compactlab, Jaeger, Wuerzburg, Germany). Patients were asked to refrain from consuming caffeine and alcohol for 12 and 24 hours prior to the experiment respectively. This study was approved by the Daw Park Repatriation General Hospital and Adelaide University Human Research and Ethics Committees. Each patient gave informed written consent to participate in the study.
Measurements and Equipment
Sleep was monitored by 2 channels of EEG (C4/A1, C3/A2), left and right electro-oculograms, submental EMG, and ECG. Sleep recordings were classified according to standard sleep staging13 and arousal scoring criteria14 by a single experienced polysomnographer who was blinded to the experimental conditions. The nostrils were decongested with xylometazoline hydrochloride nasal spray (Otrivin, Novartis Australasia, Rowville, Victoria, Australia) and anesthetized (2% lignocaine spray). Esophageal (PES) and gastric (PGA) pressures were recorded via 2 separate latex balloon catheters consisting of a 5-cm balloon (Viasys Healthcare, Hoechberg, Germany) attached to the distal end of polyethylene tubing (2.08 mm OD, 1.57 mm ID; Microtube Extrusions, North Rocks, NSW, Australia). PGA and PES catheters were advanced approximately 60 cm and 45 cm respectively through the most patent nostril. The balloons were then filled with ~0.5 mL air, and each catheter connected to solid-state pressure transducers (Spectramed DTX, Oxnard, USA). Catheter positions were adjusted until positive PGA and negative PES swings were detected during and in phase with inspiration. Epiglottic (PEPI) pressure was assessed by a thin air-perfused nasal catheter (see Hilditch et al.15 for further detail). This catheter was advanced 1–2 cm below the base of the tongue under direct visualization and connected to another pressure transducer (MP45; Validyne Engineering, Northridge, CA). All catheters were taped at the nose. Patients were fitted with a nasal mask (Series 2600, Hans Rudolph, Kansas City, MO, USA). Nasal flow and volume were measured by a pneumotachograph (PT16, Jaeger, Germany). Arterial oxyhemoglobin saturation was recorded by finger pulse oximetry (POET II model 602-3; Criticare Systems, Waukesha, WI) while end-tidal CO2 was measured at the mask (Capstar-100, CWE Inc, PA). Mask pressure (PMASK) was measured by a differential pressure transducer (MP45; Validyne Engineering, Northridge, CA) referenced to atmospheric pressure. Transdiaphragmatic pressure (PDI) was determined as PGA-PES at end-expiration. Abdominal and thoracic excursions were measured continuously using 2 pairs of magnetometer coils (Polhemus Liberty, Colchester, USA) placed in the anterior-posterior (AP) axis of the chest and abdomen. Abdominal compression was achieved by 2 large inflatable pneumatic cuffs (5082-88-1, Welch Allyn Inc, Arden, USA) with a width of 21 cm connected in series and placed externally around the abdomen. Care was taken to ensure abdominal compression did not directly restrict lower rib cage movement. This was achieved by positioning the cranial edge of the pressure cuff below the anterior lower ribs. The cuff was inflated and rapidly deflated remotely via delivery of compressed air, and via suction respectively. Cuff pressure (PCUFF) was recorded throughout each study using a solid-state pressure transducer (Spectramed DTX, Oxnard, USA) connected via a T-piece at the cuff inlet.
All conventional sleep-related signals were recorded on a Compumedics data acquisition system (E-series, Compumedics Inc., Melbourne, Australia). X, Y, and Z coordinates for each of the 4 magnetometer sensors were acquired on a second computer at a sample rate of 120 Hz. The remaining signals were recorded on a 32-channel Windaq (DI-720 DATAQ Instruments Inc, OH, USA) data acquisition system at 200 Hz. To allow accurate time-matching among the 3 recording systems, a computer activated event mark signal was simultaneously placed on all 3 acquisition systems coincident with the onset of each mask occlusion.
Nasal Continuous Positive Airway Pressure (CPAP) Apparatus and Assessment of UA Collapsibility
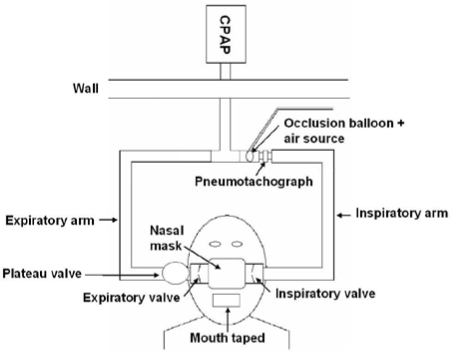
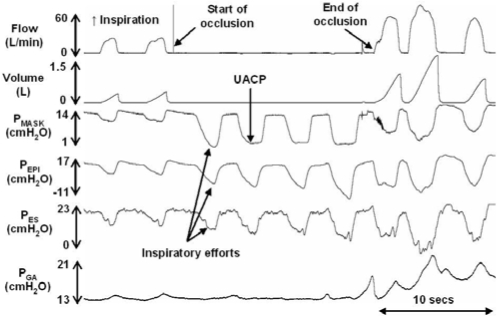
A custom designed breathing circuit (Figure 1) similar to that described by Issa and Sullivan16 was constructed to allow delivery of nasal CPAP as well as rapid external occlusion of the airway. External mask occlusion was achieved via a computer-controlled solenoid valve that delivered compressed air to rapidly inflate a balloon occlusion valve near the inspiratory port of the mask (Figure 1). Occlusions were commenced near end-expiration and were sustained until UA closure had developed or until there was clear evidence of EEG arousal. During occlusions (Figure 2), inspiratory efforts produce parallel increases in PMASK, PEPI, and PES throughout inspiration when the airway is patent. Subsequent inspiratory efforts produce progressively larger pressure deflections with increasing respiratory drive until a critical pressure is reached where PMASK ceases to change despite progressively more negative PEPI and PES. This critical pressure is indicative of UA collapse and was defined as UA closing pressure (UACP).16,17
Figure 1.
A schematic diagram of the breathing circuit used to deliver continuous positive airway pressure (CPAP) and the undertaking of external mask occlusions. CPAP was delivered to the patient via the inspiratory limb and air was expired via the plateau valve. During an external mask occlusion, a balloon located upstream of the mask was rapidly inflated near end-expiration. Occlusion termination was achieved by rapid balloon deflation.
Figure 2.
A 30-sec trace showing the events during a brief external mask occlusion in an OSA patient. Note the parallel increase in mask (PMASK), epiglottic (PEPI) and esophageal (PES) pressures during the first inspiratory effort followed by a return back to baseline. During subsequent efforts, PMASK again initially tracks PEPI and PES until a point at which it deflects away from PEPI and PES. This deflection point is indicative of UA collapse and is classified as the UACP.
Protocol
Following equipment setup and with patients supine, a trial of abdominal compression was performed during wakefulness to find a cuff inflation pressure that the patient believed was tolerable during sleep. This pressure was designated as the “inflated” cuff pressure for the remainder of the study. Patients were asked to relax and breathe nasally during 5 min of quite wakefulness for baseline ventilatory recordings prior to lights out. The patient's mouth was then taped (Sleek; Smith and Nephew, London, UK) to ensure nasal breathing and prevent mouth leaks. CPAP was commenced at the therapeutic level and the patient was allowed to fall asleep while remaining in the supine position. All subsequent experimental manipulations conducted by the researchers were undertaken from an adjacent room. Patients were monitored continuously by a video camera and polysomnograph recordings. Once asleep, the minimum level of CPAP required to abolish flow limitation was determined and then maintained throughout the remainder of the night unless adjustments became necessary (see below). Cuff state (deflated or inflated), initially chosen at random, was then initiated. Following at least one full 30-sec epoch of stable stage 2 sleep, a brief external mask occlusion was performed to assess UA collapsibility. An occlusion was terminated if the patient aroused during the occlusion or following 2-3 inspiratory efforts showing UA closure (Figure 2). At least 30 sec of stable stage 2 sleep without arousal was subsequently required before the next occlusion. Approximately 3-5 occlusions were conducted under the initial cuff condition before cuff state was alternated. This process continued throughout the remainder of the night to record as many multiple replicate measures of UACP as was possible under both cuff conditions during stage 2 sleep. In 4 patients, flow limitation recurred later in the night requiring a 1-2 cm H2O increase in CPAP. In addition, 6 patients briefly moved from the supine posture due to discomfort and allowed to return to sleep. Following ~10 min of sleep, patients were awoken and asked to shift back to the supine position. CPAP was monitored and adjusted if required before mask occlusions were again undertaken once stage 2 sleep was re-established. To control for CPAP, postural and other potential sleep stage effects, all cuff deflated vs inflated comparisons were restricted to supine stage 2 sleep, where CPAP was at the same level between cuff conditions.
Data Analysis
Chest and abdominal anterior-posterior excursions (ΔAPCHEST and ΔAPABDO) and pneumotachograph derived tidal volume (VT) during the baseline wake period were calculated breath-by-breath via custom software.18 A statistical calibration method19 was employed to solve the regression constants achieving the best fit of VT (change in lung volume from EELV to end-inspiration) versus magnetometer excursion in the following equation;
VT (mL) = ΔEELV (mL) = M*[(K*ΔAPCHEST (cm))+ΔAPABDO (cm)]
Where: K = the relative contribution of chest versus abdominal excursions and M = a scaling factor
Changes in EELV induced by external abdominal compression were calculated using the best fit regression constants K and M and the appropriate measurements of chest and abdominal displacements. ΔAPCHEST and ΔAPABDO were defined as the difference between the minimum APCHEST and APABDO dimensions (in cm) at end-expiration, for each breath in the preceding 30 sec prior to occlusions between periods with and without abdominal compression.
Respiratory data including VT, inspiratory, expiratory and total breath time (TI, TE, TTOT), breathing frequency (FB), minute ventilation (VI), peak inspiratory flow (PIF), end-expiratory PGA, PES and PDI, and RUA were calculated on a breath-by-breath basis using custom analysis software used previously.18,20 Baseline ventilatory parameters were determined by averaging breath-by-breath measures in the 30 sec prior to each occlusion. A single observer, blinded to cuff condition and PCUFF, as well as patient and occlusion number, determined UACP. The first breath clearly exhibiting PMASK flattening was identified for each trial. The pressure at which PMASK flattening began was defined as the UACP (Figure 2). Trials were excluded from data analysis if UACP was not reached (e.g., due to arousal), if the occlusion occurred other than in stage 2 sleep or if the patient was not in the supine position (confirmed by video camera) during the mask occlusion.
Statistical Analysis
Data from replicate trials were averaged within each condition in each subject and the effect of cuff state (deflated and inflated) on UACP, lung volume change and respiratory parameters analyzed using Student's paired t-tests. Relationships between single variables were examined by Pearson correlation. Multivariate linear regression analyses using backwards linear regression with P values < 0.05 to enter and P values > 0.1 to remove were undertaken to determine independent predictors of UACP. Variables entered were PGA, PDI, BMI, waist circumference, hip circumference, waist-to-hip ratio, and AHI. All data are expressed as means ± SEM. Statistical significance was inferred when P < 0.05. Statistical analyses were performed using SPSS for Windows software version 16.0 (SPSS, Inc., Chicago, IL.).
RESULTS
Anthropometric Data
Twenty-five patients were recruited, with 15 successfully completing the study. Data from the remaining 10 patients were excluded due to inability to sleep (5 patients) or mask leak and/or oral breathing despite all efforts to prevent leaks (5 patients). Of the 15 successful studies, one patient was unable to tolerate the gastric and esophageal catheters and RUA data from 2 patients were unavailable because of catheter blockage. Anthropometric measurements are displayed in Table 1. Patients were middle-aged, obese with severe OSA. Lung function was in the normal range for this group.
Table 1.
Anthropometric Data for 15 OSA Patients
| Mean ± SEM | Range | |
|---|---|---|
| Age (years) | 50.0 ± 2.6 | 33–65 |
| BMI (kg/m2) | 34.5 ± 1.1 | 29.7–41.6 |
| AHI (events/h) | 58.1 ± 6.8 | 30–114 |
| WC (cm) | 119.3 ± 3.3 | 104–136 |
| HC (cm) | 119.0 ± 2.8 | 103–130 |
| WHR | 1.0 ± 0.1 | 0.89–1.06 |
| FEV1 (% predicted) | 98.3 ± 3.1 | 82–122 |
| FVC (% predicted) | 93.7 ± 3.0 | 79–110 |
BMI = body mass index; AHI = apnea-hypopnea index; WC = waist circumference; HC = hip circumference; WHR = waist-to-hip ratio; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity
Effect of Abdominal Compression
Parameters recorded in the 30-sec period preceding each cuff deflation and inflation occlusion trial are presented in Table 2. CPAP was not different between cuff conditions. Abdominal cuff inflation produced a significant increase in FB via TE shortening. However, a concomitant VT reduction was evident such that VI remained unchanged with abdominal compression. There was a non-significant trend for a decrease in PIF with abdominal compression (P = 0.07).
Table 2.
Parameters Recorded in the 30-sec Baseline Period Preceding Airway Occlusion Trials with and without Abdominal Compression
| Cuff Deflated | Cuff Inflated | P value | |
|---|---|---|---|
| PCUFF (cm H2O) | 0 | 26.5 ± 1.3 | < 0.001 |
| CPAP (cm H2O) | 13.2 ± 1.1 | 13.3 ± 1.1 | 0.304 |
| TI (sec) | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.233 |
| TE (sec) | 2.4 ± 0.2 | 2.2 ± 0.2 | 0.023 |
| TTOT (sec) | 4.3 ± 0.2 | 4.2 ± 0.2 | 0.318 |
| FB (breaths/min) | 14.5 ± 0.6 | 14.8 ± 0.6 | 0.039 |
| VT(L) | 0.54 ± 0.02 | 0.52 ± 0.02 | 0.011 |
| VI (L/min) | 7.7 ± 0.3 | 7.6 ± 0.2 | 0.343 |
| PIF (L/min) | 43.4 ± 1.7 | 41.7 ± 1.7 | 0.070 |
| RUA (cm H2O L/s)# | 6.8 ± 1.4 | 6.5 ± 1.4 | 0.585 |
PCUFF = cuff pressure; CPAP = continuous positive airway pressure; TI, TE, TTOT = inspiratory, expiratory and total breath time respectively; FB = breathing frequency; VT = tidal volume; VI, = minute ventilation; PIF = peak inspiratory flow; RUA = upper airway airflow resistance.
Values are mean ± SEM. (N = 15,).
N = 13
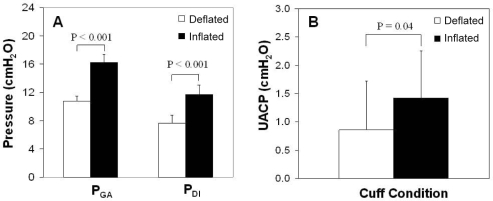
An average of 16.2 ± 3.8 (range 4–62) and 15.7 ± 4.0 (range 2–64) occlusion trials with and without cuff inflation, respectively, were successfully completed during stage 2 sleep. Abdominal compression significantly increased PGA and PDI in the order of 50% (Figure 3A, P < 0.001 for both), while there was a trend for increased end-expiratory PES (4.6 ± 0.8 vs 3.2 ± 0.8 cm H2O, P = 0.07). UACP also increased following abdominal compression (Figure 3B, 1.4 ± 0.8 vs 0.9 ± 0.9 cm H2O, P = 0.04), but RUA remained unchanged (Table 2, P = 0.59). Abdominal compression was accompanied by an abdominal volume shift of 1.1 ± 0.2 L (P < 0.001) and an increase in thoracic volume of 0.5 ± 0.1 L (P = 0.001), leading to an overall decrease in EELV by 0.5 ± 0.2 L (P = 0.045).
Figure 3.
The effect of external abdominal compression on (A) gastric pressure (PGA) and transdiaphragmatic pressure (PDI) and (B) upper airway closing pressure (UACP) Means ± SEM. (N = 14 for PGA and PDI and N = 15 for UACP).
Regression Analyses
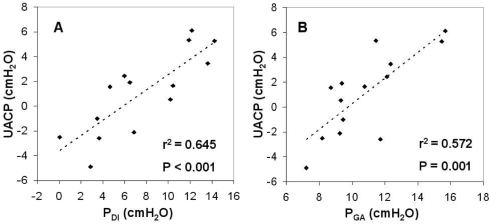
Baseline (cuff deflated) PGA was found to significantly correlate with waist circumference (r2 = 0.30, P = 0.049). No variables correlated with AHI. However, baseline UACP was strongly correlated with baseline PDI (Figure 4A) and baseline PGA (Figure 4B), r2 = 0.645 and r2 = 0.572 respectively (both P < 0.001), and there was a trend for a correlation between PGA and UACP following abdominal compression (r2 = 0.24, P = 0.08). There was no correlation between the ΔUACP with either the ΔPDI or ΔPGA. In multivariate analyses, baseline PDI was found to be the only independent predictor of baseline UACP (P < 0.001), accounting for 73% (r2 = 0.731) of the variance in UACP.
Figure 4.
Relationship between (A) upper airway closing pressure (UACP) with transdiaphragmatic pressure (PDI) and (B) with gastric pressure (PGA) without abdominal compression (N = 14).
DISCUSSION
The major finding of this study was that abdominal compression significantly increased UA collapsibility during sleep in obese OSA patients. This is the first study to demonstrate a direct effect of abdominal loading on UA collapsibility in sleep, and implicates mechanical effects of abdominal obesity as a potentially important mechanism causing increased UA collapsibility in OSA.
Effect of Abdominal Compression on UA Function and Respiratory Variables
It is not possible to determine the precise mechanisms underpinning changes in UACP with abdominal compression in this study. Potential mechanisms include (a) direct mechanical effects of increased IAP and/or lung volume mediated influences on UA compliance, and/or (b) hemodynamic effects or increased edema surrounding the UA.
There are likely to be complex interactions between variables potentially mediating increased UA collapsibility following abdominal compression, because of the nature of anatomical connections between the UA and surrounding/distal tissues, the geometry of diaphragm insertion to the rib cage and variable effects of obesity and abdominal compressive forces on chest and abdominal compartment volumes and compliances. A smaller EELV seen in obese individuals in the supine position compared to healthy-weight controls21 is likely dominated by cranial ascent of the diaphragm as a consequence of increased IAP, with further rises in IAP promoting additional cranial displacement limited by diaphragm stretch and opposing inspiratory effects of rib cage expansion.22 In the current study, abdominal compression increased PGA and PDI in the order of ~50%, produced a marginal increase in PES and decreased the abdominal AP dimension by ~1.8 cm, with slight AP chest expansion. Given that the abdominal compartment is relatively incompressible, the reduced abdominal AP dimension with abdominal compression indicates abdominal volume redistribution, equating to a volume shift of ~1 L, of which ~0.5 L was exhaled and ~0.5 L translated to an increase in rib cage AP dimensions and volume, resulting in a net fall in EELV of ~0.5 L. Increased IAP, via external loading or CO2 insufflation into the peritoneal cavity, leads to a rightward shift and a decrease in the slope of the pressure-volume curve of the total respiratory system, primarily via a fall in chest wall compliance.23–25 The expansion of the rib cage following abdominal compression has been documented by others26 and likely reflects lateral lower rib displacement at the zone of apposition of the diaphragm and rib cage (appositional effect) while passive tension generated by the diaphragm elevates the rib cage by its insertions at the costal margin (insertional effects)22,26 leading to an inspiratory effect on the rib cage.
A change in EELV is known to have profound effects on UA function. Lung inflation above resting EELV increases UA cross-sectional area,27,28 reduces airway collapsibility29 and airflow resistance.6 Conversely, lung deflation below EELV reduces UA size27,28 and produces marked increases in pharyngeal resistance.6 A study by Stanchina and colleagues29 showed greater airway collapsibility (~1.1 cm H2O increase in UA critical closing pressure, [PCRIT]) in healthy-weight individuals following a decrease in lung volume of ~0.6 L via positive extrathoracic pressure. Using an iron lung to manipulate lung volume in a group of obese OSA patients, the level of CPAP required to abolish flow limitation increased from ~12 to ~17 cm H2O following a ~0.6 L reduction in lung volume, and was reduced from ~12 to ~4 cm H2O with ~0.4 L lung inflation.7 With similar lung inflation via negative extrathoracic pressure (~0.7 L), Tagaito and colleagues30 found a reduced PCRIT in the order of ~1.2 cm H2O in anesthetized and paralyzed obese OSA patients. In the absence of neuromuscular influences, these latter findings strongly support mechanical effects of lung volume change on passive UA properties.
Lung volume effects on UA function may be mediated primarily by changes in caudal tracheal traction. This “tracheal tug” is an axial force pulling on the airway via negative intrathoracic pressure and movements of the trachea and interconnected mediastinal structures that move along with the diaphragm.31 While end-expiratory diaphragm position was not directly assessed in the present study, the observed changes in chest/abdominal AP dimensions and volumes, PDI and PES are consistent with cranial diaphragm displacement and would be expected to reduce axial tension transmitted to the UA via intrathoracic structures. Classic studies in anesthetized dogs,4 clearly show tracheal tug is a major factor promoting UA patency. Severing the esophagus and trachea caused marked pharyngeal airway narrowing or collapse. Axial tension exerted on the caudal end of the severed trachea was very strongly related to negative intrathoracic pressure and diaphragm descent. Similar findings have subsequently been reported in cats3 and most recently in pigs.32 Caudal tracheal traction has also been shown to decrease UA collapsibility and extraluminal tissue pressure in anesthetized and tracheostomized rabbits.33 These animal studies suggest mechanical interdependence between the diaphragm and intrathoracic and UA structures is critically important for maintaining pharyngeal patency.
While the mechanisms of lung volume influence on UA size and function remain unclear, thoracoabdominal distortion, through interventions specifically designed to alter lung volume or through abdominal compression performed in this study, appear likely to operate via similar mechanisms; predominantly via mechanical coupling between the upper and lower airway structures. Nevertheless, abdominal compression/mass loading and extrathoracic pressure induced changes in lung volume may have somewhat different effects on diaphragm position and UA function. Since the abdominal compartment is less compressible than the chest, extrathoracic pressure changes in an iron lung will tend to influence thoracic more than abdominal compartment volume and may have variable effects on diaphragm position, pleural pressure and tracheal traction. In contrast, raised IAP with abdominal compression or mass loading will likely produce predominantly diaphragm ascent, either with or without (i.e., counterbalanced by mass loading of the chest) concomitant chest expansion.
While abdominal compression led to a significant increase in UACP, there was no change in RUA. Diaphragm ascent and UA shortening, while potentially reducing UA wall tension and increasing UA collapsibility, may have relatively little net impact on overall UA size and consequently RUA. In support of this view, Rowley and colleagues34 found no change in RUA despite a ~2 cm H2O decrease in UA collapsibility with 1 cm tracheal stretch in anesthetized cats.
Increased central venous volume and/or edema surrounding the airway may also contribute to increased UA collapsibility. Chui and colleagues35 found an increase in neck circumference and a 102% rise in pharyngeal resistance following expulsion of leg fluid volume via lower body positive pressure (~55 cm H2O). Two subsequent studies by the same group showed similar levels of lower body positive pressure produced a decrease in UA area by ~10%,36 while UA collapsibility increased by ~20% during wakefulness.37 Reduced lung volume did not appear to explain these findings suggesting that fluid displacement into the neck was the primary cause of these effects. In addition, a recent study38 found that AHI was strongly correlated with changes in overnight leg fluid volume. Increased IAP (maximum of ~27 cm H2O) via CO2 insufflation into the peritoneal cavity has also been shown to displace blood from the abdominal compartment into the chest39 and to result in enlargement of left end-diastolic ventricular volumes40 indicative of venous congestion. Consequently, while the IAP achieved in this study was lower than in these previous studies, displacement of blood into the chest and neck cannot be discounted as a potential mechanism contributing to increased UA collapsibility with abdominal compression.
Abdominal compression significantly altered breathing patterns in these patients indicating respiratory compensatory mechanisms with abdominal loading. Both VT and TE significantly decreased whilst FB increased. On the other hand, TI and VI were unchanged, while PIF tended to be lower following cuff inflation. Similar changes in breathing pattern have been observed in obese compared to healthy weight individuals,41 and with externally applied elastic loads during wakefulness.42,43 Given abdominal compression impairs diaphragm descent, it may have similar effects to externally applied inspiratory elastic loads. While there is limited data available in sleep, ventilation has been shown to fall acutely with an elastic load, followed by partial ventilatory recovery in NREM sleep in three healthy-weight individuals.44 The changes in breathing pattern following prolonged abdominal compression during sleep in this study likely reflect similar compensatory responses.
Methodological Limitations
There are several methodological limitations that warrant brief discussion. Firstly, diaphragm/trachea displacement following abdominal compression could not be directly measured and inferences regarding diaphragm and tracheal ascent are somewhat speculative, based on changes in other interrelated measures. Imaging methods such as radiography, CT scanning, and ultrasonography, potentially useful for more direct measurements of intrathoracic changes with abdominal compression are difficult during sleep. Based on a ~3 cm decrease in abdominal AP dimension and an increase in PGA of ~23 cm H2O, Reid et al.26 inferred a 3.5-cm rise in end-expiratory diaphragm position following upright water immersion to the neck. Tokics and colleagues45 observed ~1.2 cm cranial diaphragm displacement following thoracoabdominal restriction. Following CO2 insufflation of the abdomen, 1.9 cm cephalad movements of the diaphragm46 and 1.4 cm migration of the carina towards the tip of an endotracheal tube47 have been observed. Consequently, abdominal compression almost certainly produced ascent of the diaphragm and intrathoracic structures.
The current study utilized a mask occlusion protocol16,48 rather than the more widely used PCRIT technique to assess UA collapsibility. This was chosen for two reasons: (1) it allows for more rapid and repeated measurements over a shorter time, and (2) pressure drops during PCRIT determination inevitably produce systematic reductions in lung volume that may further impact on UA collapsibility that the technique is designed to measure. With inspiratory mask occlusion, lung volume is effectively maintained at the same initial pressure. Our circuit did not occlude expiration such that some expiratory lung volume loss was possible if UA patency returned with increasing expiratory drive. However, UA closure tended to occur within 1-3 breaths following mask occlusions, before any substantial increase in ventilatory drive necessary to overcome the majority of obstructive events and therefore produce expiratory volume loss.
Magnetometer coils allow measurement of lung volume change such that further studies using body plethysmography or helium dilution measurements would be useful to assess absolute lung volume with and without abdominal loading in obese and non-obese individuals. In addition, the calibration constant K used to estimate lung volume change is likely influenced by posture.49 However, all measurements were restricted to the supine position and cuff inflated versus deflated conditions continued throughout the study such that overnight changes in K are unlikely to have importantly influenced lung volume comparisons between conditions.
The effects of acute abdominal compression may well differ from chronic mechanical effects of abdominal obesity on UA function. Increasing IAP with abdominal obesity occurs over a long time frame, potentially has different mechanical effects depending on visceral versus subcutaneous fat deposition and may be associated with other effects that may independently influence UA function, such as UA and thoracic adiposity, hormonal, metabolic and hemodynamic effects and potentially progressive respiratory compensatory changes. Nevertheless, our findings of increased airway collapsibility following acute abdominal compression demonstrate the existence of compressive effects that may well still operate with chronic natural abdominal compression with obesity.
Clinical Significance
The effect of a ~0.5 cm H2O increase in UACP on OSA severity remains unclear. However, studies investigating the relationship between PCRIT and AHI50,51 suggest that changes of this order may well be important. Sforza et al.50 found a low (r = 0.23) but significant correlation, with small differences in PCRIT associated with substantial increases in AHI in 106 patients. In 25 OSA patients, Wellman and colleagues51 demonstrated a tighter relationship (r = 0.66), with an increase in AHI of ~10 events/h with a ~0.5 cm H2O increase in PCRIT. In a study by Gold et al.,52 the average PCRIT in mild/moderate OSA patients (AHI ≥ 10 and < 40 events/h) and moderate/severe OSA patients (AHI ≥ 40 events/h) was approximately −1.6 and 2.4 cm H2O respectively, although there was considerable overlap in PCRIT between the two groups. Given the importance of UA collapsibility in OSA and relatively steep relationships between PCRIT and AHI across patients, a ~0.5 cm H2O within-patient increase in UACP appears likely to significantly impact OSA severity. Further studies are needed to establish if this is the case and the magnitude of the effect.
Obesity and male gender are well known major risk factors for OSA.1,2 However, the mechanisms via which obesity and male gender influence UA in sleep remain poorly defined. While experimental abdominal compression in the current study may not directly simulate the effects of obesity per se, increased IAP appears the most likely dominant factor underpinning increased UA collapsibility in this study. Of all available variables influenced by obesity, including BMI, waist-hip ratio, and waist and hip circumference, only PDI emerged as a significant predictor of UA collapsibility, accounting for an impressive ~73% of the variance in UACP. Consequently, compressive effects of obesity and the male propensity to an abdominal pattern of fat distribution could therefore largely underpin strong associations between obesity/male gender and OSA.
While increased neck circumference/fat deposition are known predictors of OSA severity53,54 and could directly affect UA function, they may also co-vary with other potentially more important measures of central obesity. In the Wisconsin Sleep Cohort,2 men were three times as likely as women to have OSA and six times as likely after adjusting for BMI. However, male gender was no longer predictive of OSA following adjustment for waist-hip ratio. These data are consistent with a primary underlying role of central adiposity as a cause of OSA in most patients and the gender difference in OSA prevalence. In smaller samples, observations that measures of central adiposity including waist circumference and visceral fat consistently emerge as stronger predictors of OSA severity than BMI are consistent with this view.55,56 Reports of increased snoring and OSA in the third trimester of pregnancy, despite increased ventilatory drive from hyperprogesteronemia, are also consistent with abdominal compressive forces importantly influencing UA function in sleep.57
Summary
This is the first study to show a direct effect of abdominal compression on UA collapsibility during sleep. The results support and extend previous work showing detrimental effects of reduced lung volume on UA patency and suggest that increased IAP with obesity may negatively impact on UA function. While IAP appears the most likely dominant factor underpinning these effects, further studies are needed to better elucidate the mechanisms behind these findings.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. McEvoy has received research support from Philips Respironics, ResMed, Fisher – Paykel, and Apnex and the use of equipment from Respironics, ResMed, and SomnoMed. Dr. Catcheside has received research support from Apnex and the use of equipment from Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are extremely grateful to Amanda McKenna for her valuable assistance in scoring arousals and staging the sleep studies. David Schembri and the Respiratory Function Unit staff, Repatriation General Hospital provided helpful assistance with lung function measurements.
Where Work Was Performed: Adelaide Institute for Sleep Health, Repatriation General Hospital
Support: National Health and Medical Research Council of Australia (grant number 138403)
ABBREVIATIONS
- OSA
obstructive sleep apnea
- UA
upper airway
- IAP
intra-abdominal pressure
- EELV
end-expiratory lung volume
- RUA
upper airway airflow resistance
- BMI
body mass index
- AHI
apnea-hypopnea index
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- WC
waist circumference
- HC
hip circumference
- WHR
waist-to-hip ratio
- PES
esophageal pressure
- PGA
gastric pressure
- PEPI
epiglottic pressure
- PMASK
mask pressure
- PDI
transdiaphragmatic pressure
- AP
anterior-posterior
- PCUFF
cuff pressure
- UACP
upper airway closing pressure
- ΔAPCHEST
chest excursion
- ΔAPABDO
abdominal excursion
- VT
tidal volume
- TI
inspiratory time
- TE
expiratory time
- TTOT
total breath time
- FB
breathing frequency
- VI
minute ventilation
- PIF
peak inspiratory flow
- PCRIT
upper airway critical closing pressure
- CPAP
continuous positive airway pressure
REFERENCES
- 1.Dixon JB, Schachter LM, O'Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese. Chest. 2003;123:1134–41. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–8. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 4.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 5.Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol. 1994;77:840–4. doi: 10.1152/jappl.1994.77.2.840. [DOI] [PubMed] [Google Scholar]
- 6.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–64. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 7.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Care Med. 2005;172:114–7. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol. 1984;57:1319–22. doi: 10.1152/jappl.1984.57.5.1319. [DOI] [PubMed] [Google Scholar]
- 9.Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol. 1990;68:2034–41. doi: 10.1152/jappl.1990.68.5.2034. [DOI] [PubMed] [Google Scholar]
- 10.Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–60. doi: 10.1097/00000539-199809000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Hedenstierna G, Strandberg A, Brismar B, Lundquist H, Svensson L, Tokics L. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology. 1985;62:247–54. doi: 10.1097/00000542-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–8. [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A, editors. UCLA, Los Angeles: Brain Information Service/ Brain Research Institute; 1968. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 14.ASDA. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 15.Hilditch CJ, McEvoy RD, George KE, et al. Upper airway surface tension but not upper airway collapsibility is elevated in primary Sjogren's syndrome. Sleep. 2008;31:367–74. doi: 10.1093/sleep/31.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–7. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 17.Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Care Med. 1997;155:199–204. doi: 10.1164/ajrccm.155.1.9001312. [DOI] [PubMed] [Google Scholar]
- 18.Eckert DJ, Catcheside PG, Stadler DL, McDonald R, Hlavac MC, McEvoy RD. Acute sustained hypoxia suppresses the cough reflex in healthy subjects. Am. J. Respir. Care Med. 2005;173:506–11. doi: 10.1164/rccm.200509-1455OC. [DOI] [PubMed] [Google Scholar]
- 19.Sackner MA, Watson H, Belsito AS, et al. Calibration of respiratory inductive plethysmongraph during natural breathing. J Appl Physiol. 1989;66:410–20. doi: 10.1152/jappl.1989.66.1.410. [DOI] [PubMed] [Google Scholar]
- 20.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respit Crit Care Med. 2003;168:1512–9. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 21.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005;98:512–7. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 22.Loring SH, Mead J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. J Appl Physiol. 1982;53:756–60. doi: 10.1152/jappl.1982.53.3.756. [DOI] [PubMed] [Google Scholar]
- 23.Citerio G, Vascotto E, Villa F, Celotti S, Pesenti A. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: A prospective study. Crit Care Med. 2001;29:1466–71. doi: 10.1097/00003246-200107000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Pelosi P, Foti G, Cereda M, Vicardi P, Gattinoni L. Effects of carbon dioxide insufflation for laparoscopic cholecystectomy on the respiratory system. Anaesthesia. 1996;51:744–9. doi: 10.1111/j.1365-2044.1996.tb07888.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J. Appl. Physiol. 1964;19:959–66. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- 26.Reid MB, Loring SH, Banzett RB, Mead J. Passive mechanics of upright human chest wall during immersion from hips to neck. J Appl Physiol. 1986;60:1561–70. doi: 10.1152/jappl.1986.60.5.1561. [DOI] [PubMed] [Google Scholar]
- 27.Burger CD, Stanson AW, Daniels BK, Sheedy PF, Shepard JW. Fast-CT evaluation of the effect of lung volume on upper airway size and function in normal men. Am Rev Resp Dis. 1992;146:335–9. doi: 10.1164/ajrccm/146.2.335. [DOI] [PubMed] [Google Scholar]
- 28.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Resp Dis. 1984;130:175–8. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 29.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 30.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103:1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 31.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol. 1991;70:1328–36. doi: 10.1152/jappl.1991.70.3.1328. [DOI] [PubMed] [Google Scholar]
- 32.Tuck SA, Remmers JE. Mechanical properties of the passive pharynx in Vietnamese pot-bellied pigs. I. Statics. J Appl Physiol. 2002;92:2229–35. doi: 10.1152/japplphysiol.00761.2001. [DOI] [PubMed] [Google Scholar]
- 33.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TA. Tracheal Traction Effects on Upper Airway Patency in Rabbits: The Role of Tissue Pressure. Sleep. 2006;30:179–86. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Williams BC, Smith PL, Schwartz AR. Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. Am J Respir Care Med. 1997;156:515–21. doi: 10.1164/ajrccm.156.2.9607115. [DOI] [PubMed] [Google Scholar]
- 35.Chui KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Care Med. 2006;174:1378–83. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 36.Shiota S, Ryan CM, Chiu KL, et al. Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax. 2007;62:868–72. doi: 10.1136/thx.2006.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su MC, Chiu KL, Ruttanaumpawan P, et al. Differences in upper airway collapsibility during wakefulness between men and women in response to lower body positive pressure. Clin Sci (Lond) 2008 doi: 10.1042/CS20080321. doi 10.1042/CS20080321. [DOI] [PubMed] [Google Scholar]
- 38.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and Obstructive Sleep Apnea in nonobese men. Am J Respir Care Med. 2009;179:241–6. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
- 39.Hofer CK, Zalunardo MP, Klaghofer R, Spahr T, Pasch T, Zollinger A. Changes in intrathoracic blood volume associated with pneumoperitoneum and positioning. Acta Anaesthesiol Scand. 2002;46:303–8. doi: 10.1034/j.1399-6576.2002.t01-1-460313.x. [DOI] [PubMed] [Google Scholar]
- 40.Branche PE, Duperret SL, Sagnard PE, Boulez JL, Petit PL, Viale JP. Left ventricular loading modifications induced by pneumoperitoneum: a time course echocardiographic study. Anesth Analg. 1998;86:482–7. doi: 10.1097/00000539-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol. 1983;55:1269–76. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- 42.Bland S, Lazerou L, Dyck G, Cherniack RM. The Influence Of The “Chest Wall” On Resiratory Rate And Depth. Resp Physiol. 1967;3:47–54. doi: 10.1016/0034-5687(67)90023-0. [DOI] [PubMed] [Google Scholar]
- 43.Agostino E, D'Angelo E, Piolini M. Breathing pattern in men during inspiratory elastic loads. Resp Physiol. 1978;34:279–93. doi: 10.1016/0034-5687(78)90034-8. [DOI] [PubMed] [Google Scholar]
- 44.Wilson PA, Skatrud JB, Dempsey JA. Effects of slow wave sleep on ventilatory compensation to inspiratory elastic loading. Resp Physiol. 1984;55:103–20. doi: 10.1016/0034-5687(84)90120-8. [DOI] [PubMed] [Google Scholar]
- 45.Tokics L, Hedenstierna G, Brismar B, Strandberg A, Lundquist H. Thoracoabdominal restriction in supine men: CT and lung function measurements. J Appl Physiol. 1988;64:599–604. doi: 10.1152/jappl.1988.64.2.599. [DOI] [PubMed] [Google Scholar]
- 46.Andersson LE, Baath M, Thorne A, Aspelin P, Odeberg-Wernerman S. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computer tomography. Anesthesiolgy. 2005;102:293–9. doi: 10.1097/00000542-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Lobato EB, Paige GB, Brown MM, Bennett B, Davis JD. Pneumoperitoneum as a risk factor for endobronchial intubation during laparoscopic gynecologic surgery. Anesth Analg. 1998;86:301–3. doi: 10.1097/00000539-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Ng AT, Gotsopoulos H, Qian J, Cistulli PA. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am J Respir Care Med. 2003;168:238–41. doi: 10.1164/rccm.200211-1275OC. [DOI] [PubMed] [Google Scholar]
- 49.Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol. 1995;79:2169–76. doi: 10.1152/jappl.1995.79.6.2169. [DOI] [PubMed] [Google Scholar]
- 50.Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Care Med. 1999;159:149–57. doi: 10.1164/ajrccm.159.1.9804140. [DOI] [PubMed] [Google Scholar]
- 51.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respit Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 53.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5:377–81. [PubMed] [Google Scholar]
- 54.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Care Med. 1995;152:1673–89. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 55.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 56.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 57.Edwards N, Blyton DM, Hennessey A, Sullivan CE. Severity of sleep-disordered breathing improves following parturition. Sleep. 2005;28:737–41. doi: 10.1093/sleep/28.6.737. [DOI] [PubMed] [Google Scholar]